Introduction
Resistance to trastuzumab, either primary/de novo resistance or acquired/treatment-induced resistance, is a major clinical concern facing breast oncologists today.1 Here, we describe two cases of human epidermal growth factor receptor 2 (HER2)-positive breast cancer (BC) with a mucin-producing component that were presumably resistant to trastuzumab.
Case Reports
Case 1
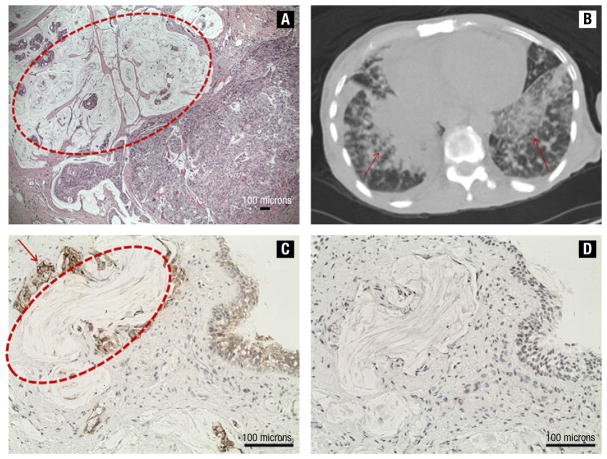
In 2004, a 57-year-old woman had a diagnosis of metastatic inflammatory BC. Biopsy of the left breast revealed infiltrating ductal carcinoma (IDC) with a mucin-producing component, histologic grade 3, estrogen receptor (ER)-positive, progesterone receptor (PR)-negative, and HER2-positive. Three liver lesions consistent with metastases were found by computed tomography (CT) scan. The patient was started on chemotherapy with carboplatin, docetaxel, and trastuzumab. After six cycles of chemotherapy, clinical and radiologic evaluation of the disease showed a complete response of the liver lesions, but a poor response in the breast and lymph nodes. A left modified radical mastectomy was performed in view of the complete resolution of the liver lesions. Pathology revealed that the entire breast, including nipple and skin, was replaced by IDC. Lymphovascular invasion was present, and 9 of 13 axillary nodes were positive for metastases. Of note, the tumor was characterized by a large colloid-producing component (Figure 1A) and was stage pT4d pN2 pMx, ER-positive, PR-negative, and HER2-positive. After surgery, the patient was treated with adjuvant radiotherapy of the chest wall and supraclavicular fossa (5040 cGy) and started on maintenance therapy with trastuzumab and anastrozole.
Figure 1.
(A) Invasive ductal carcinoma with a wide mucin-producing component (inside the red circle; scale bar: 100 μm). (B) Computed tomography scan shows multiple lung lesions (indicated by arrows). (C) and (D) Lung metastasis of HER2-positive (C, red arrow; scale bar: 100 μm) and Thyroid Transcription Factor-1-negative BC (D, scale bar: 100 μm) with colloid-producing phenotype (inside the red circle; scale bar: 100 μm)
Case 2
In 1990, a 29-year-old woman was diagnosed with a stage II IDC of the right breast. ER, PR, and HER2 expression was unknown. She was treated with lumpectomy and axillary lymph node dissection, followed by adjuvant chemotherapy with doxorubicin and cyclophosphamide and radiotherapy. When she was 41 years old, she developed a contralateral stage III (pT1c pN3), histologic grade 3, ER- and PR-positive, and HER2-negative IDC. A left modified radical mastectomy was performed, and she was started on adjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by weekly paclitaxel. Then, she received chest wall irradiation and was started on hormone therapy with tamoxifen. After 2 years, because of diffuse skeletal pain, a workup for metastatic disease was performed which revealed a diagnosis of right supraclavicular lymph node involvement and bone metastases. She underwent multiple sequential palliative treatment lines including capecitabine, weekly paclitaxel, gemcitabine, and abraxane in combination with bevacizumab. During this period she also received zolendronic acid every 3 months and goserelin monthly. After 4 years of treatment she began to experience shortness of breath and fatigue. A positron emission tomography (PET)/CT scan showed diffuse metastatic disease in bone, liver, and lung lesions. A liver biopsy was consistent with metastasis of ER- and PR-negative, HER2-positive BC. Thus, the patient was treated with carboplatin, docetaxel, and trastuzumab. A restaging PET/CT scan after four cycles showed complete resolution of skeletal metastatic tumor activity and a marked decrease in hepatic tumor activity, and stable lung disease. A decrease of tumor marker Ca 15.3 was also noted (from 1485.0 to 251.7 U/mL). Because of the mixed response to ongoing treatment, a lung transbronchial biopsy was performed and pathology revealed ER- and PR-negative, HER2-positive BC metastasis characterized by an abundant mucinous component (Figure 1C and D). Treatment with lapatinib and capecitabine was started, but the patient did not respond and died two months later from progressive disease.
Discussion
Mucinous carcinomas constitute a distinct and significantly rare pathologic entity accounting for only approximately 2% of BCs. The definition of this type of tumor requires a mucinous component of > 50% of the lesion.2,3 However, when a component of ductal carcinoma prevails over a mucinous component, the diagnosis of mixed mucinous carcinoma has to be made.4 In the first case presented, the tumor lesion was characterized by a large mucinous component and by a high grade IDC. Because mucinous carcinomas have a good prognosis, showing lymph node involvement in only 12% of cases, ER positivity in 94%, PR positivity in 81.5%,5 and HER2 overexpression in 4%–7% of tumors,6,7 the aggressive phenotype of BC, in our patient, was probably because of the IDC. In the second case, an organ-specific differentiation of BC was noted; in fact, histology of the primary tumor was different from that of the lung metastases. Changes in pathologic features have been described between primary BC and metastases,8 and before and after neoadjuvant chemotherapy.9
The two cases were HER2-positive, and, even though at different points in the natural history of the disease, they were treated with trastuzumab. In both cases, a mixed response to combined treatment with chemotherapy and trastuzumab was documented. In the first patient, a complete response of liver metastases occurred, although the treatment was ineffective in the breast and axillary lymph nodes. In the second case, there was a good response of all metastatic sites, except in the lungs. Possible molecular mechanisms of trastuzumab resistance include loss of phosphatase and tensin homolog activity, down-regulation of p27, a circulating HER2 extracellular domain, activation of insulin-like growth factor I receptor, and overexpression of mucins (MUC), especially MUC-4.10–12 MUC-4 is expressed in approximately 30% of BCs13 and can be responsible for trastuzumab resistance via two mechanisms: (1) forming complexes with members of the ErbB family and sequestering them with reduced binding of trastuzumab14,15; and (2) decreasing antibody-dependent cell-mediated cytotoxicity, one of the known mechanisms of action of trastuzumab, by blocking the accessibility of the tumor antigens to the cytotoxic immune cells.16 Staining for MUC-4 was possible only for the first case, and it was consistent with overexpression of MUC-4.
In both cases, it is possible to recognize a common feature: the large mucinous component present in the breast tissue of the first case (Figure 1A) and in lung metastases of the second case (Figure 1C and D). Our hypothesis is that the mucin acted as a barrier against trastuzumab, contributing to resistance to trastuzumab beyond the other possible mechanisms described above. In fact, mucins have a central role in maintaining homeostasis and protecting the luminal surfaces of epithelium-lined ducts in the human body.17 In our cases, this ability could represent the modality that the BC used to escape from the action of ongoing combined treatment with chemotherapy and trastuzumab.
Conclusion
The clinical problem of trastuzumab resistance is important, because patients who experience it do not have the same benefit derived from this targeted therapy compared with nonresistant patients. Early identification of these patients and an understanding of the mechanisms responsible for this resistance are imperative, so that their therapeutic options can be changed as soon as possible.
Clinical Practice Points.
The human epidermal growth factor receptor 2 (HER2) is overexpressed in 20%–25% of invasive breast cancer (BC) and is associated with a poor prognosis and resistance to certain chemotherapeutic agents. Treatment with trastuzumab, a recombinant humanized monoclonal antibody directed against the extracellular domain of the HER2 protein, improves outcomes of HER2-positive BC. However, a significant proportion of patients treated with trastuzumab either do not respond initially or relapse after experiencing a period of clinical response.
We present 2 cases of patients with metastatic HER2-positive BC, in whom the presence of a mucin-producing component impaired the effectiveness of trastuzumab.
Early identification of tumors resistant to trastuzumab and an understanding of responsible mechanisms are imperative in the care of patients with HER2-positive BC so that their therapeutic management can be changed as soon as possible. Because the presence of a mucinous component could act as a barrier against trastuzumab, surgical resection of disease should be considered early in cases of BC that have this pathologic feature. In addition, metastatic sites could become differentiated further during treatment, leading to increased production of mucin and acquired resistance to trastuzumab therapy.
Footnotes
Disclosures
All authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–47. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 2.Northridge ME, Rhoads GG, Wartenberg D, et al. The importance of histologic type on breast cancer survival. J Clin Epidemiol. 1997;50:283–90. doi: 10.1016/s0895-4356(96)00366-6. [DOI] [PubMed] [Google Scholar]
- 3.Yerushalmi R, Hayes MM, Gelmon KA. Breast carcinoma — rare types: review of the literature. Ann Oncol. 2009;20:1763–70. doi: 10.1093/annonc/mdp245. [DOI] [PubMed] [Google Scholar]
- 4.Tan PH, Tse GM, Bay BH. Mucinous breast lesions: diagnostic challenges. J Clin Pathol. 2008;61:11–9. doi: 10.1136/jcp.2006.046227. [DOI] [PubMed] [Google Scholar]
- 5.Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008;111:541–7. doi: 10.1007/s10549-007-9809-z. [DOI] [PubMed] [Google Scholar]
- 6.Barkley CR, Ligibel JA, Wong JS, et al. Mucinous breast carcinoma: a large contemporary series. Am J Surg. 2008;196:549–51. doi: 10.1016/j.amjsurg.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Diab SG, Clark GM, Osborne CK, et al. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol. 1999;17:1442–8. doi: 10.1200/JCO.1999.17.5.1442. [DOI] [PubMed] [Google Scholar]
- 8.Curigliano G, Bagnardi V, Viale G, et al. Should liver metastases of breast cancer be biopsied to improve treatment choice? Ann Oncol. 2011;22:2227–33. doi: 10.1093/annonc/mdq751. [DOI] [PubMed] [Google Scholar]
- 9.van de Ven S, Smit VT, Dekker TJ, et al. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev. 2011;37:422–30. doi: 10.1016/j.ctrv.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Le XF, Claret FX, Lammayot A, et al. The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem. 2003;278:23441–50. doi: 10.1074/jbc.M300848200. [DOI] [PubMed] [Google Scholar]
- 11.Köstler WJ, Schwab B, Singer CF, et al. Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin Cancer Res. 2004;10:1618–24. doi: 10.1158/1078-0432.ccr-0385-3. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Zi X, Zhao Y, et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–7. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 13.Carraway KL, Price-Schiavi SA, Komatsu M, et al. Muc4/sialomucin complex in the mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:323–37. doi: 10.1023/a:1011327708973. [DOI] [PubMed] [Google Scholar]
- 14.Price-Schiavi SA, Jepson S, Li P, et al. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for Herceptin resistance. Int J Cancer. 2002;99:783–91. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 15.Nagy P, Friedländer E, Tanner M, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a Herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–82. [PubMed] [Google Scholar]
- 16.Komatsu M, Yee L, Carraway KL. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999;59:2229–36. [PubMed] [Google Scholar]
- 17.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]