Abstract
Aim:
To determine whether administration of choline could attenuate brain injury in a rat model of ischemic stroke and the underlying mechanisms.
Methods:
A rat model of ischemic stroke was established through permanent middle cerebral artery occlusion (pMCAO). After the surgery, the rats were treated with choline or choline plus the specific α7 nAChR antagonist methyllycaconitine (MLA), or with the control drug nimodipine for 10 days. The neurological deficits, brain-infarct volume, pial vessel density and the number of microvessels in the cortex were assessed. Rat brain microvascular endothelial cells (rBMECs) cultured under hypoxic conditions were used in in vitro experiments.
Results:
Oral administration of choline (100 or 200 mg·kg−1·d−1) or nimodipine (20 mg·kg−1·d−1) significantly improved neurological deficits, and reduced infarct volume and nerve cell loss in the ischemic cerebral cortices in pMCAO rats. Furthermore, oral administration of choline, but not nimodipine, promoted the pial arteriogenesis and cerebral-cortical capillary angiogenesis in the ischemic regions. Moreover, oral administration of choline significantly augmented pMCAO-induced increases in the expression levels of α7 nAChR, HIF-1α and VEGF in the ischemic cerebral cortices as well as in the serum levels of VEGF. Choline-induced protective effects were prevented by co-treatment with MLA (1 mg·kg−1·d−1, ip). Treatment of rBMECs cultured under hypoxic conditions in vitro with choline (1, 10 and 100 μmol/L) dose-dependently promoted the endothelial-cell proliferation, migration and tube formation, as well as VEGF secretion, which were prevented by co-treatment with MLA (1 μmol/L) or by transfection with HIF-1α siRNA.
Conclusion:
Choline effectively attenuates brain ischemic injury in pMCAO rats, possibly by facilitating pial arteriogenesis and cerebral-cortical capillary angiogenesis via upregulating α7 nAChR levels and inducing the expression of HIF-1α and VEGF.
Keywords: ischemic stroke, pMCAO rats, choline, methyllycaconitine, neovascularization, α7 nicotinic acetylcholine receptor, HIF-1α, VEGF, angiogenesis, brain microvascular endothelial cells
Introduction
Ischemic stroke is characterized by a high incidence and high rates of mortality and disability and is severely detrimental to public health. The pathogenesis of ischemic stroke is extremely complicated, and neuroprotective drugs that counteract calcium overload, oxidation and apoptosis have not been clinically efficacious therapies for ischemic stroke1. However, producing collateral circulation in the ischemic region through the anastomosis of blood vessels or molecular bypass using the vascular endothelial growth factor (VEGF) preparations has been successfully used to treat cerebral or myocardial infarctions2,3,4. Follow-up investigations have been conducted to develop a new molecular bypass therapy using an oral drug.
Because compensatory angiogenesis occurs in the ischemic brains of stroke patients5, it would be interesting to evaluate a new brain-protective therapy that acts through an angiogenesis pathway. A series of experiments have revealed that the α7 nicotinic acetylcholine receptor (α7 nAChR) is involved in ischemia-induced angiogenesis6,7,8. Choline, a highly specific agonist of the α7 receptor9,10, has been found to promote the proliferation and tube formation of endothelial cells in vitro, and we have also observed that choline treatment increases the capillary density in the ischemic hearts of rats that suffered a myocardial infarction11. These results suggest that choline could be an important molecular bypass treatment for myocardial infarction, and therefore, we hypothesized that choline might be effective against ischemic stroke. In this study, the brain-protective efficacy of choline against ischemic stroke was evaluated, and the possible molecular pathway through which it facilitates angiogenesis in ischemic brains was also investigated.
Materials and methods
Animals and drugs
Male Sprague-Dawley (SD) rats (8 weeks old, 280±20 g and 3 weeks old, 50±10 g) were provided by the Experimental Animal Center of the Academy of Military Medical Sciences (Beijing, China). All of the animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No 80-23, revised in 1996) and were approved by the local animal care and use committee. Choline chloride (catalog number C7527), nimodipine and methyllycaconitine (MLA) citrate were purchased from Sigma-Aldrich (St Louis, MO, USA). The other reagents used were obtained from local commercial sources.
Animal groups and the permanent middle cerebral artery occlusion (pMCAO) operation
The rats were divided into the following 8 groups with 18 rats in each group: sham-operated control rats (sham); pMCAO rats (model); pMCAO rats treated with 50 mg/kg, 100 mg/kg and 200 mg/kg choline (Cho50, Cho100, and Cho200, respectively); pMCAO rats treated with 20 mg/kg nimodipine (Nim), which was used as the control drug to determine the reliability of the experimental conditions; pMCAO rats treated with 100 mg/kg choline plus 1 mg/kg MLA, a selective α7 nAChR antagonist (Cho100+MLA); and pMCAO rats treated with 1 mg/kg MLA (MLA). pMCAO was performed using a modified filament technique12. Briefly, the rats were anesthetized using chloral hydrate (300 mg/kg, intraperitoneal injection). The right common carotid artery (CCA) and external carotid artery were exposed and ligated. A 19-mm, 4-0 monofilament nylon suture (Sunbio Biotech, Beijing, China) was introduced through the right CCA into the internal carotid artery until resistance was encountered, and then the suture was tied to the right CCA. The body temperature was maintained at 37 °C using a heating pad. Choline and nimodipine were administered via gavage, whereas MLA was administered subcutaneously. The rats were treated with drugs at 4 h after surgery and then once daily at a dose of 2 mL/kg for 10 days. The sham and model groups were treated with sterile water for injection. A total of 42 rats were excluded from further assessment because 35 rats died before d 10, and 7 rats did not succeed because of the surgical operation. The possible causes of death included a subarachnoid hemorrhage or a large hemispheric infarction.
Neurological function test
The neurological functions of the rats were tested within 10 days after the pMCAO operation. The rats were evaluated daily for neurological deficits using the Bederson scoring system13. Their bilateral grasp and beam-walking behaviors were assessed as described previously14,15. Their body weights were also measured.
Infarction volume analysis
The rats were sacrificed, and their brains were quickly removed and divided into coronal sections (2-mm thickness) starting from the frontal pole. The slices were immersed in a 2% 2,3,5-triphenyltetrazolium chloride (TTC) saline solution in a Petri dish and were incubated at 37 °C for 30 min before fixation for 4 h using 4% paraformaldehyde. The infarct area was measured by a blinded observer using Image-Pro Plus software version 6.0 (IPP 6.0, Media Cybernetics, Silver Spring, MD, USA), and the area was multiplied by the section thickness to obtain the infarct volume. The infarct volume was calculated as a percentage of the contralateral hemisphere using the following formula: 100(contralateral hemisphere volume–non-infarct ipsilateral hemisphere volume)/contralateral hemisphere volume16.
Determination of the pial vessel density
The pial vessel density was determined using a modified method17. Briefly, the brains were removed and rinsed using saline to remove the blood. Stereomicroscopic images of the right ischemic hemispheres were captured. Each hemisphere was divided into 6 grids of equal areas, and the total lengths of the cortical collateral vessels of the middle cerebral arteries (MCAs) and their control regions were measured using IPP 6.0 software. The vessel density was defined as the total length of the MCA collateral vessels within the control regions (μm/mm2).
Determination of the capillary density
We applied immunohistochemistry using an antibody directed against the cluster of differentiation 34 (CD34) protein to evaluate angiogenesis in the ischemic cortical areas7. Briefly, a series of coronal sections (3 μm) of the ischemic brain regions (bregma 5.2 to 5.8 mm) were cut at 200-μm intervals. The capillary density was examined using a primary rabbit anti-rat CD34 antibody (Bioss, Beijing, China). The number of microvessels/mm2 was used to determine the capillary densities in 6 randomly selected fields within each section, which were analyzed using IPP 6.0 software.
Hematoxylin and eosin (HE) staining
Rats were anesthetized and intracardially perfused using phosphate-buffered saline followed by 4% paraformaldehyde. Their brains were then embedded in paraffin and cut into 3-μm sections. HE staining was performed to evaluate morphological changes in the nerve cells of the ischemic cerebral cortices using light microscopy.
Determination of the VEGF level
Blood was collected from the abdominal aorta and then centrifuged to obtain serum; the rat brain microvascular endothelial cells (rBMECs) were seeded in 96-well plates and treated as described in the section “Cell proliferation assay” below followed by the centrifugation of the collected medium 24 h later to obtain supernatants. The levels of VEGF in abovemention serum or supernatants were measured using a VEGF ELISA Kit (Boster Biotechnology Inc, Wuhan, China)
Real-time polymerase chain reaction analysis
Total RNA was extracted from the ischemic region of the brain cortex using TRIzol reagent (Invitrogen, CA, USA). cDNA was synthesized using qPCR RT Master Mix (TOYOBO, Osaka, Japan). Real-time PCR was performed using SYBR Green Real-time PCR Master Mix Plus (TOYOBO) and an iCycler iQ5 system (Bio-Rad, Hercules, CA, USA). The primer sequences were as follows: α7 nAChR, sense 5′-TCCCTCCAGGCATATTCAAGAGC-3′ and antisense 5′-ATTTGCAGGTCCAGTGACCACC-3′ HIF-1α, sense 5′-TGCTGGCT CCCTATATCCCAA-3′ and antisense 5′-TGGCAGTGACAGTGATGGTAG-3′ VEGF, sense 5′-TGGACCCTGGCTTTACTGCT-3′ and antisense 5′-TGAACTTCACCACTTC ATGGGC-3′ GAPDH, sense 5′-AGGGCTCATGACCACAGTCCAT-3′ and antisense 5′-ATGCCAGTGAGCTTCCCGTT-3′. The PCR amplification conditions were as follows: 95 °C for 60 s, followed by 40 cycles of 95 °C for 15 s, 60 °C for 15 s and 72 °C for 30 s. The data were quantified using the 2−ΔΔCt method, and the relative fold changes were corrected according to the level of GAPDH expression and were normalized according to the sham group values.
Western blotting analysis
The ischemic brain cortex was homogenized, and the protein concentration was determined using bicinchoninic acid (BCA) reagent (Applygen, Beijing, China). Fifty micrograms of protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred from the gel to a polyvinylidene fluoride (PVDF) membrane, and stained using the following primary antibodies prior to incubation with a secondary antibody: anti-α7 nAChR, (1:1000; Abcam, Boston, MA, USA), anti-HIF-1α (1:500, Novus, Cambridge, UK), and anti-VEGF (1:500; Santa Cruz, Dallas, TX, USA). The labeled protein bands were visualized using chemiluminescence detection reagents (Applygen, Beijing, China) and were analyzed using a chemiluminescence imaging system (Sage Creation Science, Beijing, China). All of the blots were probed with an anti-GAPDH antibody to correct for loading.
Cultivation of rBMECs
rBMECs were isolated from the brains of 3-week-old SD rats using a modified collagenase/dispase-based digestion protocol18. Briefly, the cerebral cortex was minced, homogenized and passed through 200-μm and 77-μm strainers. The tissue remaining on the strainer was digested using a 0.1% collagenase/dispase solution for 25 min at 37 °C. The pellet was separated by centrifugation at 1000×g for 20 min in 25% bovine serum albumin-Dulbecco's modified Eagle medium (DMEM). The precipitate was cultured at 37 °C in a humidified atmosphere of 95% air and 5% CO2 in complete DMEM culture medium. The cells used in the present study were passaged three times and were positive for CD34, as determined using immunocytochemistry.
HIF-1α siRNA transfer
rBMECs were seeded at a density of 2×105 cells per well in a six-well plate and were grown in antibiotic-free medium until they reached 60%–80% confluence. They were then subjected to gene silencing using small-interfering-RNA (siRNA) targeting HIF-1α or an HIF-1α scrambled control and a transfection reagent (sc-45919, sc-37007 and sc-29528, Santa Cruz) according to the manufacturer's instructions. The transfection efficiency was evaluated using Western blot analysis of HIF-1α.
Cell proliferation assay
rBMECs were seeded at a density of 5000 cells per well in 96-well plates and were cultured in a humidified atmosphere of 95% air and 5% CO2 for 24 h. Next, the cells were cultured in a humidified hypoxic incubator (CB150, Binder, Tuttlingen, Germany) containing 1% O2, 5% CO2 and 95% air for 24 h and were then exposed to the following treatments: special DMEM without choline (Merck Millipore Beijing Skywing, Beijing, China); 1, 10 or 100 μmol/L choline; 10 μmol/L choline plus 1 μmol/L MLA or 100 nmol/L HIF-1α siRNA; or 1 μmol/L MLA. After 24 h, the number of viable cells was quantified using a cell proliferation assay kit (Promega, Madison, WI, USA), which is based on a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS]. Briefly, 20 μL of the assay reagent was added to 100 μL of medium and the mixture was incubated at 37 °C for 2 h, after which the absorbance was measured at 490 nm using a multifunction microplate reader (Flexstation 3, Molecular Devices, Sunnyvale, CA, USA).
Wound healing assay
A wound-healing assay was performed as previously described with minor modifications19. Briefly, a confluent rBMEC monolayer in a 12-well plate was wounded by scraping with a pipette tip. The cell treatments were the same as described above. The cell migration distance from the wound edge to the end point was calculated at 24 h using IPP 6.0 software.
Tube formation assay
A tube formation assay was performed as previously described20. rBMECs were seeded in 96-well plates that had been pre-coated with a thin layer of Matrigel (BD Biosciences, Shanghai, China) and were allowed to form tube-like structures. The cell treatments were the same as described above. Tube formation was analyzed using the online WimTube image analysis tool and was quantified according to the total tube lengths at 10 h.
Statistical analysis
All of the data were presented as the mean values±standard error of the mean (SEM). Statistical analysis of the data was performed using the SPSS version 13.0 software package (SPSS Inc, Chicago, IL, USA). The differences were assessed using a one-way analysis of variance (ANOVA) followed by a post hoc Bonferroni's test. Differences were considered significant at a P value of less than 0.05.
Results
Choline provides brain protection against ischemic stroke
The neurological deficits, infarct volumes and necrotic neurocyte populations were determined to evaluate the extent of brain damage due to an ischemic stroke, which was imitated through surgical pMCAO in rats. The rats were randomly divided into eight groups, which are described in detail in the experimental procedures section. The extent of brain damage due to an ischemic stroke was evaluated by determining the differences between the sham-operated and pMCAO-operated rats, and the pharmacological effects of choline against ischemic stroke were evaluated by determining the differences between the pMCAO-operated rats treated with choline or its solvent. Treatment using nimodipine was designed to ensure the reliability of the methods used to evaluate the brain-protective efficacy of the tested drugs against the effects of stroke.
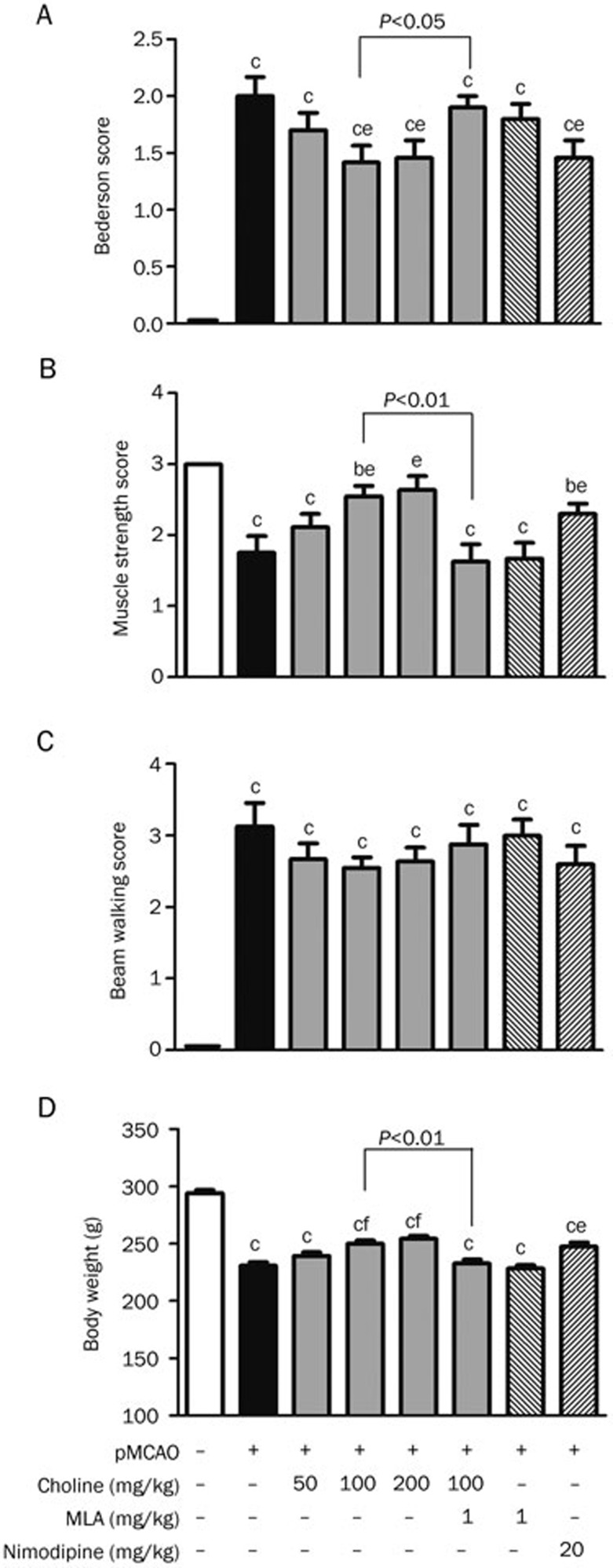
In this experiment, brain damage due to stroke was characterized by an increase in the Bederson score (Figure 1A) and beam-walking score (Figure 1C) and a decrease in the muscle-strength score (Figure 1B) and body weight (Figure 1D), all of which were attenuated by treatment with nimodipine, except the decrease in the beam-walking score. Under the same experimental conditions, after treatment with choline at doses of 100 or 200 mg·kg−1·d−1 (po) for 10 days, the body weight and muscle-strength score of the pMCAO rats increased and the Bederson score decreased, whereas the beam-walking score decreased to some extent but not significantly (P>0.05); these improvements, except that of the beam-walking score, were attenuated by treatment with MLA, a specific antagonist of the α7 nAChR, at a dose of 1 mg·kg−1·d−1 for 10 days before the oral administration of choline at 100 mg/kg.
Figure 1.
Effects of oral choline treatment on the neurologic functions and body weight of rats 10 days after the surgical permanent middle cerebral artery occlusion (pMCAO). (A) Bederson score (n=10). (B) Muscle strength score (n=10). (C) Beam walking score (n=9). (D) Body weight (n=12). Mean±SEM. bP<0.05, cP<0.01 vs sham; eP<0.05, fP<0.01 vs pMCAO.
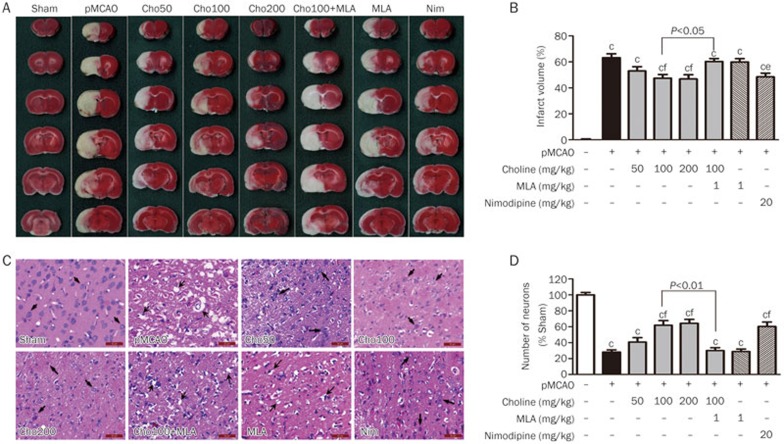
Assays were conducted to observe the pathological damage due to ischemic stroke. As shown in Figure 2, the infarct volume and the number of necrotic neurocytes in the brains of the pMCAO rats were reduced by treatment with choline at doses of 50, 100 or 200 mg·kg−1·d−1 (po) for 10 days, which were also prevented by treatment with MLA at a dose of 1 mg/kg. The above findings indicated that oral treatment with choline ameliorated the neurological deficits and alleviated the pathological damage due to ischemic stroke through the α7 receptor pathway.
Figure 2.
Effects of oral choline treatment on the infarct volume and pathological changes in the ischemic brains. (A) Macroscopic images of TTC-stained brain slices. (B) Infarct volumes (n=6). (C) Microscopic images of HE-stained ischemic cerebral cortex sections. (D) Number of neurons (n=3). Mean±SEM. bP<0.05, cP<0.01 vs sham; eP<0.05, fP<0.01 vs pMCAO. The arrows indicate neurons with different pathological changes. Scale bar=50 μm.
Choline treatment facilitated ischemia-induced angiogenesis in the stroke-damaged brains
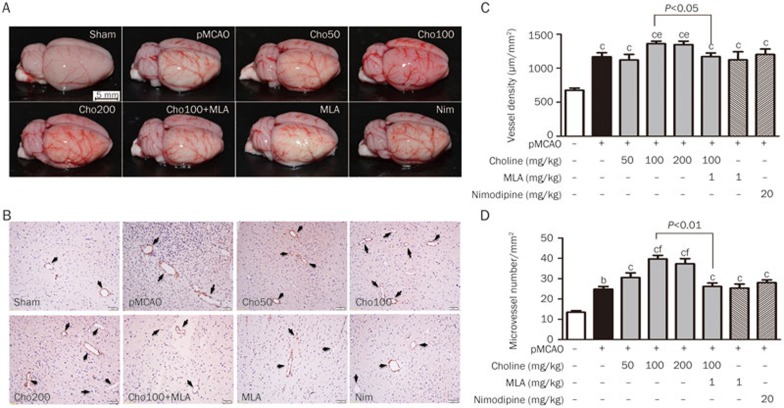
As shown in Figure 3, 10 days after the pMCAO operation, we observed ischemia-induced angiogenesis at the surface of the ischemic cortex, which was characterized by findings including increases in the blood vessel density at the cortical surface and the number of microvessels in the cortex. The choline treatments clearly facilitated ischemia-induced angiogenesis, which was prevented by treatment with MLA at a dose of 1 mg/kg. Moreover, similar facilitative effects were not observed in pMCAO-rats treated with nimodipine at a dose of 20 mg/kg.
Figure 3.
Effects of oral choline treatment on angiogenesis in the ischemic brains. (A) Macroscopic images of the pial vasculature. (B) Microscopic images of ischemic cerebral cortex sections immunohistochemically stained using an anti-CD34 antibody to analyze vessel density, scale bar=20 μm. (C) Vessel density (n=6). (D) Number of microvessels in the cortex (n=3). Mean±SEM. bP<0.05, cP<0.01 vs sham; eP<0.05, fP<0.01 vs pMCAO.
Molecular mechanisms by which choline facilitated angiogenesis in stroke-damaged brains
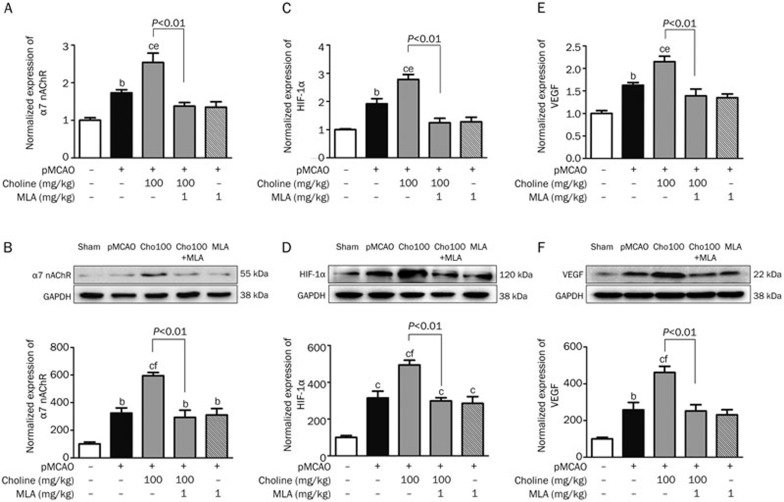
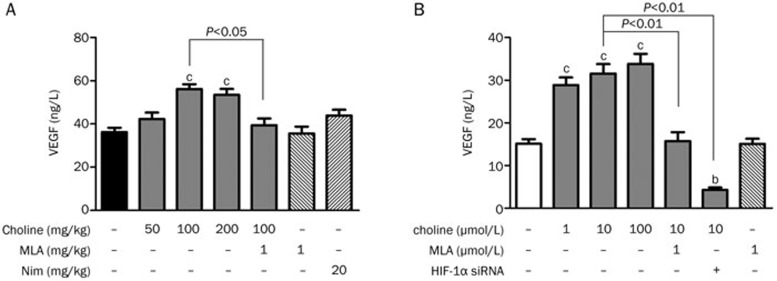
To understand the molecular pathway through which choline facilitated angiogenesis in the stroke-damaged brains, we investigated possible changes in the levels of molecular targets involved in this process. Increases in the expression of the α7 receptor, HIF-1α and VEGF at both the gene (Figure 4A) and protein (Figure 4B) levels were observed in the pMCAO-operated rats when ischemia-induced angiogenesis occurred, and the facilitative effects of choline treatment were characterized by a further increase in the expression of the α7 receptor, HIF-1α and VEGF at both the gene and protein levels, which could be prevented by MLA treatment. At the same time, the serum level of VEGF was further increased by treatment with choline at doses of 50, 100 or 200 mg·kg−1·d−1 for 10 days (Figure 5A). Based on these results, it was reasonable to suggest that choline might facilitate ischemia-induced angiogenesis in stroke-damaged brains through upregulation of α7 receptor level, which might induce the expression of HIF-1α and VEGF.
Figure 4.
Effects of oral choline treatment on the gene and protein expression levels, respectively, of α7 nAChR (A and B), HIF-1α (C and D), and VEGF (E and F) in the ischemic cerebral cortex. Mean±SEM. n=3. bP<0.05, cP<0.01 vs sham; eP<0.05, fP<0.01 vs pMCAO.
Figure 5.
Effects of choline treatment on the levels of VEGF in the sera of rats and the supernatants derived from rBMEC cultures. (A) The levels of VEGF in the sera of the pMCAO-operated rats (n=8). (B) The levels of VEGF in the supernatants derived from rBMEC cultures (n=6). Mean±SEM. In A: bP<0.05, cP<0.01 vs pMCAO. In B: bP<0.05, cP<0.01 vs control.
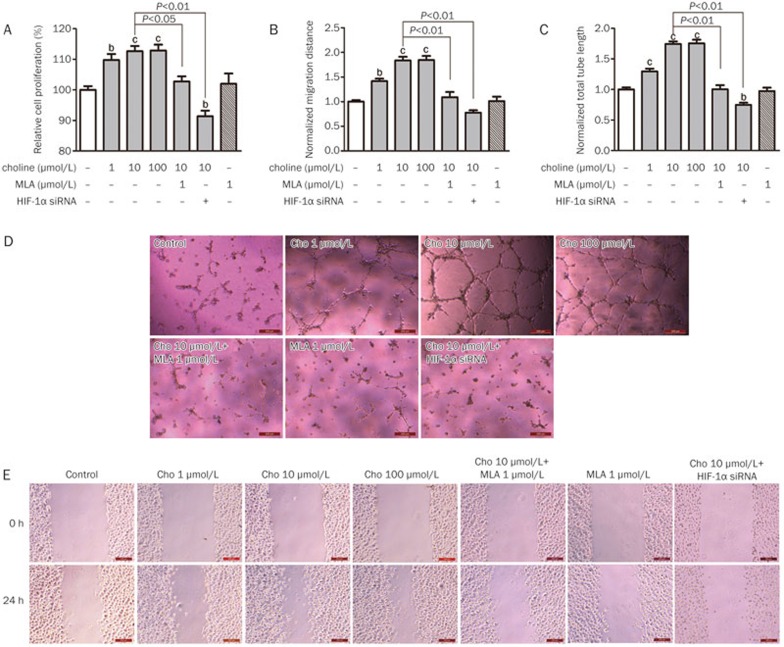
In rBMECs cultured under hypoxic conditions, the release of VEGF into the medium was stimulated by the presence of choline at concentrations of 1, 10, and 100 μmol/L in a concentration-dependent manner and prevented by the presence of MLA at 1 μmol/L or by decreasing the amount of HIF-1α with siRNA (Figure 5B; the interfering effect of siRNA was determined with Western blotting, Supplementary Figure S1). Moreover, the proliferation (Figure 6A) and migration (Figures 6B and 6E) of rBMECs, as well as their tube formation (Figures 6C and 6D), were also facilitated by the presence of choline in a concentration-dependent manner, which was also prevented by the presence of MLA at 1 μmol/L or by HIF-1α siRNA. Therefore, it was reasonable to suggest that hypoxia-induced endothelial-cell proliferation, migration and tube formation, which contribute to ischemia-induced angiogenesis, might be facilitated by choline stimulating the release of HIF-1α and VEGF from endothelial cells through the α7 receptor.
Figure 6.
Effects of choline treatment on the proliferation (A, n=4), migration (B, n=3) and tube formation (C, n=4) of rBMECs cultured under hypoxic conditions. (D) Microscopic images for the tube formation assay. (E) Microscopic images for the wound healing assay. Mean±SEM. Scale bar=200 μm. bP<0.05, cP<0.01 vs control.
Discussion
Promoting the development of collateral circulation in ischemic regions has been shown to be effective in treating vascular ischemic diseases. Blood-vessel anastomosis has been successfully used to develop collateral circulation after myocardial infarction and in patients with Moyamoya disease21. Molecular bypass using VEGF preparations has also been used to promote the development of collateral circulation after myocardial infarction and in patients with peripheral vascular ischemic diseases2,3,4. Follow-up investigations have been conducted to develop new molecular bypass therapies using oral drugs. Choline, a specific α7 receptor agonist9,10, can stimulate angiogenesis and can protect the heart from ischemic injuries by promoting angiogenesis in ischemic regions11. Thus, in this study, we tested the effects of choline treatment on brains subjected to ischemia and analyzed the molecular pathway underlying its stimulation of angiogenesis.
The results of this study indicated that choline-based brain protection against stroke is characterized by the improvement of neurologic deficits, decreases in brain-infarct volume and the number of necrotic neurocytes, and the reversal of neuronal swelling and cytoplasmic vacuole formation, which could be prevented by MLA treatment, suggesting that choline protected the brain against stroke through the α7 receptor.
This study showed that the vascular density at the brain surface and the number of microvessels in the ischemic cerebral cortex were increased at 10 d after surgical pMCAO. These findings are consistent with the ischemia-induced angiogenesis observed in the brains of stroke patients. The more extensive the ischemia-induced angiogenesis, the longer the survival of these stroke patients5. Promoting the compensatory process of ischemia-induced angiogenesis may be a therapeutic strategy for the treatment of stroke. A series of studies have demonstrated that the α7 receptor is involved in the regulation of ischemia-induced angiogenesis6,7,8. In our previous studies, choline, a selective agonist of the α7 receptor9,10, was shown to enhance angiogenesis in the chick-embryo chorioallantoic membrane22 and to promote the proliferation and tube formation of endothelial cells and increase the capillary density in the hearts of rats that suffered a myocardial infarction11. These observations suggest that ischemia-induced angiogenesis may be a target for the treatment of myocardial infarction.
This study provides additional support of the feasibility of choline for promoting angiogenesis. Our in vitro results showed that choline stimulated the proliferation, migration, tube formation, and VEGF release of cultured rBMECs grown under hypoxic conditions, which could be prevented by MLA treatment. The proliferation and migration of endothelial cells are critical processes during angiogenesis. VEGF is an important angiogenic factor23,24. Our results indicate that choline promotes rBMEC proliferation, migration, tube formation, and VEGF release, which may contribute to its stimulating angiogenesis. The results of our in vivo experiments indicated that oral choline treatment led to increases in the vascular density and the number of microvessels in the ischemic cerebral cortex, which was blocked by MLA treatment. The above-mentioned results demonstrated that choline could be used as a novel therapeutic approach to treat ischemic stroke via the α7 receptor pathway, resulting in the promotion of angiogenesis and the development of collateral circulation, thereby improving the blood supply to the ischemic region.
The expression of angiogenesis-related genes is upregulated within a few minutes after a cerebral ischemic attack25. This study showed that α7 receptor gene and protein expression were upregulated in the ischemic cerebral cortex of the pMCAO-operated rats, which was potentiated by choline treatment. Data demonstrating increases in the level of α7 nAChR expression in different brain regions after dietary choline intake support our current results26. Nicotine increased the levels of HIF-1α and VEGF expression through the α7 nAChR pathway in non-small cell lung cancer cells27. HIF-1α upregulates the expression of hypoxia-responsive genes and induces angiogenesis. VEGF, the expression of which is regulated by HIF-1α, is the most powerful angiogenic factor that has been reported to date. The expression levels of HIF-1α and of VEGF and its receptors in ischemic brains are elevated after cerebral ischemia has occurred28,29; therefore, the HIF-1α/VEGF pathway is important in the regulation of angiogenesis. Our in vivo results showed that the levels of HIF-1α and VEGF gene and protein expression were upregulated in the ischemic cerebral cortices of the pMCAO-operated rats and that these effects were potentiated by orally administered choline. Choline treatment led to an elevated serum VEGF level in the pMCAO-operated rats, which was reversed by MLA treatment. These results suggest that the molecular mechanisms of the choline-induced angiogenesis in the ischemic brain include upregulation of the expression of the α7 receptor, HIF-1, and VEGF genes and proteins in the ischemic cerebral cortex and elevation of the serum VEGF level via the α7-receptor pathway. A previous study by YU JG et al has suggested that increasing endogenous ACh through enhancing baroreflex sensitivity increases angiogenesis in ischemic myocardium and promotes expression of VEGF via activating α7 nAChR7, which also supports our results.
A battery of tests was conducted to investigate the efficacy of choline in treating ischemic stroke and the molecular mechanism underlying its effects. We suggest that choline is neuroprotective against ischemic stroke through the α7 receptor pathway, resulting in upregulation of α7 receptor, HIF-1α, and VEGF expression in the ischemic brain. Additionally, we found that nimodipine, a calcium-channel blocker that is recognized as a neuroprotective drug, showed neuroprotective activity against ischemic stroke, but did not promote neovascularization in the ischemic brains. These data suggest that the mechanism through which choline protects against ischemic stroke is different from that of the calcium-channel blocker. In this study, we observed that oral choline treatment stimulated compensatory angiogenesis in the stroke-damaged brain, although we did not eliminate other pathways through which this treatment provided brain protection. Compensatory angiogenesis and the development of collateral circulation in ischemic regions play an important role in limiting the pathogenesis and progression of the effects of stroke and are closely related to the prognosis of stroke. Thus, molecular bypass treatment using a specific α7 receptor agonist such as choline is potentially valuable for the clinical management of stroke, especially Moyamoya disease.
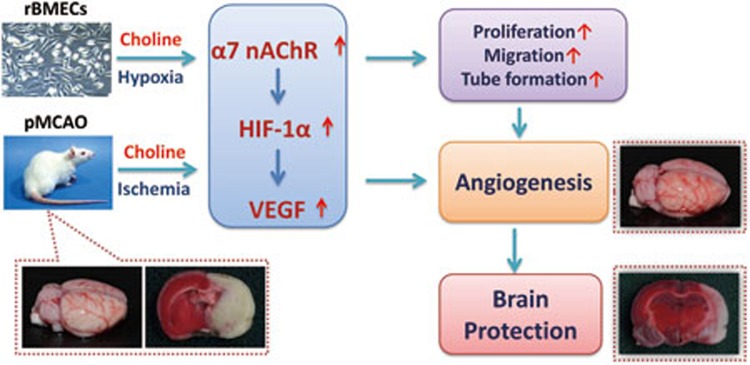
In summary, choline conferred brain protection against ischemic stroke in the pMCAO-operated rats and, as shown in Figure 7, the underlying molecular mechanisms of the novel pathway through which choline facilitated arteriogenesis of the pial vessels and angiogenesis in the cerebral cortex at the capillary level include activating the α7 receptor, up-regulating HIF-1 expression, and increasing VEGF release, resulting in endothelial cell proliferation, migration, and tube formation.
Figure 7.
Schematic illustration of a new molecular bypass therapy against ischemic stroke using choline. Choline confers brain protection against ischemic stroke in the pMCAO-operated rats possibly via a pathway that facilitates angiogenesis through mechanisms that include activating the α7 receptor, up-regulating HIF-1α expression, and increasing VEGF release, resulting in endothelial cell proliferation, migration, and tube formation.
Author contribution
Hai WANG designed the research study and wrote the manuscript; Xin JIN conducted the experiments, analyzed the data, and wrote the manuscript; Ru-huan WANG conducted some of the experiments; and Chao-liang LONG and Hui WANG participated in discussions and writing the manuscript.
Abbreviations
nAChR, nicotinic acetylcholine receptor; CCA, common carotid artery; CD34; cluster of differentiation 34; DMEM, Dulbecco's modified Eagle's medium; HE, hematoxylin and eosin; HIF, hypoxia induced factor; MLA, methyllycaconitine; PCR, polymerase chain reaction; pMCAO, permanent middle cerebral artery occlusion; rBMECs, rat brain microvascular endothelial cells; SD, Sprague–Dawley; TTC, 2,3,5-triphenyltetrazolium chloride; VEGF, vascular endothelial growth factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; PVDF, polyvinylidene fluoride
Acknowledgments
This study was supported by grants from the State 973 Research Project (2012CB518200) and the State Key Research Project of China (AWS11J003).
Footnotes
(Supplementary information is available at Acta Pharmacologica Sinica's website.
Supplementary Information
RNA interfering effect of HIF-1α siRNA.
References
- 1Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127: e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Ribatti D, Baiguera S. Phase II angiogenesis stimulators. Expert Opin Investig Drugs 2013; 22: 1157–66. [DOI] [PubMed] [Google Scholar]
- 3Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 2003; 111: 1843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Lekas M, Lekas P, Latter DA, Kutryk MB, Stewart DJ. Growth factor-induced therapeutic neovascularization for ischaemic vascular disease: time for a re-evaluation. Curr Opin Cardiol 2006; 21: 376–84. [DOI] [PubMed] [Google Scholar]
- 5Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994; 25: 1794–8. [DOI] [PubMed] [Google Scholar]
- 6Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest 2002; 110: 527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Yu JG, Song SW, Shu H, Fan SJ, Liu AJ, Liu C, et al. Baroreflex deficiency hampers angiogenesis after myocardial infarction via acetylcholine-alpha7-nicotinic ACh receptor in rats. Eur Heart J 2013; 34: 2412–20. [DOI] [PubMed] [Google Scholar]
- 8Jacobi J, Jang JJ, Sundram U, Dayoub H, Fajardo LF, Cooke JP. Nicotine accelerates angiogenesis and wound healing in genetically diabetic mice. Am J Pathol 2002; 161: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 1997; 9: 2734–42. [DOI] [PubMed] [Google Scholar]
- 10Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett 1996; 213: 201–4. [DOI] [PubMed] [Google Scholar]
- 11Li XW, Wang H. Non-neuronal nicotinic alpha 7 receptor, a new endothelial target for revascularization. Life Sci 2006; 78: 1863–70. [DOI] [PubMed] [Google Scholar]
- 12Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab 2011; 31: 1283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 1986; 17: 472–6. [DOI] [PubMed] [Google Scholar]
- 14Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC. Cognitive Deficits After Focal Cerebral Ischemia in Mice. Stroke 2000; 31: 1939–44. [DOI] [PubMed] [Google Scholar]
- 15Altumbabic M, Peeling J, Del Bigio MR. Intracerebral hemorrhage in the rat: effects of hematoma aspiration. Stroke 1998; 29: 1917–22. [DOI] [PubMed] [Google Scholar]
- 16Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 1990; 10: 290–3. [DOI] [PubMed] [Google Scholar]
- 17Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, et al. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes 2010; 59: 228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Tian S, Bai Y, Yang L, Wang X, Wu Y, Jia J, et al. Shear stress inhibits apoptosis of ischemic brain microvascular endothelial cells. Int J Mol Sci 2013; 14: 1412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Wang J, Wang Y, Wang Y, Ma Y, Lan Y, Yang X. Transforming growth factor beta-regulated microRNA-29a promotes angiogenesis through targeting the phosphatase and tensin homolog in endothelium. J Biol Chem 2013; 288: 10418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Lan Y, Liu B, Yao H, Li F, Weng T, Yang G, et al. Essential role of endothelial Smad4 in vascular remodeling and integrity. Mol Cell Biol 2007; 27: 7683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Starke RM, Komotar RJ, Connolly ES. Optimal surgical treatment for moyamoya disease in adults: direct versus indirect bypass. Neurosurg Focus 2009; 26: E8. [DOI] [PubMed] [Google Scholar]
- 22Wang H, Zeng XZ, Cui WY, Duan L. Choline promotes angiogenesis in chick embryo chorioallantoic membrane. Chin J Appl Physiol 2013; 29: 229–31. [PubMed] [Google Scholar]
- 23Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, et al. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res 2003; 92: 308–13. [DOI] [PubMed] [Google Scholar]
- 24Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 1997; 100: 3131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 2003; 23: 166–80. [DOI] [PubMed] [Google Scholar]
- 26Morley BJ, Robinson GR, Brown GB, Kemp GE, Bradley RJ. Effects of dietary choline on nicotinic acetylcholine receptors in brain. Nature 1977; 266: 848–50. [DOI] [PubMed] [Google Scholar]
- 27Zhang Q, Tang X, Zhang ZF, Velikina R, Shi S, Le AD. Nicotine induces hypoxia-inducible factor-1alpha expression in human lung cancer cells via nicotinic acetylcholine receptor-mediated signaling pathways. Clin Cancer Res 2007; 13: 4686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol (1985) 2000; 89: 1937–42. [DOI] [PubMed] [Google Scholar]
- 29Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol 2000; 156: 965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNA interfering effect of HIF-1α siRNA.