Abstract
Aim:
Transplantation of mesenchymal stem cells (MSCs) for the treatment of diabetic erectile dysfunction (ED) is hampered by apoptosis of the transplanted cells. In diabetic ED, there is increased oxidative stress and decreased NO in the corpora cavernosa, and reactive oxygen species (ROS) induce apoptosis of the transplanted cells. In this study we examined whether and how autophagy was involved in ROS-induced apoptosis of MSCs.
Methods:
Mouse C3H10 MSCs were treated with H2O2 to simulate the high oxidative condition in diabetic ED. Cell viability was measured using MTT assay. Apoptosis was analyzed by flow cytometry. Apoptosis- and autophagy-related proteins were detected with Western blot assays. Intracellular autophagosome accumulation was studied using transmission electron microscopy.
Results:
Treatment of MSCs with H2O2 (50–400 μmol/L) inhibited the cell viability in concentration- and time-dependent manners. Furthermore, H2O2 (300 μmol/L) induced apoptosis, as well as activated autophagy in MSCs. Pretreatment with lysosome inhibitor chloroquine (10 μmol/L) or PI3K inhibitor 3-methyladenine (5 mmol/L) significantly enhanced H2O2-induced cell death. Pretreatment with JNK inhibitor SP600125 (10 μmol/L) abrogated H2O2-induced accumulation of LC3-II, and attenuated H2O2-induced reduction of Bcl-2 levels in MSCs.
Conclusion:
ROS induce autophagy to counteract apoptosis in MSCs by activation of JNK. Thus, augmentation of autophagy may reduce apoptosis, prolonging MSC survival and improving MSC-based therapeutic efficacy for diabetic ED.
Keywords: mesenchymal stem cell, ROS, autophagy, apoptosis, chloroquine, 3-methyladenine, SP600125, diabetes mellitus, erectile dysfunction
Introduction
Diabetic patients suffer from a higher prevalence of erectile dysfunction (ED) and are less responsive to oral anti-ED drugs than non-diabetic individuals, which seriously affects the quality of life of diabetic patients1,2,3,4. Phosphodiesterase inhibitor 5 (PDEI5) (eg, sildenafil, vardenafil and tadalafil) represents the first-line therapeutic strategy for ED, but there are side effects of these drugs (eg, headache, flushing and diarrhea). Disappointing effects in diabetes patients compared with the general population have been reported5,6,7. Because stem cells can directionally differentiate into corpus cavernosum vascular endothelial cells and smooth muscle cells under a specific microenvironment, the transplantation of stem cells for treating diabetic ED was proposed as a potential therapeutic approach8,9,10. Mesenchymal stem cells (MSCs) have been tested in transplantation therapy for diabetic ED11,12,13. Although stem cell transplantation can improve erectile function in animal studies, the effect last only 7–28 d before gradually diminishing14,15,16. The main mechanism of these limited effects is assumed to be the apoptotic death of the transplanted stem cells. Thus, strategies that suppress apoptosis would increase stem cell survival and improve therapeutic efficacy.
Autophagy is an evolutionarily conserved catabolic process involved in the degradation and recycling of long-lived proteins and the removal of exhausted and unwanted cellular components, which is important for cell survival under various stress conditions such as nutrient starvation, reactive oxygen species (ROS) accumulation and infection. Consistent with its vital role in cell survival, proliferation and differentiation, autophagy is important in retaining the survival and function of stem cells17. Thus, modulating autophagy may be used to prolong stem cell survival and improve stem cell-based therapy.
Recent studies demonstrate that there is increased oxidative stress and decreased production of nitric oxide in the corpora cavernosa18,19. Reactive oxygen species (ROS) are toxic to transplanted stem cells, and the induction of apoptosis underlies the major mechanism of ROS-induced cytotoxicity20,21,22,23. Meanwhile, ROS can also activate autophagy in certain conditions24,25,26. The relationship between autophagy and apoptosis under oxidative conditions is complex27. Whether autophagy is activated in transplanted MSCs and the relationship between autophagy and apoptosis remain unclear.
In this report, we demonstrate that ROS kills MSCs through apoptosis. Meanwhile, it induces autophagy through JNK-mediated Bcl-2 degradation. While autophagy counteracts apoptosis, the latter is overwhelming. Therefore, our results suggest that the augmentation of autophagy could be exploited to suppress apoptosis, which may prolong transplanted MSC survival and improve MSC-based therapy for diabetic ED.
Materials and methods
Materials
Antibodies for Bcl-2 (#9941), phospho-JNK (#4668), JNK1 (#3708) and cleaved-caspase 3 (#9664) were purchased from Cell Signaling Technology (Cell Signaling Technology, Danvers, MA, USA). Anti-PARP and p62 (610497) were purchased from BD Biosciences (New York, USA). Antibodies for LC3B (L7543) and chloroquine (CQ, 50-63-5) were purchased from Sigma Aldrich (St Louis, MO, USA). The antibody against β-actin was purchased from Zoonbio Biotechnology (Nanjing, China). All secondary antibodies were purchased from Abgent (San Diego, CA, USA). The JNK inhibitor SP600125 (s1460) and pan-caspase inhibitor z-VAD were purchased from Selleckchem (Houston, TX, USA). The 3-methyladenine (3MA) (sc-205596) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Dulbecco's modified Eagle's medium/F12 (DMEM/F12) and trypsin were purchased from HyClone (Logan, UT, USA). Fetal bovine serum (FBS) was purchased from Gibco (Thermo Fisher Scientific, MA, USA).
Cell culture
The mice mesenchymal stem cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in DMEM/F12 containing 10% FBS, 1 mmol/L-glutamine, 1×105 units/L penicillin and 100 g/L streptomycin and maintained at 37 °C with 5% CO2 in a humidified atmosphere.
Measurement of cell viability
Cell viability was assessed by 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT; Beyotime, Haimen, China) assay. Briefly, cells were seeded into 96-well plates at 1×104 cells/cm2 and cultured for 24 h. The cells were then pretreated with the indicated inhibitors for 30 min by exposure to H2O2 for another 12, 24, or 48 h. After incubation, the medium was aspirated, and the cells were incubated with fresh medium containing 5 g/L MTT. After 4 h, the medium was removed, and the blue formazan crystals were dissolved in 150 μL dimethyl sulfoxide (DMSO). Absorbance (λ/nm=570) was measured using a Tecan Infinite F200/M200 type multifunction microplate reader (Tecan, Männedorf, Switzerland). The viability rate of the cells=(the OD values of treated groups/the OD values of control group)×100%.
Western blot analysis
To obtain the total protein lysates, treated cells were lysed in cold RIPA lysis buffer (Beyotime, Haimen, China) containing 1 mmol/L phenylmethane-sulfonyl fluoride (PMSF; Beyotime, Haimen, China), 1% protease inhibitor cocktail and 1% phosphatase inhibitor cocktail (Biotool, Houston, TX, USA) and centrifuged at 12 000×g for 15 min at 4 °C to remove debris. Protein concentrations were estimated using the enhanced bicinchoninic acid (BCA) protein assay kit (Beyotime, Haimen, China), and the protein extracts were heat denatured in SDS-PAGE sample loading buffer (Beyotime, Haimen, China). Equal amounts of protein (40 μg/lane) from each sample were separated by 10%–12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using the criterion system at a constant voltage of 90 V. The proteins were subsequently transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Darmstadt, Germany). After blocking with 5% non-fat dried milk for 2 h, the membrane was incubated with the primary antibodies overnight at 4 °C. Then, the immunoreactive bands were visualized by enhanced chemiluminescence using HRP-conjugated IgG secondary antibodies.
Flow cytometric (FCM) analysis of apoptosis
After treatment, the cells were trypsinized, washed with PBS and suspended in 195 μL of Annexin V-FITC binding buffer containing 5 μL Annexin V-FITC and 10 μL propidium iodide (PI; Beyotime, Haimen, China). After incubation for 10–20 min at room temperature in the dark, the cells were subjected to a FCM assay. FCM was performed using a FACSCanto 6-color flow cytometer (BD Biosciences, San Jose, CA, USA).
Transmission electron microscopy (TEM)
For the TEM analysis, treated cells were trypsinized, rinsed twice with warm PBS (37 °C) and then fixed for 1 h in 2.5% glutaraldehyde in 0.1 mol cacodylate buffer with 1% sucrose. After washing with PBS, the cells were postfixed in 1% osmium tetroxide (OsO4) and embedded in Epon; 0.1 mmol/L thin sections were stained with uranyl acetate/lead citrate and viewed using a Hitachi-7500 TEM (Tokyo, Japan).
Statistical analysis
All data are expressed as the mean±SD. The SPSS19.0 software package was used to perform all statistical analysis. Statistical comparisons were performed by one-way ANOVA. In all analysis, P<0.05 was considered statistically significant.
Results
H2O2 reduced cell viability in a dose- and time-dependent manner
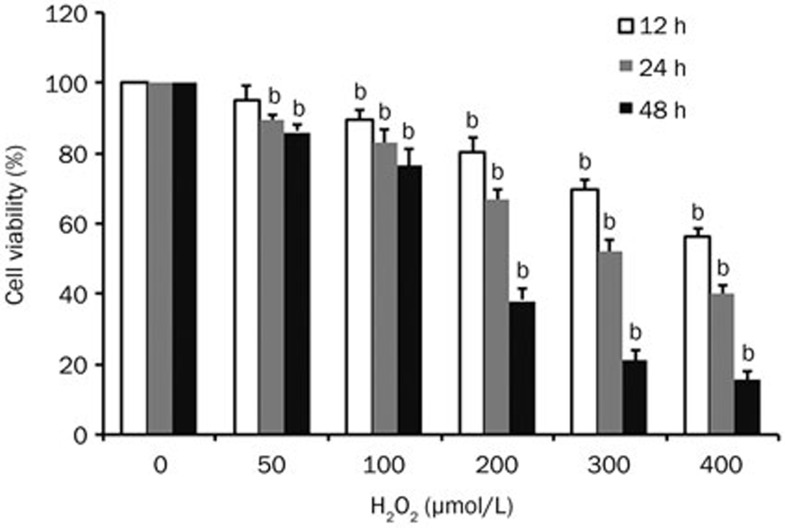
We used H2O2 to simulate a high oxidative condition. MSCs were exposed to various concentrations of H2O2 (0, 50, 100, 200, 300, and 400 μmol/L) for 12, 24, and 48 h, and cell viability was measured by the MTT assay. H2O2 reduced cell viability in a dose- and time-dependent manner (Figure 1) with an IC50 of 325.9352±35.39494 μmol/L at 24 h. Thus, 300 μmol/L of H2O2 was chosen for subsequent experiments.
Figure 1.
Assessment of the effects of H2O2 on MSC viability. Cells were treated with various concentrations of H2O2 for 12, 24, and 48 h, and cell viability was measured by the MTT assay and the cell viability ratio (%) calculated. Data from 3 independent experiments are represented as the mean±SD. bP<0.05 vs control group (0 μmol/L).
ROS induce apoptosis in MSCs
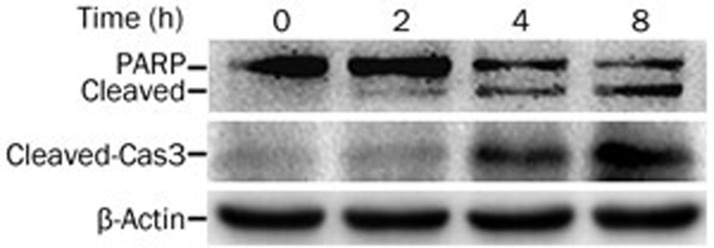
Consistent with reported studies20,21,22,23, we determined that H2O2 induced apoptosis in MSCs, which was shown by the activation of caspase 3 and cleavage of poly-ADP-ribose polymerase (PARP) (Figure 2), positive Annexin V staining (Figure 3B) and suppression of cytotoxicity by the pan-caspase inhibitor z-VAD (Figure 3A, 3B).
Figure 2.
H2O2 induces apoptosis in MSCs. The cells were treated with H2O2 (300 μmol/L) for the indicated time periods. The indicated proteins were detected by Western blot, and β-actin was detected as an input control.
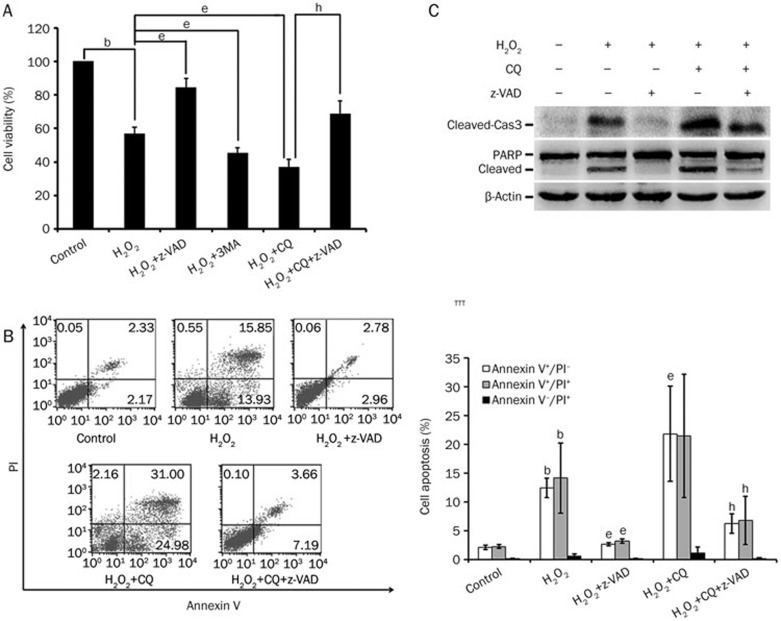
Figure 3.
Blockage of autophagy strengthens the cytotoxicity of H2O2. (A) Cells were pretreated with 3MA (5 mmol/L), CQ (10 μmol/L) or z-VAD (20 μmol/L) for 30 min and then with H2O2 (300 μmol/L) for 24 h. Cell viability was measured by MTT assay. (B) Cells were pretreated with CQ (10 μmol/L) or z-VAD (20 μmol/L) for 30 min and then with H2O2 (300 μmol/L) for 8 h. Cell apoptosis was measured by flow cytometry (FCM). Annexin V+/PI− represents the early apoptotic stage, whereas Annexin V+/PI+ represents apoptotic cells in the terminal stage. Necrotic cells display Annexin V−/PI+. The percentages of early and terminal stage apoptotic cells and necrotic cells were calculated and are shown in the histogram. (C) MSCs were pretreated with CQ (10 μmol/L) or z-VAD (20 μmol/L) for 30 min and then treated with H2O2 (300 μmol/L) for 8 h. The indicated proteins were detected by Western blot, with β-actin detected as an internal control. Mean±SD. n=3. bP<0.05 vs control group; eP<0.05 vs the H2O2 group; hP<0.05 vs the (H2O2+CQ) group.
Autophagy inhibition enhanced H2O2-induced apoptosis in MSCs
To understand the relationship between autophagy and apoptosis in high-ROS conditions, we examined the effects of autophagy inhibition on H2O2-induced apoptosis. The phosphatidylinositol 3-kinase (PI3K) inhibitor 3-MA and the lysosome inhibitor CQ, which inhibit different steps of the autophagy process, were used to pretreat the cells before H2O2 exposure. As shown in Figure 3A, cell viability was further decreased when autophagy was inhibited by 3-MA or CQ, suggesting that autophagy is cytoprotective under this condition. Consistently, the inhibition of autophagy enhanced apoptosis, as shown by increased Annexin V staining, elevated cleavage of caspase 3 and PARP in CQ pre-treated cells (Figure 3B, 3C).
H2O2 induced autophagy in MSCs
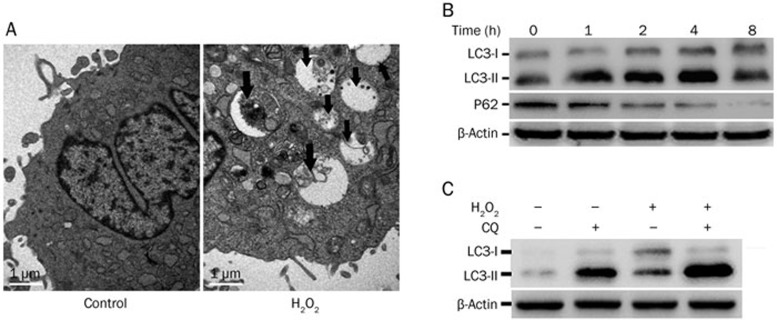
To investigate whether H2O2 was able to induce autophagy in MSCs, we used TEM to detect the autophagosome, the autophagic vacuoles that are the morphological hallmark of autophagy28. As shown in Figure 4A, autophagic vacuoles containing cellular material or membranous structures in H2O2-treated cells were detected. The conversion of LC3-I to LC3-II and the degradation of p62, markers of autophagy28,29, were also detected by Western blot (Figure 4B). Additionally, autophagy flux was detected through co-treatment with H2O2 and CQ, a lysosome inhibitor that inhibits LC3-II turnover. In the experiment, while either H2O2 or CQ alone caused moderate increases in LC3-II, the exposure of cells to both H2O2 and CQ further increased LC3-II expression (Figure 4C). Together, these results confirmed that autophagy was induced by H2O2 treatment.
Figure 4.
H2O2 induces autophagy in MSCs. (A) Cells were treated with or without H2O2 (300 μmol/L) for 4 h, and autophagosomes were detected by TEM. Black arrows indicate autophagosomes including residual digested organelles. (B) Cells were treated with H2O2 (300 μmol/L) for the indicated times. The indicated proteins were detected by Western blot, and β-actin was detected as a loading control. (C) The cells were pretreated with CQ (10 μmol/L) for 30 min and then treated with H2O2 (300 μmol/L) for another 2 h. LC3 was detected by Western blot, and β-actin was detected as a loading control.
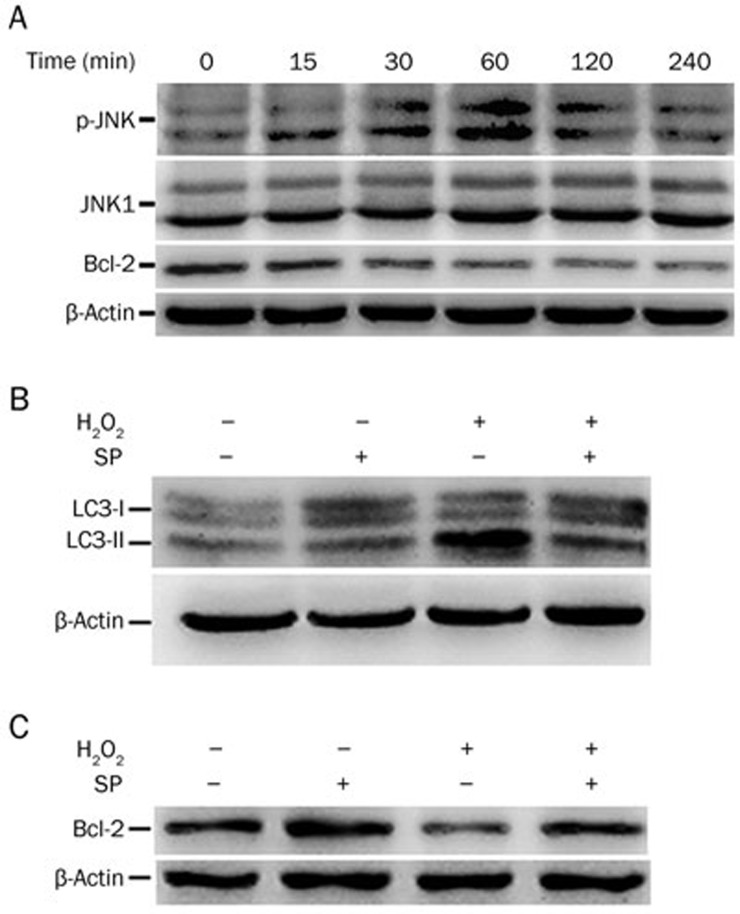
H2O2 induced autophagy was mediated by JNK activation.
Previously, it was demonstrated that autophagy can be induced through the JNK-mediated phosphorylation or degradation of Bcl-2 and Bcl-xL, which attenuates the inhibition of Bcl-2 and Bcl-xL on Beclin-130,31. To investigate whether JNK is involved in autophagy induced by H2O2, cells were treated for different time periods, and the phosphorylation of JNK, the activated form of JNK, was detected by Western blot. As shown in Figure 5A, JNK was significantly activated by H2O2. Meanwhile, the expression of Bcl-2 was decreased in a time-dependent manner.
Figure 5.
JNK activation is required for H2O2-induced autophagy. (A) Cells were exposed to H2O2 (300 μmol/L) for different durations. The indicated proteins were detected by Western blot, and β-actin was detected as a loading control. (B and C) Cells were pretreated with SP600125 (10 μmol/L) for 30 min and then treated with H202 (300 μmol/L) for an additional 2 h (for LC3) or 1 h (for Bcl-2). The indicated proteins were detected by Western blot, and β-actin was detected as a loading control.
To further determine the role of JNK in autophagy induced by H2O2, the JNK inhibitor SP600125 was used to inhibit JNK. SP600125 abrogated the effects of H2O2 on the accumulation of LC3-II, which was associated with inhibiting the decrease in Bcl-2 (Figure 5B and 5C). These results suggest that H2O2-induced autophagy involves JNK-mediated Bcl-2 suppression.
Discussion
ROS-mediated apoptosis limits the efficacy of stem cell-based therapy for diabetic ED. In this report, we investigated the role of autophagy in ROS-induced apoptosis in MSCs and found that while H2O2 killed MSCs through apoptosis, autophagy was also effectively activated by ROS. Blocking autophagy significantly increased apoptotic cytotoxicity, suggesting that ROS-activated autophagy was cytoprotective against apoptosis. Further, we found that H2O2 activated JNK and suppressed Bcl-2. These results demonstrate that while ROS kills MSCs through apoptosis, it also induces cytoprotective autophagy. It appears that the apoptosis is overwhelming, and autophagy is unable to override apoptosis. Thus, the augmentation of autophagy would reduce apoptosis and thus prolong transplanted MSC survival, which might be applicable in MSC-based therapy for diabetic ED.
The high blood glucose in diabetes mellitus puts the body in a state of high oxidative stress, with damage to the corpora cavernosa nerves and endothelial cells, as well as inactivation of neuronal and endothelial nitric oxide synthase, leading to decreased production of nitric oxide and diabetes mellitus erectile dysfunction18,19. In the rat model of diabetic ED caused by streptozotocin, the products of oxidative stress, such as malonaldehyde, were accumulated, while antioxidant substances (eg, SOD and glutathione) were decreased, resulting in increased ROS levels in the corpus cavernosum32,33. Recent investigations have demonstrated that ROS affect the self-renewal and differentiation of bone marrow mesenchymal stem cells, accelerate aging in stem cells and endothelial progenitor cells, increase apoptosis and reduce the self-healing of damaged blood vessels34,35. Consistently with the reports that ROS can induce apoptosis, we show that ROS can kill MSCs through the activation of apoptosis, which is shown by caspase 3 activation, PARP cleavage, increased Annexin V staining and suppression of ROS cytotoxicity by z-VAD. Thus, ROS-induced apoptosis plays an important role in stem cell viability and function and the pathogenesis of diabetic ED, and the manipulation of ROS-induced apoptosis may be usable to treat diabetic ED.
In addition to apoptosis, ROS also induces autophagy under certain circumstances. As a cellular catabolic process, autophagy plays an important role in stem cell differentiation and self-renewal17,36,37. In our study, we clearly demonstrate that H2O2 induces autophagy in MSCs, including increased LC3-II expression, decreased p62 expression, and autophagic flux. Autophagy can mediate cell survival or death, depending on the cell type, environment and stimulation31,38,39. The role of ROS-induced autophagy in MSCs is unclear. In our study, ROS-induced autophagy is cytoprotective, as the suppression of autophagy increases the apoptosis and cytotoxicity induced by ROS, which is consistent with a recent report on hepatocytes40.
MAPKs such as JNK are involved in the induction of autophagy through Bcl-2 phosphorylation and disruption of the Bcl-2/Beclin1 complex30. We found that ROS potently activated JNK while decreasing Bcl-2 expression in MSCs. The pharmacological inhibition of JNK significantly suppressed autophagy, implying the important role of JNK in ROS-induced autophagy in MSCs. Further, JNK inhibitor attenuated Bcl-2 reduction. While previous studies have shown that Bcl-2 phosphorylation can disrupt the Bcl-2/Beclin1 complex for autophagy activation30, we recently found that the JNK-mediated degradation of Bcl-2 also leads to the induction of autophagy31. Thus, it is possible to conclude that JNK-mediated Bcl-2 degradation results in Beclin1-mediated autophagy activation in ROS-treated MSCs.
In summary, we demonstrate that while ROS kills MSCs through apoptosis, it also induces cytoprotective autophagy. Because the apoptosis is overwhelming, autophagy is unable to override apoptosis. Therefore, the augmentation of autophagy could reduce apoptosis and prolong transplanted MSC survival, improving MSC-based therapy for diabetic ED. Further in vivo studies are needed to test this hypothesis.
Author contribution
Xin GOU, Wei-yang HE and Yong LIN designed the research; Guan-yu LIU and Xiao-xue JIANG performed the experiments; Guan-yu LIU, Xiao-xue JIANG, You-lin KUANG and Ke REN analyzed the data; and Yong LIN, Guan-yu LIU and Xin ZHU wrote the paper.
Abbreviations
3-MA, 3-methyladenine; Bcl-2, B-cell lymphoma 2; CQ, chloroquine; DMSO, dimethyl sulfoxide; ED, erectile dysfunction; FCM, flow cytometric; JNK, c-Jun N-terminal kinase; LC3, microtubule associated protein 1 light chain 3; MAPKs, mitogen-activated protein kinases; MSCs, mesenchymal stem cells; MTT, methyl thiazolyl tetrazolium; OD value, optical density value; PARP, poly(ADP-ribose) polymerase; PDEI5, phosphodiesterase type-5 inhibitors; PMSF, phenylmethylsulphonyl fluoride; PVDF, polyvinylidene fluoride; ROS, reactive oxygen species; TEM, transmission electron microscopy.
Acknowledgments
This study was partly supported by grants from the National Natural Science Foundation of China (81370706 and 81372758), the Natural Science Foundation of Chongqing, China (CSTC2013JCYJA10058), the Human Resources and Social Security Bureau of Chongqing (2013011) and the Chongqing Health Bureau (2012–2–001).
References
- 1Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994; 15: 54–61. [DOI] [PubMed] [Google Scholar]
- 2Penson DF, Latini DM, Lubeck DP, Wallace KL, Henning JM, Lue TF. Do impotent men with diabetes have more severe erectile dysfunction and worse quality of life than the general population of impotent patients? Results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction (ExCEED) database. Diabetes Care 2003; 26: 1093–9. [DOI] [PubMed] [Google Scholar]
- 3Thorve VS, Kshirsagar AD, Vyawahare NS, Joshi VS, Ingale KG, Mohite RJ. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications 2011; 25: 129–36. [DOI] [PubMed] [Google Scholar]
- 4De Berardis G, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, Kaplan SH, et al. Erectile dysfunction and quality of life in type 2 diabetic patients: a serious problem too often overlooked. Diabetes Care 2002; 25: 284–91. [DOI] [PubMed] [Google Scholar]
- 5Phé V, Rouprêt M. Erectile dysfunction and diabetes: A review of the current evidence-based medicine and a synthesis of the main available therapies. Diabetes Metab 2012; 38: 1–13. [DOI] [PubMed] [Google Scholar]
- 6Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med 2009; 6: 1232–47. [DOI] [PubMed] [Google Scholar]
- 7Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA 1999; 281: 421–6. [DOI] [PubMed] [Google Scholar]
- 8Wingate K, Bonani W, Tan Y, Bryant SJ, Tan W. Compressive elasticity of three-dimensional nanofiber matrix directs mesenchymal stem cell differentiation to vascular cells with endothelial or smooth muscle cell markers. Acta Biomater 2012; 8: 1440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Lozito TP, Taboas JM, Kuo CK, Tuan RS. Mesenchymal stem cell modification of endothelial matrix regulates their vascular differentiation. J Cell Biochem 2009; 107: 706–13. [DOI] [PubMed] [Google Scholar]
- 10Song YS, Lee HJ, Park IH, Kim WK, Ku JH, Kim SU. Potential differentiation of human mesenchymal stem cell transplanted in rat corpus cavernosum toward endothelial or smooth muscle cells. Int J Impot Res 2007; 19: 378–85. [DOI] [PubMed] [Google Scholar]
- 11Qiu X, Sun C, Yu W, Lin H, Sun Z, Chen Y, et al. Combined strategy of mesenchymal stem cell injection with vascular endothelial growth factor gene therapy for the treatment of diabetes-associated erectile dysfunction. J Androl 2012; 33: 37–44. [DOI] [PubMed] [Google Scholar]
- 12He Y, He W, Qin G, Luo J, Xiao M. Transplantation KCNMA1 modified bone marrow-mesenchymal stem cell therapy for diabetes mellitus-induced erectile dysfunction. Andrologia 2014; 46: 479–86. [DOI] [PubMed] [Google Scholar]
- 13Qiu X, Lin H, Wang Y, Yu W, Chen Y, Wang R, et al. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med 2011; 8: 427–36. [DOI] [PubMed] [Google Scholar]
- 14Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, et al. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol 2012; 61: 201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Gou X, He WY, Xiao MZ, Qiu M, Wang M, Deng YZ, et al. Transplantation of endothelial progenitor cells transfected with VEGF165 to restore erectile function in diabetic rats. Asian J Androl 2011; 13: 332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Bivalacqua TJ, Deng W, Kendirci M, Usta MF, Robinson C, Taylor BK, et al. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol 2007; 292: H1278–90. [DOI] [PubMed] [Google Scholar]
- 17Guan JL, Simon AK, Prescott M, Menendez JA, Liu F, Wang F, et al. Autophagy in stem cells. Autophagy 2013; 9: 830–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Dunsmuir WD, Holmes SA. The aetiology and management of erectile ejaculatory and fertility problems in men with diabetes mellitus. Diabet Med 1996; 13: 700–8. [DOI] [PubMed] [Google Scholar]
- 19Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science 1992; 257: 401–3. [DOI] [PubMed] [Google Scholar]
- 20Thubagere A, Reinhard BM. Nanoparticle-induced apoptosis propagates through hydrogen-peroxide-mediated bystander killing: insights from a human intestinal epithelium in vitro model. ACS Nano 2010; 4: 3611–22. [DOI] [PubMed] [Google Scholar]
- 21Huang Q, Zhou HJ, Zhang H, Huang Y, Hinojosa-Kirschenbaum F, Fan P, et al. Thioredoxin-2 inhibits mitochondrial reactive oxygen species generation and apoptosis stress kinase-1 activity to maintain cardiac function. Circulation 2015; 131: 1082–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Wang Q, Zhang M, Ding Y, Wang Q, Zhang W, Song P, et al. Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circ Res 2014; 114: 480–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Honda F, Kano H, Kanegane H, Nonoyama S, Kim ES, Lee SK, et al. The kinase Btk negatively regulates the production of reactive oxygen species and stimulation-induced apoptosis in human neutrophils. Nat Immunol 2012; 13: 369–78. [DOI] [PubMed] [Google Scholar]
- 24Xie X, Le L, Fan Y, Lv L, Zhang J. Autophagy is induced through the ROS-TP53-DRAM1 pathway in response to mitochondrial protein synthesis inhibition. Autophagy 2012; 8: 1071–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci 2011; 36: 30–8. [DOI] [PubMed] [Google Scholar]
- 26Zhang T, Li Y, Park KA, Byun HS, Won M, Jeon J, et al. Cucurbitacin induces autophagy through mitochondrial ROS production which counteracts to limit caspase-dependent apoptosis. Autophagy 2012; 8: 559–76. [DOI] [PubMed] [Google Scholar]
- 27Kaminskyy VO, Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid Redox Signal 2014; 21: 86–102. [DOI] [PubMed] [Google Scholar]
- 28Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012; 8: 445–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Jiang P, Mizushima N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015; 75: 13–8. [DOI] [PubMed] [Google Scholar]
- 30Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 2008; 30: 678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31He W, Wang Q, Xu J, Xu X, Padilla MT, Ren G, et al. Attenuation of TNFSF10/TRAIL-induced apoptosis by an autophagic survival pathway involving TRAF2-and RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy 2012; 8: 1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Long T, Liu G, Wang Y, Chen Y, Zhang Y, Qin D. TNF-α, erectile dysfunction, and NADPH oxidase-mediated ROS generation in corpus cavernosum in high-fat diet/streptozotocin-induced diabetic rats. J Sex Med 2012; 9: 1801–14. [DOI] [PubMed] [Google Scholar]
- 33Li M, Zhuan L, Wang T, Rao K, Yang J, Yang J, et al. Apocynin improves erectile function in diabetic rats through regulation of NADPH oxidase expression. J Sex Med 2012; 9: 3041–50. [DOI] [PubMed] [Google Scholar]
- 34Yao EH, Yu Y, Fukuda N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Curr Pharm Biotechnol 2006; 7: 101–8. [DOI] [PubMed] [Google Scholar]
- 35Xiao Y, Li X, Cui Y, Zhang J, Liu L, Xie X, et al. Hydrogen peroxide inhibits proliferation and endothelial differentiation of bone marrow stem cells partially via reactive oxygen species generation. Life Sci 2014; 112: 33–40. [DOI] [PubMed] [Google Scholar]
- 36Mortensen M, Soilleux EJ, Djordjevic G, Tripp R, Lutteropp M, Sadighi-Akha E, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med 2011; 208: 455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Mortensen M, Watson AS, Simon AK. Lack of autophagy in the hematopoietic system leads to loss of hematopoietic stem cell function and dysregulated myeloid proliferation. Autophagy 2011; 7: 1069–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38He W, Wang Q, Srinivasan B, Xu J, Padilla MT, Li Z, et al. A JNK-mediated autophagy pathway that triggers c-IAP degradation and necroptosis for anticancer chemotherapy. Oncogene 2014; 33: 3004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Chen W, Sun Y, Liu K, Sun X. Autophagy: a double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res 2014; 9: 1210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Bhogal RH, Weston CJ, Curbishley SM, Adams DH, Afford SC. Autophagy: a cyto-protective mechanism which prevents primary human hepatocyte apoptosis during oxidative stress. Autophagy 2012; 8: 545–58. [DOI] [PMC free article] [PubMed] [Google Scholar]