Abstract
Cardiac complications after hematopoietic stem cell transplantation (HSCT) can lead to significant morbidity and mortality. Cardiac evaluation during the first 100 days after HSCT is usually performed only if clinically indicated, and no studies have examined whether routine screening is beneficial in this patient population at high risk for tissue injury. We conducted a single-center prospective clinical study to screen for cardiac complications in pediatric and young adult patients. One hundred consecutive HSCT patients underwent scheduled echocardiographic screening on day +7 after transplantation, independent of their clinical condition. At least 1 abnormality was identified in 30% of cases. Seventeen children had a pericardial effusion, 13 elevated right ventricular pressure, and 3 reduced left ventricular function. Survival was reduced in children with any echocardiographic abnormality at day 7 (67% versus 80% in those with and without, respectively, abnormality, P = .073). Moreover, raised right ventricular pressure at day +7 was significantly associated with transplant-associated thrombotic microangiopathy (TA-TMA; P = .004) and may indicate early vascular injury in the lungs. These data suggest that echocardiography 7 days after HSCT can detect early cardiac complications of HSCT and may identify early vascular injury associated with TA-TMA.
Keywords: Pulmonary hypertension, Pericardial effusion, Left ventricle depression, Thrombotic microangiopathy, Echocardiography, Hematopoietic stem cell, transplantation
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) is an important and effective treatment strategy for many malignancies, marrow failure syndromes, and immunodeficiencies in children and young adults. The chemotherapy and radiation used in HSCT may cause significant cardiac and vascular endothelial toxicity, resulting in complications after transplant, such as pericardial effusion (PEF), left ventricular (LV) dysfunction, and pulmonary hypertension (PH). Moreover, heart disease is a major cause of long-term morbidity and mortality in survivors of HSCT in childhood [1].
Predicting the impact of cardiac complications in pediatric patients after HSCT is challenging because of the lack of prospective studies evaluating these factors. PEF, if left untreated, can cause cardiac tamponade, acutely decreasing cardiac function [2–6]. The reported incidence of PEF in retrospective cohorts has varied from 0.2% to 19% in patients after HSCT [7,8]. In our institution, we found a high incidence of PEFs in patients with transplant-associated thrombotic microangiopathy (TA-TMA) [9]. LV dysfunction has been reported in patients after high-dose cyclophosphamide and anthracyclines, and patients with LV dysfunction can initially be asymptomatic [10–12]. PH is associated with increased pulmonary vascular resistance and subsequent elevation in pulmonary artery pressures [13–15]. If undiagnosed, increased pulmonary artery pressure leads to elevated right ventricular (RV) pressure, cardiac failure, and death [14,16,17]. The initial symptoms of PH can be vague, and respiratory complications after HSCT are common, making the diagnosis of PH difficult [14,18]. Jodele et al. [19] reported a 2.3% incidence of PH with 80% mortality in a retrospective analysis of patients transplanted at our institution. All 5 patients who developed PH had histologic evidence of TMA in pulmonary arterioles, suggesting TA-TMA might be involved in pathogenesis of PH after HSCT.
We conducted a prospective single-institution study to determine the value of early post-transplant echocardiographic screening for PH, LV dysfunction, and PEF 7 days after HSCT in children and young adults. We hypothesized that scheduled post-transplant echocardiographic screening at day +7 would identify patients at risk for cardiac complications, leading to early clinical interventions and improved outcomes.
METHODS
In January 2012 we established uniform screening guidelines to monitor for acute cardiac complications after HSCT in pediatric and young adult patients at Cincinnati Children’s Medical Center. All patients undergoing HSCT had echocardiographic evaluation within 30 days before starting their HSCT conditioning regimen (baseline echocardiography) and repeat echocardiography on day +7 independent of clinical condition. Patients with abnormal echocardiography on day +7 had follow-up echocardiography in 7- to 14-day intervals. Patients who were admitted to the pediatric intensive care unit (PICU) for cardiorespiratory failure or TA-TMA or had signs or symptoms of shock were additionally evaluated with echocardiography on arrival to the PICU. The pediatric cardiology service evaluated patients with PEF or LV dysfunction on echocardiography, whereas the PH service evaluated those with elevated RV pressures or other signs of PH.
The aim of this analysis was to determine the utility of the scheduled patient screening on day +7 after HSCT in detecting elevated RV pressure, PEF, and LV dysfunction and predicting adverse outcomes. Our secondary aim was to identify risk factors associated with the development of PEF, elevated RV pressure, and LV dysfunction.
Study Population
The study population consisted of 100 consecutive children and young adults who received HSCT at Cincinnati Children’s Hospital Medical Center from January 2012 to March 2013. Data were collected prospectively after institutional review board approval. Data collected included patient demographics, echocardiography data, disease and therapy characteristics, transplant complications, and therapy outcomes. Currently accepted clinical criteria were used for diagnosis of acute graft-versus-host disease [20], veno-occlusive disease of the liver [21], viremias, and transplant-related mortality [22]. Respiratory failure was diagnosed in patients requiring endotracheal intubation and mechanical ventilation. One-year overall survival (OS) was counted from day 0 (stem cell infusion) to 1-year post-transplant or death. Finally, we evaluated oxygen requirement, respiratory failure, and hypertension during the first 100 days. Patients were categorized as having severe systemic hypertension if they required a continuous antihypertensive infusion and/or 3 or more antihypertensive medications to maintain systolic blood pressure below 95 percentile for age and height [23].
Diagnosis of TA-TMA
Patients were diagnosed with TA-TMA if the following laboratory criteria occurred concurrently and were documented on at least 2 tests: (1) elevated lactate dehydrogenase above the upper limit of normal, (2) new-onset thrombocytopenia less than 50 × 109/L or a greater than 50% decrease in the platelet count, (3) evidence of schistocytes in the peripheral blood, (4) new-onset anemia below the lower limit of normal, (5) negative Coombs test, and (6) the absence of coagulopathy. The date of TA-TMA diagnosis was defined as the first date when all diagnostic criteria were fulfilled [24–27].
Echocardiography Screening Protocol
Each HSCT patient received a comprehensive echocardiographic study at the time points listed above. Echocardiography screening included assessment of LV systolic function, PEF, and evaluation for PH. PH assessment was performed using the PH protocol as previously described [17].
A licensed technician performed all echocardiographic studies, and pediatric cardiologists reviewed all evaluations. A dedicated pediatric PH specialist reviewed abnormal PH-specific echocardiograms. Pulmonary artery pressure was estimated from a trans-tricuspid valve gradient calculated from the maximum velocity of continuous Doppler tricuspid regurgitation, using a modified Bernoulli equation and assuming right central venous pressure of 5 mm Hg [28,29]. PEF was identified from the separation of pericardial layers detected on echocardiography [4,6]. LV function was evaluated by assessment of the LV ejection fraction and/or shortening fraction [11].
Elevated RV pressure was defined as RV pressure greater than 35% of the patient’s systolic blood pressure at time of echocardiography [17,28,30,31]. Intraventricular septal flattening was also evaluated in the determination of elevated RV pressure [32]. Echocardiography has a high sensitivity in predicting PH; however, the specificity is low until the estimated RV pressure approaches 50% systemic [29–31,33]. Therefore, patients who had a documented increase in RV pressures after HSCT to 35% to 49% of systemic were classified as “at risk for PH,” and those with RV pressures of at least 50% of systemic were diagnosed with PH [17].
Patients were diagnosed with PEF if a new or enlarging PEF was found after transplant. PEFs were clinically classified as “small” (no interventions), “moderate to large” (medical interventions required), and “tamponade” (surgical intervention required) per standard guidelines [4]. Cardiac tamponade was diagnosed when echocardiography demonstrated diastolic collapse of the anterior RV free wall, right atrial collapse, left atrial, and/or LV collapse [5,34].
LV function was measured by ejection fraction, which represents the volumetric fraction of blood pumped out of the ventricle, and fractional shortening, an additional sensitive and specific measurement to assess LV function. Patients found to have an ejection fraction of 50% or less and/or a fractional shortening level less than 2 standard deviations below the age-adjusted mean [11] were determined to have LV dysfunction. Patients were identified as having an abnormal echocardiogram at day +7 if they were found to have at least 1 of the above-mentioned outcome measures: elevated RV pressure, PEF, and/or LV dysfunction.
Statistical Analysis
Descriptive statistics were reported as medians, interquartile ranges, and frequencies. Differences in categorical and continuous variables were assessed with the Fisher exact and Wilcoxon rank sum tests, respectively. Associated odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were calculated using the Mantel-Haenszel method. Multivariate analysis was performed to evaluate associations between abnormal echocardiography findings at day +7 and patient age, underlying disease, conditioning regiment, graft-versus-host disease prophylaxis, and autologous versus allogeneic HSCT. The sensitivity, specificity, and positive and negative predictive values of elevated RV pressure and/or PEF at day +7 at diagnosing TA-TMA was estimated.
One-year OS comparing patients with abnormal echocardiography with those without was calculated with Kaplan-Meier analysis with the associated P values calculated by log-rank analysis. Cumulative incidence of TA-TMA with death as a competing variable was calculated by Gray’s method [35]. All patients were diagnosed with TA-TMA before 100 days post-HSCT, so relapse was not considered as a competing risk for TA-TMA because all patients relapsed after this time.
All statistical test conducted were 2-sided, and P < .05 was considered significant. Cumulative incidence was calculated in R [36,37]. All other data analyses were performed using SPSS version 20.0 (SPSS, Inc., Chicago, IL).
RESULTS
Patient Demographics
We analyzed echocardiographic screening data from 100 consecutive HSCT patients; demographics of this patient population are shown in Table 1. Most study patients were white, with a median age of 5.4 years. Sixty-two percent received transplantation for nonmalignant disorders, mainly primary immune deficiencies (36%) and bone marrow failure syndromes (21%). Eighty-four percent of patients underwent allogeneic HSCT, and 86% of the allogeneic grafts were from unrelated donors. Bone marrow was the most common stem cell source, used in 59% of patients. Peripheral blood stem cells were used mainly for autologous stem cell transplantation (n = 16) and patients with Fanconi anemia as an ex vivo T cell–depleted graft (n = 11). Cord blood was used when a suitable bone marrow donor was not available. The conditioning regimen was myeloablative in 42% and reduced intensity in 58% of patients; 9% of patients received total body radiation.
Table 1.
Demographics of Patients Who Underwent Echocardiographic Screening on Day +7
| Characteristic | Abnormal Day +7 Echocardiography (n = 30) |
Normal Day +7 Echocardiography (n = 70) |
P |
|---|---|---|---|
| Male | 18 (60%) | 47 (67%) | .765 |
| Median age at day 0, yr (IQR) | 6.8 (3.7–12.1) | 5.2 (2.4–10.4) | .098 |
| Myeloablative preparative regimen | 9 (30%) | 33 (47%) | .127 |
| Diagnosis | .414 | ||
| Malignancy | 10 (33%) | 28 (40%) | |
| Immunodeficiency | 12 (40%) | 24 (34%) | |
| Bone marrow failure | 5 (17%) | 16 (23%) | |
| Genetic/metabolic | 2 (7%) | 2 (3%) | |
| Benign hematology | 1 (3%) | 0 | |
| Stem cell source | .338 | ||
| Bone marrow | 21 (70%) | 38 (54%) | |
| Cord | 2 (7%) | 8 (11%) | |
| PBSCs | 7 (23%) | 24 (34%) | |
| Donor type | .198 | ||
| Autologous | 2 (7%) | 14 (20%) | |
| Related donor | 3 (10%) | 9 (13%) | |
| Unrelated donor | 25 (83%) | 47 (67%) |
IQR indicates interquartile range; PBSCs, peripheral blood stem cells. Demographics with associated outcomes: elevated RV pressure, PEF, and LV dysfunction. Statistics were performed with Wilcoxon ranked sums test and Fisher exact test.
OS of Patients with Abnormal Echocardiography at Day +7
Thirty of 100 screened patients (30%) had abnormal echocardiography at day +7 (Figure 1). Most patients with abnormal echocardiography were asymptomatic, and abnormal echocardiography was not associated with fluid retention. Thirteen patients had elevated RV pressure (all categorized as at risk for PH), 17 patients had a PEF, and 3 patients had decreased LV function. Three patients had both elevated RV pressure and a PEF at day +7. Patients with abnormal echocardiography at day +7 had a 67% OS rate at 1 year compared with 80% in patients with normal echocardiography (P = .073). Two of the 3 patients with 2 abnormal findings on day +7 died before 1 year. Twenty-seven percent of patients (8 of 30) with abnormal echocardiography at day +7 had an oxygen requirement at the time of echocardiography, compared with 4% (3 of 70) with normal echocardiography at day +7 (P =.0025).
Figure 1.
Survival among study subjects with and without abnormal echocardiography at day +7 after HSCT. The Kaplan-Meier estimate for overall survival at 1-year was 67% ± 21% in subjects with abnormal echocardiography versus 80% ± 8% in those without abnormal echocardiography at day +7 (P = 0.073) by the log rank test.
Outcomes of Abnormalities and Associated Risk Factors
Elevated RV pressure
Two of thirteen patients (15%) with elevated RV pressure at day +7 were diagnosed with PH by day +30 (days 26 and 29) (Table 2). One other patient who did not have elevated RV pressure at day +7 developed PH, which was diagnosed and treated on admission into the PICU at day +39. Patients with PH were not catheterized for direct pressure measurement because of concerns for morbidity. All 3 patients with PH were diagnosed and treated for TA-TMA before day +100 and were treated with PH-specific therapy, including nitric oxide, bosentan, and/or sildenafil. Two patients had a good response to intervention, and 1 patient with PH died from cardiopulmonary failure. Three of 13 patients at risk for PH at day +7 had elevated RV pressures before transplant. None of the 3 developed PH after HSCT, and all had resolution of their increased RV pressures by day +100. All patients determined to be “at risk for PH” were followed clinically with repeat echocardiography and close monitoring of clinical status, and no intervention was done unless the patient was diagnosed with PH.
Table 2.
Echocardiography Results and Associated Outcomes and Risk Factors
| Elevated RV Pressure (n = 13) |
Normal RV Pressure (n = 87) |
P | PEF (n = 17) |
No PEF (n = 83) |
P | Decreased LV Function (n = 3) |
Normal LV Function (n = 97) |
P | |
|---|---|---|---|---|---|---|---|---|---|
| aGVHD (n = 33) | 2 (15%) | 31 (36%) | .210 | 6 (35%) | 27 (32%) | 1.000 | 3 (100%) | 30 (31%) | .034 |
| cGVHD (n = 4) | 1 (8%) | 3 (3%) | .432 | 0 | 4 (5%) | 1.000 | 0 | 4 (4%) | 1.000 |
| VOD (n = 1) | 0 | 1 (1%) | 1.000 | 0 | 1 (1%) | 1.000 | 0 | 1 (1%) | 1.000 |
| TA-TMA (n = 32) | 9 (69%) | 23 (26%) | .004 | 9 (52%) | 23 (28%) | .051 | 0 | 32 (33%) | .549 |
| BK virus (n = 36) | 5 (39%) | 31 (36%) | 1.000 | 10 (59%) | 26 (31%) | .050 | 3 (100%) | 33 (34%) | .044 |
| Adenovirus (n = 19) | 1 (8%) | 18 (21%) | .452 | 6 (35%) | 13 (16%) | .086 | 1 (33%) | 18 (19%) | .472 |
| CMV (n = 25) | 2 (15%) | 23 (26%) | .508 | 5 (29%) | 20 (24%) | .759 | 1 (33%) | 24 (25%) | 1.000 |
| EBV (n = 41) | 6 (46%) | 35 (40%) | .766 | 6 (35%) | 35 (42%) | .788 | 2 (67%) | 39 (40%) | .566 |
| Severe hypertension (n = 25) | 6 (46%) | 20 (23%) | .094 | 7 (41%) | 19 (23%) | .136 | 2 (66%) | 24 (25%) | .165 |
| Oxygen during first 100 d (n = 33) | 10 (77%) | 23 (26%) | .001 | 9 (53%) | 24 (29%) | .087 | 1 (33%) | 32 (33%) | .984 |
| Respiratory failure (n = 22) | 4 (31%) | 18 (21%) | .475 | 7 (41%) | 15 (18%) | .053 | 1 (33%) | 21 (22%) | .530 |
| 1-Year TRM (n = 15) | 3 (23%) | 12 (14%) | .407 | 4 (24%) | 11 (13%) | .279 | 0 | 15 (16%) | 1.000 |
| Death in first year (n = 24) | 5 (39%) | 19 (22%) | .293 | 6 (35%) | 18 (22%) | .231 | 1 (33%) | 23 (24%) | .565 |
aGVHD indicates acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; VOD, veno-occlusive disease; CMV, cytomegalovirus; EBV, Epstein-Barr virus; TRM, transplant-related mortality.
Outcome associations: elevated RV pressure, PEF, and LV dysfunction. Statistics were performed with Wilcoxon ranked sums test and Fisher exact test.
Our data analysis showed that patients with elevated RV pressure at day +7 were significantly more likely to have TA-TMA (OR, 6.3; 95% CI, 1.8 to 22.3) and an oxygen requirement in the first 100 days (OR, 9.3; 95% CI, 2.3 to 36.7) (Table 2). One-year OS was decreased (61%) in 13 patients with elevated RV pressure compared with those without (78%) (P =.29).
Pericardial effusion
Seventeen patients (17%) had a PEF at day +7. Six of 17 patients (35%) had a small PEF before transplantation. One patient was diagnosed with a moderate to large PEF at day +7, and 3 patients (18%) with small PEFs at day +7 developed a moderate to large PEF within 30 days. All 4 patients with a moderate to large PEF received medical intervention based on screening results, and 1 required pericardiocentesis for cardiac tamponade. TA-TMA, respiratory failure, and BK viremia were all more common in the first 100 days after HSCT in patients with a PEF at day +7 (Table 2).
Decreased LV function
Two of 3 patients with decreased LV function at day +7 had decreased function before transplantation. All 3 patients with decreased LV function developed aGVHD and BK viremia. Finally, we explored several multivariable models to examine independent risk factors for the development of abnormal echocardiography at day +7, but none identified significant associations.
TA-TMA and Day +7 Echocardiography
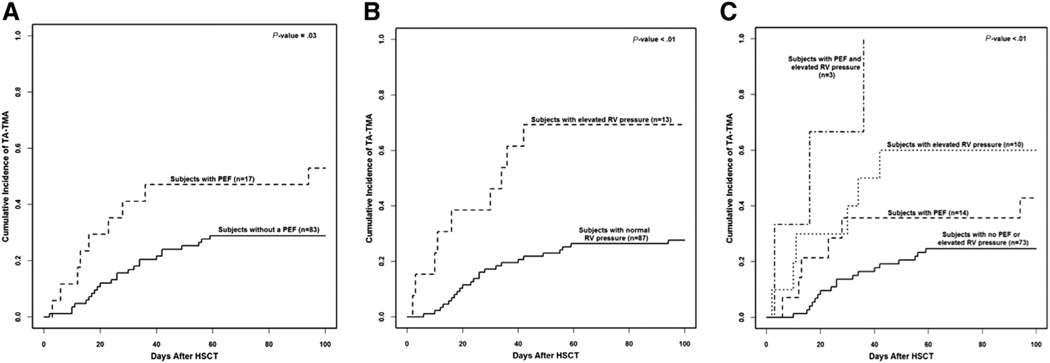
Thirty-two patients (32%) were diagnosed with TA-TMA. The cumulative incidence of TA-TMA within 100 days was higher in patients with elevated RV pressure (P = .004) and PEF (P = .051) at day +7 than in patients without these findings (Figure 2). All 3 patients (100%) with PEF and elevated RV pressure, 6 of 10 patients (60%) with elevated RV pressure alone, and 6 of 14 patients (43%) with PEF alone on day +7 developed TA-TMA compared with 17 of 73 patients (23%) with no PEF or elevated RV pressure at day +7 (P =.0003). Elevated RV pressure and/or PEF had a sensitivity of 46.9% (95% CI, 29.1 to 65.3), specificity of 82.4% (95% CI, 71.2 to 90.5), positive predictive value of 55.6% (95% CI, 35.3 to 74.5), and negative predictive value of 76.7% (95% CI, 65.4 to 85.8). TA-TMA was diagnosed at a median of 24 days after HSCT (interquartile range, 15 to 36 days), and all but 2 patients developed TA-TMA after day +7 echocardiography. The other patients with abnormal echocardiography that went on to develop TA-TMA were asymptomatic at day +7. Twelve of 15 patients (67%) with abnormal echocardiography and TA-TMA continued to have abnormal echocardiography 30 days after HSCT.
Figure 2.
Gray’s competing risk method was used to obtain cumulative incidence of TA-TMA in the first 100 days after HSCT, with death as a competing risk. Thirty-two patients were diagnosed with TA-TMA. (A) 52% (9/17) of patients with PEF were diagnosed with TA-TMA compared to 28% (23/83). (B) 69% (9/13) patients with elevated RV pressure were diagnosed with TA-TMA compared to 26% (23/87) of patients without elevated RV pressure at day +7. (C) All three patients (100%) with PEF and elevated RV pressure developed TA-TMA. Sixty percent (6/10) of patients with elevated RV pressure and 43% (6/14) of patients with PEF alone on day +7 developed TA-TMA. Nineteen percent (14/73) of patients with no PEF or elevated RV pressure at day +7 developed TA-TMA after HSCT. HSCT indicates hematopoietic stem cell transplant; PEF, Pericardial effusion; RV, right ventricular pressure; TA-TMA, transplant associated thrombotic microangiopathy.
DISCUSSION
This is the first study to prospectively investigate the incidence of cardiac complications in pediatric and young adult patients shortly after undergoing HSCT. We performed echocardiographic screening in all HSCT patients on day +7 after transplantation independent of their clinical condition and documented a much higher incidence of echocardiographic abnormalities than previously reported by retrospective analyses. Thirty percent of HSCT patients had at least 1 echocardiographic abnormality at day +7, and 1-year post-transplant survival was lower in these children compared with patients without abnormalities. Thirteen percent of patients had elevated RV pressure at day +7. Three patients developed PH in the first 30 days after transplant, and 2 of them had elevated RV pressure at day +7 screening. We believe that PH is underdiagnosed in the HSCT population. Echocardiographic finding of RV pressure of 35% to 49% of systemic has sensitivity of 83% and specificity of 72% of diagnosing PH, likely missing less severe cases [32]. Cardiac catheterization is the gold standard diagnostic test for PH but is rarely used in fragile post-transplant patients and, indeed, was not used in our cases. It is possible that early and more aggressive use of this technique might improve diagnosis and management and could be considered in severe or complex cases [17].
Seventeen patients were diagnosed with PEF on day +7, and 4 patients (24%) progressed to a moderate to large PEF within 30 days and received prompt medical or surgical treatment. These findings suggest that day +7 echocardiography identifies a significant number of patients who will develop clinically significant PEF after HSCT where acute clinical decompensation can be avoided by close patient monitoring.
An interesting finding in our study was that 69% of patients with an elevated RV pressure alone and all patients with elevated RV pressure and PEF on day 7 after HSCT were diagnosed with TA-TMA by markers of microangiopathic hemolysis and organ injury. The incidence of TMA we observed is higher than that reported by others, likely because we prospectively monitored all patients in a uniform manner, in contrast to retrospective reports that likely captured only the most severe cases. The incidence of TA-TMA in this study is similar to that previously reported by our group in a TA-TMA prospective study [38]. In the current study the association of elevated RV pressure on day +7 after HSCT and high incidence of TA-TMA in those patients may indicate that pulmonary vascular injury occurs very early after transplantation but is not evident by currently used TA-TMA laboratory diagnostic criteria until later [25]. Also, many patients with elevated RV pressure and TA-TMA received TA-TMA–specific intervention, which may have reduced the development of severe PH.
TA-TMA remains a very severe transplant complication in which vascular endothelial injury affects small vessels in the organs, resulting in thrombosis and tissue injury [22]. Early diagnosis of TA-TMA by using current diagnostic criteria is challenging, and elevated RV pressure may be associated with early presentation of TA-TMA. Further research may determine if these patients can benefit from close scrutiny and early therapy for TA-TMA. Multivisceral TA-TMA can present with acute severe PH associated with very high mortality after HSCT. Moreover, HSCT patients with pulmonary microangiopathy decompensate very quickly with additional stressors like hypoxia and often cannot be successfully resuscitated [22]. Our group previously documented PH and PEF in patients with prolonged symptoms of TA-TMA and speculated that untreated TMA may affect pulmonary or cardiac vessels, resulting in organ injury and high mortality [19]. Our current prospective screening data suggest that PEF and elevated RV pressure may be very early indicators of TA-TMA and occur because of vascular injury from chemotherapy, radiation, or donor graft. Echocardiography might be a more sensitive tool to detect early vascular changes in the lungs before the evidence of microangiopathic hemolysis in the blood. Current TA-TMA diagnostic criteria like elevated lactate dehydrogenase and schistocytes in peripheral blood may be late markers of vascular injury after HSCT.
Our data suggest that cardiac and pulmonary vascular pathology leading to significant post-transplant complications is likely underestimated in HSCT population, especially in children, and may contribute to transplant-related mortality if not treated. The association we identified between PH, PEF, and TA-TMA should be further explored to identify markers of vascular injury that might elucidate the mechanism of pulmonary vascular and cardiac injury after HSCT. Such studies are currently underway.
In summary, our data indicate that comprehensive echocardiographic screening including RV pressure estimation in allogeneic transplant recipients on day +7 after transplantation yields a high frequency of important abnormalities [3,17,28,30]. Repeat echocardiography should be considered in patients with abnormal day +7 findings and in any patient with a new oxygen requirement, respiratory failure, or TA-TMA. Patients with elevated RV pressure, PEF, or LV depression should undergo prompt evaluation by cardiologists and/or PH specialists and close subsequent monitoring. Future prospective studies are needed to validate cardiac screening algorithms and to identify novel biomarkers that can aid in early diagnosis of cardiac complications after HSCT, and serve as potential therapeutic targets.
Acknowledgments
The authors thank the physicians, nurses, care managers, transplant coordinators, echocardiography technicians, and other care providers and staff at Cincinnati Children’s Hospital Medical Center and especially the patients and their families. Dr. Dandoy performed this study as part of the Master of Science degree in Clinical and Translational Research, University of Cincinnati College of Medicine.
Footnotes
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: C.D., S.J., and S.M.D. designed the study, enrolled study subjects, performed research, and wrote the paper. C.D. and A.L. performed statistical analyses and prepared figures. R.H., M.C., and T.D.R. reviewed echocardiographic studies, provided conceptual insights for study design, and edited the manuscript. R.S.C., Z.P., J.E., and K.C.M. provided vital conceptual insights for study design and edited the manuscript.
REFERENCES
- 1.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 2.Bodson L, Vieillard-Baron A. Cardiac tamponade. Curr Opin Crit Care. 2011;17:416–424. doi: 10.1097/MCC.0b013e3283491f27. [DOI] [PubMed] [Google Scholar]
- 3.Jung H-O. Pericardial effusion and pericardiocentesis: role of echocardiography. Kor Circ J. 2012;42:725–734. doi: 10.4070/kcj.2012.42.11.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maisch B, Seferović PM, Ristić AD, et al. Guidelines on the Diagnosis and Management of Pericardial Diseases Executive Summary: The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Roy CL, Minor MA, Brookhart M, Choudhry NK. Does this patient with a pericardial effusion have cardiac tamponade? JAMA. 2007;297:1810–1818. doi: 10.1001/jama.297.16.1810. [DOI] [PubMed] [Google Scholar]
- 6.Sagristà-Sauleda J, Soler-Soler J. Diagnosis and management of pericardial effusion. World J Cardiol. 2011;3:135–143. doi: 10.4330/wjc.v3.i5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murdych T. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977–1997. Bone Marrow Transplant. 2001;28:283–287. doi: 10.1038/sj.bmt.1703133. [DOI] [PubMed] [Google Scholar]
- 8.Aldoss O, Gruenstein DH, Bass JL, et al. Pericardial effusion after pediatric hematopoietic cell transplant. Pediatr Transplant. 2013;17:294–299. doi: 10.1111/petr.12062. [DOI] [PubMed] [Google Scholar]
- 9.Lerner D, Dandoy CE, Hirsch R, et al. Pericardial effusion in pediatric SCT recipients with thrombotic microangiopathy. Bone Marrow Transplant. 2014;49:862–863. doi: 10.1038/bmt.2014.40. [DOI] [PubMed] [Google Scholar]
- 10.Fujimaki K, Maruta A, Yoshida M, et al. Severe cardiac toxicity in hematological stem cell transplantation: predictive value of reduced left ventricular ejection fraction. Bone Marrow Transplant. 2001;27:307–310. doi: 10.1038/sj.bmt.1702783. [DOI] [PubMed] [Google Scholar]
- 11.Kantor PF, Lougheed J, Dancea A, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2013;29:1535–1552. doi: 10.1016/j.cjca.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Gottdiener JS, Appelbaum FR, Ferrans VJ, et al. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med. 1981;141:758–763. [PubMed] [Google Scholar]
- 13.Humbert M, Gerry Coghlan J, Khanna D. Early detection and management of pulmonary arterial hypertension. Eur Respir Rev. 2012;21:306–312. doi: 10.1183/09059180.00005112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 15.Roofthooft MT, Hillege HL, ten Harkel AD, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755–1764. doi: 10.1161/CIRCULATIONAHA.110.969584. [DOI] [PubMed] [Google Scholar]
- 16.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 17.Dandoy CE, Hirsch R, Chima R, et al. Pulmonary hypertension after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1546–1556. doi: 10.1016/j.bbmt.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Soubani AO, Hassoun PM. Pulmonary complications of bone marrow transplantation. Chest. 1996;109:1066–1077. doi: 10.1378/chest.109.4.1066. [DOI] [PubMed] [Google Scholar]
- 19.Jodele S, Hirsch R, Laskin B, et al. Pulmonary arterial hypertension in pediatric patients with hematopoietic stem cell transplant–associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2013;19:202–207. doi: 10.1016/j.bbmt.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Myers KC, Lawrence J, Marsh RA, et al. High-dose methylprednisolone for veno-occlusive disease of the liver in pediatric hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2013;19:500–503. doi: 10.1016/j.bbmt.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith AR, Majhail NS, MacMillan ML, et al. Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood. 2011;117:2728–2734. doi: 10.1182/blood-2010-08-303263. [DOI] [PubMed] [Google Scholar]
- 23.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 24.Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90:918–926. doi: 10.1097/TP.0b013e3181f24e8d. [DOI] [PubMed] [Google Scholar]
- 25.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118:1452–1462. doi: 10.1182/blood-2011-02-321315. [DOI] [PubMed] [Google Scholar]
- 26.Ho VT, Cutler C, Carter S, et al. Blood and Marrow Transplant Clinical Trials Network Toxicity Committee Consensus Summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:571–575. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Ruutu T, Barosi G, Benjamin RJ, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92:95–100. doi: 10.3324/haematol.10699. [DOI] [PubMed] [Google Scholar]
- 28.Kirkpatrick EC. Echocardiography in pediatric pulmonary hypertension. Paediatr Respir Rev. 2013;14:157–164. doi: 10.1016/j.prrv.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Forfia PR, Vachiéry J-L. Echocardiography in pulmonary arterial hypertension. Am J Cardiol. 2012;110:S16–S24. doi: 10.1016/j.amjcard.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Bossone E, D’Andrea A, D’Alto M, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr. 2013;26:1–14. doi: 10.1016/j.echo.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Taleb M, Khuder S, Tinkel J, Khouri SJ. The diagnostic accuracy of Doppler echocardiography in assessment of pulmonary artery systolic pressure: a meta-analysis. Echocardiography. 2013;30:258–265. doi: 10.1111/echo.12061. [DOI] [PubMed] [Google Scholar]
- 32.Haddad F, Guihaire J, Skhiri M, et al. Septal curvature is marker of hemodynamic, anatomical, and electromechanical ventricular interdependence in patients with pulmonary arterial hypertension. Echocardiography. 2014;31:699–707. doi: 10.1111/echo.12468. [DOI] [PubMed] [Google Scholar]
- 33.Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365:44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 34.Sparano DM, Ward RP. Pericarditis and pericardial effusion: management update. Curr Treat Options Cardiovasc Med. 2011;13:543–555. doi: 10.1007/s11936-011-0151-8. [DOI] [PubMed] [Google Scholar]
- 35.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 36.Team RDC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 37.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 38.Jodele S, Davies S, Lane A, et al. Refined diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a prospective study in children and young adults. Blood. 2014;124:645–653. doi: 10.1182/blood-2014-03-564997. [DOI] [PMC free article] [PubMed] [Google Scholar]