Abstract
Metastasizing prostate tumor cells invade along nerves innervating the encapsulated human prostate gland in a process known as perineural invasion. The extacellular matrix laminin class of proteins line the neural route and tumor cells escaping from the gland express the laminin binding integrin α6β1 as a prominent cell surface receptor. Integrin α6β1 promotes aggressive disease and supports prostate tumor cell metastasis to bone. Laminins and their integrin receptors are necessary for the development and maintenance of the peripheral nervous system, indicating the potential role for integrin receptors in directing prostate tumor cell invasion on nerves during perineural invasion.
A general model for metastasis consists of a multi-step process involving tumor cell interactions with the surrounding environment. These steps include a loss of cellular adhesion and detachment, local invasion of the host extracellular matrix, intravasation into the vasculature or lymphatics, and extravasation into the parenchyma of distant tissues. Neurotropic cancers however, including prostate, pancreatic, head and neck, and colorectal cancer exhibit remarkable affinity for and utilization of the complex neuroanatomy of highly innervated organs as a means for primary tumor cell escape (Liebig et al., 2009). Prostate tumor cell migration and invasion in prostatic nerves and neurovascular bundles, a process known as perineural invasion (PNI), is a major route for extracapsular extension during prostate cancer metastasis (Villers et al., 1989). Accordingly, the clinical significance of perineural invasion as a major factor contributing to prostate cancer metastasis and as a predictor for poor patient outcome has been documented (Harnden et al., 2007; Lee et al., 2007; Liebig et al., 2009).
Prostate cancer is multifocal and heterogeneous, and is a slow growing solid tumor (Johansson et al., 1997, 2004). Confined and localized prostate cancer is considered curable while invasion through the prostate capsule and development of metastases is associated with poor prognosis. Current diagnostic procedures do not provide accurate early detection or define individuals who will develop aggressive and incurable metastatic disease. This is a significant clinical problem considering prostate carcinoma is the most commonly diagnosed visceral neoplasm and second leading cause of death in men (Jemal et al., 2007). Discovery of novel prognostic indicators or molecular targets which directly relate to tumor cell metastasis along nerves will enable a dramatic shift in the approaches used to currently treat the disease, including potentially improving nerve sparing radical prostatectomy approaches.
The laminin class of extracellular matrix proteins are present in the neural route (Ryschich et al., 2009) and prostate tumor cells expressing the α6β1 integrin invade along prostatic nerves. The laminin receptor integrin α6β1 is essential during peripheral nerve development and our group has demonstrated its major role in regulating prostate tumor cell migration and invasion (Pawar et al., 2007) and prostate tumor metastasis to bone (Ports et al., 2009; Sroka et al., 2009). This review will discuss the novel role of integrin α6 in directing prostate tumor cell adhesion and invasion on nerves during perineural invasion. Establishing a comprehensive understanding of the interdependence between increased laminin dependent invasion of tumor cells and nerves during PNI will provide framework for developing pertinent biomarkers or targeted therapies for isolating and treating patients exhibiting aggressive disease. Inhibiting tumor cell escape along nerves in the prostate will be instrumental for future clinical control of the disease.
Laminin Adhesion and Prostate Cancer Progression
Integrins are non-covalently bound heterodimeric cell surface receptors which play a major role in cell adhesion and migration. The integrins are heterodimer receptors consisting of 24 members comprised of 18α and 8β subunits all encoded by distinct genes (van der Flier and Sonnenberg, 2001; Hynes, 2002). These receptors are tissue specific and regulate cell locomotion and intracellular signaling events by binding to their respective ligands in the extracellular environment while simultaneously interacting with intracellular signaling components. The laminin binding integrin alpha subunits including α6 (CD49f), α3 (CD49C), and α7 are among the most highly conserved of all integrin family receptors (Hughes, 2001; Huhtala et al., 2005) and play a significant role in both normal and pathological conditions.
The role of laminin binding integrins and laminin extracellular matrix proteins in regulating cell adhesion and migration events during prostate cancer progression has been extensively studied by our group and others. Altered expression or function of extracellular matrix and adhesion molecules including laminins and integrins is accompanied by acquisition of motility and invasiveness in prostate cancer and is therefore a significant event during the natural progression of the disease (Nagle et al., 1995). The major expression patterns of integrin family members and their role in directing cellular signaling in normal and neoplastic prostate glands have been reviewed (Goel et al., 2008, 2009). We and others have shown that several integrins including the α2β1 collagen receptor, α3β1 and α6β1 laminin receptors, the α5β1 fibronectin receptor, the αvβ1 vitronectin receptor and the α6β4 hemidesmosome laminin receptor are all expressed in normal prostate glands (Bonkhoff et al., 1993; Knox et al., 1994; Nagle et al., 1995; Davis et al., 2001), while only the laminin binding integrins α6β1 and α3β1 are expressed in prostate carcinoma (Schmelz et al., 2002). Attachment of epithelial cells to the substratum in normal prostate cells primarily occurs via integrin α6β4 to laminin-332 in highly adhesive hemidesmosome structures. The disruption of α6β4 adhesions between quiescent prostatic basal cells and the substratum in prostate cancer occurs from the absence of the integrin β4 subunit. This enables engagement of the α6β1 and α3β1 laminin receptors and the pro-migratory phenotype of luminal epithelial cells during prostate cancer progression.
Coincident with expression changes observed with laminin binding integrins, the laminin extracellular matrix proteins are also modified during prostate cancer progression. Laminins are a family of heterotrimeric glycoproteins consisting of one α, β, and γ subunit. In vertebrates, five α, three β, and three γ chains have been identified and all isoforms are encoded by distinct genes. The α chains are encoded by the LAMA gene family, the β chains by the LAMB family and the γ chains by the LAMC family. Our group has identified that laminin-332 (α3β3γ2) and -511 (α5β1γ1) are expressed in normal prostate glands while only laminin-511 persists in prostate cancer (Hao et al., 1996; Bair et al., 2005). The crucial role of laminin-332 in maintaining basal lamina integrity and epidermal adhesion was initially identified in the severe and lethal blistering skin disease Herlitz’s junctional epidermolysis bullosa (JEB) (Aberdam et al., 1994; Pulkkinen et al., 1994; Uitto et al., 1997; Nakano et al., 2000). Specific mutations in any of the three laminin-332 genes (LAMA3 (α3), LAMB3 (β3), and LAMC2 (γ2)) results in complete loss of laminin-332 leading to defective adhesion structures and the blistering phenotype (Pulkkinen and Uitto, 1999). The decrease in laminin-332 and integrin β4 expression and the persistent expression of its ligand laminin-511 illustrate the significance of α6β1 regulated laminin adhesion events in prostate cancer invasion.
The retention of α6β1expression in human prostate cancer implicates a potential role for laminin dependent adhesion in prostate cancer progression. Indeed, our group has identified a novel structural variant of α6β1, α6pβ1, specific to human cancer tissue and tumor cell lines (Davis et al., 2001; Demetriou et al., 2004). The structural variant is formed on the surface of tumor cells through proteolytic cleavage of the extracellular laminin binding domain by the serine protease urokinase plasminogen activator (uPA) at amino acid residues R594 and R595 (Davis et al., 2001; Demetriou and Cress, 2004; Demetriou et al., 2004; Pawar et al., 2007). This post-translational modification of integrin α6β1 increases prostate tumor cell migration and invasion on laminin substrates (Pawar et al., 2007) and promotes metastasis to the laminin rich bone microenvironment (Ports et al., 2009). Extracellular domain cleavage of α6 to α6p is necessary for normal development in Xenopus laevi (Demetriou et al., 2008) implicating the role for post-translational cleavage of α6 in both normal and pathological conditions.
Laminin Receptors and the Peripheral Nervous System
Substantial evidence exists for the role of laminins and laminin receptors in the peripheral nervous system (PNS) (Colognato et al., 2005; Feltri and Wrabetz, 2005). Laminins are one of the most abundant extracellular matrix proteins in the PNS. Specific laminin family members play a significant role in neuronal regeneration and development. Studies have identified laminin-211, -411, and -511 expression in the PNS and each are required for peripheral nerve fiber formation (Feltri and Wrabetz, 2005). Laminins initiate signaling and regulate cellular functions on Schwann cells which sort and myelinate neurons for proper function of the nervous system (Chernousov et al., 2008). Laminin α4 deficient mice demonstrate defects in hypomyelination and axonal sorting of peripheral nerves, and mutations in the laminin α2 gene cause peripheral neuropathy (Wallquist et al., 2005; Yang et al., 2005). It has also been shown that inhibition of Schwann cell specific expression of the laminin γ1 chain in an in vivo model disrupts Schwann cell myelination and axonal sorting (Chen and Strickland, 2003).
Engagement of laminin through integrin receptors is a requirement for Schwann cell regulated myelination of axons in the peripheral nervous system during various stages of development (Yu et al., 2007). Specifically, the α6β1 and α6β4 integrins are laminin receptors in Schwann cells and the nerve sheath (Niessen et al., 1994; Terpe et al., 1994; Dubovy et al., 1999) and the α6β1 receptor is necessary for axonal sorting by Schwann cells (Feltri and Wrabetz, 2005) and early nervous system development (Lallier et al., 1996). Studies using Schwann cell specific knock out of the β4 subunit in vivo demonstrated that the receptor is necessary for nerve regeneration and myelination (Van der Zee et al., 2008). Interestingly, recent studies have shown that a significant percentage of human neural progenitor cells express the α6 integrin subunit (Flanagan et al., 2006; Hall et al., 2006; Mueller et al., 2006), and the significance of α6β1 and laminin dependent adhesion of neuronal stem cells and neural progenitor expansion has also been reported (Ma et al., 2008). In this study, human embryonic cell-derived progenitor expansion, migration and differentiation was most responsive to laminin extracellular matrix and was abrogated when α6 integrin function was blocked.
Perineural Invasion in Prostate Cancer
The human prostate gland is a complex tubulo-alveolar gland with regions defined by concentric zones including the anterior fibromuscular stroma, the central zone, the peripheral zone, and the transition zone (McNeal, 1981; Timms, 2008). The prostate gland is completely surrounded by a smooth muscle casing known as the prostate capsule. The duct-acinar system of the prostate is comprised of luminal, neuroendocrine and underlying basal cells surrounded by a thin basal lamina (Ware, 1994). The substratum surrounding normal acini is comprised of laminin-332, laminin-511, collagen IV, and collagen VII, and forms a contiguous lining which surrounds the basal cell layer (Knox et al., 1994; Nagle et al., 1995). The smooth muscle stroma of the human prostate gland is permeated by the cavernous nerve and neurovascular formations of the pelvic plexus which are comprised of autonomic nerves (Dunzendorfer et al., 1976; Vaalasti and Hervonen, 1980; Gil-Vernet, 1996; Schwalenberg et al., 2009). Anatomic variation does exist regarding the neuroanatomical structures of the male pelvis and prostate gland (Schwalenberg et al., 2009). However, it has been identified that there is significant nerve innervation in the peripheral zone of the prostate gland, which is the predominant site for prostate tumor development (Chang et al., 1998; Powell et al., 2005).
Perineural invasion is a major route for extraprostatic extension during prostate cancer metastasis (Liebig et al., 2009; Marchesi et al., 2010). Prostate tumor cells migrate and invade along all three structural components of the prostatic nerves, including the perineurium, epineurium and the innermost endoneurial sheath during PNI (Fig. 1). The clinical implication of PNI as a reliable prognostic indicator for disease recurrence has been noted (Freeman, 2009) and several studies have identified PNI as an independent and significant prognostic factor for the presence of tumor cell extension beyond the prostate (Ukimura et al., 1998; Vargas et al., 1999; de la Taille et al., 1999). Recent evidence has also shown that patients with prostate cancer have increased axonal and neurite outgrowth in cancerous regions, implying that tumor cell motility and invasiveness along nerves is not a tumor cell-autonomous process, but a reciprocal relationship between nerves and tumor cells to promote invasion (Ayala et al., 2008).
Fig. 1.
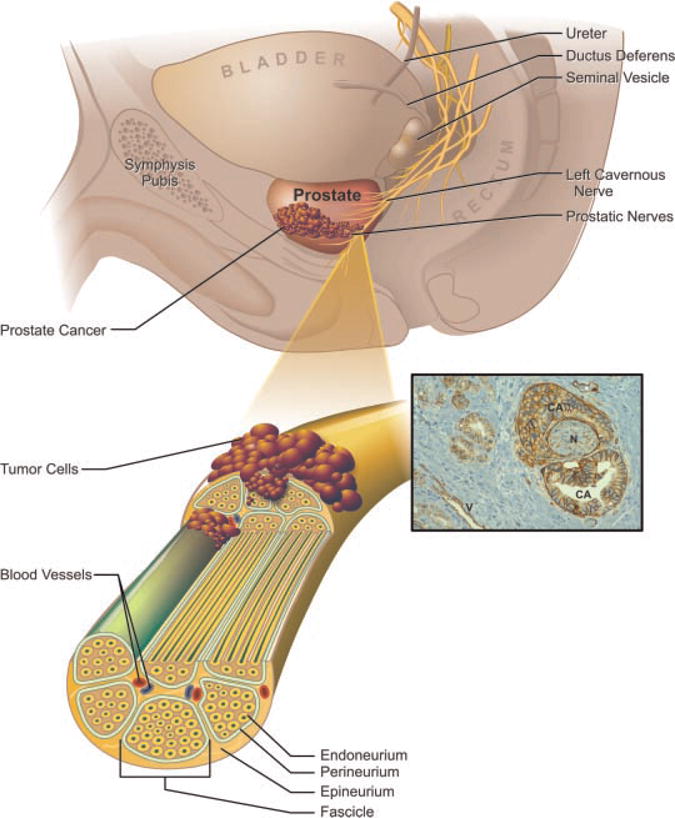
The prostatic nerve plexus breaches the prostate capsule and penetrates into the peripheral zone of the prostate gland where most prostate tumors develop. The layers of the nerve sheath include the epineurium, perineurium and endoneurium from outside in, respectively. The perineurium encompasses a bundle of nerves called a fascicle. Perineural and endoneural invasion of prostate tumor cells along the nerve route promotes extra prostatic escape. Inset: During tumor invasion on prostatic nerves, human prostate tumor cells express high levels of the α6 integrin (brown). Note the absence of cancer cell (CA) invasion along vessels (V) when compared to significant invasion of the nerve (N). Integrin α6 is expressed around vessels as expected. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.
Since the description by Walsh et al. (1983) of anatomical nerve sparing radical retropubic prostatectomy the ability of urologists to preserve erectile function has improved. The posterolateral and extraprostatic location of the neurovascular bundles should not compromise surgical margins in cases in which the cancer is truly organ confined. Pathological evidence of cancer extending through the prostatic capsule (pT3) is associated with an increased risk of positive surgical margins, and potential disease recurrence. Thus in some pT3 cases preservation of the neurovascular bundles may adversely affect surgical margin status. Examination of the prognostic role of perineural invasion in prostatectomy specimens has demonstrated a significant correlation between perineural invasion and extraprostatic extension (Vargas et al., 1999). While the clinical utility of this prognostic information remains controversial, many urologic surgeons are using this information to determine extent of resection at the time of prostatectomy.
Molecular Mechanisms of Prostate Cancer Perineural Invasion
Detailed molecular mechanisms describing the characteristics of tumor cells and nerves in the prostate cancer PNI microenvironment have been hampered due to difficulties in creating an accurate representation of the disease state in in vitro and in vivo models. However, several groups have established unique model systems in which analyses of tumor and nerve interactions can be observed. Ayala et al. established that nerves and prostate tumor cells exhibit increased growth and reciprocal interactions in a co-culture model (Ayala et al., 2001). In this model, prostate tumor cells were grown in Matrigel with adult mouse dorsal root ganglia. Directed neurite outgrowth toward tumor cells and tumor cell migration along penetrating neurite extensions was observed. Their group also identified increased expression of anti-apoptotic regulators such as nuclear factor kappa β and Pim-2 in prostate tumor cells growing adjacent to nerves (Ayala et al., 2004; Dai et al., 2005) in prostate tumor cells growing adjacent to nerves. Further work by Cornell et al. identified the requirement of prostate stroma in mediating reciprocal interactions between malignant prostate cells and nerves during PNI using the mouse dorsal root ganglia and Matrigel model (Cornell et al., 2003). Other groups have identified increased expression of the mir-244 miRNA in tumor cells associated with prostatic nerves (Prueitt et al., 2008) and increased expression of the chemokine CXCL12 and its associated receptor CXCR4 (Zhang et al., 2008).
The nerve rich microenvironment may also provide a niche for tumor cells to adapt and alter expression of genes necessary for maintaining survival and an aggressive phenotype prior to executing the metastatic program. Indeed, a recent study has shown that prostate tumor cells undergoing active PNI harbor a specific transcriptome signature (Prueitt et al., 2008). The gene expression changes noted in this study associated with PNI were related to changes in cell metabolism and mitochondrial function, while earlier studies demonstrate decreased apoptosis and increased survival in tumor cells undergoing PNI (Yang et al., 1996; Ayala et al., 2004, 2006a). Specific gene expression changes have also been noted during PNI in pancreatic cancer using both in vitro and in vivo models (Koide et al., 2006; Dai et al., 2007). These studies along with the finding that prostate cancer leads to increased neurogenesis (Ayala et al., 2008) established that the PNI microenvironment facilitates a growth and survival advantage for both nerves and prostate tumor cells migrating along nerves.
Cell Adhesion Molecules and Perineural Invasion
The role of tumor cell adhesion molecules in regulating PNI during disease progression has been most extensively studied in pancreatic PNI models. Recent work by Marchesi et al. (2008) identified the role of the transmembrane chemokine CX3CL1 and its receptor CX3CR1 in the adhesion and migration of pancreatic tumor cells on nerves during pancreatic cancer perineural invasion. In this study, activation of β1 integrins and focal adhesion kinase (FAK) was necessary for CX3CR1 mediated pancreatic tumor cell neurotropism. Additional work by Gil et al. (2010) determined the role of the glial cell-derived neurotrophic factor (GDNF), a chemoattractant secreted by nerves, in regulating pancreatic tumor cell adherence and invasion on nerves. The transmembrane mucin known as MUC1 expressed on pancreatic tumor cells also plays a role in pancreatic tumor cell adhesion during PNI by binding to myelin-associated glycoprotein (MAG) on Schwann cells (Swanson et al., 2007). Ryschich et al. identified expression of laminin in the perineural space of nerves in pancreatic cancer and increased migration and invasion of pancreatic cells on laminin substrates was observed during pancreatic cancer PNI (Ryschich et al., 2009). Recent work has also demonstrated that inhibition of melanoma cell adhesion molecule (MCAM or CD146) using short hairpin silencing RNA in an adenoid cystic carcinoma cell line decreased perineural invasion in vitro (Chen et al., 2009).
Studies specifically defining the role of adhesion molecules in prostate cancer PNI are needed. We hypothesize that prostate tumor cells depend on tumor cell adhesion to laminin rich nerves during PNI for promoting tumor cell invasion on nerves. Sinha et al. previously demonstrated laminin expression in prostatic nerves in human prostate cancer tissue (Sinha et al., 1989). In Table 1, we also summarize our findings regarding laminin chain expression in human prostate, including normal, cancer, and regions of PNI. Interestingly, the prominent laminin chains expressed in neoplastic lesions and PNI comprise the ligands of integrin α6, the laminin α5, α4, and γ1 chains (Table 1). Further, we have identified remarkable expression of the integrin α6 receptor in tumor cells actively invading prostatic tumors during PNI (Figs. 1 and 2A,B). Importantly, Figure 2A,B illustrates remarkable differences in the manner in which tumor cells actively invade along the prostatic nerves. Tumor cells can be observed invading both the endoneurium and perineurium (2A) and integrin α6 expression is observed in tumor cells undergoing both perineural and endoneural invasion. It is currently unknown whether tumor cell invasion in the perineural versus endoneural regions of nerves reflects differences in their metastatic phenotype. We speculate that the metastatic advancement of more differentiated glandular structures (Fig. 2B) varies greatly from less differentiated tumors which invade both the endoneurium and perineurium (Fig. 2A). Future studies to dissect the importance of the variability in how tumor cells invade along nerves and the role laminin dependent adhesion plays in this process will be the focus of ongoing studies in our laboratory.
TABLE 1.
Laminin chain expression in prostate cancer and PNI
| Laminin chain | Normal prostate glandular tissue | Prostate cancer | PNI prostatic nerves | Antibody clone |
|---|---|---|---|---|
| α3 | +++ | ++ | ND | BM165 |
| α4 | +++ | ++ | +++ | FC10 |
| α5 | +++ | +++ | +++ | 4C7 |
| β2 | ++++ | − | − | C4 |
| β3 | ++ | − | − | Clone 17 |
| γ1 | +++ | ++ | +++ | 2E8 |
| γ2 | +++ | − | − | GB3 |
Code: +++ high expression, ++ moderate expression, − absence of expression, ND, not determined. Frozen human prostate cancer specimens were reacted with laminin chain specific antibodies as indicated followed by incubation with Alexa Fluor secondary antibodies (Molecular Probes, Eugene, OR). Immunofluorescence was observed with a confocal laser scanning microscope (Carl Zeiss, Germany). The laminin α3 chain BM165 antibody has been described previously (Sroka et al., 2008), the α4 chain monoclonal antibody FC10 was a kind gift from Dr. I. Virtanen (University of Helsinki, Finland). The α5 (4C7), β2 (C4) and γ1 (2E8) antibodies were kind gifts from Dr. E. Engvall (The Burnham Institute, La Jolla, CA). The γ2 monoclonal antibody GB3 was from Serotec (Oxford, UK) and the β3 chain monoclonal antibody Clone 17 was from BD Bioscience (San Jose, CA).
Fig. 2.
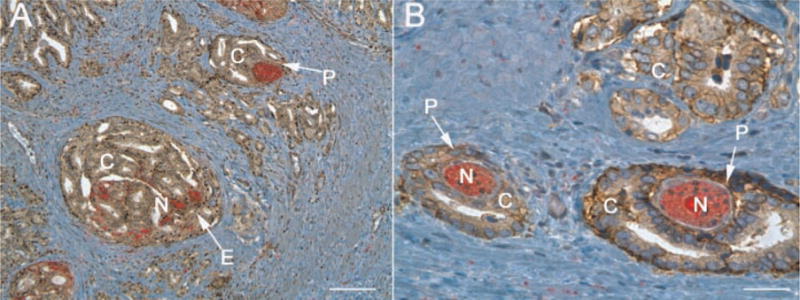
Integrin α6 expression in endoneural and perineural invasion in human prostate cancer. Human prostate cancer tissue was stained with the AA6NT polyclonal antibody specific for the α6 integrin (Ports et al., 2009) (brown) and the neural specific marker PGP 9.5 (red). A: Tumor cell (C) invasion of the endoneurium (E) and perineurium (P) of nerves (N). B: Higher magnification of the α6 integrin in PNI during prostate tumor cell invasion of the perineurium of nerves (P). Note the polarized glandular structures developed by tumor cells invading the perineurium in (B) versus the less differentiated structures observed in tumor cells invading the endoneurium in (A). Whitebar, 80 μm (A) and 40 μm (B). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.
Concluding Remarks
The mechanisms directing perineural invasion in neurotropic cancers are currently poorly defined although the clinical significance of the disease has been established (Liebig et al., 2009). Determining how tumor and nerve interactions orchestrate and promote metastatic disease is necessary for combating extraprostatic extension during prostate cancer. The laminin rich prostatic nerves and the persistent expression of the pro-metastatic laminin binding α6 integrin in prostate tumor cells undergoing PNI may be potential targets for therapeutic intervention or diagnostic procedures. The deleterious effects of metastatic prostate cancer warrant increased study in the field of perineural invasion.
Acknowledgments
We would like to thank the dedicated staff of the Biomedical Communications Service of the University of Arizona Health Sciences Center and the Tissue Acquisition Shared Service (TACMASS) located in the Arizona Cancer Center.
Contract grant sponsor: NIH;
Contract grant numbers: P30 CA23074, PO1 CA56666.
Literature Cited
- Aberdam D, Galliano MF, Vailly J, Pulkkinen L, Bonifas J, Christiano AM, Tryggvason K, Uitto J, Epstein EH, Jr, Ortonne JP, et al. Herlitz’s junctional epidermolysis bullosa is linked to mutations in the gene (LAMC2) for the gamma 2 subunit of nicein/kalinin (LAMININ-5) Nat Genet. 1994;6:299–304. doi: 10.1038/ng0394-299. [DOI] [PubMed] [Google Scholar]
- Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, Rowley D. In vitro dorsal root ganglia and human prostate cell line interaction: Redefining perineural invasion in prostate cancer. Prostate. 2001;49:213–223. doi: 10.1002/pros.1137. [DOI] [PubMed] [Google Scholar]
- Ayala GE, Dai H, Ittmann M, Li R, Powell M, Frolov A, Wheeler TM, Thompson TC, Rowley D. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64:6082–6090. doi: 10.1158/0008-5472.CAN-04-0838. [DOI] [PubMed] [Google Scholar]
- Ayala GE, Dai H, Li R, Ittmann M, Thompson TC, Rowley D, Wheeler TM. Bystin in perineural invasion of prostate cancer. Prostate. 2006a;66:266–272. doi: 10.1002/pros.20323. [DOI] [PubMed] [Google Scholar]
- Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, Ittmann MM, Rowley D. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14:7593–7603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- Bair EL, Chen ML, McDaniel K, Sekiguchi K, Cress AE, Nagle RB, Bowden GT. Membrane type 1 matrix metalloprotease cleaves laminin-10 and promotes prostate cancer cell migration. Neoplasia. 2005;7:380–389. doi: 10.1593/neo.04619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkhoff H, Stein U, Remberger K. Differential expression of alpha 6 and alpha 2 very late antigen integrins in the normal, hyperplastic, and neoplastic prostate: Simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum Pathol. 1993;24:243–248. doi: 10.1016/0046-8177(93)90033-d. [DOI] [PubMed] [Google Scholar]
- Chang JJ, Shinohara K, Bhargava V, Presti JC., Jr Prospective evaluation of lateral biopsies of the peripheral zone for prostate cancer detection. J Urol. 1998;160:2111–2114. doi: 10.1097/00005392-199812010-00044. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol. 2003;163:889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhang HL, Jiang YG, Li JH, Liu BL, Sun MY. Inhibition of CD146 gene expression via RNA interference reduces in vitro perineural invasion on ACC-M cell. J Oral Pathol Med. 2009;38:198–205. doi: 10.1111/j.1600-0714.2008.00706.x. [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S. Regulation of Schwann cell function by the extracellular matrix. Glia. 2008;56:1498–1507. doi: 10.1002/glia.20740. [DOI] [PubMed] [Google Scholar]
- Colognato H, ffrench-Constant C, Feltri ML. Human diseases reveal novel roles for neural laminins. Trends Neurosci. 2005;28:480–486. doi: 10.1016/j.tins.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Cornell RJ, Rowley D, Wheeler T, Ali N, Ayala G. Neuroepithelial interactions in prostate cancer are enhanced in the presence of prostatic stroma. Urology. 2003;61:870–875. doi: 10.1016/s0090-4295(02)02426-3. [DOI] [PubMed] [Google Scholar]
- Dai H, Li R, Wheeler T, Diaz de Vivar A, Frolov A, Tahir S, Agoulnik I, Thompson T, Rowley D, Ayala G. Pim-2 upregulation: Biological implications associated with disease progression and perinueral invasion in prostate cancer. Prostate. 2005;65:276–286. doi: 10.1002/pros.20294. [DOI] [PubMed] [Google Scholar]
- Dai H, Li R, Wheeler T, Ozen M, Ittmann M, Anderson M, Wang Y, Rowley D, Younes M, Ayala GE. Enhanced survival in perineural invasion of pancreatic cancer: An in vitro approach. Hum Pathol. 2007;38:299–307. doi: 10.1016/j.humpath.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Davis TL, Cress AE, Dalkin BL, Nagle RB. Unique expression pattern of the alpha6beta4 integrin and laminin-5 in human prostate carcinoma. Prostate. 2001;46:240–248. doi: 10.1002/1097-0045(20010215)46:3<240::aid-pros1029>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Taille A, Katz A, Bagiella E, Olsson CA, O’Toole KM, Rubin MA. Perineural invasion on prostate needle biopsy: An independent predictor of final pathologic stage. Urology. 1999;54:1039–1043. doi: 10.1016/s0090-4295(99)00350-7. [DOI] [PubMed] [Google Scholar]
- Demetriou MC, Cress AE. Integrin clipping: A novel adhesion switch? J Cell Biochem. 2004;91:26–35. doi: 10.1002/jcb.10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou MC, Pennington ME, Nagle RB, Cress AE. Extracellular alpha 6 integrin cleavage by urokinase-type plasminogen activator in human prostate cancer. Exp Cell Res. 2004;294:550–558. doi: 10.1016/j.yexcr.2003.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou MC, Stylianou P, Andreou M, Yiannikouri O, Tsaprailis G, Cress AE, Skourides P. Spatially and temporally regulated alpha6 integrin cleavage during Xenopus laevis development. Biochem Biophys Res Commun. 2008;366:779–785. doi: 10.1016/j.bbrc.2007.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovy P, Svizenska I, Jancalek R, Klusakova I, Houstava L, Haninec P, Zitkova A. Immunohistochemical localization of laminin-1 in the acellular nerve grafts is associated with migrating Schwann cells which display corresponding integrin receptors. Gen Physiol Biophys. 1999;18:63–65. [PubMed] [Google Scholar]
- Dunzendorfer U, Jonas D, Weber W. The autonomic innervation of the human prostate. Histochemistry of acetylcholinesterase in the normal and pathologic states. Urol Res. 1976;4:29–31. doi: 10.1007/BF00256133. [DOI] [PubMed] [Google Scholar]
- Feltri ML, Wrabetz L. Laminins and their receptors in Schwann cells and hereditary neuropathies. J Peripher Nerv Syst. 2005;10:128–143. doi: 10.1111/j.1085-9489.2005.0010204.x. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Rebaza LM, Derzic S, Schwartz PH, Monuki ES. Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res. 2006;83:845–856. doi: 10.1002/jnr.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. Perineural and lymphovascular invasion on prostatic biopsy: Pathological assessment and significance. Surg Oncol. 2009;18:200–202. doi: 10.1016/j.suronc.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Gil Z, Cavel O, Kelly K, Brader P, Rein A, Gao SP, Carlson DL, Shah JP, Fong Y, Wong RJ. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;2:107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Vernet JM. Prostate cancer: Anatomical and surgical considerations. Br J Urol. 1996;78:161–168. doi: 10.1046/j.1464-410x.1996.00841.x. [DOI] [PubMed] [Google Scholar]
- Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;15:657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Alam N, Johnson IN, Languino LR. Integrin signaling aberrations in prostate cancer. Am J Transl Res. 2009;1:211–220. [PMC free article] [PubMed] [Google Scholar]
- Hall PE, Lathia JD, Miller NG, Caldwell MA, ffrench-Constant C. Integrins are markers of human neural stem cells. Stem Cells. 2006;24:2078–2084. doi: 10.1634/stemcells.2005-0595. [DOI] [PubMed] [Google Scholar]
- Hao J, Yang Y, McDaniel KM, Dalkin BL, Cress AE, Nagle RB. Differential expression of laminin 5 (alpha 3 beta 3 gamma 2) by human malignant and normal prostate. Am J Pathol. 1996;149:1341–1349. [PMC free article] [PubMed] [Google Scholar]
- Harnden P, Shelley MD, Clements H, Coles B, Tyndale-Biscoe RS, Naylor B, Mason MD. The prognostic significance of perineural invasion in prostatic cancer biopsies: A systematic review. Cancer. 2007;109:13–24. doi: 10.1002/cncr.22388. [DOI] [PubMed] [Google Scholar]
- Hughes AL. Evolution of the integrin alpha and beta protein families. J Mol Evol. 2001;52:63–72. doi: 10.1007/s002390010134. [DOI] [PubMed] [Google Scholar]
- Huhtala M, Heino J, Casciari D, de Luise A, Johnson MS. Integrin evolution: Insights from ascidian and teleost fish genomes. Matrix Biol. 2005;24:83–95. doi: 10.1016/j.matbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Johansson JE, Holmberg L, Johansson S, Bergstrom R, Adami HO. Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA. 1997;277:467–471. [PubMed] [Google Scholar]
- Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, Adami HO. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- Knox JD, Cress AE, Clark V, Manriquez L, Affinito KS, Dalkin BL, Nagle RB. Differential expression of extracellular matrix molecules and the alpha 6-integrins in the normal and neoplastic prostate. Am J Pathol. 1994;145:167–174. [PMC free article] [PubMed] [Google Scholar]
- Koide N, Yamada T, Shibata R, Mori T, Fukuma M, Yamazaki K, Aiura K, Shimazu M, Hirohashi S, Nimura Y, Sakamoto M. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer. Clin Cancer Res. 2006;12:2419–2426. doi: 10.1158/1078-0432.CCR-05-1852. [DOI] [PubMed] [Google Scholar]
- Lallier TE, Whittaker CA, DeSimone DW. Integrin alpha 6 expression is required for early nervous system development in Xenopus laevis. Development. 1996;122:2539–2554. doi: 10.1242/dev.122.8.2539. [DOI] [PubMed] [Google Scholar]
- Lee IH, Roberts R, Shah RB, Wojno KJ, Wei JT, Sandler HM. Perineural invasion is a marker for pathologically advanced disease in localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:1059–1064. doi: 10.1016/j.ijrobp.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: A review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi F, Piemonti L, Fedele G, Destro A, Roncalli M, Albarello L, Doglioni C, Anselmo A, Doni A, Bianchi P, Laghi L, Malesci A, Cervo L, Malosio M, Reni M, Zerbi A, Di Carlo V, Mantovani A, Allavena P. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68:9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 1:77–82. doi: 10.1016/j.cytogfr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2:35–49. doi: 10.1002/pros.2990020105. [DOI] [PubMed] [Google Scholar]
- Mueller FJ, Serobyan N, Schraufstatter IU, DiScipio R, Wakeman D, Loring JF, Snyder EY, Khaldoyanidi SK. Adhesive interactions between human neural stem cells and inflamed human vascular endothelium are mediated by integrins. Stem Cells. 2006;24:2367–2372. doi: 10.1634/stemcells.2005-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol. 1995;146:1498–1507. [PMC free article] [PubMed] [Google Scholar]
- Nakano A, Pfendner E, Hashimoto I, Uitto J. Herlitz junctional epidermolysis bullosa: Novel and recurrent mutations in the LAMB3 gene and the population carrier frequency. J Invest Dermatol. 2000;115:493–498. doi: 10.1046/j.1523-1747.2000.00086.x. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Cremona O, Daams H, Ferraresi S, Sonnenberg A, Marchisio PC. Expression of the integrin alpha 6 beta 4 in peripheral nerves: Localization in Schwann and perineural cells and different variants of the beta 4 subunit. J Cell Sci. 1994;107:543–552. doi: 10.1242/jcs.107.2.543. [DOI] [PubMed] [Google Scholar]
- Pawar SC, Demetriou MC, Nagle RB, Bowden GT, Cress AE. Integrin alpha6 cleavage: A novel modification to modulate cell migration. Exp Cell Res. 2007;313:1080–1089. doi: 10.1016/j.yexcr.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ports MO, Nagle RB, Pond GD, Cress AE. Extracellular engagement of alpha6 integrin inhibited urokinase-type plasminogen activator-mediated cleavage and delayed human prostate bone metastasis. Cancer Res. 2009;69:5007–5014. doi: 10.1158/0008-5472.CAN-09-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell MS, Li R, Dai H, Sayeeduddin M, Wheeler TM, Ayala GE. Neuroanatomy of the normal prostate. Prostate. 2005;65:52–57. doi: 10.1002/pros.20245. [DOI] [PubMed] [Google Scholar]
- Prueitt RL, Yi M, Hudson RS, Wallace TA, Howe TM, Yfantis HG, Lee DH, Stephens RM, Liu CG, Calin GA, Croce CM, Ambs S. Expression of microRNAs and protein-coding genes associated with perineural invasion in prostate cancer. Prostate. 2008;68:1152–1164. doi: 10.1002/pros.20786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen L, Uitto J. Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol. 1999;18:29–42. doi: 10.1016/s0945-053x(98)00005-5. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L, Christiano AM, Gerecke D, Wagman DW, Burgeson RE, Pittelkow MR, Uitto J. A homozygous nonsense mutation in the beta 3 chain gene of laminin 5 (LAMB3) in Herlitz junctional epidermolysis bullosa. Genomics. 1994;24:357–360. doi: 10.1006/geno.1994.1627. [DOI] [PubMed] [Google Scholar]
- Ryschich E, Khamidjanov A, Kerkadze V, Buchler MW, Zoller M, Schmidt J. Promotion of tumor cell migration by extracellular matrix proteins in human pancreatic cancer. Pancreas. 2009;38:804–810. doi: 10.1097/MPA.0b013e3181b9dfda. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Cress AE, Scott KM, Burger F, Cui H, Sallam K, McDaniel KM, Dalkin BL, Nagle RB. Different phenotypes in human prostate cancer: Alpha6 or alpha3 integrin in cell-extracellular adhesion sites. Neoplasia. 2002;4:243–254. doi: 10.1038/sj.neo.7900223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalenberg T, Neuhaus J, Liatsikos E, Winkler M, Loffler S, Stolzenburg JU. Neuroanatomy of the male pelvis in respect to radical prostatectomy including three-dimensional visualization. BJU Int. 2009;1:21–27. doi: 10.1111/j.1464-410X.2009.08702.x. [DOI] [PubMed] [Google Scholar]
- Sinha AA, Gleason DF, Wilson MJ, Staley NA, Furcht LT, Palm SL, Reddy PK, Sibley RK, Martinez-Hernandez A. Immunohistochemical localization of laminin in the basement membranes of normal, hyperplastic, and neoplastic human prostate. Prostate. 1989;15:299–313. doi: 10.1002/pros.2990150403. [DOI] [PubMed] [Google Scholar]
- Sroka IC, Chen ML, Cress AE. Simplified purification procedure of laminin-332 and laminin-511 from human cell lines. Biochem Biophys Res Commun. 2008;3:410–413. doi: 10.1016/j.bbrc.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroka IC, Pond GD, Nagle RB, Porreca F, King T, Pestano G, Futscher B, Gard JM, Riley J, Sathyanarayana UG, Cress AE. Human cell surface receptors as molecular imaging candidates for metastatic prostate cancer. Open Prostate Cancer J. 2009;2:59–66. doi: 10.2174/1876822900902010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67:10222–10229. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- Terpe HJ, Stark H, Ruiz P, Imhof BA. Alpha 6 integrin distribution in human embryonic and adult tissues. Histochemistry. 1994;101:41–49. doi: 10.1007/BF00315830. [DOI] [PubMed] [Google Scholar]
- Timms BG. Prostate development: A historical perspective. Differentiation. 2008;76:565–577. doi: 10.1111/j.1432-0436.2008.00278.x. [DOI] [PubMed] [Google Scholar]
- Uitto J, Pulkkinen L, McLean WH. Epidermolysis bullosa: A spectrum of clinical phenotypes explained by molecular heterogeneity. Mol Med Today. 1997;3:457–465. doi: 10.1016/s1357-4310(97)01112-x. [DOI] [PubMed] [Google Scholar]
- Ukimura O, Troncoso P, Ramirez EI, Babaian RJ. Prostate cancer staging: Correlation between ultrasound determined tumor contact length and pathologically confirmed extraprostatic extension. J Urol. 1998;159:1251–1259. doi: 10.1016/s0022-5347(01)63575-4. [DOI] [PubMed] [Google Scholar]
- Vaalasti A, Hervonen A. Nerve endings in the human prostate. Am J Anat. 1980;157:41–47. doi: 10.1002/aja.1001570105. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- Van der Zee CE, Kreft M, Beckers G, Kuipers A, Sonnenberg A. Conditional deletion of the Itgb4 integrin gene in Schwann cells leads to delayed peripheral nerve regeneration. J Neurosci. 2008;28:11292–11303. doi: 10.1523/JNEUROSCI.3068-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas SO, Jiroutek M, Welch WR, Nucci MR, D’Amico AV, Renshaw AA. Perineural invasion in prostate needle biopsy specimens. Correlation with extraprostatic extension at resection. Am J Clin Pathol. 1999;111:223–228. doi: 10.1093/ajcp/111.2.223. [DOI] [PubMed] [Google Scholar]
- Villers A, McNeal JE, Redwine EA, Freiha FS, Stamey TA. The role of perineural space invasion in the local spread of prostatic adenocarcinoma. J Urol. 1989;142:763–768. doi: 10.1016/s0022-5347(17)38881-x. [DOI] [PubMed] [Google Scholar]
- Wallquist W, Plantman S, Thams S, Thyboll J, Kortesmaa J, Lannergren J, Domogatskaya A, Ogren SO, Risling M, Hammarberg H, Tryggvason K, Cullheim S. Impeded interaction between Schwann cells and axons in the absence of laminin alpha4. J Neurosci. 2005;25:3692–3700. doi: 10.1523/JNEUROSCI.5225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: Anatomical and pathological considerations. Prostate. 1983;4:473–485. doi: 10.1002/pros.2990040506. [DOI] [PubMed] [Google Scholar]
- Ware JL. Prostate cancer progression. Implications of histopathology. Am J Pathol. 1994;145:983–993. [PMC free article] [PubMed] [Google Scholar]
- Yang G, Wheeler TM, Kattan MW, Scardino PT, Thompson TC. Perineural invasion of prostate carcinoma cells is associated with reduced apoptotic index. Cancer. 1996;78:1267–1271. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1267::AID-CNCR15>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Yang D, Bierman J, Tarumi YS, Zhong YP, Rangwala R, Proctor TM, Miyagoe-Suzuki Y, Takeda S, Miner JH, Sherman LS, Gold BG, Patton BL. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J Cell Biol. 2005;168:655–666. doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Yu H, Chen ZL. Laminins in peripheral nerve development and muscular dystrophy. Mol Neurobiol. 2007;35:288–297. doi: 10.1007/s12035-007-0026-x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Qi L, Li M, Zhang D, Xu S, Wang N, Sun B. Chemokine CXCL12 and its receptor CXCR4 expression are associated with perineural invasion of prostate cancer. J Exp Clin Cancer Res. 2008;27:62. doi: 10.1186/1756-9966-27-62. [DOI] [PMC free article] [PubMed] [Google Scholar]


