Abstract
A variety of cognitive disorders are worsened by stress exposure and involve dysfunction of the newly evolved prefrontal cortex (PFC). Exposure to acute, uncontrollable stress increases catecholamine release in PFC, reducing neuronal firing and impairing cognitive abilities. High levels of noradrenergic α1-adrenoceptor and dopaminergic D1 receptor stimulation activate feedforward calcium–protein kinase C and cyclic AMP–protein kinase A signaling, which open potassium channels to weaken synaptic efficacy in spines. In contrast, high levels of catecholamines strengthen the primary sensory cortices, amygdala and striatum, rapidly flipping the brain from reflective to reflexive control of behavior. These mechanisms are exaggerated by chronic stress exposure, where architectural changes lead to persistent loss of PFC function. Understanding these mechanisms has led to the successful translation of prazosin and guanfacine for treating stress-related disorders. Dysregulation of stress signaling pathways by genetic insults likely contributes to PFC deficits in schizophrenia, while age-related insults initiate interacting vicious cycles that increase vulnerability to Alzheimer’s degeneration.
Exposure to uncontrollable stress rapidly evokes chemical changes in brain that impair the higher cognitive functions of the PFC while strengthening primitive brain reactions. This flip from reflective to reflexive brain state may have survival value when we are in danger, but it can be ruinous for life in the Information Age, when we need higher cognitive abilities to thrive. It has been appreciated for decades that uncontrollable stress drives mental illness, including cognitive disorders such as schizophrenia, and new evidence suggests it may also contribute to the cognitive deterioration of Alzheimer’s disease. These disorders particularly afflict the most newly evolved pyramidal cell circuits in association cortex, circuits that are uniquely regulated at the molecular level. The following reviews the effects of stress on PFC circuits and its relevance to degenerative changes in stress-related cognitive disorders.
The newly evolved prefrontal cortex
The evolution and organization of the PFC
The PFC subserves our highest order cognitive abilities, generating the mental representations that are the foundation of abstract thought and the basis for flexible, goal-directed behavior. In primates, the PFC is topographically organized: the dorsolateral PFC (dlPFC) guides thoughts, attention and actions1, while the orbital and ventromedial PFC (vmPFC) regulate emotion2 (Fig. 1a). The dlPFC has extensive connections with the association cortices and the dorsal aspects of the striatum1 for the regulation of thought and action. In contrast, the most caudal and medial aspects of the PFC (for example, Brodmann’s areas 24 and 25, also called the anterior cingulate cortex and the subgenual cortex, respectively) project to limbic structures such as the amygdala, ventral striatum, hypothalamus and brainstem for control of the autonomic nervous system2 (Fig. 1b). These PFC areas, along with the insular cortex, are thought to be critical for the mental suffering aspects of pain3. These areas receive projections from more rostral and lateral PFC, providing opportunities for the integration of cognitive and emotional processing. PFC circuits are usually positioned to either facilitate or inhibit processing, thus allowing flexible, top-down control. Data from humans suggest that the right hemisphere may be particularly important for inhibitory control3.
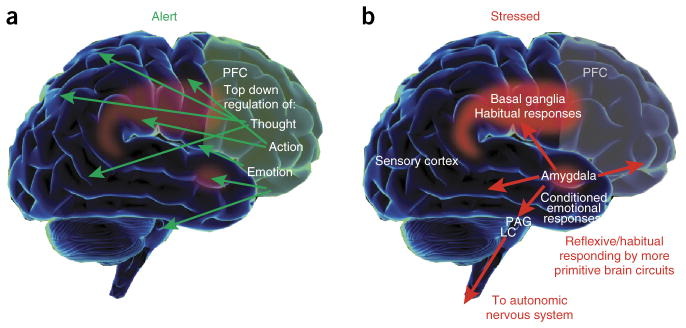
Figure 1.
Changes in brain systems controlling behavior under conditions of alert safety versus uncontrollable stress. (a) Under conditions when a subject feels alert, safe and interested, phasic release of catecholamines strengthens the higher cognitive functioning of the PFC, thus allowing top-down regulation of thought, action and emotion. In primates, the PFC is topographically organized, with the dorsal and lateral surfaces mediating attention, thought and action while the ventral and medial aspects mediate emotion. The anatomical projections of these areas reflect these specializations. (b) During stress exposure, high levels of catecholamines take the PFC ‘off-line’ while strengthening the functions of more primitive circuits—for example, the conditioned emotional responses of the amygdala and the habitual actions of the basal ganglia. The amygdala activates brainstem stress systems, which in turn activate the sympathetic nervous system.
The topographic organization of the PFC in humans is reflected in the sites of dysfunction in neuropsychiatric disorders. For example, there is loss of dlPFC gray matter in schizophrenia4,5 and Alzheimer’s disease6, while changes in more ventral and medial PFC regions are evident in mood disorders7 and in post-traumatic-stress disorder (PTSD)8. In bipolar disorder, the disinhibitory symptoms of mania are associated with dysfunction of the right hemisphere7, consistent with the specialized inhibitory role of this hemisphere.
The integrity of dlPFC function is often tested in working memory tasks, where information must be held in mind and constantly updated to guide accurate, flexible responding. Studies of nonhuman primate PFC have shown that the pyramidal cell microcircuits that subserve visual spatial working memory reside in deep layer III of the dlPFC1 (Fig. 2). These are the circuits that have expanded most in mammalian evolution, with increasing numbers of basal dendrites and spines9. This huge increase in dendritic spines allows the extraordinary number of neural connections needed for high-order cognition, where representations of representations expand the repertoire of cognitive abilities9.
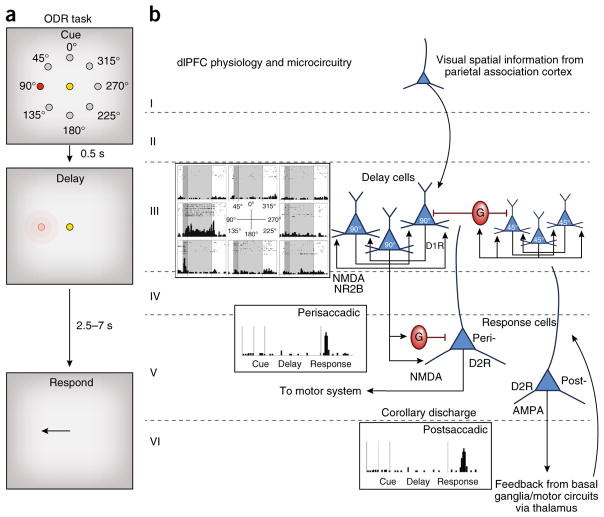
Figure 2.
The cellular basis of working memory, as discovered by Goldman-Rakic. (a) The oculomotor delayed response (ODR) task. A monkey fixates on a central spot while a cue is briefly lit at one of eight locations. The monkey must remember that location over a delay period while maintaining fixation. At the end of the delay, the fixation point is extinguished and the monkey can move its eyes to the remembered location for juice reward. The cue location constantly changes over hundreds of trials, requiring the constant updating of working memory. (b) The physiology and microcircuitry of the primate dlPFC. Delay cells maintain persistent firing across the delay period for their preferred location, but not other locations. The persistent firing is generated by the recurrent excitation of pyramidal cells with shared preferred directions, likely receiving their information from area 7 of the parietal association cortex. These pyramidal cells excite each other via NMDAR NR2B synapses on spines; there are only subtle influences of AMPARs. The spatial tuning of delay cells is enhanced via lateral inhibition from GABA (G) interneurons. Delay-cell microcircuits reside in deep layer III and possibly superficial layer V. Delay cells are modulated by dopamine actions at D1R but not D2R. In contrast, response cells are modulated by D2R but not D1R and likely reside in layer V. Perisaccadic response cells fire immediately before the motor response and likely convey orders to the motor system, while postsaccadic response cells convey feedback (corollary discharge) about the response. Some response cells show both pre- and postsaccadic firing; that is, both motor and feedback characteristics. Postsaccadic response cell firing relies on AMPAR as well as NMDAR stimulation. Response cells are what are most common in rodent PFC, which has a very large layer V.
Microcircuits for the generation of mental representations in primate dlPFC
‘Delay cells’ in the primate dlPFC are able to generate mental representations in the absence of sensory stimulation1: for example, the representation of the 90° direction from a central fixation point (Fig. 2). This persistent firing across a delay period arises from the recurrent excitation of pyramidal cells with shared spatial tuning—for example, a group of cells that all receive information from the parietal association cortex for the location 90°, their ‘preferred direction’. The spatial tuning of delay cells is refined by lateral inhibition from GABAergic interneurons (Fig. 2). Pyramidal cells interconnect on dendritic spines through glutamatergic NMDA receptor (NMDAR) type NR2B synapses10 (Fig. 3). The permissive depolarization of the postsynaptic density needed for NMDAR opening is provided by cholinergic stimulation of nicotinic α7 receptors in the postsynaptic density11 with only minor contributions from AMPA-type glutamate receptors (AMPAR)10, consistent with the lower expression of AMPAR in layer III (ref. 12).
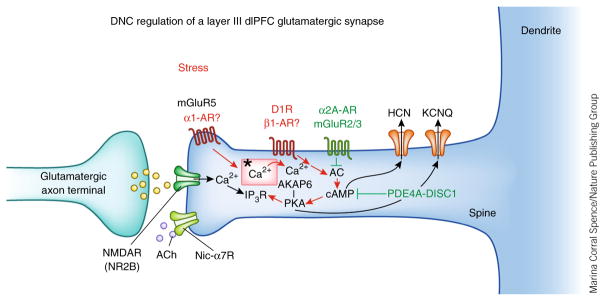
Figure 3.
Dynamic network connectivity (DNC) in the primate dlPFC. Layer III NMDAR synapses on spines in the primate dlPFC are powerfully modulated by the arousal systems (acetylcholine (ACh), norepinephrine, dopamine). ACh has permissive effects on NMDAR opening via nicotinic α7 receptors (nic-α7R) in the synapse. Feedforward Ca2+–cAMP signaling, as driven by stress exposure, can rapidly weaken synaptic efficacy and network connectivity by opening K+ channels (HCN, KCNQ) near the synapse and in the spine neck (red). Conversely, inhibition of feedforward Ca2+–cAMP signaling strengthens connections (green). The ultrastructural locations of α1-AR and β1-AR in primate dlPFC are not yet known. Asterisk indicates the spine apparatus, the extension of the smooth endoplasmic reticulum into the spine. AC, adenylyl cyclase.
The functional strength of these NMDAR synapses is dynamically modulated to rapidly enhance or weaken connections and thus helps to shape the contents and strength of working memory (Fig. 3). These very rapid changes in synapse strength, called dynamic network connectivity13, are mediated by feedforward, cAMP–Ca2+ signaling, which opens K+ channels (HCN, KCNQ) near the synapse to weaken the connection. A constellation of cAMP-related proteins are observed next to the Ca2+-containing spine apparatus, where they can increase or decrease feedforward, cAMP–Ca2+ signaling14 (Fig. 3)
The dlPFC also contains response cells, neurons that fire just before or during the motor response (Fig. 2). These neurons are modulated in a more classical manner—for example, with a reliance on AMPAR actions10—consistent with the higher expression of AMPAR in layer V of monkey dlPFC12. Layer V response-like cells appear to be the type of neuron most common in rodent PFC15. Thus, even within the dlPFC, delay cells have distinct molecular signatures compared to surrounding neurons that make them especially vulnerable to stress exposure.
Acute stress exposure rapidly impairs higher PFC functions in animals and humans
Exposure to uncontrollable stress impairs the higher cognitive functions of the PFC
The study of stress effects on cognitive abilities began after the Second World War, when it was realized that highly skilled pilots crashed their planes in the stress of battle as a result of mental errors (reviewed in ref. 16). A key aspect of these findings was that the subject had to feel a lack of control over the stressor17, a factor also found in animal studies18. Later research in animals demonstrated that exposure to acute, uncontrollable stress impairs the working memory abilities of the PFC19,20, while tasks that rely on the habitual functions of basal ganglia circuits, for example20,21, or the emotional conditioning of the amygdala22 are spared or even enhanced by stress exposure (Fig. 1b).
A variety of stressors have been used to observe how stress affects functioning in the rodent brain. Early studies often used restraint stress23 and/or inescapable shock18, as well as conditioned fear (for example, a tone previously paired with shock)24. Biochemical and then behavioral studies also used a pharmacological stressor, FG7142, a benzodiazepine inverse agonist (that is, a compound with an action opposite to that of Valium) that generates a classic glucocorticoid response and increases catecholamine release in the PFC19. Studies of stress effects in monkeys as well have employed FG7142, or loud white noise, a stressor used in early studies of humans20. More recent stress research in humans has employed a variety of stressors, including social stress, watching an upsetting video and listening to an account of stressful effects in one’s own life.
Exposure to an acute, uncontrollable stress impairs the performance of PFC cognitive tasks in rodents, monkeys and humans25. For example, rats exposed to either 2 h of restraint stress or administered the pharmacological stressor, FG7142, are impaired on the spatial delayed alternation task, a test of spatial working memory that depends on the medial PFC19,26. Performance of this task also requires decision-making capabilities and the ability to inhibit a prepotent but inappropriate response, functions linked to the PFC. Stressed rats make more perseverative errors on the task, consistent with the inflexible behavior patterns that often occur under conditions of PFC dysfunction19. In contrast, the performance of a visual spatial discrimination task with similar sensory, motor and motivational demands, but no need for PFC abilities, is unchanged by stress exposure19. A similar pattern is seen in monkeys, where acute exposure to loud white noise stress20 or FG7142 (ref. 19) impairs performance of a spatial delayed response working memory task, but has no effect on performance of a spatial discrimination task. Human subjects exposed to an acute social stress also exhibit impairments in working memory and attention, for example27, indicating that this effect is found across many species.
The effects of acute stress on hippocampal physiology and function are more complex. Acute stress appears to enhance hippocampus-dependent fear-related memory consolidation (for example, contextual fear conditioning), but impairs spatial learning that is unrelated to the fear-inducing conditions28. The severity of the acute stressor also appears to influence whether hippocampal physiology is affected. In many studies, acute, mild restraint stress had subtle or no effects on LTP23, or if brief, could even enhance LTP29. However, the addition of inescapable shocks to the restraint paradigm impairs LTP28. As restraint stress alone is sufficient to impair the spatial working memory functions of the PFC26, it appears that the hippocampus is less sensitive to impairment by acute stress exposure than is the PFC.
With the advent of brain imaging, stress studies have now begun to examine the neural circuit activity altered by acute stress in humans30,31. Functional MRI studies have shown that listening to a stressful account of one’s own life, compared to listening to a neutral passage, increases the blood oxygen level–dependent (BOLD) response in the medial PFC (anterior cingulate cortex), especially in the right hemisphere30. These results are consistent with the role of the anterior cingulate in processing mental suffering3. Studies have also shown evidence of acute stress impairing dlPFC function in humans. Subjects who watched an upsetting video showed impaired performance of an N-back working memory task and reduced BOLD activity over the dlPFC31. This study also found that acute stress exposure diminished the normal deactivation of the default mode network, including relative increases in the BOLD signal in the vmPFC and insula, circuits that normally deactivate during cognition and activate with stress31. The stress-induced impairment in working memory performance and reduction in dlPFC activity were particularly evident in subjects with greater catecholamine actions; that is, in those subjects with a methionine substitution in the catabolic enzyme, COMT, which weakens catecholamine degradation. These results are consistent with stress-induced catecholamine release impairing dlPFC working memory function32 (see below). Stress-induced impairment of working memory during an N-back task has also been linked to electrophysiological signs of PFC dysfunction: cognitive impairment correlated with reduced PFC theta activity33.
In contrast to the impairments in dlPFC working memory, an earlier study showed that watching an upsetting video enhanced the memory consolidation of the emotionally charged events in the film34. This improvement in memory consolidation correlated with increased activity in the amygdala while the subject watched the video34. The increased activity in the amygdala also involved increased catecholamine actions35 (see below), accentuating how chemical changes during acute stress exposure can switch neural orchestration of behavior from top-down to more primitive brain states (Fig. 1b).
Relevance to mental disorders
It has been appreciated for many years that stress exacerbates mental illness36—for example, the initial descent into schizophrenia37 or the switch from euthymia to illness in bipolar disorder38. Prolonged or traumatic stress exposure can lead to depression or PTSD, disorders that are more prevalent in women39,40. Data from animal studies indicate that estrogen can exaggerate stress-induced PFC dysfunction in female rats26,41. Similar mechanisms in humans may contribute to the increased vulnerability of women of cycling age for stress-induced mental disorders42. Notably, there is recent evidence that women exposed to serious stressors in middle age have an increased incidence of Alzheimer’s disease 20 years later43. This study is consistent with others showing that distress may hasten dementia44. Thus, stress exposure may increase risk of a variety of mental or cognitive disorders.
Rapid molecular events with acute stress exposure
Increased catecholamine release in PFC
Exposure to acute, uncontrollable stress induces a number of chemical changes in brain that rapidly impair PFC function. In addition to global increases in glucocorticoids, stress increases catecholamine release in PFC19,24,45. In primates, even a very mild stress can activate the dopaminergic ‘salience’ neurons that respond to both aversive and rewarding events46 and can increase dopamine release in dlPFC47. Stress also activates the noradrenergic neurons of the locus coeruleus via stimulation by the amygdala of corticotropin-releasing factor (CRF) receptors on locus coeruleus neurons48, increasing norepinephrine release in PFC49. Indeed, a subset of locus coeruleus neurons project selectively to PFC50, which may accentuate the stress response in this region. Catecholamine levels are further increased by glucocorticoids, which block the transporters on glia that normally remove catecholamines from the extracellular space51. These catecholamine actions may be increased in females by estrogen. For example, CRF activation of the locus coeruleus is accentuated in females52 and dopamine in the PFC is increased by estrogen53, suggesting mechanisms that may underlie the increased vulnerability of females to stress exposure.
High levels of catecholamine release in PFC lead to cognitive deficits. For example, the degree of cognitive impairment during stress exposure correlates with levels of dopamine release in the rat PFC19. In both rats and monkeys, stress-induced PFC dysfunction can be blocked by dopamine D1 receptor (D1R) or norepinephrine α1-adrenoceptor (AR) antagonists19,54, and conversely, it can be mimicked by high levels of D1R55 or α1-AR56,57 stimulation in PFC. For example, infusion of an α1-AR agonist into the monkey dlPFC or rat medial PFC produces a marked impairment in spatial working memory performance56.
Higher catecholamine levels have been linked to stress-induced impairment of PFC function and changes in brain state in humans as well. As mentioned above, those with a methionine substitution in COMT have weaker enzymatic activity and thus higher levels of catecholamines. These people show much greater working memory impairment and dlPFC hypoactivity during stress than those subjects with the more effective enzyme32. High levels of norepinephrine β-AR stimulation during acute stress increase the coupling of the vmPFC to subcortical limbic areas58 and enhance the memory consolidation processing of the amygdala35. High levels of norepinephrine combined with glucocorticoids have also been shown to promote habitual responding and reduce the sensitivity of the vmPFC to changes in outcome value59. Thus, the importance of norepinephrine in switching control from reflective, dlPFC circuits to more reflexive subcortical circuits can be seen in humans as well as in animals.
Intracellular signaling pathways that weaken PFC function
We have begun to understand the intracellular actions that impair PFC function during stress16 (Fig. 3). Norepinephrine α1-ARs activate Ca2+–protein kinase C (PKC) signaling, which reduces delay-cell firing in the primate dlPFC57, while high levels of dopamine D1R stimulation reduce dlPFC delay-cell firing by increasing cAMP–protein kinase A (PKA) signaling60. Norepinephrine may also drive cAMP signaling via the β1-AR61, although this pathway requires further study. Physiological, behavioral and immunoelectron microscopic evidence suggest that these pathways interact: feedforward Ca2+–cAMP signaling opens nearby HCN and KCNQ K+ channels to weaken the efficacy of nearby NMDAR synaptic connections13. This reduces the persistent firing of the dlPFC neurons that generate the mental representations needed for working memory and top-down control. Conversely, inhibition of Ca2+–PKC or cAMP–PKA signaling, or blockade of HCN channels, can rescue PFC delay-cell firing and working memory functions57,60,62.
In contrast to delay cells, which reduce firing with high levels of dopamine D1R stimulation, layer V sensory-motor response cells in dlPFC show increased firing with high levels of dopamine D2 receptor stimulation63. As response cells are inhibited by delay cells during the delay epoch, they also may become disinhibited as a result of loss of this top-down regulation. As layer V response-like cells appear to predominate in rodents, recordings from rodent PFC may give a misleading view of what occurs in primate dlPFC, where the higher cognitive circuits in layer III show reduced rather than elevated levels of firing with high levels of catecholamines.
In contrast to that in PFC, high levels of catecholamines strengthen the affective responses of the amygdala22,64, the habitual or compulsive responses of the striatum65 and sensory processing in the primary somatosensory cortex66. Similarly, PKC signaling excites sensory processing in the barrel cortex67 and reinforces fear conditioning in the amygdala68. Glucocorticoids have been shown to accentuate the effects of catecholamines in both the PFC and the amygdala69, thus coordinating and exaggerating the switch from thoughtful to habitual responding during exposure to stress (Fig. 1).
Exaggeration of changes with chronic stress exposure
Circuit-specific, architectural changes with chronic stress
Chronic stress exposure accentuates many of the effects of acute stress exposure, as architectural changes exaggerate the switch from highly evolved to more primitive brain circuits. Sustained stress exposure induces loss of dendrites and spines in layer II/III pyramidal cells of rodent PFC70–73 and loss of the dendritic tufts of layer V pyramidal cells74. Dendritic spine loss from layer II/III pyramidal cells in the prelimbic medial PFC correlates with impaired working memory on the delayed alternation task75. Similarly, dendritic retraction from layer II/III pyramidal cells in the dorsal medial PFC correlates with weaker attentional flexibility on a perceptual set-shifting task71. These findings indicate that architectural changes have functional relevance. In young adult rodents, layer II/III PFC pyramidal cell dendrites can regrow with sufficient time spent under safe conditions, but this plasticity is lost with advanced age76.
The changes in dendrites and spines with chronic stress are circuit specific. In contrast to the PFC, chronic stress exposure increases dendritic growth in the amygdala77, thus accentuating the imbalance of amygdala over PFC function. Even within the PFC, there are circuit-specific alterations that lead to amygdala dominance with chronic stress: the subset of PFC neurons that activate the amygdala do not atrophy during stress (indeed, in females, these dendrites can be extended with stress), while the PFC neurons engaged in cortico-cortical connections show the expected loss of dendritic material73. Similarly, the dendrites of pyramidal cells in the rodent orbital PFC extend rather than retract with chronic stress71. Chronic stress has no effect on performance of a reward reversal task that depends on orbital PFC function in rats71, further delineating this dissociation. Overall, a simplistic interpretation of this body of work is that pyramidal cells in cognitive circuits lose dendrites with chronic stress while those in emotional circuits are unchanged or strengthened. In contrast to pyramidal cells, the dendrites of GABAergic Martinotti interneurons hypertrophy with chronic stress in mouse PFC78, which may further reduce pyramidal cell excitation in cognitive circuits.
The loss of PFC gray matter with chronic stress has also been documented in humans. Structural imaging has shown that lower PFC gray matter volume correlates with exposure to adverse events79. Chronic stress has also been shown to weaken PFC functional connectivity80 and PFC regulation of the amygdala81, and to increase the volume of the putamen, thus accentuating the switch from flexible goal-directed to habitual responding82. Thus, sustained stress exposure in both animals and humans maintains the brain in a more primitive, reactive state.
Molecular changes with chronic stress that contribute to spine loss
The actions of norepinephrine are exaggerated with chronic stress exposure: there is increased expression of the synthetic enzymes tyrosine hydroxylase and dopamine β-hydroxylase in noradrenergic neurons and axons in both rats83–85 and primates86. Chronic stress also increases the tonic firing of locus coeruleus neurons via increased CRF–PKA activation of pacemaker cation channels87. Interestingly, CRF is increased in the locus coeruleus of patients with depression88, suggesting that this mechanism may be central to a chronic stress response in humans as well. Physical exercise can be protective during stress by increasing the expression of galanin in the locus coeruleus, which reduces locus coeruleus firing, decreases stress-induced catecholamine release and protects PFC spines89. In contrast to noradrenergic neurons, the dopaminergic axons projecting to rodent PFC become depleted with chronic stress exposure90,91. However, remaining dopamine release appears sufficient for detrimental actions, as D1R blockade during chronic stress prevents dendritic retraction in rat PFC92.
The mechanisms underlying stress-induced spine loss are just beginning to be understood and are an important arena for further research given their relevance to cognitive disorders. How do stress pathways interact with the normal processes of spine pruning, for example, during adolescence93? Are they related to spine loss with advancing age94? More specifically, how do the feedforward Ca2+–cAMP signaling mechanisms induced by stress exposure interact with inflammatory events and with signaling pathways that regulate actin dynamics in spines? Studies of the developing visual system show that activation of complement signaling induces phagocytosis of spines and synapses by astroctyes95, but this may be a ‘cleanup’ system that works in tandem with other mechanisms—for example, mechanisms that actively disassemble the actin cytoskeleton.
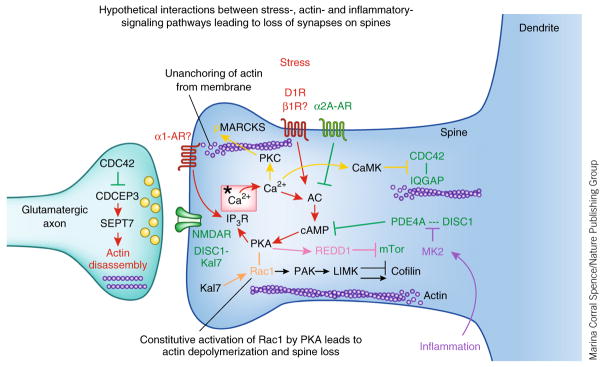
One possible link between stress signaling pathways and actin regulation involves PKC phosphorylation of MARCKS (myristoylated, alanine-rich C-kinase substrate), which normally anchors the actin skeleton to the cell membrane. In vitro studies of hippocampal pyramidal cell cultures have shown that PKC phosphorylation of MARCKS induces collapse of the actin cytoskeleton by disconnecting actin from the neuronal membrane96 (Fig. 4, gold). Inhibition of PKC signaling before daily stress exposure in rats prevents the loss of spines from layer II/III PFC pyramidal cells normally observed with chronic stress75. The protection of dendritic spines correlates with preserved working memory function75. Future studies could examine whether the preservation of spines involves MARCKS stabilization of the actin cytoskeleton and whether medications that similarly inhibit PKC signaling (for example, lithium, valproic acid, atypical anti-psychotics) similarly rescue PFC dendritic spines from the effects of stress exposure.
Figure 4.
Hypothetical interactions between the intracellular signaling pathways activated by stress exposure and pathways that regulate actin dynamics and inflammation. Stress signaling pathways are shown in red, regulatory pathways and mechanisms that strengthen connectivity are shown in green. Inflammatory pathways are shown in purple; calcium-related signaling events are shown in yellow; Rac1 constitutive activation by PKA is shown in gold; REDD1 inhibition of mTor signaling is shown in pink. Note that the regulation of actin is often studied in cultured neurons and rarely in PFC neurons. Thus, future research will be needed to see stress signaling events alter spine number in PFC pyramidal cells through activation of these pathways.
A more recent study found that inhibition of cAMP–PKA signaling with the α2A-AR agonist guanfacine is also protective of PFC dendritic spines and cognitive function in rats97. Guanfacine’s beneficial effects during chronic stress likely arise from a number of interrelated mechanisms. Guanfacine strengthens dlPFC connectivity via stimulation of postsynaptic α2A-ARs on layer III dendritic spines, inhibiting cAMP opening of HCN channels near the synapse98 (Fig. 4). Guanfacine may also diminish the harmful effects of stress through actions outside the PFC. Stimulation of α2A-ARs weakens amygdala function99, reduces stress-induced dopamine release in the PFC100 and reduces the tonic firing of locus coeruleus neurons and thus reduces norepinephrine release87,101. Guanfacine may also prevent spine loss by reducing inflammation in the brain (Fig. 4, purple). Microglia and astrocytes are activated by β-AR stimulation, while activated microglia are deactivated by α2A-AR stimulation102. As guanfacine is approved by the US Food and Drug Administration for use in adolescents103, it may offer a practical approach for reducing the inflammatory response and gray matter loss found in prodromal schizophrenia5.
Stress may also reduce the number of dendritic spines in the PFC by suppressing new spine formation. Recent studies have shown that mTor (mammalian target of rapamycin) signaling increases spine generation in the apical tuft of layer V pyramidal cells in the rat PFC and that stress exposure inhibits this effect by increasing the expression of REDD1 (regulated in development and DNA damage responses 1) (Fig. 4, pink)74. Norepinephrine stimulation of β-AR–cAMP–PKA signaling increases the expression of REDD1 in macrophages104. Similar events in PFC neurons could provide a bridge between cat-echolamine-induced increases in cAMP and reductions in mTor signaling during stress exposure. It is not known, however, whether the mechanisms underlying spine loss are universal or are specific to particular brain regions or circuits, or why stress causes dendritic expansion in some neurons and atrophy in others. These are important areas for future research.
Emerging data also suggest that different kinds of stress (physiological or psychological) may evoke similar signaling pathways to lead to PFC dysfunction and spine loss. For example, hypoxia increases REDD1 expression105 and also induces spine loss in PFC and impaired PFC cognitive function106. As with psychological stressors, these effects are prevented by treatment with guanfacine106. Similarly, traumatic brain injury (TBI) induces elevated catecholamine signaling in the PFC107 and elevated α1-AR expression that contributes to working memory impairment108. As TBI increases the risk of PTSD109 and Alzheimer’s disease110, these data may help us understand the factors that make higher brain circuits so vulnerable to insult.
Translation to humans
At least some of these mechanisms studied in animals are immediately relevant to stress-related disorders in humans. For example, increases in REDD1 have been found in the dlPFC of depressed patients, which is similar to what is seen in the stressed rat PFC74. Notably, there is evidence that treatment strategies arising from basic research are effective in stress-related disorders111. The α1-AR antagonist prazosin is now in widespread use to treat PTSD in veterans, active duty soldiers and civilians (reviewed in ref. 111). Prazosin reduces flashbacks, improves concentration and thinking, and reduces substance abuse, signs of improved PFC function.
Guanfacine is now in widespread use for the treatment of PFC disorders on the basis of research in animals, and it has been shown to improve PFC functions and reduce cravings in subjects with stress-induced substance abuse112,113. Guanfacine also appears to help children who have been traumatized, one of the few medications helpful in this arena114. The positive findings with guanfacine and prazosin are reassuring, as they validate the mission of basic research.
Potential relevance to spine loss in mental disorders
A major goal of current research is to understand how activation of stress signaling pathways in PFC contributes to psychiatric symptoms and to dendritic spine loss in mental illness. There is evidence of vmPFC gray matter loss in mood disorders7,115,116, which implies dendritic atrophy. However, there have been no direct studies of changes in spine numbers in these circuits. Similarly, there have been no studies of the molecular regulation of vmPFC circuits in primates, and so we do not know whether these circuits are modulated in a manner similar to that of dlPFC. These are both important arenas for future research. However, there have been several studies of dendritic spine changes in the dlPFC in schizophrenia. Neuronal cell bodies are preserved, but there is extensive loss of dendrites and spines from layers III and possibly layer V pyramidal cells4,117,118. Indeed, the onset of schizophrenia is accompanied by waves of PFC gray matter loss, as well as increased signs of inflammation5. The loss of spines from newly evolved cognitive circuits in schizophrenia likely contributes to their profound hypoactivity119. Understanding the causes of dendritic spine loss may help identify treatments to slow or prevent the descent into disease.
Clues from DISC1
Emerging data indicate that the scaffolding protein Disrupted In Schizophrenia 1 (DISC1) is critical for regulation of the stress response in PFC, suggesting that genetic insults that interfere with the function of this protein increase the risk for stress-related psychiatric disorders. Mutations in DISC1 are associated with high rates of mental illness120,121, and more subtle polymorphisms are associated with decreased PFC gray matter and impaired working memory122,123. DISC1 anchors many proteins and thus regulates their functional localization and molecular interactions124. Particularly relevant to stress signaling in PFC, DISC1 anchors the phosphodiesterases (PDE4s) that catabolize cAMP and regulate its signaling121,125. Immunoelectron microscopy studies of human126 and rhesus monkey13,127 dlPFC show that DISC1 is located in layer III spines, where it anchors PDE4A next to the spine apparatus, critically positioned to regulate feedforward cAMP–Ca2+ stress signaling pathways13,127. Notably, genetic insults in PDE4A are also linked to schizophrenia128. The onset of schizophrenia is associated with signs of increased inflammation and PFC gray matter loss5, and biochemical studies in vitro have shown that inflammation reduces the ability of DISC1 to anchor PDE4A via increases in MK2 signaling125 (Fig. 4, purple). Loss of DISC1 anchoring of PDE4A due to inflammation or genetic insults would thus disinhibit the stress response and lower the threshold for stress-induced PFC dysfunction.
Studies in rodents with genetic alterations of DISC1 are consistent with this hypothesis. Knockdown of DISC1 in rodent PFC increases cAMP signaling in PFC neurons129 and increases sensitivity to stress-induced PFC cognitive deficits130. DISC1 also regulates the integrity of PFC spines by anchoring kalirin-7 (Kal7, the rodent homolog of Duo) and preventing its stimulation of Rac1 signaling131. Loss of DISC1 leads to constituent activation of Rac1 signaling and spine loss via p21-activated kinase (PAK) signaling131,132 (Fig. 4, orange). Interestingly, PKA can form a complex with Rac1 that induces constitutive Rac1 activity133, a mechanism that may contribute to stress-induced spine loss. As loss-of-function mutations in DISC1 are associated with a variety of mental illnesses, especially an increased incidence of depression120, loss of spines may contribute to a variety of disorders, with symptoms related to the subcircuit(s) most affected—for example, changes in vmPFC increasing risk of depression and impairment of dlPFC circuits increasing risk of schizophrenia.
Molecular differences in the dlPFC of patients with schizophrenia
Molecular analyses of the dlPFC from patients with schizophrenia have also begun to provide clues regarding potential mechanisms driving dendritic spine loss. Tissue analyses have found reductions in mRNAs for CDC42 and Duo that correlate with decreased spines134. A later study found increased expression of the CDC42 effector protein CDC42EP3 specifically in layer III, as well as reduced septin-7 (SEPT7), suggesting altered regulation of septin filaments in layer III synapses135. In vitro data indicate that high levels of Ca2+– calmodulin signaling can disrupt CDC42–IQGAP interactions needed for actin regulation136 (Fig. 4, gold), suggesting another possible link between stress signaling and actin dynamics. However, it is not known whether such interactions occur in layer III dlPFC spines. Bridging signaling events in dlPFC neuronal circuits with molecular changes in the neurons of patients with mental illness is an important goal for further research.
Potential relevance to degeneration in Alzheimer’s disease
Dysregulation of stress signaling pathways with advancing age may also increase vulnerability to degeneration in Alzheimer’s disease—for example, due to an age-related loss of PDE4A. Alzheimer’s disease is characterized by amyloid-β (Aβ) plaques and by neurofibrillary tangles composed of hyperphosphorylated tau (pTau). Cognitive impairment correlates with the number of neurofibrillary tangles137, which selectively affect highly connected pyramidal cells in association cortex but not in primary sensory cortex6,138,139. Research is beginning to uncover why pyramidal cells in association cortex are so vulnerable, why advancing age is such a large risk factor for neurodegeneration and why stress may drive disease.
Although the largest risk factor for Alzheimer’s disease is advanced age, TBI is also an established risk factor140, and new evidence suggests that psychological distress43 and female sex141 are also risk factors for Alzheimer’s degeneration. Indeed, the increased risk of Alzheimer’s disease associated with the E4 allele of the APOE gene is especially pronounced in women and is associated with increased pTau141. As described above, TBI and psychological distress share signaling events in PFC, and females have an exaggerated stress response. Intriguingly, animal studies have shown that stress exposure increases the phosphorylation of tau142. Thus, these seemingly disparate risk factors may share underlying molecular mechanisms that confer risk of degeneration.
Feedforward stress signaling pathways are dysregulated by advancing age
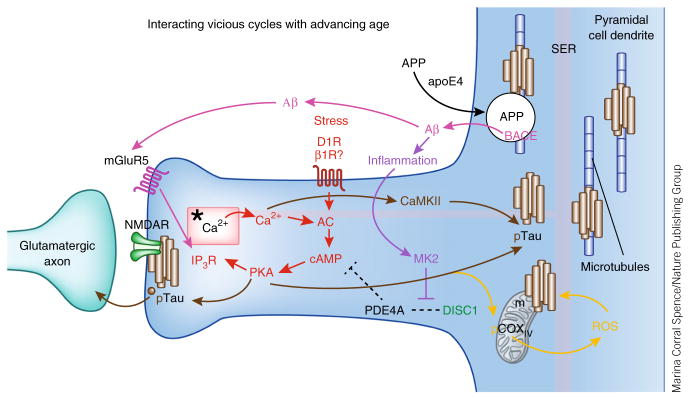
The phosphodiesterase PDE4A is critically positioned to regulate stress signaling pathways in the dlPFC pyramidal cell circuits needed for higher cognition14. PDE4A is anchored to the spine apparatus, where it can catabolize cAMP and reduce feed-forward Ca2+–cAMP signaling in spines (Figs. 3–5). Studies of the aging monkey cortex have found that PDE4A is lost from these spines with advancing age, perhaps as a result of age-related increases in inflammation that may unanchor PDE4A125. Age-related reductions in PDE4A are associated with increased pTau in the dlPFC but not the primary visual cortex, a pattern similar to the pattern of neurofibrillary tangles in Alzheimer’s disease14. Increased tau phosphorylation occurs at sites phosphorylated by PKA and by Ca2+-activated kinases14 (Fig. 5, brown). pTau accumulates over the spine apparatus and in the postsynaptic density of putative glutamatergic-like (but not inhibitory) synapses on spines, where there is evidence of pTau trafficking in vesicles. In the nearby dendrite, pTau aggregates on microtubules, where it may interfere with intracellular trafficking, including the trafficking of amyloid precursor protein (APP).
Figure 5.
The multiple, interacting, feedforward vicious cycles that may be disinhibited in the aging dlPFC, contributing to increased vulnerability to degeneration. Red: stress activates feedforward Ca2+–cAMP signaling pathways near the glutamate NMDAR synapses on spines. In the young adult dlPFC, the phosphodiesterase PDE4A is anchored by DISC1 next to the spine apparatus (*), an extension of the smooth endoplasmic reticulum (SER), critically positioned to regulate feedforward Ca2+–cAMP signaling in dlPFC spines. PDE4A is lost from spines with advancing age, dysregulating Ca2+–cAMP signaling and increasing the activation of kinases (for example, PKA and calcium/calmodulin-dependent kinase II (CaMKII)) that phosphorylate tau14. IP3R, inositol-1,4,5-trisphosphate receptor. Brown: pTau aggregates over the spine apparatus, at glutamatergic synapses, and over microtubules in dendrites and traffics in vesicles between neurons14. The aggregation of pTau on microtubules in dendrites likely interferes with intracellular trafficking, including the trafficking of APP, the precursor to Aβ. Magenta: APP is cleaved to Aβ when it is trapped in endosomes that contain β-secretase (BACE)—for example, when there is interference with APP endosomal trafficking143. Indeed, the increased risk of Alzheimer’s disease conferred by the apoE4 variant is thought to involve increased localization of APP into endosomes145. The aggregation of pTau on microtubules may similarly trap APP-containing endosomes and lead to the increased generation of Aβ oligomers. The generation of Aβ oligomers can drive additional vicious cycles by stimulating mGluR5 (ref. 147). mGluR5 are localized near the synapse on spines in dlPFC, positioned to activate feedforward Ca2+–cAMP signaling and thus drive more tau phosphorylation. Purple: Aβ fibrils drive inflammation148, which can unanchor residual PDE4A125 and further disinhibit stress signaling pathways. Orange: increased stress signaling may also dysregulate mitochondrial function, as PKA can phosphorylate cyclooxygenase IV (COXIV) to increase reactive oxygen species (ROS)149, which also increase tau phosphorylation and Aβ production150, leading to additional mitochondrial dysfunction. Thus, dysregulation of stress signaling pathways in the dlPFC with advancing age may contribute to many deleterious molecular events that increase vulnerability to degeneration. Alzheimer’s disease pathology may begin anywhere along these pathways (for example, genetic alterations in APP processing or environmental stressors promoting pTau) and, by driving these interacting cycles, lead to the same degenerative phenotype.
Multiple, interacting vicious cycles increase risk of neuro-degeneration
Dysregulation of feedforward Ca2+–cAMP signaling in dlPFC spines could drive multiple, interacting, vicious cycles that increase vulnerability to degeneration (Fig. 5, red). APP can be cleaved to Aβ (Fig. 5, magenta) when it is trapped in endosomes that contain β-secretase (BACE)143, a process exacerbated by the APOE E4 genotype144,145. The aggregation of pTau on microtubules may similarly trap APP-containing endosomes and lead to the generation of Aβ oligomers. Aβ oligomers can drive additional vicious cycles by stimulating metabotropic glutamate receptor 5 (mGluR5)146,147, which activates feedforward Ca2+–cAMP signaling and drive tau phosphorylation (Fig. 5, brown). Aβ fibrils also increase inflammation148 (Fig. 5, purple), which may unanchor residual PDE4A125, and further disinhibit stress signaling pathways. Increased stress signaling may also dysregulate mitochondrial function, which also leads to tau phosphorylation149 and Aβ production150 (Fig. 5, orange). These in turn cause more mitochondrial dysfunction, thus feeding yet another intracellular vicious cycle.
The presence of so many interacting vicious cycles suggests that the degenerative process could be initiated by a variety of precipitating events, any of which could set the entire process in motion. For example, genetic errors in APP processing such as presenilin mutations can increase the production of Aβ early in life and thus cause early-onset illness, or the loss of PDE4A regulation of the stress response with advancing age can drive the phosphorylation of tau and lead to late-onset disease. Future research may determine whether this ‘signature of vulnerability’ observed in the dlPFC is also evident in other association cortices that degenerate in Alzheimer’s disease (for example, entorhinal cortex, parietal association cortex) and whether inhibition of stress signaling events (for example, with α2A-AR or mGluR3 agonists, or mGluR5 antagonists) can provide strategies for prevention.
Closing
Studies of the molecular pathways activated by stress exposure have begun to explain why PFC circuits deteriorate in so many cognitive disorders. The presence of intrinsic mechanisms to actively weaken connections during stress exposure in these newly evolved circuits renders them particularly vulnerable when they are dysregulated owing to genetic or environmental insults. This contrasts with the stress effects on subcortical regions such as the amygdala that are strengthened by stress exposure, thus switching the brain into a more primitive, reflexive state. Much more research is needed to understand the mechanics of spine loss, the generality of the stress response to other high-order association cortices, and how genetic insults interact with stress signaling pathways to hasten disease. However, the benefits of this basic research are already evident in new, effective treatments for stress-related cognitive disorders.
Acknowledgments
Gratitude to S. Katrancha for discussions regarding spine dynamics. A.F.T.A. is supported by a US National Institutes of Health Director’s Pioneer Award DP1AG047744-01, R01AG043430-01A1 and RO1MH100064-01A1.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares competing financial interests: details are available in the online version of the paper.
References
- 1.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. This is an excellent review of the dlPFC microcircuits that underlie spatial working memory in primates and of how they generate the mental representations that are the foundation of abstract thought. [DOI] [PubMed] [Google Scholar]
- 2.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. This is an excellent review of the connections and functions of the ventral and medial PFC in primates. [DOI] [PubMed] [Google Scholar]
- 3.Opler LA, Opler MG, Arnsten AFT. Ameliorating treatment-refractory depression with intranasal ketamine: potential NMDA receptor actions in the pain circuitry representing mental anguish. CNS Spectr. 2015 Jan 26; doi: 10.1017/S1092852914000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 5.Cannon TD, et al. Progressive reduction in cortical thickness as psychosis develops: A multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bussière T, et al. Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: stereologic analysis of prefrontal cortex area 9. J Comp Neurol. 2003;463:281–302. doi: 10.1002/cne.10760. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg HP, et al. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Kühn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73:70–74. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Elston GN, et al. Specializations of the granular prefrontal cortex of primates: implications for cognitive processing. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:26–35. doi: 10.1002/ar.a.20278. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, et al. NMDA receptors subserve working memory persistent neuronal firing In dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, et al. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc Natl Acad Sci USA. 2013;110:12078–12083. doi: 10.1073/pnas.1307849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta D, Arion D, Lewis DA. Developmental expression patterns of GABAA receptor subunits in layer 3 and 5 pyramidal cells of monkey prefrontal cortex. Cereb Cortex. 2015;25:2295–2305. doi: 10.1093/cercor/bhu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnsten AFT, Wang M, Paspalas CD. Neuromodulation of thought: Flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlyle BC, et al. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc Natl Acad Sci USA. 2014;111:5036–5041. doi: 10.1073/pnas.1322360111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caetano MS, et al. Lost in transition: Aging-related changes in executive control by the medial prefrontal cortex. J Neurosci. 2012;32:3765–3777. doi: 10.1523/JNEUROSCI.6011-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnsten AFT. Stress signaling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass DC, Reim B, Singer JE. Behavioral consequences of adaptation to controllable and uncontrollable noise. J Exp Soc Psychol. 1971;7:244–257. [Google Scholar]
- 18.Minor TR, Jackson RL, Maier SF. Effects of task-irrelevant cues and reinforcement delay on choice-escape learning following inescapable shock: evidence for a deficit in selective attention. J Exp Psychol Anim Behav Process. 1984;10:543–556. [PubMed] [Google Scholar]
- 19.Murphy BL, Arnsten AFT, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnsten AFT, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. This study and ref. 19 above were the first to show that stress exposure impairs PFC function in animals and that it is caused by high levels of stress-induced catecholamine release in the PFC. [DOI] [PubMed] [Google Scholar]
- 21.Elliott AE, Packard MG. Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiol Learn Mem. 2008;90:616–623. doi: 10.1016/j.nlm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K, McEwen BS, Pavlides C. Site and time dependent effects of acute stress on hippocampal long-term potentiation in freely behaving rats. Exp Brain Res. 2003;152:52–59. doi: 10.1007/s00221-003-1519-0. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein LE, Rasmusson AM, Bunney SB, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnsten AFT. The biology of feeling frazzled. Science. 1998;280:1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- 26.Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav Brain Funct. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olver JS, Pinney M, Maruff P, Norman TR. Impairments of spatial working memory and attention following acute psychosocial stress. Stress Health. 2015;31:115–123. doi: 10.1002/smi.2533. [DOI] [PubMed] [Google Scholar]
- 28.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 29.Spyrka J, Danielewicz J, Hess G. Brief neck restraint stress enhances long-term potentiation and suppresses long-term depression in the dentate gyrus of the mouse. Brain Res Bull. 2011;85:363–367. doi: 10.1016/j.brainresbull.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Sinha R, Lacadie CM, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Ann NY Acad Sci. 2004;1032:254–257. doi: 10.1196/annals.1314.032. [DOI] [PubMed] [Google Scholar]
- 31.Qin S, Hermans EJ, van Marle HJF, Lou J, Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006. This study was the first to show that acute stress exposure impairs the working memory functions of the dlPFC in human subjects, using fMRI to document the pattern of brain activity changes that occur with exposure to chronic stress. Its companion study (ref. 32) showed that stress-induced cognitive impairment in dlPFC is associated with genetic signatures of higher catecholamine levels, thus bridging the research in animals and human subjects. [DOI] [PubMed] [Google Scholar]
- 32.Qin S, et al. The effect of moderate acute psychological stress on working memory-related neural activity is modulated by a genetic variation in catecholaminergic function in humans. Front Integr Neurosci. 2012;6:16. doi: 10.3389/fnint.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gärtner M, Rohde-Liebenau L, Grimm S, Bajbouj M. Working memory-related frontal theta activity is decreased under acute stress. Psychoneuro-endocrinology. 2014;43:105–113. doi: 10.1016/j.psyneuen.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Cahill L, McGaugh JL. Modulation of memory storage. Curr Opin Neurobiol. 1996;6:237–242. doi: 10.1016/s0959-4388(96)80078-x. [DOI] [PubMed] [Google Scholar]
- 35.Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 36.Mazure CM, editor. Does Stress Cause Psychiatric Illness? American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- 37.Raune D, Kuipers E, Bebbington P. Stressful and intrusive life events preceding first episode psychosis. Epidemiol Psichiatr Soc. 2009;18:221–228. [PubMed] [Google Scholar]
- 38.Hammen C, Gitlin M. Stress reactivity in bipolar patients and its relation to prior history of disorder. Am J Psychiatry. 1997;154:856–857. doi: 10.1176/ajp.154.6.856. [DOI] [PubMed] [Google Scholar]
- 39.Weissman MM, et al. Cross-national epidemiology of major depression and bipolar disorder. J Am Med Assoc. 1996;276:293–299. [PubMed] [Google Scholar]
- 40.Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- 41.Shansky RM, et al. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- 42.Bebbington PE, et al. The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity. Psychol Med. 1998;28:9–19. doi: 10.1017/s0033291797006077. [DOI] [PubMed] [Google Scholar]
- 43.Johansson L, et al. Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer’s disease: a 38-year longitudinal population study. BMJ Open. 2013;3:e003142. doi: 10.1136/bmjopen-2013-003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson RS, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- 45.Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–402. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- 46.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kodama T, Hikosaka K, Honda Y, Kojima T, Watanabe M. Higher dopamine release induced by less rather than more preferred reward during a working memory task in the primate prefrontal cortex. Behav Brain Res. 2014;266:104–107. doi: 10.1016/j.bbr.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Van Bockstaele EJ, Colago EE, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 49.Nakane H, Shimizu N, Hori T. Stress-induced norepinephrine release in the rat prefrontal cortex measured by microdialysis. Am J Physiol. 1994;267:R1559–R1566. doi: 10.1152/ajpregu.1994.267.6.R1559. [DOI] [PubMed] [Google Scholar]
- 50.Chandler DJ, Gao WJ, Waterhouse BD. Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci USA. 2014;111:6816–6821. doi: 10.1073/pnas.1320827111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gründemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the cortisone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- 52.Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32:709–723. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kritzer MF, Kohama SG. Ovarian hormones influence morphology, distribution and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1998;395:1–17. [PubMed] [Google Scholar]
- 54.Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AFT. A role for norepinephrine in stress-induced cognitive deficits: α-1-adrenoceptor mediation in prefrontal cortex. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 55.Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of dopamine D1 receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arnsten AFT, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 57.Birnbaum SG, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 58.Hermans EJ, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- 59.Schwabe L, Tegenthoff M, Höffken O, Wolf OT. Simultaneous glucocorticoid and noradrenergic activity disrupts the neural basis of goal-directed action in the human brain. J Neurosci. 2012;32:10146–10155. doi: 10.1523/JNEUROSCI.1304-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vijayraghavan S, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 61.Ramos BP, et al. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 62.Gamo NJ, et al. Stress impairs prefrontal cortical function via D1 dopamine receptor interactions with HCN channels. Biol Psychiatry. 2015 Feb 4; doi: 10.1016/j.biopsych.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 64.Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between β- and α1-adrenoceptors. J Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Everitt BJ, et al. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Phil Trans R Soc Lond B. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waterhouse BD, Moises HC, Woodward DJ. Noradrenergic modulation of somatosensory cortical neuronal responses to iontophoretically applied putative transmitters. Exp Neurol. 1980;69:30–49. doi: 10.1016/0014-4886(80)90141-7. [DOI] [PubMed] [Google Scholar]
- 67.Mouradian RD, Seller FM, Waterhouse BD. Noradrenergic potentiation of excitatory transmitter action in cerebrocortical slices: evidence of mediation by an alpha1-receptor-linked second messenger pathway. Brain Res. 1991;546:83–95. doi: 10.1016/0006-8993(91)91162-t. [DOI] [PubMed] [Google Scholar]
- 68.Bonini JS, Cammarota M, Kerr DS, Bevilaqua LR, Izquierdo I. Inhibition of PKC in basolateral amygdala and posterior parietal cortex impairs consolidation of inhibitory avoidance memory. Pharmacol Biochem Behav. 2005;80:63–67. doi: 10.1016/j.pbb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci USA. 2010;107:16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seib LM, Wellman CL. Daily injections alter spine density in rat medial prefrontal cortex. Neurosci Lett. 2003;337:29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- 71.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radley JJ, et al. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 73.Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–2484. doi: 10.1093/cercor/bhp003. This study used tract tracing to be able to identify the connections of neurons in rat medial PFC and showed that those positioned to excite the amygdala had expanded dendrites following chronic stress, while those with cortical projections showed the expected atrophy with chronic stress exposure. These effects were particularly prevalent in females, consistent with the greater stress response in females with circulating estrogen. This study thus revealed an important heterogeneity within the PFC, reminding us that some circuits within the PFC actually promote the stress response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ota KT, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med. 2014;20:531–535. doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hains AB, et al. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci USA. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bloss EB, et al. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gilabert-Juan J, Castillo-Gomez E, Guirado R, Moltó MD, Nacher J. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct Funct. 2013;218:1591–1605. doi: 10.1007/s00429-012-0479-1. [DOI] [PubMed] [Google Scholar]
- 79.Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim P, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci USA. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soares JM, et al. Stress-induced changes in human decision-making are reversible. Transl Psychiatry. 2012;2:e131. doi: 10.1038/tp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Melia KR, et al. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem. 1992;58:494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- 84.Miner LH, et al. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci. 2006;26:1571–1578. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan Y, Chen P, Li Y, Zhu MY. Effects of chronic social defeat on expression of dopamine β-hydroxylase in rat brains. Synapse. 2013;67:300–312. doi: 10.1002/syn.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bethea CL, Kim A, Cameron JL. Function and innervation of the locus ceruleus in a macaque model of functional hypothalamic amenorrhea. Neurobiol Dis. 2013;50:96–106. doi: 10.1016/j.nbd.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biol Psychiatry. 1999;46:1131–1139. doi: 10.1016/s0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- 88.Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28:1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- 89.Sciolino NR, et al. Galanin mediates features of neural and behavioral stress resilience afforded by exercise. Neuropharmacology. 2015;89:255–264. doi: 10.1016/j.neuropharm.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mizoguchi K, et al. Chronic stress induces impairment of spatial working memory due to prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matuszewich L, McFadden LM, Friedman RD, Frye CA. Neurochemical and behavioral effects of chronic unpredictable stress. Behav Pharmacol. 2014;25:557–566. doi: 10.1097/FBP.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin GL, Borders CB, Lundewall LJ, Wellman CL. D1 receptors regulate dendritic morphology in normal and stressed prelimbic cortex. Psychoneuroendocrinology. 2015;51:101–111. doi: 10.1016/j.psyneuen.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 94.Dumitriu D, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chung WS, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 97.Hains AB, Yabe Y, Arnsten AFT. Chronic stimulation of alpha-2A-adrenoceptors with guanfacine protects rodent prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Neurobiol Stress. 2015;2:1–9. doi: 10.1016/j.ynstr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang M, et al. α2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 99.DeBock F, et al. α2-adrenoreceptor activation inhibits LTP and LTD in the basolateral amygdala: involvement of Gi/o-protein-mediated modulation of Ca2+-channels and inwardly rectifying K+-channels in LTD. Eur J Neurosci. 2003;17:1411–1424. doi: 10.1046/j.1460-9568.2003.02544.x. [DOI] [PubMed] [Google Scholar]
- 100.Morrow BA, George TP, Roth RH. Noradrenergic α-2 agonists have anxiolytic-like actions on stress-related behavior and mesoprefrontal dopamine biochemistry. Brain Res. 2004;1027:173–178. doi: 10.1016/j.brainres.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 101.Engberg G, Eriksson E. Effects of alpha 2-adrenoceptor agonists on locus coeruleus firing rate and brain noradrenaline turnover in N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ)-treated rats. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:472–477. doi: 10.1007/BF00169548. [DOI] [PubMed] [Google Scholar]
- 102.Gyoneva S, Traynelis SF. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J Biol Chem. 2013;288:15291–15302. doi: 10.1074/jbc.M113.458901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Childress AC, Berry SA. Pharmacotherapy of attention-deficit hyperactivity disorder in adolescents. Drugs. 2012;72:309–325. doi: 10.2165/11599580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 104.Yanagawa Y, Hiraide S, Matsumoto M, Togashi H. Rapid induction of REDD1 gene expression in macrophages in response to stress-related catecholamines. Immunol Lett. 2014;158:109–115. doi: 10.1016/j.imlet.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 105.Katiyar S, et al. REDD1, an inhibitor of mTOR signalling, is regulated by the CUL4A–DDB1 ubiquitin ligase. EMBO Rep. 2009;10:866–872. doi: 10.1038/embor.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kauser H, Sahu S, Kumar S, Panjwani U. Guanfacine is an effective countermeasure for hypobaric hypoxia-induced cognitive decline. Neuroscience. 2013;254:110–119. doi: 10.1016/j.neuroscience.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 107.Kobori N, Clifton GL, Dash PK. Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J Neurotrauma. 2006;23:1094–1102. doi: 10.1089/neu.2006.23.1094. [DOI] [PubMed] [Google Scholar]
- 108.Kobori N, Hu B, Dash PK. Altered adrenergic receptor signaling following traumatic brain injury contributes to working memory dysfunction. Neuroscience. 2011;172:293–302. doi: 10.1016/j.neuroscience.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bryant R. Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clin Neurosci. 2011;13:251–262. doi: 10.31887/DCNS.2011.13.2/rbryant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer’s disease. Neurosci Biobehav Rev. 2012;36:1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 111.Arnsten AFT, Raskind M, Taylor FB, Connor DF. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89–99. doi: 10.1016/j.ynstr.2014.10.002. This paper reviews how studies of stress effects in animals have led to successful treatments for PTSD in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McKee S, et al. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J Psychopharmacol. 2015;29:300–311. doi: 10.1177/0269881114562091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fox H, Sofuoglu M, Sinha R. Guanfacine enhances inhibitory control and attentional shifting in early abstinent cocaine-dependent individuals. J Psychopharmacol. 2015 Jan 7;29:312–323. doi: 10.1177/0269881114562464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Connor DF, Grasso DJ, Slivinsky MD, Pearson GS, Banga A. An open-label study of guanfacine extended release for traumatic stress related symptoms in children and adolescents. J Child Adolesc Psychopharmacol. 2013;23:244–251. doi: 10.1089/cap.2012.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Licznerski P, Duman RS. Remodeling of axospinous synapses in the pathophysiology and treatment of depression. Neuroscience. 2013;251:33–50. doi: 10.1016/j.neuroscience.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Savitz JB, Price JL, Drevets WC. Neuropathological and neuromorphometric abnormalities in bipolar disorder: view from the medial prefrontal cortical network. Neurosci Biobehav Rev. 2014;42:132–147. doi: 10.1016/j.neubiorev.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 117.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 118.Black JE, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- 119.Arion D, et al. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry. 2015 Jan 6; doi: 10.1038/mp.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- 121.Millar JK, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 122.Cannon TD, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry. 2005;62:1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- 123.Szeszko PR, et al. DISC1 is associated with prefrontal cortical gray matter and positive symptoms in schizophrenia. Biol Psychol. 2008;79:103–110. doi: 10.1016/j.biopsycho.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Camargo LM, et al. Disrupted in Schizophrenia 1 interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- 125.MacKenzie KF, et al. Phosphorylation of cAMP-specific PDE4A5 (phosphodiesterase-4A5) by MK2 (MAPKAPK2) attenuates its activation through protein kinase A phosphorylation. Biochem J. 2011;435:755–769. doi: 10.1042/BJ20101184. [DOI] [PubMed] [Google Scholar]
- 126.Kirkpatrick B, et al. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J Comp Neurol. 2006;497:436–450. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- 127.Paspalas CD, Min Wang M, Arnsten AFT. Constellation of HCN channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex—potential substrate for working memory deficits in schizophrenia. Cereb Cortex. 2013;23:1643–1654. doi: 10.1093/cercor/bhs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deng X, et al. Positive association of phencyclidine-responsive genes, PDE4A and PLAT, with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:850–858. doi: 10.1002/ajmg.b.31233. [DOI] [PubMed] [Google Scholar]
- 129.El-Hassar L, et al. Disrupted in Schizophrenia 1 modulates medial prefrontal cortex pyramidal neuron activity through cAMP regulation of transient receptor potential C and small-conductance K+ channels. Biol Psychiatry. 2014;76:476–485. doi: 10.1016/j.biopsych.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gamo NJ, et al. Role of Disrupted in Schizophrenia 1 (DISC1) in stress-induced prefrontal cognitive dysfunction. Transl Psychiatry. 2013;3:e328. doi: 10.1038/tp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hayashi-Takagi A, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hayashi-Takagi A, et al. PAKs inhibitors ameliorate schizophrenia-associated dendritic spine deterioration in vitro and in vivo during late adolescence. Proc Natl Acad Sci USA. 2014;111:6461–6466. doi: 10.1073/pnas.1321109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bachmann VA, Bister K, Stefan E. Interplay of PKA and Rac: fine-tuning of Rac localization and signaling. Small GTPases. 2013;4:247–251. doi: 10.4161/sgtp.27281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- 135.Ide M, Lewis DA. Altered cortical CDC42 signaling pathways in schizophrenia: implications for dendritic spine deficits. Biol Psychiatry. 2010;68:25–32. doi: 10.1016/j.biopsych.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Briggs MW, Sacks DB. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 2003;542:7–11. doi: 10.1016/s0014-5793(03)00333-8. [DOI] [PubMed] [Google Scholar]
- 137.Giannakopoulos P, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 138.Pearson RCA, Esiri MM, Hiorns RW, Wilcock GK, Powell TPS. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA. 1985;82:4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lewis DA, Campbell MJ, Terry RD, Morrison JH. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer’s disease: a quantitative study of visual and auditory cortices. J Neurosci. 1987;7:1799–1808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guo Z, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 141.Altmann A, Tian L, Henderson VW, Greicius MD & Alzheimer’s Disease Neuroimaging Initiative Investigators. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Okawa Y, Ishiguro K, Fujita SC. Stress-induced hyperphosphorylation of tau in the mouse brain. FEBS Lett. 2003;535:183–189. doi: 10.1016/s0014-5793(02)03883-8. [DOI] [PubMed] [Google Scholar]
- 143.Bhalla A, et al. The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport. Neurobiol Dis. 2012;47:126–134. doi: 10.1016/j.nbd.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cam JA, Bu G. Modulation of β-amyloid precursor protein trafficking and processing by the low density lipoprotein receptor family. Mol Neurodegener. 2006;1:8. doi: 10.1186/1750-1326-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yajima R, et al. ApoE-isoform-dependent cellular uptake of amyloid-β is mediated by lipoprotein receptor LR11/SorLA. Biochem Biophys Res Commun. 2015;456:482–488. doi: 10.1016/j.bbrc.2014.11.111. [DOI] [PubMed] [Google Scholar]
- 146.Renner M, et al. Deleterious effects of amyloid β oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66:739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Um JW, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron. 2013;79:887–902. doi: 10.1016/j.neuron.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Doens D, Fernández PL. Microglia receptors and their implications in the response to amyloid β for Alzheimer’s disease pathogenesis. J Neuroinflammation. 2014;11:48. doi: 10.1186/1742-2094-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Acin-Perez R, Gatti DL, Bai Y, Manfredi G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 2011;13:712–719. doi: 10.1016/j.cmet.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Melov S, et al. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]