Abstract
Recent studies on non-structural carbohydrate (NSC) reserves in trees focused on xylem NSC reserves, while still little is known about changes in phloem carbohydrate pools, where NSC charging might be significantly different. To gain insight on NSC dynamics in xylem and phloem, we monitored NSC concentrations in stems and roots of Pinus cembra and Larix decidua growing at the alpine timberline throughout 2011. Species-specific differences affected tree phenology and carbon allocation in the course of the year. After a delayed start in spring, NSC concentrations in Larix decidua were significantly higher in all sampled tissues from August until end of growing season. In both species NSC concentrations were five to seven times higher in phloem than in xylem. However, significant correlations between xylem and phloem starch content found for both species indicate a close linkage between long term carbon reserves in both tissues. In Larix decidua also free sugar concentrations in xylem and phloem were significantly correlated throughout the year, while missing correlations between xylem and phloem free sugar pools in Pinus cembra indicate a decline of phloem soluble carbohydrate pools during periods of high sink demand.
Keywords: carbohydrates, Central Alps, Larix decidua, NSC, phloem, Pinus cembra, storage, xylem
Introduction
Non-structural carbohydrate (NSC) reserves are considered a measure for tree carbon surplus or shortage (Körner 2003) and are used as an indicator for vitality and sink source balance of trees growing in extreme environments. High NSC concentration found in trees growing at the alpine treeline (cf. Hoch et al. 2002; Hoch and Körner 2003) support the growth limitation hypothesis (Körner 1998), while NSC starvation is discussed as mechanism for the worldwide phenomenon of drought-related tree mortality (e.g. McDowell et al. 2008; Sala et al. 2012).
In the temperate zone NSC reserves play an important role in the annual cycle of trees. Carbohydrate pools fuel respiration during winter and in periods of low photosynthetic activity, they initiate tree growth after dormancy, but also provide energy for adaptive responses to pathogen attacks and soil water deficits (Lyr 1992; Barbaroux et al. 2003). Allocation of tree NSC reserves during the year is determined by phenological changes (Hoch et al. 2002, 2003; Gruber et al. 2012). Therefore, repeated evaluation of NSC concentrations throughout the year is necessary, to gain insight into tree carbon supply status (Woodruff and Meinzer 2011). However, during the last years studies focused on xylem NSC reserves, whereas only little attention has been put on the NSC content of phloem during the growing season. Giovannelli et al. (2011) sampled cambial region and differentiating phloem in Populus × canadensis (Mönch) and found higher concentrations than in xylem, as did Landhäuser and Lieffers (2003) and Anderegg et al. (2012), when measuring NSC content in bark of Populus tremuloides (Michx.).
The phloem plays a key role in long distance carbohydrate and signal transport in trees and during the last years much effort has been made for better understanding the complex mechanisms linking source and sink (for review see van Bel 2003; Minchin and Lacointe 2004; Turgeon and Wolf 2009). While sieve cell and sieve element/companion cell complexes are the major pathway for osmotically driven transport (Münch 1930), phloem parenchyma fulfil functions in defence, wound healing (cf. Franceschi et al. 2005;; Nagy et al. 2004) and storage (Zimmermann and Brown 1971; Evert 2006). Carbohydrates are also transported via transpiration stream. Xylem transport of carbohydrates contributed for up to 28% of the total C budget of leaves in Populus canescens (AITON.) SM. (Mayrhofer et. al., 2004) and the significance of xylem-carbohydrate transport was also proofed in labelling experiments (Kreuzwieser et al., 2002, Ghirardo et al., 2011). It’s established that there is exchange and lateral transport between phloem and xylem ray parenchyma (van Bell 1990; Zwieniecki et al. 2004), and a within plant cycling of carbohydrates via phloem and xylem transport as hypothesized by Heizmann et al. (2001) could be another effective pathway linking xylem and phloem carbohydrate reserves. However, still little is known about changes in phloem carbohydrate reserves throughout the year and carbohydrate charging might be significantly different in phloem than in xylem. To gain insight on NSC dynamics in xylem and phloem, we monitored NSC concentrations in stems and roots of two conifer species growing at the alpine timberline throughout the year. We hypothesized, that (i) there is a close correlation between long term carbon reserves (starch) in phloem and xylem in both species and organs (stem and root), whereas (ii) differences in turnover rates will lead to missing correlation between phloem vs. xylem free sugar content.
Materials and Methods
Study site and climatic conditions
The study was conducted at the timberline (1950 m a.s.l.) of Mt. Patscherkofel (2246 m a.s.l.) near Innsbruck, in western Austria (47.120 N and 11.270 E). Mt. Patscherkofel is located in the Central Austrian Alps within an inner-alpine dry zone. During the period 1967–2004, mean annual precipitation at the top of Mt. Patscherkofel was 890 mm with a maximum during summer (June–August: 357 mm) and minimum in winter (December–February: 147 mm). Air and soil temperatures at 5 cm soil depths (CS 215 and T107 temperature probes, Campbell Scientific, Shepshed; UK) were monitored at the sampling site. Data were collected automatically at 30-min intervals using a CR 10× Datalogger (Campbell Scientific, Shepshed; UK). Daily precipitation was recorded at the meteorological station on top of Mt. Patscherkofel. The geology at the Mt. Patscherkofel is dominated by gneisses and schists. According to the World Base for Soil Resources (FAO 1998), the soil at the study site is classified as a haplic podzol.
Sampling for NSC analysis
The study was conducted on Pinus cembra (L.) and Larix decidua (Mill.). Pinus cembra is the dominant conifer species at the timberline of the eastern central Alps, whereas Larix decidua is scattered at some sites. Samples were taken from six pre-selected dominant trees from each species (Tab. 1), on six sampling dates (March 30, May 23, June 27, August 2, September 13, November 3) in 2011. At each sampling date, the following tissues were collected: (i) sapwood of stem xylem, (ii) stem cambium and phloem (hereafter called stem phloem), (iii) xylem from coarse roots and (iv) root cambium and phloem (hereafter called root phloem). Samples (cubes with a side length of approximately 1 cm) were cut out of the trees using a sharp chisel. Stem xylem was sampled at breast height at the north facing site of the trees following a spiral trajectory up the stem. A distance of c. 4 cm in tangential and longitudinal direction was kept to avoid lateral influence of wound reactions on adjacent sampling positions. Root tissue was sampled from 5 to 10 cm soil depths, 0.5 to 1 m in distance from the stem. All sampling spots were sealed with a tree wound dressing to minimize effects on the trees. Samples were always collected around noon to minimise possible effects of diurnal fluctuations. The sampled material was stored in a cool box immediately after collection. Within 3 h, enzymes in the samples were denatured by heating the samples in a microwave at 600 W for 90 s (Hoch et al. 2002; Hoch and Körner 2003). The sampled tissues were carefully separated under the reflecting microscope using a scalpel. Due to suberisation and changes in cell structure the outer dead bark showed a darker pigmentation than the living inner bark. This change in colour was used to separate the tissues. Afterwards, the sampled tissues were dried to weight constancy at 60°C, ground to powder and stored dry until they were analysed. Mean radial width of living phloem was calculated from four micro-cores (diameter 2.5 mm) taken at different expositions of each sampling tree (Tab. 1).
Table 1.
Characteristics of Larix decidua and Pinus cembra trees selected for sampling (n=6) throughout 2011. Mean values ± standard deviations; (RW = ring width).
| Diameter1 (cm) | Age2 (yr) | RW3 (mm) | Sapwood width (mm) | Phloem width4 (mm) | |
|---|---|---|---|---|---|
| Larix decidua | 22.0 ± 1.6 | 56.8 ± 11.3 | 1.76 ± 0.47 | 29.2 ± 6.5 | 2.73 ± 0.36 |
| Pinus cembra | 30.5 ± 8.0 | 52.6 ± 8.1 | 3.02 ± 0.78 | 52.8 ± 19.9 | 2.34 ± 0.37 |
Range of tree diameter measured at sampling height.
Number of counted tree rings from increment cores taken at c.1.3 m.
Mean ring width during the period 2000-2010.
Width of phloem in March 2011
Analysis of NSC
For binding plant phenols, 0.5 mg polyvinylpyrrolidone was added to approximately 10 mg of finely ground plant material. Soluble carbohydrates were extracted from the samples twice in 80% (v/v) acetone for 15 min at 50°C. After vaporising the acetone, the residue of the soluble fraction was resolved in distilled water. After resolving, glucose was converted to glucose-6-phosphate catalysed by hexokinase. The concentration of glucose was determined photometrical at 340 nm, as NADPH+ H+ formation during enzymatic conversion of glucose-6-phosphate to gluconate-6-phosphate by the enzyme glucose-6- phosphate dehydrogenase. Aliquots of the resolved extract were treated with hexokinase and phosphoglucose isomerase, to convert fructose to fructose-6-phosphate and subsequently to glucose-6- phosphate, which was then measured as described above. Sucrose was hydrolysed by the enzyme β-fructosidase to glucose and fructose. The sucrose content was calculated from the difference of glucose concentrations before and after enzymatic inversion. For starch measurements, extraction was carried out by incubating the insoluble fraction with hydrochloric acid for 2 h at 60°C. After pH adjustment (pH 4.6), amyloglucosidase was used to hydrolyse starch to glucose, which was subsequently measured as described above. The photometric analyses were conducted using test solutions from Boehringer Mannheim (Mannheim, Germany).
Radial growth
For tracing the radial stem growth electronic band dendrometers (DMS dendrometer type D-6 with measuring amplifier t8.MV, UMS, Munich, Germany) had been installed at breast height at two trees per selected species. Dead outermost layers (periderm) of the bark were slightly removed to reduce the influence of hygroscopic swelling and shrinkage of the bark and to ensure close contact with the stem (cf. Zweifel and Häsler 2001). Data were recorded with a DT-6 data logger (Delta-T Devices Ltd., Cambridge, U.K.) every 30 minutes. Daily means were calculated by averaging all daily measurements (48 values/day). Dendrometer traces were set to zero on April 16, when after rehydration (first soil thawing occurred on April 8, Fig. 1), soil stayed permanently unfrozen. Time of maximum radial growth was determined by applying Gompertz modelled growth functions (e.g. Zeide 1993; Deslauriers and Morin 2005).
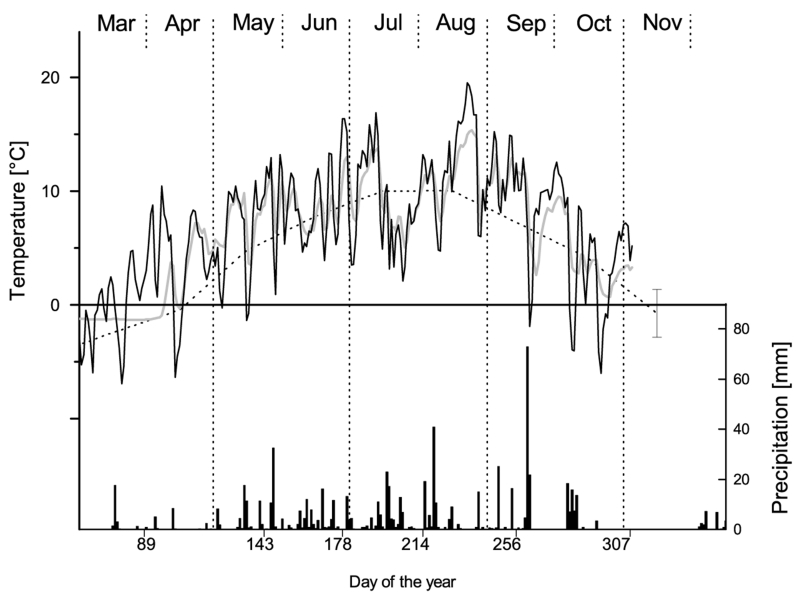
Fig. 1.
Mean daily air temperature (black line), mean daily soil temperature (gray line) and daily precipitation sums (bars) during the growing season 2011 and long term mean monthly air temperatures (1969 -2004) (dotted line), within the study area.
Statistical analyses
Differences in NSC concentrations between sampling dates were tested for significance by applying post-hoc multiple comparison (Tukey HSD test). Differences in NSC concentrations in respective tissues of Larix decidua and Pinus cembra during the year were tested for significance using repeated measures analysis applying a Bonferroni correction. The correlations between NSC concentrations in phloem and xylem were tested for significance by calculating Pearson correlation coefficients. All tests were performed using SPSS 18 (IBM, New York, NY, USA).
Results
Environmental variables during growing season 2011
At the study site, climate during growing season 2011 was characterised by exceptional high temperatures (Fig. 1). From April to November mean air temperatures exceeded long term means (LTM) by more than 4°C. Soil (at 5 cm soil depths) was unfreezing early in spring (permanently unfrozen from April 16) and was not freezing again till begin of December. Spring precipitation was rather low (March and April 40.4 mm, LTM 125.2 mm), while from May to October precipitation was around LTM. Even though there was no precipitation from October 19 till beginning of December, we could not detect soil dryness at the study site during this period.
Radial growth
Radial growth in Pinus cembra started around April 20, more than two weeks earlier than in Larix decidua (around May 7) (Fig. 2C and 3C). Maximum increment growth was reached on May 23 and June 17 in Pinus cembra and Larix decidua, respectively. Pinus cembra had produced 90% of annual increment on July 1, Larix decidua one month later on July 31. Ring width in the sampled trees during the last ten years was significantly higher in Pinus cembra than in Larix decidua (Tab. 1).
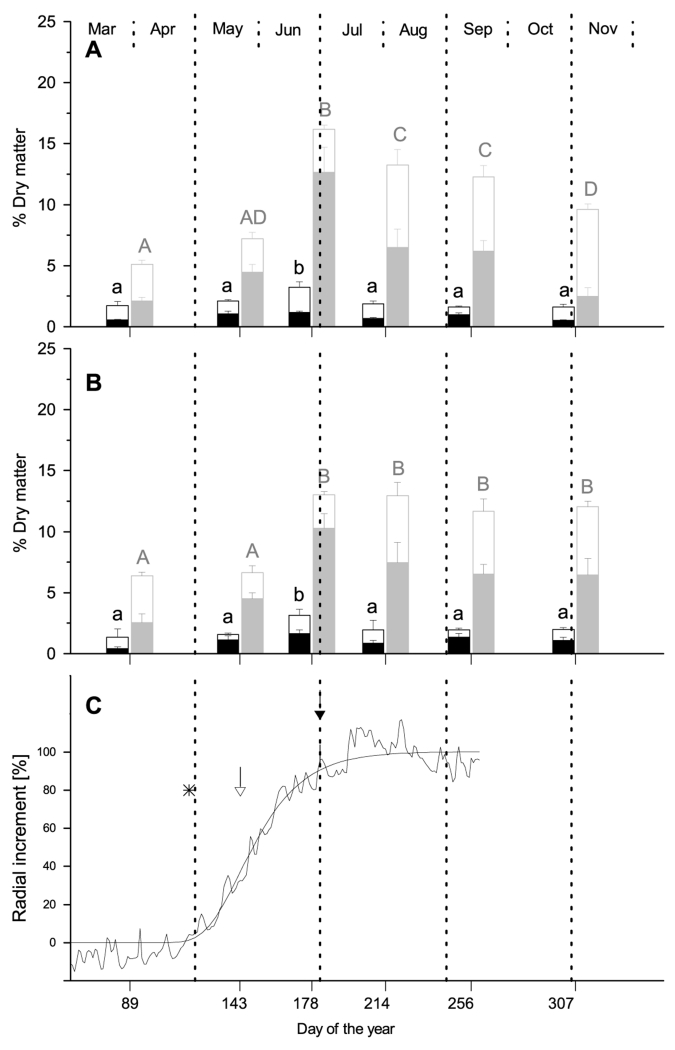
Fig. 2.
Mean concentrations of free sugars (open sections of bars) and starch (filled sections of bars) in xylem (black bars) and phloem (gray bars) of stems (A) and coarse roots (B) of Pinus cembra, at six sampling dates during 2011. Different letters indicate significantly different NSC concentrations among sampling dates (P < 0.05, lowercase letters: xylem; upper case letters: phloem). (C) Dynamics of xylem growth in 2011 modelled using the Gompertz function. The open arrow denotes time of maximal radial growth. The filled arrow denotes the time when 90% of increment were produced. Asterisks indicate time of bud break.
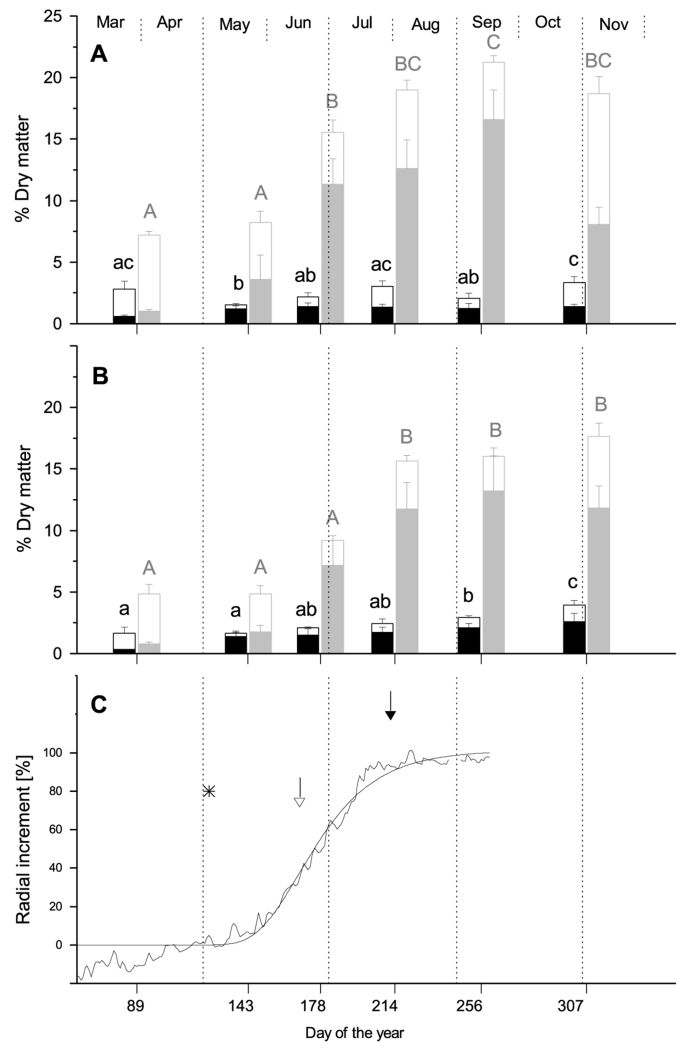
Fig. 3.
Mean concentrations of free sugars (open sections of bars) and starch (filled sections of bars) in xylem (black bars) and phloem (gray bars) of stems (A) and coarse roots (B) of Larix decidua, at six sampling dates during 2011. Different letters indicate significantly different NSC concentrations among sampling dates (P < 0.05, lowercase letters: xylem, upper case letters: phloem). (C) Dynamics of xylem growth in 2011 modelled using the Gompertz function. The open arrow denotes time of maximal radial growth. The filled arrow denotes the time when 90% of increment were produced. Asterisks indicate time of bud break.
NSC concentrations in Larix decidua and Pinus cembra
Concentrations of free sugars (i.e. sucrose, glucose and fructose) and starch changed significantly during the year in all sampled tissues (p< 0,001 for all tissues, Figs. 2 and 3). In March NSC reserves in stem xylem and phloem were significantly higher in Larix decidua than in Pinus cembra (2.8 and 1.7 % of dry matter in xylem and 7.2 and 5.1% in phloem of Larix decidua and Pinus cembra, respectively), while in roots there were no significant differences. At this time, all sampled tissues show high fractions of free sugars. In May NSC concentrations had not increased significantly in any of the sampled tissues in both species. At the end of June NSC values peaked in Pinus cembra in all sampled tissues (3.2 and 3.1% of dry matter in stem and root xylem; 16.2 and 13.0 in stem and root phloem). At this time NSC concentrations in Pinus cembra where significantly higher than in Larix decidua, except in stem phloem where values were at same level. After June NSC concentrations in Pinus cembra were decreasing while in Larix decidua values kept rising. In Larix decidua NSC concentrations in stem phloem were highest in September (21.2 % of dry matter), while in all other tissues maximum concentrations were reached at beginning of November (3.3 and 4.0% of dry matter in xylem of stem and roots; and 17.6 % in root phloem). From August till November NSC concentrations in Larix decidua were significantly higher in all sampled tissues (Tab. 2). This can mainly be assigned to accumulation of starch in tissues of Larix decidua.
Table 2.
P-values from repeated measures analyses for effects of species (Pinus cembra versus Larix decidua) on the NSC concentrations in stem and root, xylem and phloem. Mean carbohydrate concentrations were higher in Larix decidua, except mean concentrations of free sugars in root tissues (gray fields); (ns = not significant).
| March - November |
August - November |
||||
|---|---|---|---|---|---|
| xylem | phloem | xylem | phloem | ||
| stem | free sugars | ns | <0.001 | 0.001 | ns |
| starch | 0.001 | <0.001 | <0.001 | <0.001 | |
| NSC | 0.003 | <0.001 | 0.001 | <0.001 | |
|
| |||||
| root | free sugars | ns | ns | ns | <0.001 |
|
|
|||||
| starch | ns | ns | 0.001 | <0.001 | |
| NSC | ns | ns | 0.001 | 0.004 | |
Relation between xylem and phloem NSC concentrations
NSC concentrations were significantly higher in phloem than in xylem, in both studied species and at all sampling dates (Figs. 2 and 3). In Pinus cembra NSC concentration in stem and root phloem was about five times higher than in xylem throughout the year. In Larix decidua average phloem content was around six times higher in stems and five times higher in roots. Highest differences between phloem and xylem during the year were found for stem starch concentrations (7.0 and 7.4 times higher in Pinus cembra and Larix decidua, respectively), while differences in free sugars where considerable smaller in both species.
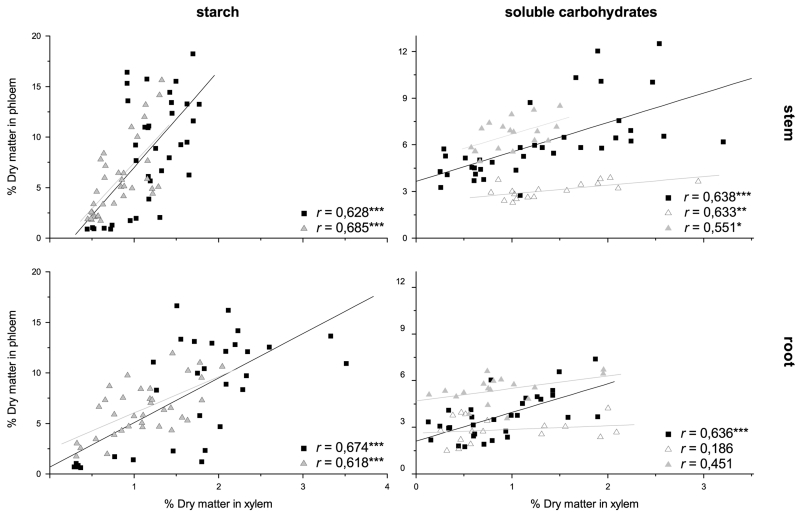
In both studied species xylem and phloem starch concentrations throughout the year were significantly correlated in stems and roots (Fig. 4). In Larix decidua also concentrations of free sugars in xylem and phloem were significantly correlated throughout the year, while in Pinus cembra no significant correlation over all sampling dates could be detected. However, xylem and phloem free sugar concentrations in stems were significantly correlated in the period from March to end of June and from beginning of August to November, whereas no significant correlation was detected for roots. The increased concentrations of free sugars in phloem of Pinus cembra after the third sampling date (Fig. 3), have their reason in higher glucose and fructose concentrations from forth sampling date till end of season (data not shown).
Fig. 4.
Pearson correlations between carbohydrate concentrations (free sugars and starch) in xylem and phloem for stems and roots of Pinus cembra (grey triangles) and Larix decidua (black squares). Regarding soluble carbohydrate content in Pinus cembra, correlations were separately calculated for the first and last three sampling dates (open and filled triangles, respectively). Significant correlations are denoted by asterisks (***p < 0.001; ** p < 0.01; * p < 0.05.)
Discussion
Species-specific differences and strategies affect carbon allocation and NSC reserves in the course of the year. In spring, Larix decidua has to set its priority on the production of new needles which starts three- to four weeks before radial growth commences (Moser et al. 2010). Accordingly, the onset of radial growth in spring is delayed for several weeks if compared with evergreen Pinus cembra. (Figs. 2c and 3c, Rossi et al. 2006). For deciduous Larix decidua, NSC reserves in spring are crucial for leaf development (Kagawa et al. 2006a), while in evergreen conifers like Pinus cembra, bud break and sprouting is supplied by the recent photosynthates (Hansen and Beck 1994), which makes them less dependent on preceding year’s reserves. However, the production of masses of thin layered photosynthetic very active needles, with up to twofold greater photosynthetic rates (Gower and Richards 1990), gives larch a competitive advantage over other conifer species.
Phenological changes trigger the allocation of tree NSC reserves during the year (Hoch et al. 2002, 2003; Gruber et al. 2012). When first samples were taken in March, both species were still dormant and high fractions of free sugars can be associated with winter freezing tolerance (e.g. Oleksyn et al. 2000; Hoch et al. 2002). High consumption during leaf flush and onset of radial growth in Larix decidua as well as maximal radial growth in Pinus cembra, impede an accumulation of NSCs till May (Kagawa 2006a, 2006b; Begum et al. 2010). The reduction of growth investments at the end of June led to an accumulation of carbohydrates and a peak of NSC concentrations in Pinus cembra (cf. Gruber et al. 2012), while in Larix decidua still active radial growth mitigated the increase of carbohydrate reserves. The high NSC concentrations in Larix decidua during late summer, especially the increase of starch concentrations, indicate an accumulation of reserves for winter and spring, while evergreen species like Pinus cembra appear to be less dependent on stored carbohydrates (Kuptz et al. 2011). However aside of higher photosynthetic carbon gain (Matyssek 1986; Wieser et al. 2009) also reduced above and below ground carbon investment (e.g. Gower and Richards 1990; Montague and Givnish 1996, Kajimoto et al. 1999) enable Larix decidua to establish this substantial reserves. The high percentage of free sugars in the above ground tissues in November indicates the transition to dormancy and development of winter freezing tolerance (e.g. Hoch et al. 2002; Gruber et al. 2012), while root growth might have continued until soil freezing (Oleksyn et al. 2000).
Only few studies have evaluated the carbohydrate concentrations in both phloem and xylem of trees. Landhäuser and Lieffers (2003), found about ten fold higher NSC concentrations in phloem (including bark) of Populus tremuloides and the significantly higher NSC concentrations in bark of Pinus cembra branches found by Li et al. (2002) confirm the high differences in NSC concentration between xylem and phloem measured in our study. These high phloem NSC concentrations can be ascribed to the high density of phloem storage tissue (Kramer and Kozlowski 1979), while in xylem NSC-storage is restricted to scattered ray and axial parenchyma. NSC concentrations in xylem differ strongly from the cambium towards the pith (Hoch et al. 2003) which makes an estimation of whole tree xylem carbohydrate reserves difficult. However, considering a trees total amount of xylem and phloem (cf. Tab. 1) xylem carbohydrate pools might still represent a major part of whole tree carbohydrate reserves.
As xylem and phloem long term carbon reserves follow the same whole tree source sink balance a coupling of xylem and phloem NSC reserves could be expected. Besides, there is active exchange between these tissues and parts of the carbohydrates accumulating in phloem are transported to xylem and vice versa (for review see van Bel 1990). The significant correlations between starch content in xylem and phloem found in both species during this study (Fig. 3) support this hypothesis of an active exchange and a close coupling of long term carbon reserves. Accordingly, Landhäuser and Lieffers (2003) found similar seasonal patterns of starch concentrations in stem xylem and phloem of Populus tremuloides.
A significant amount of phloem transported carbohydrates might be translocated to the transpiration stream via phloem-xylem exchange along the stem, to balance fluctuations of photosynthetic incomes (Heizmann 2001). However, the missing correlation for free sugar content in xylem and phloem of Pinus cembra throughout the year indicate differences in phloem and xylem carbohydrate loading and mobilisation. As the lack of correlation was caused by low glucose and fructose levels in phloem from first to third sampling date, we suppose that high sink demand during radial increment and respiration during winter reduced glucose and fructose reserves in phloem parenchyma located adjacent to the cambium, while sucrose levels were less affected due to fast turnover by phloem transport, where sucrose is the only carbohydrate translocated in conifers (Lyr et al. 1992). Nevertheless, a coupling of xylem and phloem free sugar pools is not only indicated by the significant correlations found for Larix decidua throughout the year, but also by significant correlations between free sugars in stem xylem and phloem of Pinus cembra calculated for the first three sampling dates and again after replenishing of free sugar pools.
The high starch reserves throughout the year confirm that both examined timberline species face no risk of carbohydrate depletion (e.g. Hoch et al. 2002). On the other hand, periods of high sink demand can lead to a decline of phloem soluble carbohydrate pools which can not be compensated by xylem-phloem exchange or carbohydrate transport. Today, vascular transport is thought to be associated with continuous and simultaneous leakage and reloading along the transport pathway (Minchin and Lacointe 2004; Turgeon 2010). Therefore, depending on season and phenology, only parts of the carbohydrates measured in phloem and xylem can be considered long term reserves while others may be in transition or stored in short-term storage pools with changing half-lives (Lehmaier et al. 2008). Labelling experiments (e.g. Hansen and Beck 1990, 1994; Kuptz et al. 2011) together with a determination of phloem and xylem sap carbohydrate contents (Heizmann et al. 2001) will be necessary to gain insight on the changing carbohydrate turnover rates in phloem and xylem during the year.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF Project No. P22836-B16, “Growth response of Pinus cembra to experimentally modified soil temperatures at the treeline”).
References
- Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, Field CB. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. PNAS. 2012;109:233–237. doi: 10.1073/pnas.1107891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaroux C, Bréda N, Dufrêne E. Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica) New Phytol. 2003;157:605–615. doi: 10.1046/j.1469-8137.2003.00681.x. [DOI] [PubMed] [Google Scholar]
- Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Changes in the localization and levels of starch and lipids in cambium and phloem during cambial reactivation by artificial heating of main stems of Cryptomeria japonica trees. Ann Bot. 2010;106:885–895. doi: 10.1093/aob/mcq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bel AJE. The phloem, a miracle of ingenuity. Plant Cell Environ. 2003;26:125–149. [Google Scholar]
- van Bel AJE. Xylem-phloem exchange via the rays: The undervalued route of transport. J Exp Bot. 1990;41:631–644. [Google Scholar]
- Deslauriers A, Morin H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees. 2005;19:402–408. [Google Scholar]
- Evert RF. Esau’s Plant Anatomy. 3th Edition Wiley; New Jersey: 2006. [Google Scholar]
- FAO . World reference base for soil resources. FAO; Rome: 1998. [Google Scholar]
- Franceschi VR, Krokene P, Christiansen E, Krekling T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytologist. 2005;167:353–376. doi: 10.1111/j.1469-8137.2005.01436.x. [DOI] [PubMed] [Google Scholar]
- Ghirardo A, Gutknecht J, Zimmer I, Brüggemann N, Schnitzler JP. Biogenic volatile organic compound and respiratory CO2 emissions after 13C-labeling: online tracing of C translocation dynamics in poplar plants. PLoS One. 2011;6:e17393. doi: 10.1371/journal.pone.0017393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli A, Emiliani G, Traversi ML, Deslauriers A, Rossi S. Sampling cambial region and mature xylem for non structural carbohydrates and starch analyses. Dendrochron. 2011;29:177–182. [Google Scholar]
- Gower ST, Richards JH. Larches: deciduous conifers in an evergreen world. Bioscience. 1990;40:818–826. [Google Scholar]
- Gruber A, Pirkebner D, Florian C, Oberhuber W. No evidence for depletion of carbohydrate pools in Scots pine (Pinus sylvestris L) under drought stress. Plant Biol. 2012;14:142–148. doi: 10.1111/j.1438-8677.2011.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Beck E. The fate and path of assimilation products in the stem of 8-year-old Scots pine (Pinus sylvestris L) trees. Trees. 1990;4:16–21. [Google Scholar]
- Hansen J, Beck E. Seasonal changes in the utilization and turnover of assimilation products in 8-year-old Scots pine (Pinus sylvestris L) trees. Trees. 1994;8:172–182. [Google Scholar]
- Heizmann U, Kreuzwieser J, Schnitzler J-P, Brüggemann N, Rennenberg H. Assimilate transport in the xylem sap of pedunculate oak (Quercus robur) saplings. Plant Biol. 2001;3:132–138. [Google Scholar]
- Hoch G, Körner C. The carbon charging of pines at the climatic treeline: a global comparison. Oecologia. 2003;135:10–21. doi: 10.1007/s00442-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Hoch G, Popp M, Körner C. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos. 2002;98:361–374. [Google Scholar]
- Hoch G, Richter A, Körner C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2003;26:1067–1081. [Google Scholar]
- Kajimoto T, Matsuura Y, Sofronov MA, Volokitina AV, Mori S, Osawa A, Abaimov AP. Above- and belowground biomass and net primary productivity of a Larix gmelinii stand near Tura, central Siberia. Tree Physiol. 1999;19:815–822. doi: 10.1093/treephys/19.12.815. [DOI] [PubMed] [Google Scholar]
- Kagawa A, Sugimoto A, Maximov TC. Seasonal course of translocation, storage and remobilization of 13C pulse-labeled photoassimilate in naturally growing Larix gmelinii saplings. New Phytol. 2006a;171:793–804. doi: 10.1111/j.1469-8137.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- Kagawa A, Sugimoto A, Maximov TC. 13CO2 pulse-labelling of photoassimilates reveals carbon allocation within and between tree rings. Plant, Cell Environ. 2006b;29:1571–1584. doi: 10.1111/j.1365-3040.2006.01533.x. [DOI] [PubMed] [Google Scholar]
- Körner C. A re-assessment of high elevation treeline positions and their explanation. Oecologia. 1998;115:445–459. doi: 10.1007/s004420050540. [DOI] [PubMed] [Google Scholar]
- Körner C. Carbon limitation in trees. J Ecol. 2003;91:4–17. [Google Scholar]
- Kramer PJ, Kozlowski TT. Physiology of Woody Plants. Academic Press; New York, San Francisco, London: 1979. [Google Scholar]
- Kreuzwieser J, Graus M, Wisthaler A, Hansel A, Rennenberg H, Schnitzler J-P. Xylem-transported glucose as an additional carbon source for leaf isoprene formation in Quercus robur. New Phytol. 2002;156:171–178. doi: 10.1046/j.1469-8137.2002.00516.x. [DOI] [PubMed] [Google Scholar]
- Kuptz D, Fleischmann F, Matyssek R, Grams TEE. Seasonal patterns of carbon allocation to respiratory pools in 60-yr old deciduous (Fagus sylvatica) and evergreen (Picea abies) trees assessed via whole-tree stable carbon isotope labeling. New Phytol. 2011;191:160–172. doi: 10.1111/j.1469-8137.2011.03676.x. [DOI] [PubMed] [Google Scholar]
- Landhäusser SM, Lieffers VJ. Seasonal changes in carbohydrate reserves in mature northern Populus tremuloides clones. Trees. 2003;17:471–476. [Google Scholar]
- Lehmeier CA, Lattanzi FA, Schäufele R, Wild M, Schnyder H. Root and shoot respiration of perennial ryegrass are supplied by the same substrate pools: assessment by dynamic 13C labeling and compartmental analysis of tracer kinetics. Plant Physiol. 2008;148:1148–1158. doi: 10.1104/pp.108.127324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Hoch G, Körner C. Source/sink removal affects mobile carbohydrates in Pinus cembra at the Swiss treeline. Trees. 2002;16:331–337. [Google Scholar]
- Lyr H, Fiedler HJ, Tranquillini W. Physiologie und Ökologie der Gehölze. Gustav Fischer Publ; Jena, Germany: 1992. P. [Google Scholar]
- Matyssek R. Carbon, water and nutrient relations in evergreen and deciduous conifers. Tree Physiol. 1986;2:177–187. doi: 10.1093/treephys/2.1-2-3.177. [DOI] [PubMed] [Google Scholar]
- Mayrhofer S, Heizmann U, Magel E, Eiblmeier M, Muller A, Rennenberg H, Hampp R, Schnitzler J-P, Kreuzwieser J. Carbon balance in the leaves of young poplar trees. Plant Biol. 2004;6:730–745. doi: 10.1055/s-2004-821268. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Pockman W, Allen C, Breshears D, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams D, Yepez EA. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb? New Phytol. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- Minchin PEH, Lacointe A. New understanding on phloem physiology and possible consequences for modelling long-distance carbon transport. New Phytol. 2004;166:771–779. doi: 10.1111/j.1469-8137.2005.01323.x. [DOI] [PubMed] [Google Scholar]
- Montague TG, Givnish TJ. Distribution of black spruce vs eastern larch along peatland gradients: relationship to relative stature, growth rate, and shade tolerance. Can J Bot. 1996;74:1514–1532. [Google Scholar]
- Moser L, Fonti P, Büntgen U, Esper J, Luterbacher J, Franzen J, Frank D. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol. 2010;30:225–233. doi: 10.1093/treephys/tpp108. [DOI] [PubMed] [Google Scholar]
- Münch E. Die Stoffbewegungen in Der Pflanze. Gustav Fischer; Jena, Germany: 1930. [Google Scholar]
- Nagy NE, Fossdal CG, Krokene P, Krekling T, Lønneborg A, Solheim H. Induced responses to pathogen infection in Norway spruce phloem: changes in polyphenolic parenchyma cells, chalcone synthase transcript levels and peroxidase activity. Tree Physiol. 2004;24:505–515. doi: 10.1093/treephys/24.5.505. [DOI] [PubMed] [Google Scholar]
- Oleksyn J, Zytkowiak R, Karolewski P, Reich PB, Tjoelker MG. Genetic and environmental control of seasonal carbohydrate dynamics in trees of diverse Pinus sylvestris populations. Tree Physiol. 2000;20:837–847. doi: 10.1093/treephys/20.12.837. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauners A, Anfodillo T. Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the Alpine timberline. IAWA J. 2006;27:383–394. [Google Scholar]
- Sala A, Woodruff DR, Meinzer FC. Carbon dynamics in trees: feast or famine? Tree Physiol. 2012;32:764–775. doi: 10.1093/treephys/tpr143. [DOI] [PubMed] [Google Scholar]
- Turgeon R. The role of phloem loading reconsidered. Plant Physiol. 2010;152:1817–1823. doi: 10.1104/pp.110.153023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Wolf S. Phloem transport: cellular pathways and molecular trafficking. Ann Rev Plant Biol. 2009;60:207–221. doi: 10.1146/annurev.arplant.043008.092045. [DOI] [PubMed] [Google Scholar]
- Wieser G, Matyssek R, Luzian R, Zwerger P, Pindur P, Oberhuber W, Gruber A. Effects of atmospheric and climate change at the timberline of the Central European Alps. Ann Forest Sci. 2009;66:402–413. doi: 10.1051/forest/2009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff DR, Meinzer FC. Water stress, shoot growth and storage of non-structural carbohydrates along a tree height gradient in a tall conifer. Plant Cell Environ. 2011;34:1920–1930. doi: 10.1111/j.1365-3040.2011.02388.x. [DOI] [PubMed] [Google Scholar]
- Zeide B. Analysis of growth equations. Forest Sci. 1993;39:594–616. [Google Scholar]
- Zweifel R, Häsler R. Dynamics of water storage in mature subalpine Picea abies: temporal and spatial patterns of change in stem radius. Tree Physiol. 2001;21:561–569. doi: 10.1093/treephys/21.9.561. [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Melcher PJ, Feild TS, Holbrook NM. Apotential role for xylem–phloem interactions in the hydraulic architecture of trees: effects of phloem girdling on xylem hydraulic conductance. Tree Physiol. 2004;24:911–917. doi: 10.1093/treephys/24.8.911. [DOI] [PubMed] [Google Scholar]
- Zimmermann MH, Brown CL. Trees: Structure and function. Springer-Verlag; New York: 1971. [Google Scholar]