Abstract
Aims
To examine long-term healthcare utilization and costs of patients with stable coronary artery disease (SCAD).
Methods and results
Linked cohort study of 94 966 patients with SCAD in England, 1 January 2001 to 31 March 2010, identified from primary care, secondary care, disease, and death registries. Resource use and costs, and cost predictors by time and 5-year cardiovascular disease (CVD) risk profile were estimated using generalized linear models. Coronary heart disease hospitalizations were 20.5% in the first year and 66% in the year following a non-fatal (myocardial infarction, ischaemic or haemorrhagic stroke) event. Mean healthcare costs were £3133 per patient in the first year and £10 377 in the year following a non-fatal event. First-year predictors of cost included sex (mean cost £549 lower in females), SCAD diagnosis (non-ST-elevation myocardial infarction cost £656 more than stable angina), and co-morbidities (heart failure cost £657 more per patient). Compared with lower risk patients (5-year CVD risk 3.5%), those of higher risk (5-year CVD risk 44.2%) had higher 5-year costs (£23 393 vs. £9335) and lower lifetime costs (£43 020 vs. £116 888).
Conclusion
Patients with SCAD incur substantial healthcare utilization and costs, which varies and may be predicted by 5-year CVD risk profile. Higher risk patients have higher initial but lower lifetime costs than lower risk patients as a result of shorter life expectancy. Improved cardiovascular survivorship among an ageing CVD population is likely to require stratified care in anticipation of the burgeoning demand.
Keywords: Stable coronary artery disease, Electronic health records, Costs, Resource use
Introduction
Improved survival coupled with a decline in the incidence of acute myocardial infarction (AMI)1,2 has dramatically changed the pattern of healthcare use over recent years.3,4 Nowadays, patients with stable coronary artery disease (SCAD), including patients with stable angina and those who have become stable after acute coronary syndrome (ACS),5,6 are older and living longer and so make greater use of healthcare resources. Patients with SCAD might be considered to have ‘fallen off the radar’ of clinical interest: no longer in cardiac rehabilitation [mainly offered to those immediately after AMI or coronary artery bypass grafting (CABG)], discharged from ongoing specialist care, and with suboptimal drug compliance, adherence, and persistence.7 Such patients, however, vary widely in their risk of subsequent AMI or coronary death (∼10-fold, between top and bottom deciles of risk),8 which will clearly have differential resource implications.
While previous studies of resource use and cost have taken as a starting point AMI,9,10 there is a paucity of information about the contemporary use and associated costs of healthcare beyond the initial hospital stay. In addition, existing studies in the area have been limited in a number of ways. First, as a result of the nature of their samples, they are not population based and do not reflect contemporary and routine clinical practice.11 Second, they use overly simplistic models, typically restricting their analysis to a subset of SCAD index events and not evaluating how the pattern of healthcare resource use changes following a first post-SCAD myocardial infarction (MI) or stroke.9,10 Third, no study has evaluated resource utilization and costs according to baseline cardiovascular risk, despite the importance of this information in improving decision-making and ensuring more efficient use of limited healthcare resources. Fourth, longer term and particularly lifetime implications of SCAD have not been quantified.
These knowledge gaps have a number of important ramifications. They create uncertainty for those who need to forecast future care needs, restrain the research and development of new technologies and treatments for SCAD, and limit informed clinical decision-making. To address these limitations, our study aimed to (i) determine healthcare utilization and the associated costs in the first year with SCAD and in the year following a first non-fatal event (i.e. AMI, ischaemic or haemorrhagic stroke), (ii) study predictors of costs in the first year of SCAD, and (iii) estimate the 5-year and lifetime costs among patients at low and high risk of subsequent events and coronary heart disease (CHD) death.
Methods
Data set and patient population
The ClinicAl research using Linked Bespoke studies and Electronic Records (CALIBER) e-health database was the data resource for this study. CALIBER links patient records from four different data sources: Clinical Practice Research Database (CPRD), Myocardial Ischaemia National Audit Project (MINAP) registry, Hospital Episodes Statistics (HES), and the Office for National Statistics. The data of CPRD were used to obtain heart rate measurements, demographic variables, and other risk factors. Primary care practices that provide valuable data to CPRD and cover ∼4% of UK population are representative in terms of demographic parameters such as gender, age, and ethnicity12,13 and overall mortality14 and have been validated for epidemiological research. A description of the CALIBER approach has been presented elsewhere.15 Classification algorithms combining Read, International Classification of Disease 10 (ICD-10), drug, and procedure codes to define risk factors and endpoints are available at http://www.caliberresearch.org/portal/.
Eligible patients were those with a diagnosis of stable angina, patients with a diagnosis of ACS within the study period (unstable angina or AMI), or those with a diagnosis of CHD in which there is no further specification of whether it is angina or MI (other CHD). Study start date was calculated from the date of diagnosis of stable angina or other CHD or from 6 months after an ACS or coronary intervention. The period of 6 months was chosen to differentiate long-term prognosis from the high-risk period that typically follows an ACS or revascularization. Diagnoses were identified in CPRD, HES, or MINAP records according to definitions given in the CALIBER data manual.15 Patients were only eligible for the study during the period they were actively registered at a CPRD practice that was collecting up-to-standard data (according to CPRD measures of data quality and completeness), with follow-up ending if a patient transferred out of a CPRD practice. Full details of the cohort are available elsewhere.8
Healthcare utilization
Healthcare utilization extracted from the data set included primary care consultations, pharmaceutical prescriptions, inpatient stays, and diagnostic tests. Primary care consultations included all contacts between the patient and healthcare professionals captured in the CPRD data set. Prescription data were available from the CPRD data set and distinguished between drugs that were cardiovascular disease (CVD)-related and those that were not. Inpatient stays extracted from HES were based on Health Resource Group (HRG) codes and defined as CHD, CVD (including CHD and broader HRGs), or non-CVD related based on ICD-10 codes. Diagnostic tests as recorded in the primary care data set but not outpatient consultations were also extracted, the latter being due to the absence of outpatient HES data linkage.
Costs
All costs were calculated from the perspective of the UK National Health Service (NHS) in pound sterling based on 2011/12 prices. Costs were calculated by combining healthcare utilization data from CALIBER with associated unit costs that were taken from published UK sources.16–18 For hospitalizations, costs are calculated based on finished consultant episodes in HES. Costs are presented in terms of total healthcare costs (all costs incurred), CHD costs (all CHD-related hospitalization costs, CVD-related drugs, and all primary care and diagnostic costs), and CVD costs (all CVD-related hospitalization costs, CVD-related drugs, and all primary care and diagnostic costs).
Analytical methods
Estimates of healthcare utilization and costs were calculated for the first year in the SCAD cohort and the first year following a non-fatal event during the follow-up period (AMI, ischaemic or haemorrhagic stroke) with results reported as means and standard deviations, with medians and interquartile ranges reported in the appendices. Observations that were right censored for any reason other than mortality (i.e. those for which the data are incomplete for the year of interest, either first year with SCAD or first year following an event, but the reason for incompleteness was not death) were excluded from the analysis.
A generalized linear model with a log link and gamma distribution was used to estimate the impact of baseline covariates on costs in the first year in the SCAD cohort to account for the non-linear impact of covariates and the right skewness in cost data. Covariates were based on those used by Rapsomaniki et al.,8 which developed a prognostic model for SCAD patients, on the assumption that predictors of costs were likely linked to prognostic indicators. The covariates included the baseline diagnosis for entry to SCAD, co-morbidities, age, gender, smoking status, and biomarkers. Models were fitted on five multiply-imputed data sets and estimates combined using Rubin's rules.19 The impact of covariates has been transformed back onto the natural scale to allow for ease of interpretation.
Panel data methods with time invariant covariates were used to estimate patient costs over each 90-day period. Also estimated were the impact of events (non-fatal AMI, ischaemic and haemorrhagic stroke, CVD- and non-CVD-related death) on the costs in the period of the event and subsequent periods if the event was non-fatal. These costs were then combined with a state transition Markov model to estimate costs over a longer period. The model estimated the probabilities of, and mean times to, the first events of non-fatal AMI, ischaemic or haemorrhagic stroke, CVD- and non-CVD-related death, and subsequent CVD or non-CVD death following a non-fatal first event. These estimates were conditional on baseline covariates and were inferred from patient-level costs, covariates, survival, and events experienced. Full details of this model are available elsewhere with a brief description given in the appendix.20 For the purpose of this article, the results of costs over time are presented for patients based on average covariate patterns for the 5-year risk deciles of a cardiovascular event. Discounted costs are also presented using a discount rate of 3.5% per annum.21
Results
Cohort
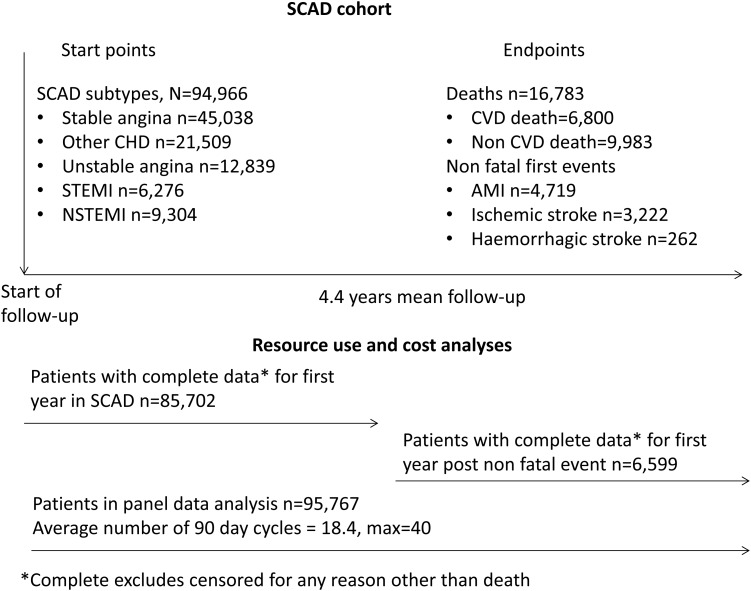
In total, 94 966 patients were identified who met the inclusion criteria, of which 44% were female. The mean age of men and women included were 67 and 72 years, respectively. For the primary SCAD diagnosis, 47.4% of patients had stable angina, 13.5% unstable angina, 6.7% ST-elevation MI (STEMI), 9.7% non-STEMI (NSTEMI), and 22.6% had CHD not otherwise specified. Full details of the cohort can be found in Appendix Table A1.
Figure 1 summarizes the SCAD cohort and the patient numbers used for each analysis.
Figure 1.
Stable coronary artery disease cohort.
Healthcare utilization
Table 1 reports healthcare utilization in the first year in the SCAD cohort and in the first year following a non-fatal event during the follow-up period (AMI, ischaemic or haemorrhagic stroke). In the first year in the SCAD cohort, 20.5% of patients (n = 17 532) were hospitalized for CVD, and those who were hospitalized had a mean 1.9 stays (spells) in hospital with a mean length of stay of 4.6 days. In the year following a non-fatal event during follow-up, 66% of patients (n = 4354) were hospitalized for CHD. These patients spent a mean of 2.2 stays in hospital with a mean length of stay of 6.5 days. In the first year in SCAD, patients had a mean of 10.8 primary care appointments, and this increased to 13.7 in the first year following a non-fatal event. In the first year of SCAD, 88.2% of patients were taking cardiovascular medication, which decreased to 83.6% in the year following a non-fatal event. In the first year of SCAD, 5.7% of patients had a revascularization procedure, increasing to 13.5% of patients in the first year following a non-fatal event.
Table 1.
Healthcare utilization in first year in the stable coronary artery disease cohort and first year following a non-fatal event during follow-up (myocardial infarction, ischaemic or haemorrhagic stroke)
| Resource use in first year (n= 85 702) | Resource in first year following an event (n = 6599) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Hospitalizations | ||
| Hospitalized (%) | 37.6 (0.484) | 83.5 (0.371) |
| Hospitalized for CVD (%) | 27 (0.444) | 80.2 (0.399) |
| Hospitalized for CHD (%) | 20.5 (0.403) | 66 (0.474) |
| Inpatient stays | 0.875 (4.421) | 2.364 (6.425) |
| Inpatient stays for CVD | 0.503 (2.545) | 1.931 (5.284) |
| Inpatient stays for CHD | 0.343 (1.321) | 1.434 (3.315) |
| With any hospitalization | n = 32 242 | n = 5512 |
| Inpatient stays | 2.325 (6.971) | 2.83 (6.935) |
| Length of stay | 6.717 (17.583) | 14.857 (20.349) |
| With any hospitalization for CVD | n = 23 160 | n = 5291 |
| Inpatient stays for CVD | 1.862 (4.631) | 2.408 (5.803) |
| Length of stay | 7.516 (12.757) | 15.541 (20.939) |
| With any hospitalization for CHD | n = 17 532 | n = 4354 |
| Inpatient stays for CHD | 1.674 (2.51) | 2.174 (3.88) |
| Length of stay | 4.579 (7.617) | 6.515 (10.857) |
| Revascularizations | ||
| Any revascularization (%) | 5.7 (0.232) | 13.5 (0.341) |
| PCI (%) | 3 (0.17) | 9.1 (0.287) |
| CABG (%) | 2.9 (0.169) | 5 (0.218) |
| Primary care consultations | 10.768 (10.857) | 13.671 (15.407) |
| Drugs | ||
| Patients on any drugs (%) | 91.3 (0.281) | 85.1 (0.356) |
| Patients on CVD drugs (%) | 88.2 (0.322) | 83.6 (0.37) |
| Patients on anticoagulants (%) | 8.7 (0.282) | 14.7 (0.354) |
| Patients on ACEi or ARB (%) | 47.7 (0.499) | 61.5 (0.487) |
| Patients on anti-platelets (%) | 65.6 (0.475) | 76.3 (0.425) |
| Patients on β-blockers (%) | 46 (0.498) | 50.1 (0.5) |
| Patients on calcium channel blockers (%) | 31.5 (0.464) | 32.6 (0.469) |
Costs
Table 2 reports costs for hospitalizations, primary care appointments, diagnostic tests, and drugs in patients in the first year in the SCAD cohort, and in the first year following a non-fatal event during follow-up (MI, ischaemic or haemorrhagic stroke). The mean total healthcare costs in the first year in the SCAD cohort were £3133 per patient, of which 56.8% (£1780) and 70.2% (£2199) were related to CHD and CVD, respectively. This estimate increased to £10 377 per patient in the year following a non-fatal event during follow-up, of which 66.2% (£6869) and 85.9% (£8916) were related to CHD and CVD, respectively. The majority of healthcare costs were driven by hospitalizations (64.4% in the first year in the SCAD cohort and 84.2% in the year following a non-fatal event during follow-up).
Table 2.
Costs in first year in the stable coronary artery disease cohort and in first year following an event during follow-up (myocardial infarction, ischaemic or haemorrhagic stroke)
| Costs in first year (n = 85 702) | Costs in first year following an event (n = 6599) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Total costs | ||
| Total cost (£) | 3133 (6101) | 10 377 (12 260) |
| Total CVD cost (£) | 2199 (4632) | 8916 (10 930) |
| Total CHD costs (£) | 1780 (3686) | 6869 (9467) |
| Hospitalizations | ||
| Inpatient costs (£) | 2018 (5632) | 8744 (11 554) |
| Inpatient CVD costs (£) | 1487 (4493) | 7957 (10 796) |
| Inpatient CHD costs (£) | 1067 (3548) | 5910 (9338) |
| Primary care costs (£) | 466 (463) | 589 (629) |
| Diagnostic test costs (£) | 141 (232) | 228 (311) |
| Drugs | ||
| All drug costs (£) | 508 (1548) | 816 (3135) |
| CVD drug costs (£) | 105 (113) | 142 (175) |
Cost predictors in the first year in the stable coronary artery disease cohort
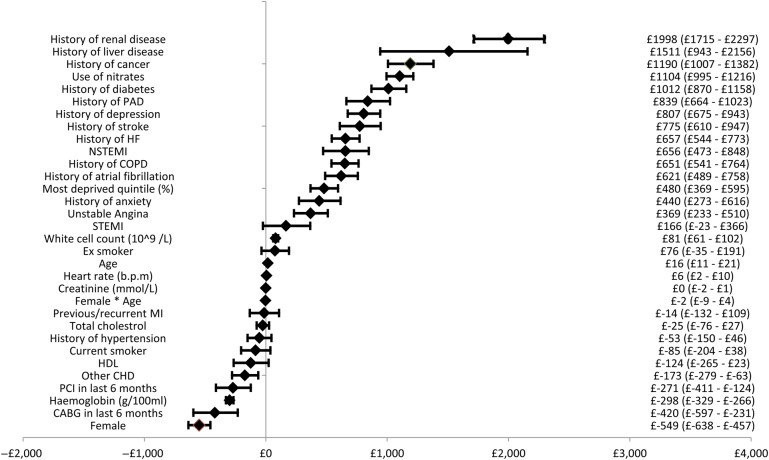
Figure 2 presents the results of the regression analysis showing the incremental costs associated with different covariates and the associated 95% confidence intervals (CI) for the first year in the SCAD cohort. Non-CVD-related co-morbidities had the largest impact on costs, with a history of renal disease associated with the largest increment of £1998 per patient (95% CI £1715–£2297). A history of heart failure resulted in an additional cost of £802 per patient (95% CI £683–£920). Of the baseline diagnoses for entry to the SCAD cohort, NSTEMI had the largest impact on cost with those patients with NSTEMI costing an additional £656 per patient (95% CI £473–£848) when compared with those with stable angina. Females were significantly less costly than males, being female was associated with a cost decrement of £549 per patient (95% CI −£638 to −£457). Figures A1 and A2 in the appendix present the same results for CVD- and CHD-related costs, respectively.
Figure 2.
Forest plot of incremental costs associated with covariates.
Estimated 90-day period and event costs
Tables A4, A5, and A6 in the appendix provide estimates of total healthcare costs, CVD-related costs, and CHD-related costs, respectively, for a 90-day period based on a range of baseline characteristics. The tables also show the incremental costs in the period of an event and in subsequent periods for non-fatal events. For example, the background total healthcare costs for a male, mean age 69 years, with no co-morbidities would be £341 in the first 90 days, increasing by £10 for each subsequent 90-day period. If the patient had a non-fatal AMI, he would incur an incremental cost (on top of the normal period cost of £372) of £5028 in the 90 days following the AMI with the incremental costs decreasing in subsequent trimesters until 360 days after which there is an incremental cost of £521 per 90 days suggesting significant ongoing lifetime costs of events. Also of note, the incremental costs in the period of death from CVD- and non-CVD-related causes were £2008 and £2240, respectively.
Estimated costs over time for stable coronary artery disease
Table 3 presents estimates of 5-year and lifetime costs (total and CVD related, undiscounted and discounted) of the representative patients for each risk group. The covariate profiles used are shown in Appendix Table A7.
Table 3.
Mean 5-year and lifetime costs for stable coronary artery disease patient population by cardiovascular risk decile
| Results | Risk group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 5-year riska (%) | 3.46 | 5.43 | 6.95 | 8.53 | 10.36 | 12.57 | 15.64 | 20.07 | 27.23 | 44.18 |
| Average age (years) | 52 | 59 | 62 | 65 | 68 | 70 | 73 | 76 | 80 | 84 |
| Life expectancy (years) | 26.81 | 19.62 | 17.34 | 15.63 | 14.26 | 13.03 | 11.92 | 10.48 | 8.52 | 5.51 |
| Total 5-year costs (£) | 9335 | 11 200 | 12 308 | 13 512 | 14 644 | 15 930 | 17 660 | 19 609 | 21 617 | 23 391 |
| Discounted total 5-year costsb (£) | 8495 | 10 204 | 11 221 | 12 327 | 13 368 | 14 554 | 16 153 | 17 963 | 19 853 | 21 620 |
| Total 5-year CVD costs (£) | 5306 | 6959 | 7941 | 8954 | 9874 | 10 904 | 12 242 | 13 742 | 15 380 | 17 050 |
| Discounted total 5-year CVD costsb (£) | 4823 | 6338 | 7238 | 8168 | 9014 | 9962 | 11 197 | 12 589 | 14 126 | 15 759 |
| Total 5-year CHD costs (£) | 4172 | 5543 | 6354 | 7153 | 7867 | 8583 | 9449 | 10 385 | 11 376 | 12 392 |
| Discounted total 5-year CHD costsb (£) | 3801 | 5057 | 5801 | 6534 | 7191 | 7850 | 8651 | 9521 | 10 455 | 11 459 |
| Total lifetime costs (£) | 116 888 | 81 490 | 73 057 | 68 102 | 64 521 | 62 034 | 61 435 | 59 446 | 54 345 | 43 020 |
| Discounted total lifetime costsb | 62 210 | 50 864 | 48 046 | 46 535 | 45 429 | 44 785 | 45 283 | 44 903 | 42 436 | 35 549 |
| Total lifetime CVD costs (£) | 71 943 | 52 034 | 47 681 | 45 251 | 43 438 | 42 266 | 42 301 | 41 366 | 38 410 | 31 199 |
| Discounted total lifetime CVD costsb (£) | 37 857 | 32 331 | 31 288 | 30 896 | 30 584 | 30 531 | 31 211 | 31 281 | 30 024 | 25 801 |
| Total lifetime CHD costs (£) | 46 921 | 36 069 | 33 892 | 32 693 | 31 741 | 30 944 | 30 793 | 29 885 | 27 533 | 22 324 |
| Discounted total lifetime CHD costsb (£) | 25 316 | 22 868 | 22 639 | 22 672 | 22 657 | 22 619 | 22 946 | 22 778 | 21 646 | 18 522 |
aOf AMI, ischaemic or haemorrhagic stroke, or fatal CVD.
bDiscounted at a rate of 3.5% per annum to calculate the present value of the costs.
A patient with SCAD representative of the lowest risk decile (5-year cardiovascular risk of 3.5% and a life expectancy of 26.8 years) would have expected undiscounted costs of £9335 over 5 years, of which 44.7 and 56.8% would be CHD and CVD related, respectively; and undiscounted lifetime costs of £116 888, of which 40.1 and 61.5% would be CHD and CVD related, respectively. A representative patient of the highest risk decile (5-year cardiovascular risk of 44.2% and a life expectancy of 5.51 years) would have expected undiscounted costs of £23 391 over 5 years, of which 53.0 and 72.9% would be CHD and CVD related, respectively; and lifetime undiscounted costs of £43 020, of which 51.9 and 72.5% would be CHD and CVD related, respectively.
Figure 3 shows the predicted total, CVD-related, and CHD-related costs and the survival curves over a 25-year period for representative patients of risk deciles 1 (lowest risk), 4, 7, and 10 (highest risk). Higher risk patients with SCAD have higher initial costs, which are overtaken by the lower risk patients as a result of higher mortality in the higher risk groups resulting in less time to accrue costs (5-year survivorship differs from 98.4% in risk decile 1 to 40.7% in risk decile 10; and 25-year survivorship differs from 58.2% in risk decile 1 to 0.5% in risk decile 10).
Figure 3.
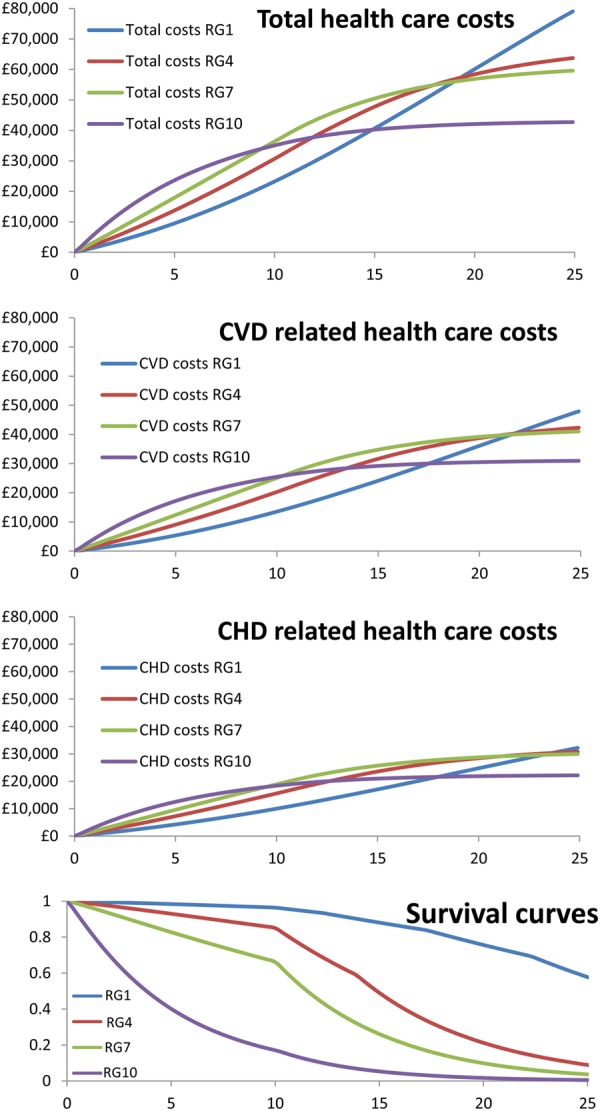
Expected costs and survival over time for patients representative of risk deciles 1, 4, 7, and 10. CHD, coronary heart disease; CVD, cardiovascular disease; RG, risk decile.
Discussion
This study addresses a fundamental gap in knowledge relevant to clinicians and policymakers: what is the clinical, health service, and cost burden associated with SCAD? Using data from a large, contemporary, and representative population of patients from the England with SCAD, the analysis has shown that substantial healthcare costs are likely to be incurred as a result of improved ACS survivorship and the ageing population. Moreover, 5-year and lifetime costs vary according to CVD risk, which may be readily predicted from the baseline characteristics of patients that are routinely collected. High-risk patients have considerably higher costs over the initial 5 years but lower lifetime costs than lower risk patients as a result of shorter life expectancy.
Patients with SCAD might be considered to have ‘fallen off the radar’ of clinical interest. Current guidelines give little information about how frequently and where such patients should be followed up or if and how they should be risk stratified.5,6 Our results clearly highlight the unmet need and the shortfalls of current approaches—with high use of primary care and frequent hospitalizations, there are considerable ongoing costs. The number of primary care consultations, a mean of 10.8 per patient in the first year in the SCAD cohort, is higher than previous estimates for the overall population (5.5 per year).22 In the first year in the SCAD cohort, over a third of patients were hospitalized (and 20.5% for CHD reasons). This is substantially higher than recent estimates for the general population in one area of the UK (14.9%),23 and markedly higher than that in the general population for a similar age (23.4 and 21.2% of 60- to 74-year-old males and females, respectively, based on HES data).
Healthcare utilization is, however, insufficient as a metric of the impact on the healthcare system. It is also important to consider the cost imposed on the NHS associated with those resources as this indicates the value of resources that cannot be devoted to health-enhancing activities for other patients. Mean costs of £3133 in the first year in the SCAD cohort are much higher than those in patients without chronic conditions (£293), but comparable with other chronic conditions (e.g. diabetes £3036).23 Very high costs in the first year following a non-fatal event during follow-up (MI, ischaemic or haemorrhagic stroke) (£10 377) are reflective of the healthcare utilization required to treat that event.
Patients were stratified by risk to understand the costs accrued in greater detail. This higher resolution analysis allows the identification of where novel treatments and health service interventions have the greatest potential to be cost-effective. Non-CVD co-morbidities were common and had a major influence on costs with e.g. renal disease resulting in a mean extra cost of £1998 per patient in the first year of SCAD. This is an important finding when the presence of multiple co-morbidities has been shown to increase costs23 yet clinicians tend to focus only on single diseases.24
The panel data analysis examined the average cost per 90-day period with the disease and also the costs of events. The estimated incremental cost of a non-fatal MI over 1 year (£7677 ignoring mortality risk) was lower than some previous estimates from trial populations (e.g. among patients with stable angina the estimate of £9775 from the EUROPA trial).11 However, this lower estimate may be reflective of less intensive use of healthcare resources in non-trial settings and should provide a more accurate representation of costs of these events in routine clinical practice. Estimated stroke costs in the first year of the event (£8902 for ischaemic and £10 477 for haemorrhagic stroke incremental to background costs) were similar to those seen in other studies. For example, the OXVASC study estimated mean total healthcare costs per patient in the first year following stroke at £10524.25
Furthermore, a study of costs in the first year of SCAD and the first year following an event is of limited use to decision-makers who require more detailed information on the long-term costs and consequences of SCAD. This can be seen from the panel data analysis, which suggested ongoing long-term costs as a result of non-fatal events. By estimating 5-year and lifetime costs by CVD risk group, it was possible to examine the long-term cost implications as a result of the disease and future events. In the shorter term of 5 years, which is shorter than the life expectancy of even the highest risk group (although survivorship in this group was only 40.7% at 5 years), costs increased with cardiovascular risk, and were largely driven by the high number of fatal and non-fatal events among these patients. Over a lifetime, however, patients in the lower risk groups eventually had substantially higher costs than higher risk patients, primarily driven by greater life expectancy and, therefore, costs being incurred over a much longer period. This is a key finding of our research: increased survivorship as well as an increasingly co-morbid and older population will result in significant future healthcare costs. In turn, this has implications for the cost and therefore the cost-effectiveness of established and new SCAD treatments.
Many studies have attempted to address the burden of disease in terms of health losses but fewer have examined the impact on financial costs of disease. Our research used SCAD as an exemplar in estimating resource use and costs from ‘real world’ electronic health record data. The methods used here could be readily applied to other chronic diseases to help produce evidence of their resource and cost implications to better inform clinicians and decision-makers. This would reduce uncertainty for those who need to forecast future care needs and allow for better focused research and development of new technologies and treatments for these chronic diseases as well as resulting in better informed clinical decision-making.
Limitations
While our study has a number of strengths including the multi-source electronic health record linkage, there are a number of limitations. In addition to being censored at 2010, after which there have been further improvements in the care and survivorship of SCAD, a key weakness of this study was the exclusion of outpatient appointment costs that cannot currently be linked from HES. As a result, the total healthcare costs of this population are underestimated. Further disaggregation of primary care costs into CHD and CVD related was not possible and therefore in each category all primary care costs have been included and therefore are likely overestimated. The estimation of lifetime costs has also involved extrapolation over a longer time period than is currently observed in the CALIBER data. This extrapolation is subject to considerable uncertainty. The SCAD population is also inherently heterogeneous, and there may be value in further disaggregation of the population in future research.
Conclusions
Using a multi-source electronic health record approach, this study provides, for the first time, estimates and predictors of contemporary national healthcare utilization and costs in the first year of SCAD and the first year following an event. It reveals that patients with SCAD incur substantial healthcare utilization and costs, which vary and may be predicted by 5-year CVD risk profiles. While high-risk patients incur substantially higher costs over the short term (5 years), low-risk patients incur higher lifetime costs as a result of greater life expectancy. Improved cardiovascular survivorship and an ageing UK population will require stratified care in anticipation of the burgeoning economic demand. The methods used here could be readily applied to other chronic diseases to better inform clinical decision-making.
Authors’ contributions
All authors contributed to the interpretation of the data and several drafts of the manuscript. S.W., M.A., A.M., S.P., and M.S. conducted the statistical analysis and developed the model used to estimate long-term costs. A.D.S., K.A., M.C., C.P.G., A.T., and H.H. provided advice throughout the process. A.T. and H.H. were responsible for the overall grant from the NIHR.
Acknowledgements
We acknowledge the help of the authors of the original prognostic model, namely, Eleni Rapsomaniki, Pablo Perel, Spiros Denaxas, Julie George, Owen Nicholas, Ruzan Udumyan, Gene Feder, Aroon Hingorani, and Liam Smeeth. Further we acknowledge those responsible for the development of the CALIBER data set including Spiros Denaxas, Julie George, Emily Herrett, Dipak Kalra, Aroon Hingorani, Mike Kivimaki, and Liam Smeeth.
Appendix
Model description
A state transition Markov model was used to capture the natural history of SCAD patients. The model predicts time to first non-fatal events (MI, ischaemic stroke or haemorrhagic stroke) and CVD and non-CVD mortality (as a first event or following a non-fatal first event). The model has a lifetime horizon and uses a 90-day cycle length to capture the time varying and age-dependent nature of risks. Risks of the five primary clinical endpoints (non-fatal MI, non-fatal ischaemic stroke, non-fatal haemorrhagic stroke, and CVD and non-CVD mortality) were estimated based on key prognostic factors, including demographic measures, SCAD subtype, use of long-acting nitrates, whether CABG or PCI was performed in the 6 months following CAD diagnosis, previous MI, smoking, blood pressure, diagnosis of hypertension, diabetes, lipids, CVD co-morbidities, non-CVD co-morbidities, psychosocial factors, and clinically assessed biomarkers. Risk equations for the six subsequent events, namely CVD and non-CVD mortality following non-fatal MI, ischaemic stroke, and haemorrhagic stroke were estimated in a similar way. However, due to the greatly reduced numbers of events observed, these were based only on sex and age at time of non-fatal event. Non-CVD mortality beyond the maximum follow-up in the CALIBER data set (10 years) was based on age–sex-specific non-CVD mortality from national life tables. Costs are accrued based on baseline covariate, events experienced, and time alive with the costs based on the results detailed in this article. Full details can be found in Asaria et al.20
Table A1.
Patient characteristics
| Socio-demographic characteristics | |
|---|---|
| Sex (% female) | 44 |
| Age (if male) (years) | 67 |
| Age (if female) (years) | 72 |
| Most deprived quintile (%) | 20 |
| Baseline diagnoses for entry to the SCAD cohort | |
| NSTEMI (%) | 10 |
| STEMI (%) | 7 |
| Unstable angina (%) | 14 |
| Stable angina (%) | 47 |
| CHD not otherwise specified (%) | 23 |
| CVD risk factors | |
| Current smoker (%) | 35 |
| Ex-smoker (%) | 32 |
| Never smoked (%) | 33 |
| Hypertension (%) | 76 |
| Diabetes (%) | 16 |
| CVD co-morbidities | |
| Heart failure (%) | 26 |
| Atrial fibrillation (%) | 15 |
| Peripheral arterial disease (%) | 8 |
| Stroke (%) | 9 |
Table A2.
Healthcare utilization in first year with stable coronary artery disease and first year following a non-fatal event (myocardial infarction, ischaemic or haemorrhagic stroke)
| Mean (SD) | Median (IQR) | |
|---|---|---|
| Resource use in first year (n = 85 702) | ||
| Hospitalizations | ||
| Hospitalized (%) | 37.6 (0.484) | 0 (0–1) |
| Hospitalized for CVD (%) | 27 (0.444) | 0 (0–1) |
| Hospitalized for CHD (%) | 20.5 (0.403) | 0 (0–1) |
| Inpatient stays | 0.875 (4.421) | 0 (0–3) |
| Inpatient stays for CVD | 0.503 (2.545) | 0 (0–2) |
| Inpatient stays for CHD | 0.343 (1.321) | 0 (0–2) |
| With any hospitalization (n = 32 242) | ||
| Inpatient stays | 2.325 (6.971) | 1 (1–5) |
| Length of stay | 6.717 (17.583) | 3 (1–23) |
| With any hospitalization for CVD (n = 23 160) | ||
| Inpatient stays for CVD | 1.862 (4.631) | 1 (1–4) |
| Length of stay | 7.516 (12.757) | 3.667 (1–26) |
| With any hospitalization for CHD (n = 17 532) | ||
| Inpatient stays for CHD | 1.674 (2.51) | 1 (1–4) |
| Length of stay | 4.579 (7.617) | 2 (1–14.75) |
| Revascularizations | ||
| Any revascularization (%) | 5.7 (0.232) | 0 (0–1) |
| PCI (%) | 3 (0.17) | 0 (0–0) |
| CABG (%) | 2.9 (0.169) | 0 (0–0) |
| Primary care consultations | 10.768 (10.857) | 8 (0–30) |
| Drugs | ||
| Patients on any drugs (%) | 91.3 (0.281) | 1 (0–1) |
| Patients on CVD drugs (%) | 88.2 (0.322) | 1 (0–1) |
| Patients on anticoagulants (%) | 8.7 (0.282) | 0 (0–1) |
| Patients on ACEi or ARB (%) | 47.7 (0.499) | 0 (0–1) |
| Patients on anti-platelets (%) | 65.6 (0.475) | 1 (0–1) |
| Patients on β-blockers (%) | 46 (0.498) | 0 (0–1) |
| Patients on calcium channel blockers (%) | 31.5 (0.464) | 0 (0–1) |
| Resource in first year following an event (n = 6599) | ||
| Hospitalizations | ||
| Hospitalized (%) | 83.5 (0.371) | 1 (0–1) |
| Hospitalized for CVD (%) | 80.2 (0.399) | 1 (0–1) |
| Hospitalized for CHD (%) | 66 (0.474) | 1 (0–1) |
| Inpatient stays | 2.364 (6.425) | 1 (0–6) |
| Inpatient stays for CVD | 1.931 (5.284) | 1 (0–5) |
| Inpatient stays for CHD | 1.434 (3.315) | 1 (0–4) |
| With any hospitalization (n = 5512) | ||
| Inpatient stays | 2.83 (6.935) | 2 (1–6) |
| Length of stay | 14.857 (20.349) | 8.5 (1.5–50.5) |
| With any hospitalization for CVD (n = 5291) | ||
| Inpatient stays for CVD | 2.408 (5.803) | 2 (1–5) |
| Length of stay | 15.541 (20.939) | 9 (2–52) |
| With any hospitalization for CHD (n = 4354) | ||
| Inpatient stays for CHD | 2.174 (3.88) | 1 (1–5) |
| Length of stay | 6.515 (10.857) | 3.5 (1–21.5) |
| Revascularizations | ||
| Any revascularization (%) | 13.5 (0.341) | 0 (0–1) |
| PCI (%) | 9.1 (0.287) | 0 (0–1) |
| CABG (%) | 5 (0.218) | 0 (0–1) |
| Primary care consultations | 13.671 (15.407) | 11 (0–38) |
| Drugs | ||
| Patients on any drugs (%) | 85.1 (0.356) | 1 (0–1) |
| Patients on CVD drugs (%) | 83.6 (0.37) | 1 (0–1) |
| Patients on anticoagulants (%) | 14.7 (0.354) | 0 (0–1) |
| Patients on ACEi or ARB (%) | 61.5 (0.487) | 1 (0–1) |
| Patients on anti-platelets (%) | 76.3 (0.425) | 1 (0–1) |
| Patients on β-blockers (%) | 50.1 (0.5) | 1 (0–1) |
| Patients on calcium channel blockers (%) | 32.6 (0.469) | 0 (0–1) |
Table A3.
Costs in first year with stable coronary artery disease and in first year following an event (myocardial infarction, ischaemic of haemorrhagic stroke)
| Mean (SD) (£) | Median (IQR) (£) | |
|---|---|---|
| Costs in first year (n = 85 702) | ||
| Total costs | ||
| Total cost | 3133 (6101) | 1149 (43–12 641) |
| Total CVD cost | 2199 (4632) | 735 (0–10 274) |
| Total CHD costs | 1780 (3686) | 685 (0–8413) |
| Hospitalizations | ||
| Inpatient costs | 2018 (5632) | 0 (0–10 603) |
| Inpatient CVD costs | 1487 (4493) | 0 (0–9333) |
| Inpatient CHD costs | 1067 (3548) | 0 (0–7284) |
| Primary care costs | 466 (463) | 361 (0–1291) |
| Diagnostic test costs | 141 (232) | 70 (0–538) |
| Drugs | ||
| All drug costs | 508 (1548) | 209 (0–1698) |
| CVD drug costs | 105 (113) | 84 (0–272) |
| Costs in first year following an event (n = 6599) | ||
| Total costs | ||
| Total cost | 10 377 (12 260) | 6855 (580–30 755) |
| Total CVD cost | 8916 (10 930) | 5819 (384–27 279) |
| Total CHD costs | 6869 (9467) | 3972 (159–23 336) |
| Hospitalizations | ||
| Inpatient costs | 8744 (11 554) | 5421 (0–27 436) |
| Inpatient CVD costs | 7957 (10 796) | 4871 (0–25 843) |
| Inpatient CHD costs | 5910 (9338) | 3127 (0–22 020) |
| Primary care costs | 589 (629) | 456 (0–1664) |
| Diagnostic test costs | 228 (311) | 131 (0–806) |
| Drugs | ||
| All drug costs | 816 (3135) | 293 (0–2924) |
| CVD drug costs | 142 (175) | 110 (0–376) |
Table A4.
Total healthcare costs per 90-day period
| Coefficient (£) | Standard error | Lower 95% CI (£) | Upper 95% CI (£) | |
|---|---|---|---|---|
| Background cost per 90 days | ||||
| Baseline (for a man aged 69 with stable angina and no other co-morbidities) | 341 | 10.15 | 322 | 361 |
| Baseline age (centred) | 7 | 0.42 | 6 | 7 |
| Female | −7 | 10.09 | −26 | 13 |
| Increase per 90 days | 10 | 0.18 | 10 | 11 |
| SCAD diagnosis (relative to stable angina) | ||||
| Other CHD | −20 | 12.40 | −44 | 5 |
| NSTEMI | 157 | 18.37 | 121 | 193 |
| STEMI | −26 | 21.48 | −68 | 17 |
| Unstable angina | 153 | 14.92 | 124 | 183 |
| CVD co-morbidities | ||||
| History of heart failure | 364 | 12.23 | 340 | 388 |
| History of atrial fibrillation | 186 | 14.32 | 158 | 214 |
| History of PAD | 327 | 17.80 | 292 | 362 |
| Non-CVD co-morbidities | ||||
| Diabetes | 338 | 13.71 | 312 | 365 |
| History of liver disease | 530 | 50.70 | 431 | 629 |
| History of COPD | 231 | 11.58 | 208 | 254 |
| History of cancer | 331 | 17.51 | 296 | 365 |
| History of renal disease | 756 | 20.93 | 715 | 797 |
| Incremental cost of non-fatal MI | ||||
| Cost in first 90-day period | 5028 | 34.41 | 4961 | £5096 |
| Cost in second 90-day period | 1282 | 36.84 | 1210 | £1354 |
| Cost in third 90-day period | 675 | 39.15 | 598 | £751 |
| Cost in fourth 90-day period | 692 | 40.84 | 612 | £772 |
| Cost in subsequent 90-day periods | 521 | 18.64 | 484 | £557 |
| Additional incremental cost of non-fatal MI for patients with diabetes | ||||
| Additional cost in first 90-day period | 776 | 75.79 | 627 | 924 |
| Cost in second 90-day period | 1100 | 81.56 | 940 | 1260 |
| Cost in third 90-day period | 785 | 87.01 | 614 | 955 |
| Cost in fourth 90-day period | 550 | 90.87 | 372 | 728 |
| Cost in subsequent 90-day periods | 369 | 42.16 | 286 | 451 |
| Incremental cost of non-fatal ischaemic stroke | ||||
| Cost in first 90-day period | 6215 | 36.01 | 6144 | 6285 |
| Cost in second 90-day period | 1239 | 38.03 | 1164 | 1313 |
| Cost in third 90-day period | 795 | 41.05 | 714 | 875 |
| Cost in fourth 90-day period | 654 | 43.12 | 569 | 738 |
| Cost in subsequent 90-day periods | 564 | 19.97 | 525 | 604 |
| Incremental cost of non-fatal haemorrhagic stroke | ||||
| Cost in first 90-day period | 7011 | 125.41 | 6765 | 7257 |
| Cost in second 90-day period | 1767 | 138.80 | 1495 | 2039 |
| Cost in third 90-day period | 947 | 155.22 | 643 | 1252 |
| Cost in fourth 90-day period | 751 | 165.14 | 428 | 1075 |
| Cost in subsequent 90-day periods | 927 | 74.20 | 782 | 1073 |
| Cost of fatal events | ||||
| Fatal CVD event | 2008 | 24.88 | 1960 | 2057 |
| Fatal non-CVD event | 2240 | 20.16 | 2201 | 2280 |
Table A5.
Total cardiovascular disease-related healthcare costs
| Coefficient (£) | Standard error | Lower 95% CI (£) | Upper 95% CI (£) | |
|---|---|---|---|---|
| Background cost per 90 days | ||||
| Baseline (for a man aged 69 with stable angina and no other co-morbidities) | 224 | 7.74 | 209 | 239 |
| Baseline age (centred) | 6 | 0.32 | 6 | 7 |
| Female | −23 | 7.65 | −38 | −8 |
| Increase per 90 days | 7 | 0.15 | 6 | 7 |
| SCAD diagnosis (relative to stable angina) | ||||
| Other CHD | 2 | 9.39 | −16 | 21 |
| NSTEMI | 145 | 13.66 | 119 | 172 |
| STEMI | 29 | 15.77 | −2 | 60 |
| Unstable angina | 125 | 11.32 | 103 | 147 |
| CVD co-morbidities | ||||
| History of heart failure | 248 | 9.29 | 230 | 266 |
| History of atrial fibrillation | 221 | 10.90 | 199 | 242 |
| History of PAD | 242 | 13.53 | 216 | 269 |
| Non-CVD co-morbidities | ||||
| Diabetes | 194 | 10.43 | 173 | 214 |
| History of liver disease | 279 | 38.68 | 203 | 355 |
| History of COPD | 142 | 8.79 | 124 | 159 |
| History of cancer | 154 | 13.34 | 128 | 180 |
| History of renal disease | 418 | 16.07 | 386 | 449 |
| Incremental cost of non-fatal MI | ||||
| Cost in first 90-day period | 4854 | 29.55 | 4796 | 4911 |
| Cost in second 90-day period | 1209 | 31.64 | 1147 | 1271 |
| Cost in third 90-day period | 640 | 33.63 | 574 | 706 |
| Cost in fourth 90-day period | 675 | 35.08 | 606 | 744 |
| Cost in subsequent 90-day periods | 481 | 15.64 | 451 | 512 |
| Additional incremental cost of non-fatal MI for patients with diabetes | ||||
| Additional cost in first 90-day period | 674 | 65.05 | 546 | 801 |
| Cost in second 90-day period | 1042 | 70.02 | 904 | 1179 |
| Cost in third 90-day period | 660 | 74.71 | 514 | 807 |
| Cost in fourth 90-day period | 403 | 78.04 | 250 | 556 |
| Cost in subsequent 90-day periods | 280 | 35.33 | 210 | 349 |
| Incremental cost of non-fatal ischaemic stroke | ||||
| Cost in first 90-day period | 5957 | 30.95 | 5897 | 6018 |
| Cost in second 90-day period | 1151 | 32.69 | 1087 | 1215 |
| Cost in third 90-day period | 675 | 35.29 | 606 | 744 |
| Cost in fourth 90-day period | 539 | 37.08 | 466 | 612 |
| Cost in subsequent 90-day periods | 448 | 16.86 | 415 | 481 |
| Incremental cost of non-fatal haemorrhagic stroke | ||||
| Cost in first 90-day period | 6836 | 107.73 | 6625 | 7047 |
| Cost in second 90-day period | 1517 | 119.28 | 1284 | 1751 |
| Cost in third 90-day period | 585 | 133.45 | 324 | 847 |
| Cost in fourth 90-day period | 393 | 142.02 | 115 | 671 |
| Cost in subsequent 90-day periods | 670 | 62.69 | 547 | 792 |
| Cost of fatal events | ||||
| Fatal CVD event | 2071 | 21.30 | 2029 | 2113 |
| Fatal non-CVD event | 1737 | 17.28 | 1703 | 1771 |
Table A6.
Total coronary heart disease-related healthcare costs
| Coefficient (£) | Standard error | Lower 95% CI (£) | Upper 95% CI (£) | |
|---|---|---|---|---|
| Background cost per 90 days | ||||
| Baseline (for a man aged 69 with stable angina and no other co-morbidities) | 179 | 5.93 | 167 | 191 |
| Baseline age (centred) | 4 | 0.24 | 4 | 5 |
| Female | −23 | 5.85 | −35 | −12 |
| Increase per 90 days | 4 | 0.12 | 3 | 4 |
| SCAD diagnosis (relative to stable angina) | ||||
| Other CHD | 82 | 7.17 | 68 | 97 |
| NSTEMI | 219 | 10.49 | 198 | 239 |
| STEMI | 111 | 12.04 | 88 | 135 |
| Unstable angina | 163 | 8.65 | 146 | 179 |
| CVD co-morbidities | ||||
| History of heart failure | 143 | 7.11 | 129 | 157 |
| History of atrial fibrillation | 84 | 8.34 | 67 | 100 |
| History of PAD | 161 | 10.35 | 141 | 181 |
| Non-CVD co-morbidities | ||||
| Diabetes | 144 | 7.98 | 128 | 160 |
| History of liver disease | 198 | 29.62 | 140 | 256 |
| History of COPD | 117 | 6.72 | 104 | 131 |
| History of cancer | 107 | 10.21 | 87 | 127 |
| History of renal disease | 201 | 12.34 | 176 | 225 |
| Incremental cost of non-fatal MI | ||||
| Cost in first 90-day period | 4658 | 23.69 | 4612 | 4705 |
| Cost in second 90-day period | 1166 | 25.36 | 1116 | 1215 |
| Cost in third 90-day period | 590 | 26.96 | 538 | 643 |
| Cost in fourth 90-day period | 642 | 28.13 | 587 | 697 |
| Cost in subsequent 90-day periods | 475 | 12.40 | 451 | 500 |
| Additional incremental cost of non-fatal MI for patients with dibetes | ||||
| Additional cost in first 90-day period | 643 | 52.14 | 541 | 745 |
| Cost in second 90-day period | 792 | 56.13 | 682 | 902 |
| Cost in third 90-day period | 701 | 59.90 | 584 | 819 |
| Cost in fourth 90-day period | 330 | 62.57 | 207 | 453 |
| Cost in subsequent 90-day periods | 269 | 28.01 | 215 | 324 |
| Incremental cost of non-fatal ischaemic stroke | ||||
| Cost in first 90-day period | 3029 | 24.82 | 2981 | 3078 |
| Cost in second 90-day period | 620 | 26.22 | 568 | 671 |
| Cost in third 90-day period | 415 | 28.31 | 359 | 470 |
| Cost in fourth 90-day period | 262 | 29.74 | 203 | 320 |
| Cost in subsequent 90-day periods | 256 | 13.42 | 230 | 282 |
| Incremental cost of non-fatal haemorrhagic stroke | ||||
| Cost in first 90-day period | 2874 | 86.38 | 2704 | 3043 |
| Cost in second 90-day period | 790 | 95.66 | 602 | 977 |
| Cost in third 90-day period | 218 | 107.04 | 8 | 428 |
| Cost in fourth 90-day period | 301 | 113.92 | 77 | 524 |
| Cost in subsequent 90-day periods | 251 | 49.89 | 154 | 349 |
| Cost of fatal events | ||||
| Fatal CVD event | 1407 | 17.06 | 1374 | 1441 |
| Fatal non-CVD event | 1068 | 13.84 | 1040 | 1095 |
Table A7.
Covariate profiles based on deciles of 5-year risk
| Patient average covariate profiles based on deciles of 5-year risk of composite CVD first event | ||||||||||
| Risk decile | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 5-year risk (average) | 3.69% | 5.70% | 7.37% | 9.15% | 11.20% | 13.71% | 17.14% | 22.14% | 30.42% | 52.37% |
| Re-calculated 5-year risk | 3.46% | 5.43% | 6.95% | 8.53% | 10.36% | 12.57% | 15.64% | 20.07% | 27.23% | 44.18% |
| Socio-demographic characteristics | ||||||||||
| Sex (female) | 64% | 48% | 42% | 39% | 37% | 37% | 38% | 42% | 44% | 46% |
| Age (if male) | 49 | 55 | 59 | 62 | 65 | 67 | 71 | 74 | 77 | 81 |
| Age (if female) | 53 | 62 | 67 | 70 | 73 | 75 | 78 | 80 | 83 | 87 |
| Age (weighted average) | 52 | 59 | 62 | 65 | 68 | 70 | 73 | 76 | 80 | 84 |
| Most deprived quintile | 15% | 17% | 18% | 19% | 20% | 21% | 21% | 22% | 22% | 24% |
| SCAD diagnosis and severity | ||||||||||
| Other CHD | 11% | 17% | 20% | 22% | 24% | 24% | 25% | 26% | 25% | 20% |
| NSTEMI | 0% | 1% | 3% | 5% | 8% | 10% | 12% | 17% | 23% | 43% |
| STEMI | 1% | 4% | 8% | 12% | 13% | 14% | 13% | 9% | 6% | 4% |
| Unstable angina | 10% | 13% | 12% | 12% | 12% | 12% | 13% | 15% | 17% | 15% |
| Stable angina | 78% | 65% | 56% | 49% | 43% | 39% | 37% | 34% | 29% | 18% |
| PCI in last 6 months | 9% | 12% | 13% | 14% | 13% | 13% | 11% | 9% | 6% | 4% |
| CABG in last 6 months | 9% | 7% | 6% | 5% | 5% | 4% | 4% | 3% | 2% | 1% |
| Previous/recurrent MI | 2% | 6% | 10% | 14% | 18% | 23% | 26% | 29% | 32% | 43% |
| Use of nitrates | 10% | 16% | 19% | 21% | 24% | 28% | 33% | 37% | 43% | 56% |
| CVD risk factors | ||||||||||
| Current smoker | 31% | 35% | 36% | 37% | 38% | 38% | 37% | 35% | 32% | 30% |
| Ex-smoker | 27% | 30% | 31% | 32% | 32% | 33% | 34% | 34% | 34% | 34% |
| Never smoked | 41% | 35% | 33% | 31% | 30% | 29% | 29% | 31% | 33% | 36% |
| Hypertension | 69% | 70% | 71% | 71% | 72% | 74% | 76% | 79% | 83% | 87% |
| Diabetes | 4% | 8% | 10% | 12% | 14% | 16% | 18% | 21% | 24% | 32% |
| Total cholesterol (mmol/L) | 4.95 | 4.91 | 4.84 | 4.79 | 4.74 | 4.74 | 4.70 | 4.68 | 4.64 | 4.54 |
| HDL (mmol/L) | 1.41 | 1.37 | 1.35 | 1.35 | 1.35 | 1.35 | 1.36 | 1.37 | 1.37 | 1.35 |
| CVD co-morbidities | ||||||||||
| Heart failure | 5% | 7% | 9% | 12% | 15% | 19% | 27% | 37% | 52% | 73% |
| Peripheral arterial disease | 1% | 2% | 3% | 4% | 6% | 8% | 10% | 13% | 16% | 25% |
| Atrial fibrillation | 3% | 5% | 7% | 9% | 10% | 13% | 16% | 21% | 29% | 43% |
| Stroke | 0% | 1% | 1% | 2% | 3% | 5% | 8% | 14% | 22% | 39% |
| Non-CVD co-morbidities | ||||||||||
| Chronic kidney disease | 2% | 2% | 3% | 4% | 4% | 5% | 7% | 9% | 12% | 20% |
| Chronic obstructive pulmonary disease | 20% | 20% | 20% | 21% | 22% | 23% | 25% | 27% | 28% | 30% |
| Cancer | 4% | 5% | 6% | 7% | 8% | 9% | 11% | 13% | 14% | 12% |
| Chronic liver disease | 0% | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 1% |
| Psychosocial characteristics | ||||||||||
| Depression at diagnosis | 20% | 17% | 15% | 15% | 14% | 14% | 15% | 17% | 18% | 21% |
| Anxiety at diagnosis | 7% | 6% | 6% | 7% | 7% | 7% | 8% | 8% | 10% | 12% |
| Biomarkers | ||||||||||
| Heart rate (b.p.m.) | 72 | 71 | 71 | 71 | 71 | 71 | 72 | 73 | 74 | 76 |
| Creatinine (mmol/L) | 88 | 92 | 95 | 96 | 98 | 100 | 101 | 104 | 109 | 125 |
| White cell count (109/L) | 6.81 | 7.05 | 7.19 | 7.31 | 7.44 | 7.54 | 7.62 | 7.76 | 7.88 | 8.22 |
| Haemoglobin (g/100 mL) | 14.26 | 14.26 | 14.16 | 14.05 | 13.88 | 13.70 | 13.48 | 13.16 | 12.81 | 12.20 |
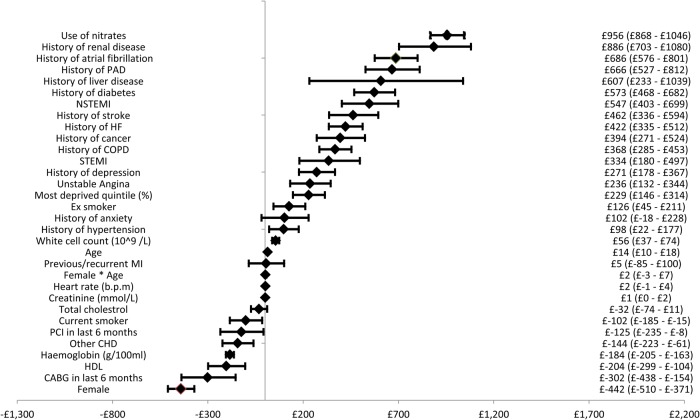
Figure A1.
Forest plot of cardiovascular disease costs.
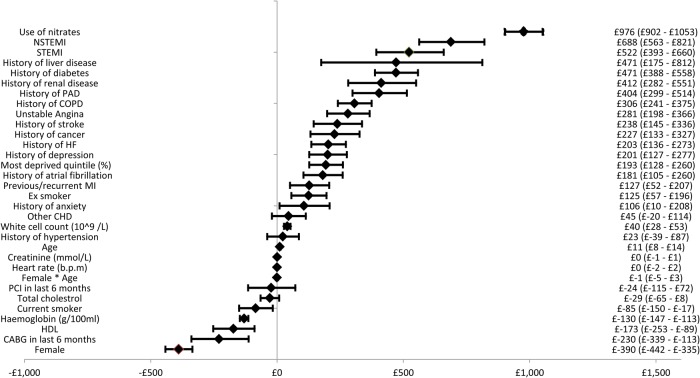
Figure A2.
Forest plot of coronary heart disease costs.
Funding
The study was funded by the UK National Institute for Health Research (NIHR) (RP-PG-0407-10314) and the Wellcome Trust (WT 086091/Z/08/Z) and was supported by the Farr Institute of Health Informatics Research, funded by The Medical Research Council (K006584/1) in partnership with Arthritis Research UK, the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Engineering and Physical Sciences Research Council, the NIHR, the National Institute for Social Care and Health Research (Welsh Assembly Government), the Chief Scientist Office (Scottish Government Health Directorates), and the Wellcome Trust. The views and opinions expressed within the paper do not necessarily reflect those of the NIHR or the UK Department of Health. A.D.S. is supported by a Wellcome Trust clinical research training fellowship (0938/30/Z/10/Z). C.P.G. is funded by the National Institute for Health Research (NIHR-CTF-2014-03-03) as Associate Professor and Honorary Consultant Cardiologist. This work made use of the facilities of N8 HPC provided and funded by the N8 consortium and EPSRC (Grant No. EP/K000225/1). The Centre is co-ordinated by the Universities of Leeds and Manchester. Funding to pay the Open Access publication charges for this article was provided by University College London.
Conflict of interest: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that S.W., M.A., S.P., C.P.G., A.D.S., M.C., and H.H. have nothing to disclose. A.M. reports and currently sits on one of the NICE Technology Appraisal Committees. M.S. received grant funding for the work reported in this paper from the National Institute for Health Research. Outside of the published work, M.S. has received personal fees from various pharmaceutical and medical device companies, some of which have products used in cardiovascular disease. A.T. reports personal fees from Menarini Pharmaceuticals, other from Servier, outside the submitted work. K.A. reports personal fees from ABPI, Roche, Novo Nordisk, AstraZeneca, Janssen, and Allergan, outside the submitted work.
References
- 1. Gale CP, Cattle BA, Woolston A, Baxter PD, West TH, Simms AD, Blaxill J, Greenwood DC, Fox KAA, West RM. Resolving inequalities in care? Reduced mortality in the elderly after acute coronary syndromes. The Myocardial Ischaemia National Audit Project 2003–2010. Eur Heart J 2012;33:630–639. [DOI] [PubMed] [Google Scholar]
- 2. Hall M, Alabas O, Dondon T, Jernberg T, Gale C. Use of relative survival to evaluate non ST-elevation myocardial infarction quality of care and clinical outcomes. EHJQCCO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gale CP, Allan V, Cattle BA, Hall AS, West RM, Timmis A, Gray HH, Deanfield J, Fox KAA, Feltbower R. Trends in hospital treatments, including revascularisation, following acute myocardial infarction, 2003–2010: a multilevel and relative survival analysis for the National Institute for Cardiovascular Outcomes Research (NICOR). Heart 2014;100:582–589. [DOI] [PubMed] [Google Scholar]
- 4. Alabas OA, Allan V, McLenachan JM, Feltbower R, Gale CP. Age-dependent improvements in survival after hospitalisation with acute myocardial infarction: an analysis of the Myocardial Ischemia National Audit Project (MINAP). Age Ageing 2014;43:779–785. [DOI] [PubMed] [Google Scholar]
- 5. Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; ESC Committee for Practice Guidelines, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 6. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, Kligfield PD, Krumholz HM, Kwong RYK, Lim MJ, Linderbaum A, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Smith SC, Spertus JA, Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60:e44–e164. [DOI] [PubMed] [Google Scholar]
- 7. Kolandaivelu K, Leiden BB, O'Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J 2014;35:3267–3276. [DOI] [PubMed] [Google Scholar]
- 8. Rapsomaniki E, Shah A, Perel P, Denaxas S, George J, Nicholas O, Udumyan R, Feder GS, Hingorani AD, Timmis A, Smeeth L, Hemingway H. Prognostic models for stable coronary artery disease based on electronic health record cohort of 102 023 patients. Eur Heart J 2014;35:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mark DB, Naylor CD, Hlatky MA, Califf RM, Topol EJ, Granger CB, Knight JD, Nelson CL, Lee KL, Clapp-Channing NE, Sutherland W, Pilote L, Armstrong PW. Use of medical resources and quality of life after acute myocardial infarction in Canada and the United States. N Engl J Med 1994;331:1130–1135. [DOI] [PubMed] [Google Scholar]
- 10. Reed SD, Radeva JI, Weinfurt KP, McMurray JJ, Pfeffer MA, Velazquez EJ, Allsbrook JS, Masselink LE, Sellers MA, Califf RM, Schulman KA, Valiant I. Resource use, costs, and quality of life among patients in the multinational Valsartan in Acute Myocardial Infarction Trial (VALIANT). Am Heart J 2005;150:323–329. [DOI] [PubMed] [Google Scholar]
- 11. Briggs A, Mihaylova B, Sculpher M, Hall A, Wolstenholme J, Simoons M, Deckers J, Ferrari R, Remme WJ, Bertrand M, Fox K. Cost effectiveness of perindopril in reducing cardiovascular events in patients with stable coronary artery disease using data from the EUROPA study. Heart 2007;93:1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gallagher A, Puri S, van Staa T. Linkage of the general practice research database (GPRD) with other data sources. Pharmacoepidemiol Drug Saf 2011;20:S230–S367. [Google Scholar]
- 13. Mathur R, Bhaskaran K, Chaturvedi N, Leon DA, van Staa T, Grundy E, Smeeth L. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J Public Health (Oxf) 2014;36:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrett E, Shah AD, Boggon R, Denaxas S, Smeeth L, van Staa T, Timmis A, Hemingway H. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ 2013;346:f2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Denaxas SC, George J, Herrett E, Shah AD, Kalra D, Hingorani AD, Kivimaki M, Timmis AD, Smeeth L, Hemingway H. Data resource profile: cardiovascular disease research using linked bespoke studies and electronic health records (CALIBER). Int J Epidemiol 2012;41:1625–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curtis L. Unit Costs of Health and Social Care 2012. http://www.pssru.ac.uk/project-pages/unit-costs/2012/. [Google Scholar]
- 17. Department of Health. NHS reference costs: financial year 2011 to 2012. https://www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012. [Google Scholar]
- 18. Health and Social Care Information Centre. Prescription cost analysis - England, 2012. http://www.hscic.gov.uk/catalogue/PUB10610. [Google Scholar]
- 19. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 20. Asaria M, Walker S, Palmer S, Gale CP, Shah AD, Abrams K, Crowther M, Manca A, Timmis A, Hemingway H, Sculpher M. Using electronic health records to predict costs and outcomes in chronic disease using the example of stable coronary artery disease. Heart (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. NICE. Guide to the methods of technology appraisal. Regulation 2008;26:76. [DOI] [PubMed] [Google Scholar]
- 22. Hippisley-Cox J, Vinogradova Y. Trends in Consultation Rates in General Practice 1995 to 2008: Analysis of the QRESEARCH Database. Nottingham, UK: QRESEARCH; 2009. p1–23. http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Trends+in+Consultation+Rates+in+General+Practice+1995+to+2008+:+Analysis+of+the+QResearch+®#0(24 November 2014). [Google Scholar]
- 23. Kasteridis P, Street A, Dolman M, Gallier L, Hudson K, Martin J, Wyer I. The importance of multimorbidity in explaining utilisation and costs across health and social care settings: evidence from South Somersets Symphony Project. CHE Res Pap 96. 2014. http://ideas.repec.org/p/chy/respap/96cherp.html(24 November 2014). [Google Scholar]
- 24. Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, Blumenthal D. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med 2007;22(Suppl 3):391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luengo-Fernandez R, Gray AM, Rothwell PM, Oxford Vascular S. A population-based study of hospital care costs during 5 years after transient ischemic attack and stroke. Stroke 2012;43:3343–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]