Abstract
Radiosynthesis and in vitro evaluation of [18F]-2-(4-bromo-2,5-dimethoxyphenyl)-N-(2-(2-fluoroethoxy)benzyl)ethanamine, ([18F]FECIMBI-36) or ([18F]1), a potential agonist PET imaging agent for 5-HT2A/2C receptors is described. Syntheses of reference standard 1 and the corresponding des-fluoroethyl radiolabeling precursor (2) were achieved with 75% and 65% yields, respectively. In vitro pharmacology assay of FECIMBI-36 by [3H]-ketanserin competition binding assay obtained from NIMH-PDSP showed high affinities to 5-HT2AR (Ki = 1 nM) and 5-HT2CR (Ki = 1.7 nM). Radiolabeling of FECIMBI-36 was achieved from the boc-protected precursor 2 using [18F]-fluoroethyltosylate in presence of Cs2CO3 in DMSO followed by removal of the protective group. [18F]1 was isolated using RP-HPLC in 25 ± 5% yield, purity ≥95% and specific activity 1–2 Ci/μmol (N = 6). In vitro autoradiography studies demonstrate that [18F]1 selectively label 5-HT2A and 5-HT2C receptors in slide-mounted sections of postmortem human brain using phosphor imaging. Our results indicate the potential of [18F]1 for imaging 5-HT2A/2C receptors in the high affinity state in vivo using PET imaging.
Keywords: 5-HT, 5HT2A/2CR, Agonist, PET, Radiotracer
5-HT2Rs belong to the class of G-protein coupled receptors (GPCRs) and have three 3 subtypes, namely 5-HT2AR, 5-HT2BR and 5-HT2CR with 70–80% sequence homology.1–4 5-HT2AR is the most abundant excitatory 5-HT receptor in human brain and plays an essential role in a number of physiological processes and psychiatric disorders including dementia, schizophrenia, suicidal behavior and major depression.1–8 5-HT2Rs are also implicated in the therapeutic effects of some antidepressants as well as antipsychotics.9–11 5-HT2BR is less abundant in the central nervous system (CNS), whereas 5-HT2CR is abundant in the choroid plexus, and at a low level in the striatum, hippocampus, neocortex and amygdala.1,10 5-HT2R can be present in a high or low agonist affinity binding state.1 In the CNS, 5-HT2AR are found in large amounts in the forebrain and neocortex and low levels in brainstem nuclei and hippocampus.12,13
Although there are several radioligands available for in vivo imaging of 5-HT2AR, none can detect changes in the high affinity state in pathological conditions. Whether any alteration of 5-HT2AR in major CNS disorders involves the high-affinity conformation is not known.14–18 An agonist positron emission tomography (PET) radiotracer would have many advantages for 5-HT2AR imaging because agonists bind the functionally relevant high-affinity sub-population of GPCRs.14–18 The availability of a well-characterized agonist PET tracer would facilitate the measurement of minimally effective dose and in addition, a combination of antagonist and agonist PET data would allow separate quantification of the high- and low-affinity conformational states of the 5-HT2R.
[11C]CIMBI-5 (Fig. 1); 5-HT2AR Ki = 0.044 nM, Emax = 85%, 5-HT2BR Ki = 73 nM and 5-HT2CR Ki = 2 nM,12 is the first 5-HT2AR agonist PET tracer studied by others and us in vivo in pigs and nonhuman primates.12–16 More recently, [11C]CIMBI-36, a bromoanalog of CIMBI-5, was developed by Ettrup et al. and evaluated in pig and nonhuman primates.17,18 [11C]CIMBI-36 is currently being examined in human subjects for its ability to quantify 5HT2AR by PET imaging.17–19 PDSP data for CIMBI-36 shows that it has a 5HT2AR Ki = 0.5 nM and Emax = 87%, 5HT2BR Ki = 0.5 nM, and 5-HT2CR Ki = 1.7 nM.17 PET studies in nonhuman primates suggest that cortical binding of [11C]CIMBI-36 is mostly due to 5-HT2AR, whereas, tracer uptake in choroid plexus can be attributed to the binding to 5-HT2CR population. PET blocking studies of [11C]CIMBI-36 with the 5HT2AR antagonist ketanserin in human subjects reveal a cortex to cerebellum ratio <2.17,18 Furthermore, the radioligand has slow washout, particularly in cortex, a brain region of high receptor density.19 The slow kinetics make accurate kinetic modeling difficult, and this would be a serious limitation for research use of [11C]CIMBI-36. To overcome this disadvantage, PET imaging of 5-HT2AR using [18F] radioligands (t1/2 = 110 min) would allow longer duration scanning and enable valid modeling.15,16 Additionally, [F-18] tracers due to their 110 min half-life, permit deliveries to PET Centers that lack an onsite cyclotron and thereby allow cost effective multicenter clinical studies. We identified the fluoroethyl version of CIMBI-36 (FECIMBI-36, 1) as a potential agonist ligand for 5-HT2AR [18F]-tracer development. Herein we describe the synthesis, pharmacology assessment, radiolabeling and in vitro evaluation of [18F]FECIMBI-36 in slide-mounted sections of postmortem human brain.
Figure 1.
Chemical structures of CIMBI-5, CIMBI-36 and FECIMBI-36.
The synthesis of desfluoroethyl CIMBI-36 (2) was achieved according to minor modifications of a published procedure.14,20 The synthesis of reference standard FECIMBI-36 (1) was achieved in 75% yield by reacting the corresponding boc-protected precursor (2) with 1-bromo-2-fluoroethane and NaH, followed by removal of the protective group with trifluoroacetic acid at room temperature (Scheme 1).21 The affinities of FECIMBI-36 for 5-HT2AR and various other biogenic amine receptors and transporters and functional assays were determined through the National Institute of Mental Health Psychoactive Drug Screening Program (NIMH-PDSP) (Table 1).
Scheme 1.
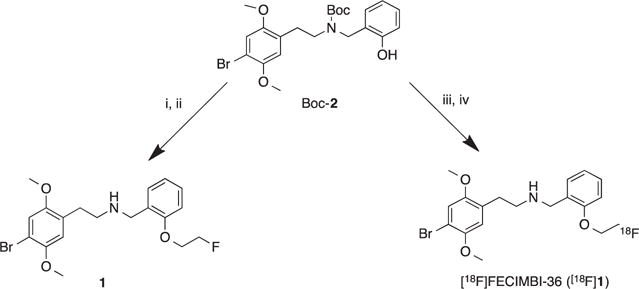
Synthesis of FECIMBI-36 and radiosynthesis of [18F]FECIMBI-36. Reagents and conditions: (i) Fluoroethyl bromide, NaH, DMF, 110 °C, 12 h; (ii)TFA/DCM, 15 min, 75%, (iii) [18F]FEOTs, Cs2CO3, DMSO, 120°C, 20min; (iv) TFA/CAN (1:1), 80°C, 10min.
Table 1.
Affinity and selectivity of FECIMBI-36
| Targets | Ki (nM) | Targets | Ki (nM) |
|---|---|---|---|
| 5-HT1A | 1433 | D1 | >10,000 |
| 5-HT1B | >10,000 | D2 | 1394 |
| 5-HT1D | >10,000 | D3 | 1076 |
| 5-HT1E | >10,000 | KAR | >10,000 |
| 5-HT2A | 1.0 | D4 | >1000 |
| 5-HT2B | 2.8 | D5 | >10,000 |
| 5-HT2C | 1.7 | DAT | >10,000 |
| 5-HT3 | >10,000 | DOR | >10,000 |
| 5-HT7 | >10,000 | EP | >10,000 |
| Adenosine | >10,000 | GABA | >10,000 |
| α1A–1C | >10,000 | H4 | 2126 |
| α2A | 551 | HERG | >10,000 |
| α2B | 1256 | KOR | 558 |
| α2C | 656 | M | >10,000 |
| β1 | >10,000 | H3, H4 | >10,000 |
| β2 | 3220 | MDR1 | >10,000 |
| β3 | >10,000 | H2 | 1983 |
| BZP | >10,000 | H1 | 150 |
| Ca++ | >10,000 | MOR | 149 |
| AMPA | >10,000 | mGluR | >10,000 |
| NET | 208 | NMDA | >10,000 |
| NK | >10,000 | SERT | 284 |
| Sigma1 | 499 | Sigma2 | 51 |
| Na+ | >10,000 | NT1 | >10,000 |
| CB1, CB2 | >10,000 | I | >10,000 |
| mGluRs | >10,000 | VMAT | >10,000 |
| NOP | >10,000 | NMDA | >10,000 |
| PBR | 876 | NT | >10,000 |
| Oxytocin | >10,000 | PKC | >10,000 |
| Smoothened | >10,000 | V1–2 | >10,000 |
| Agonist assays | 5-HT2AR | 5-HT2BR | 5-HT2CR |
| Emax: 63.4% | Emax: 18.4% | Emax: 100% |
Ki values measured against [3H]ketanserin show that FECIMBI-36 has high affinity for 5-HT2Rs (Table 1). FECIMBI-36 showed no significant affinities for other tested brain targets, pGP and MDRs and selected affinities are shown in Table 1. Agonist potency assays revealed that Emax values of FECIMBI-36 for 5-HT2AR, 5-HT2BR and 5-HT2CR are 63.4%, 18.4% and 100%, respectively, with no antagonistic properties (Table 1).
Radiosynthesis of [18F]FECIMBI-36 was achieved by a one pot, two step reaction with the phenolate of 2 (Scheme 1). In summary, reaction of [18F]FEOTs with precursor 2 in the presence of Cs2CO3 afforded [18F]1 in 25 ± 5% yield and >95% chemical and radiochemical purities (N = 6).22 The [18F]FEOTs in turn was synthesized by a procedure previously reported by us.23 Total synthesis and purification time required for the radiosynthesis was 50 min at EOS (N = 6). The specific activity of the radioproduct was 1–2 Ci/μmol at EOS. Partition coefficient (logP) of [18F]1 was 4, indicating that the radioligand should have adequate lipophilicity for passive brain entry.24 The radioproduct was stable in saline–ethanol (9:1) for up to 6 h based on analytical HPLC analyses and no detectable [18F]radiofluorination was found over 6 h in the formulated solution. We determined the potential of [18F]CIMBI-36 to label binding sites in sections of human brain using modifications of a method described by Finnema et al.18,25 Sections (20 μm) of prefrontal cortex Brodmann area (BA9), hippocampus, temporal cortex and choroid plexus from a coronal slab of hemisphere, and for comparison, sections of cerebellum from the same 37 year old male case, were incubated with [18F]1 for 60 min at 37 °C The 5-HT2AR antagonist ketanserin (10 μM) was used to define nonspecific binding. Following incubation, sections were rapidly dried and exposed to phosphorimaging screens for 60 min.
As is evident from Figure 2, there is binding of [18F]1 to 5HT2R in prefrontal cortex, temporal cortex, hippocampus and choroid plexus. The nonspecific binding was reduced when the washing time was increased from 6 min to 20 min (data not shown). Binding was observed throughout the gray matter of the prefrontal cortex. Specific binding (total binding − nonspecific binding × 100) is approximately 40% of total binding in prefrontal cortex (Fig. 3). The distribution of specific binding corresponded to the distribution of the 5-HT2A antagonist ligand [3H]ketanserin,26,27 suggesting that the [18F]FECIMBI-36 specific binding is largely to the 5-HT2AR in gray matter of the prefrontal cortex and other regions including the temporal cortex and hippocampus. As shown in Figure 2, specific binding is also found in the choroid plexus, a region where the highest concentration of 5HT2CR is present. No appreciable binding was found in cerebellum, which is consistent with the known distribution of 5-HT2R in human brain. The cerebellar binding of [18F]1 is lower than [11C]CIMBI-36 and has better target to non-target ratio in vitro.
Figure 2.
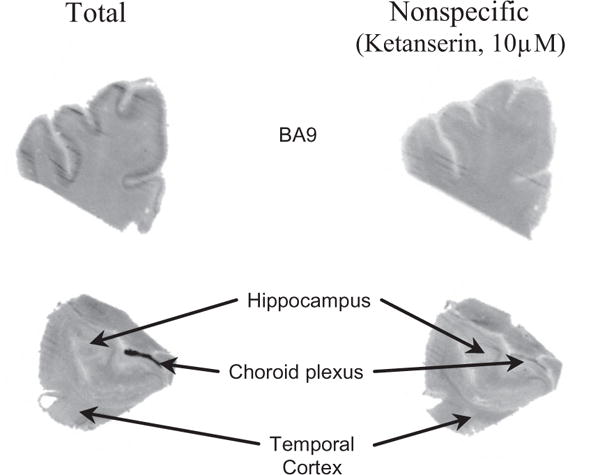
Phosphor images of [18F]1 in postmortem human brain sections. Total (left upper and lower images) and nonspecific (right upper and lower images) binding of [18F]FECIMBI-36 in human brain sections. Nonspecific binding was determined by ligand displacement with 10 μM ketanserin. Note there is more binding in gray matter than in white matter and there is binding and displacement in the choroid plexus.
Figure 3.
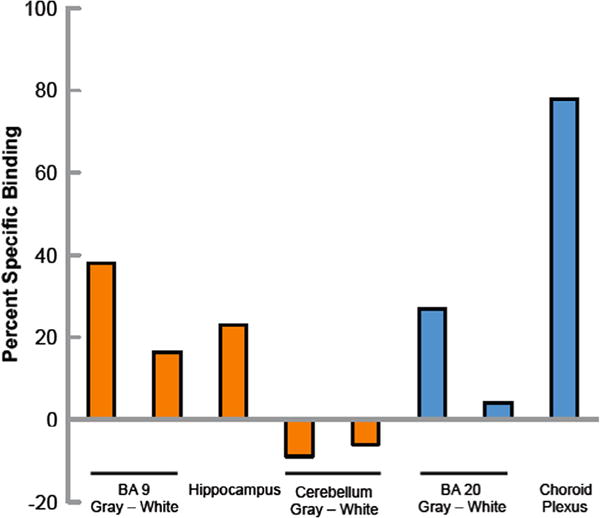
Specific binding of [18F]1 in postmortem human brain sections. Percent specific binding of [18F]FECIMBI-36. Orange bars are the mean of measurements from two IDs and blue bars are measurements from one ID. All regions were assayed in triplicate within an experiment.
In summary, we synthesized FECIMBI-36 and determined its binding affinity and selectivity to the 5HT2R and a variety of other receptors and transporters. The radiosynthesis of [18F]1 was performed successfully in 25 ± 5% yield and the total time required for radiosynthesis was 50 min from EOS with excellent chemical and radiochemical purities and high specific activity. Autoradiography studies show high binding of [18F]1 to 5-HT2A/2C receptors in various brain regions of postmortem human brain sections from nonpsychiatric controls. Our results indicate [18F]FECIMBI-36 as a potential agonist radiotracer with 110 min half-life for sensitive and accurate in vivo detection of 5HT2A/2CR in neuropsychiatric disorders, and to measure in vivo occupancies of novel drugs under development.
Acknowledgments
The authors thank Dr. Bryan Roth and the NIMH-PDSP program for the competitive receptor, transporter binding and functional assays. This work is a continuation of our previous studies supported by the NIMH – United States Grant MH 091470.
References and notes
- 1.Nichols DE, Nichols CD. Chem Rev. 2008;108:1614. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 2.Hoyer D, Hannon JP, Martin GR. Pharmacol Biochem Behav. 2002;71:533. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 3.Nicholas DE. Pharmacol Ther. 2004;101:131. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Meltzer HY, Matsubara S, Lee JC. J Pharmacol Exp Ther. 1989;251:238. [PubMed] [Google Scholar]
- 5.Glennon RA, Dukat M. In: Foye’s Medicinal Chemistry, Chapter 11 Serotonin Receptors and Drugs Affecting Serotonergic Neurotransmission. Williams DA, Zito SW, Lemke TL, Roche VF, editors. Lippincott, Williams, & Wilkins; Philadelphia: 2012. [Google Scholar]
- 6.Zimmer L, Le Bars D. J Labelled Compd Radiopharm. 2013;56:105. doi: 10.1002/jlcr.3001. [DOI] [PubMed] [Google Scholar]
- 7.Paterson LM, Tyacke RJ, Nutt DJ, Knudsen GM. J Cereb Blood Flow Metab. 2010;30:1682. doi: 10.1038/jcbfm.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, Reis DJ, Mann JJ. Arch Gen Psychiatry. 1990;47:1038. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- 9.Saulin A, Savli M, Lanzenberger R. Amino Acids. 2012;42:2039. doi: 10.1007/s00726-011-1078-9. [DOI] [PubMed] [Google Scholar]
- 10.Nichols DE, Frescas SP, Chemel BR, Rehder KS, Zhong D, Lewin AH. Bioorg Med Chem Lett. 2008;16:6116. doi: 10.1016/j.bmc.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonhardt S, Gorospe E, Hoffman BJ, Teitler M. Mol Pharmacol. 1992;42:328. [PubMed] [Google Scholar]
- 12.Ettrup A, Palner M, Gillings N, Santini MA, Hansen M, Kornum BR, Rasmussen LK, Nagren K, Madsen J, Begtrup M, Knudsen GM. J Nucl Med. 2010;51:1763. doi: 10.2967/jnumed.109.074021. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakaran J, Majo V, Milak M, Gillings N, Ettrup A, Prem S, Mann JJ, Parsey RV, Knudsen GM, Kumar JSD. J Nucl Med. 2012;53:1876. [Google Scholar]
- 14.Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, Kristensen J, Begtrup M, Knudsen GM. Eur J Nucl Med Mol Imaging. 2011;38:681. doi: 10.1007/s00259-010-1686-8. [DOI] [PubMed] [Google Scholar]
- 15.McKenna DJ, Peroutka SJ. J Neurosci. 1989;9:3482. doi: 10.1523/JNEUROSCI.09-10-03482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palacios JM, Vilaró MT, Mengod G. Exp Brain Res. 2013;230:395. doi: 10.1007/s00221-013-3636-8. [DOI] [PubMed] [Google Scholar]
- 17.Ettrup A, Holm S, Hansen M, Wasim M, Santini MA, Palner M, Madsen J, Svarer C, Kristensen JL, Knudsen GM. Mol Imaging Biol. 2013;15:376. doi: 10.1007/s11307-012-0609-4. [DOI] [PubMed] [Google Scholar]
- 18.Finnema SJ, Stepanov V, Ettrup A, Nakao R, Amini N, Svedberg M, Lehmann C, Hansen M, Knudsen GM, Halldin C. Neuroimage. 2014:1–84. 342. doi: 10.1016/j.neuroimage.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 19.Ettrup A, Bang S-C, McMahon B, Lehel S, Dyssegaard A, Skibsted AW, Jørgensen LM, Hansen M, Baandrup AO, Bache S, Svarer1 C, Kristensen JL, Gilling N, Madsen J, Knudsen GM. J Cereb Blood Flow Metab. 2014;34:1188. doi: 10.1038/jcbfm.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.tert-Butyl 4-bromo-2,5-dimethoxyphenethyl(2-hydroxybenzyl) carbamate (radiolabeling precursor, 2: 1H NMR (300 MHz, CDCl3): 9.6 (br s, 1H), 7.5 (m, 1H), 7.4 (m, 1H), 7.3 (s, 1H), 7.2 (d, 1H, J = 8.2), 7.0 (m, 1H), 6.7 (s, 1H), 4.5 (s, 2H), 4.0 (s, 6H), 3.6 (m, 2H), 2.8 (m, 2H), 1.6 (s, 9H); HRMS calculated for C22H28BrNO5, 465.1151; found, 465.1164 (M+).
- 21.tert-Butyl4-bromo-2,5-dimethoxyphenethyl(2-(2-fluoroethoxy) benzyl)-carbamate (nonradioactive standard (1): In a two necked RB flask containing sodium hydride (30.72 mg, 4 mmol (60% emulsion)), a solution of tert-butyl 4-bromo-2,5-dimethoxy-phenethyl(2-hydroxybenzyl) carbamate (2 150 mg, 0.32 mmol) dissolved in 1 mL of anhydrous DMF was added slowly. The mixture was stirred at room temperature for 15 min followed by the addition of 1-bromo-2-fluoroethane (51 mg, 0.4 mmol) and the contents were stirred for 18 h. After completion of the reaction, as indicated by TLC (25% ethyl acetate in hexane), a 1:1 mixture of trifluoroacetic acid and dichloromethane (1 mL) was added to the above solution and the contents were stirred for an additional 15 min. When the reaction was completed according to TLC, the solution was diluted with ethyl acetate (25 mL) and washed with saturated aqueous sodium bicarbonate, followed by water and brine. The combined organic phase was extracted and dried over anhydrous magnesium sulfate. Evaporation of ethyl acetate in a rotary evaporator under vacuum provided the crude product, which was then purified by silica gel column chromatography using 20% ethyl acetate in hexane. The product fraction was isolated and the combined fractions were evaporated and dried under high vacuum to obtain the final product in 75% yield (98 mg). 1H NMR (300 MHz, CDCl3): 7.3 (m, 2H), 6.9 (m, 4H), 4.7 (d, J = 8.5,2H), 4.2 (dm, 2H), 3.9-3.7 (m, 8H), 2.9 (m, 4H); HRMS calculated for C19H23BrFNO3, 411.0845; found, 412.0924 (MH+).
- 22.Radiosynthesis of [18F]1 Approximately 1 mg of 1a in 200 μL DMSO was added to a freshly prepared and dried [18F]FEOTs in presence of Cs2CO3. The reaction mixture was heated in a sealed vial for 20 min at 110 °C. The mixture was allowed to cool to room temperature, added 200 μL of TFA/acetonitrile (1:1) and the solution was heated at 80 °C for 10 min. The crude product was directly injected into a semi preparative RP-HPLC (Phenomenex C18, 10 × 250 mm, 10 μ) and the product was eluted with acetonitrile: 0.1 M ammonium formate solution containing 0.5% acetic acid (35:65) at a flow rate of 10mL/min. The radioproduct fraction with a retention time of 9–10 min based on γ-detector was collected, diluted with 50 mL of deionized water, and passed through a C-18 Sep-Pak® cartridge. Reconstitution of the product in 1 mL of absolute ethanol afforded [18F]1 (25 ± 5% yield, based on [18F]F− at EOS. A portion of the ethanol solution was analyzed by analytical RP-HPLC (Phenomenex, Prodigy ODS(3) 4.6 × 250 mm, 5 μ; mobile phase: acetonitrile/0.1 M ammonium formate containing 0.5% acetic acid; 40–60, flow rate: 2 mL/min, retention time: 6 min, wavelength: 254 nm) to determine the radiochemical purity and specific activity. The ethanol solution of [18F]1 was used for further studies.
- 23.Majo VJ, Prabhakaran J, Milak MS, Mali P, Parsey RV, Mann JJ, Kumar JSD. Bioorg Med Chem. 2013;17:5598. doi: 10.1016/j.bmc.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson AA, Jin L, Garcia A, DaSilva JN, Houle S. Appl Radiat Isot. 2001;54:203. doi: 10.1016/s0969-8043(00)00269-4. Briefly, partition coefficient (logP) of [18F]1 is measured to estimate its lipophilicity by mixing 0.1 mL of the radioligand formulation with 5 g each of 1-octanol and freshly prepared PBS buffer (pH = 7.4) in a culture tube as described by Wilson et al. Radioactivity per 0.5 g each of 1-octanol and aqueous layer was measured using a well counter. The partition coefficient is determined by calculating the ratio of counts/g of 1-octanol to that of buffer. [DOI] [PubMed] [Google Scholar]
- 25.In vitro autoradiography experiments in postmortem human brain sections using [18F]1 In vitro autoradiography experiments were performed using postmortem human brain sections adopting a method described by Finnema et al17 Three sections (20 μm) each for total and nonspecific binding, per brain region, were assayed Slide-mounted sections of cortex, hippocampus, choroid plexus and cerebellum were brought to 22 °C and incubated in 50 mM Tris HCl (pH 7.4) containing 120 mM NaCl, 5 mM KCl, 2 mM CaCl2 and 1 mM MgCl2 and 5–10 nM of radioligand (2.0 mCi in 1200 mL of buffer) for 60 min (37 °C). Adjacent sections were incubated in the same buffer with 10 μM ketanserin to determine nonspecific binding. Sections were washed in same buffer at 4 °C for 6 or 20 min and briefly dipped in ice-cold water to remove salts. Slides were quickly dried under a stream of cold air and exposed to ST-phosphor-imaging screens (Packard, wrapped in Mylar film) for 60 min. Screens were scanned with a Packard Cyclone phosphor-imaging system and analyzed with MCID Analysis 7.0 revision 1. All assays were performed in triplicate.
- 26.Hoyer D, Vos P, Closse A, Pazos A, Palacios JM, Davies H. Naunyn Schmiedebergs Arch Pharmacol. 1987;335:226. doi: 10.1007/BF00172788. [DOI] [PubMed] [Google Scholar]
- 27.Marazziti D, Rossi A, Palego L, Giannaccini G, Naccarato A, Lucacchini A, Cassano GB. Neurochem Res. 1997;22:753. doi: 10.1023/a:1027366413289. [DOI] [PubMed] [Google Scholar]


