Abstract
Aim:
The mitochondrial targeted 2C-type serine/threonine protein phosphatase (PP2Cm) is encoded by the gene PPM1K and is highly conserved among vertebrates. PP2Cm plays a critical role in branched-chain amino acid catabolism and regulates cell survival. Its expression is dynamically regulated by the nutrient environment and pathological stresses. However, little is known about the molecular mechanism underlying the regulation of PPM1K gene expression. In this study, we aimed to reveal how PPM1K expression is affected by miRNA-mediated post-transcriptional regulation.
Methods:
Computational analysis based on conserved miRNA binding motifs was applied to predict the candidate miRNAs that potentially affect PPM1K expression. Dual-luciferase reporter assay was performed to verify the miRNAs' binding sites in the PPM1K gene and their influence on PPM1K 3′UTR activity. We further over-expressed the mimics of these miRNAs in human and mouse cells to examine whether miRNAs affected the mRNA level of PPM1K.
Results:
Computational analysis identified numerous miRNAs potentially targeting PPM1K. Luciferase reporter assays demonstrated that the 3′UTR of PPM1K gene contained the recognition sites of miR-204 and miR-211. Overexpression of these miRNAs in human and mouse cells diminished the 3′UTR activity and the endogenous mRNA level of PPM1K. However, the miR-22 binding site was found only in human and not mouse PPM1K 3′UTR. Accordingly, PPM1K 3′UTR activity was suppressed by miR-22 overexpression in human but not mouse cells.
Conclusion:
These data suggest that different miRNAs contribute to the regulation of PP2Cm expression in a species-specific manner. miR-204 and miR-211 are efficient in both mouse and human cells, while miR-22 regulates PP2Cm expression only in human cells.
Keywords: PP2Cm, PPM1K, miR-204, miR-211, miR-22, species-specific
Introduction
Protein phosphatase 2C in mitochondria (PP2Cm) is a mitochondrial matrix-targeted serine/threonine protein phosphatase encoded by the gene PPM1K1,2. It is a member of the protein phosphatase 2C family and is highly conserved among vertebrates. PP2Cm expression is enriched in brain, heart, and liver3. PP2Cm protein is exclusively located in the mitochondrial matrix soluble fraction and modulates mitochondrial function4. In our previous work, we have shown that PP2Cm regulates mitochondrial membrane permeability transition pore (MPTP) opening and is critical in cell death regulation. The loss of PP2Cm leads to apoptosis and abnormal cardiac development in zebrafish1.
PP2Cm is a key regulator of the catabolism of branched-chain amino acids (leucine, isoleucine, and valine, collectively referred as BCAA). The first step of the BCAA catabolic pathway is the conversion of BCAA into branched-chain α-keto acids (BCKA) by a branched-chain amino-transferase (BCAT). Then, BCKA is oxidized by branched-chain α-keto acid dehydrogenase (BCKD) complex, the rate-limiting enzyme in BCAA catabolism. BCKD's expression and activity control BCAA homeostasis5,6, and its defect causes Maple Syrup Urine Disease. BCKD activity is negatively regulated by Ser293 phosphorylation of the E1α subunit. Previously, we demonstrated that PP2Cm binds to BCKD complex and dephosphorylates Ser293 of the E1α subunit to activate BCKD3,7. Ppm1k-deficient mice develop impaired BCAA catabolism and elevated plasma concentrations of BCAA and BCKA7. Recently, disrupted BCAA homeostasis has been linked to metabolic, neurological, and cardiovascular diseases8,9,10, although the role of PP2Cm in the pathogenesis of these diseases remains to be established.
MicroRNAs (miRNAs) are small noncoding RNAs generated by the nuclear genome and contribute to targeted gene regulation at post-transcriptional or translational level by pairing to the target transcript's 3′-untranslated region (3′UTR)11,12,13. The pairing between the target sequence and the seed sequence (6–8 nt) of the miRNA is regarded as the most important feature for target recognition by miRNAs in mammals14. The targeting sites can be highly conserved where they match the miRNA seed sequence perfectly, or less conserved with one or two mismatches. Links have been found between miRNAs and numerous developmental and pathological processes13,15. In particular, miR-204 and miR-211 belong to the same miRNA sub-family and share the same seed sequence: miR-204 is embedded in intron 6 of the TRPM3 gene (transient receptor potential melastatin 3) and has been shown to be a key regulator in metabolism, cell death, and cardiovascular disease16,17,18,19,20, and miR-211 is encoded by intron 6 of the TRPM1 gene17,21 and also been shown to play important roles in cell survival and cancer22,23,24. miR-204 is completely conserved between human and mouse. However, mouse miR-211 has a similar mature sequence to its human counterpart with only one nucleotide difference in the non-seed region (www.mirbase.org). Finally, miR-22 is fully conserved between human and mouse and has been reported to be involved in cardiovascular diseases and metabolic regulation25,26,27.
Although PP2Cm performs crucial roles in cells, little is known about the regulatory mechanism of PPM1K gene expression. In a previous study, we observed the nutrient-dependent transcriptional regulation of PP2Cm in response to BCAA availability3. In this study, we revealed a contribution of miRNAs to PP2Cm regulation at the post-transcriptional level in cells from different species.
Materials and methods
Cell culture
HepG2, NIH 3T3, and HeLa cells were cultured in Dulbecco's modified Eagle's medium (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, Gibco BRL, Gaithersburg, MD, USA), penicillin (100 IU/mL) and streptomycin (100 μg/mL) in a humidified 5% CO2-95% air incubator at 37 °C.
Plasmids, miRNA mimics and transfection
The 3′UTR of mouse Ppm1k (4216 bp, chr6:57,456,496-57,460,711) and human PPM1K (2214 bp, chr4:89,181,531-89,183,744) were identified using the UCSC Genome Browser (http://genome.ucsc.edu/) and cloned into the psiCHECK_2 plasmid (Promega) with Xho I and Not I sites, respectively. The 3′UTR fragment was located in the 3′ flanking region of the synthetic Renilla luciferase gene. Thus, Renilla luciferase expression can be regulated by the downstream 3′UTR activity28. The PPM1K 3′UTR of miR-204, miR-211 and miR-22 binding site deletion mutants were generated by PCR-mediated mutagenesis and selected by Dpn I (TOYOBO) digestion. Deletion was confirmed by DNA sequencing.
The miRNA sequences were identified from the miRBase database, and all the miRNA mimics were purchased from GenePharma (Shanghai, China) with the following sequences: negative control (miR-NC): 5′-UUCUCCGAACGUGUCACGUTT-3′, miR-204: 5′-UUCCCUUUGUCAUCCUAUGCCU-3′, miR-211: 5′-UUCCCUUUGUCAUCCUUUGCCU-3′, and miR-22: 5′-AAGCUGCCAGUUGAAGAACUGU-3′.
Plasmids and miRNA mimics were transfected using lipofectimine2000 (Thermo Fisher) according to the manufacturer's protocol. For miRNA mimics transfected into mammalian cells, Life Technologies Reverse Transfection Protocol was performed.
Identification of candidate miRNAs targeting PPM1K
Two computational target prediction programs (MiRanda, TargetScan) were applied to analyze miRNAs that potentially targeted the PPM1K gene29. TargetScan was used to predict the conserved binding region, which is perfectly complementary to bases 2-8 of the miRNA (from 5′ to 3′)30. The human and mouse 3′UTR sequences of PPM1K were analyzed.
Luciferase reporter assays
Luciferase reporter assays were performed by co-transfecting miRNA mimics and psiCHECK_2 constructs containing wild-type and mutated 3′UTRs into HeLa cells. The transfected cells were rinsed once with cold PBS and lysed with passive lysis buffer (Promega Beijing, Beijing, China) after transfection for 24 h. Luciferase activity was measured using the GloMax-Multi Detection System (Promega) and the Dual-Luciferase Reporter Assay System (Promega). Renilla luciferase expression was normalized to the expression of firefly luciferase. Data are represented as mean±SEM from three biological replicates representing three independent experiments.
RNA extraction, reverse transcription and real-time PCR analysis
Total RNA was extracted using TRIzol Reagent according to the manufacturer's protocol. Then, total RNA was reverse transcribed into first-strand cDNA using M-MLV Reverse Transcriptase (Promega). The cDNA transcripts were quantified on a Step-One Plus Real-Time PCR System (Thermo Fisher) using SYBR Green (Thermo Fisher) with the following primer sequences: human PPM1K_F: 5′-CACAGATTGGCAAACGGAAA-3′, human PPM1K_R: 5′-GCAGGTCCACCGTGTCCAT-3′, mouse Ppm1k_F: 5′-TCTCATTGGCAAACGGAAAG-3′, and mouse Ppm1k_R: 5′-CAGACAGGTGGGCATAACTCG-3′.
For reverse transcription and real-time PCR of miR-204, miR-211 and miR-22, a miRcute miRNA first-strand cDNA synthesis kit and a miRcute miRNA qPCR detection kit (TIANGEN, Shanghai, China) were used according to the manufacturer's protocol. The miRNA-specific forward primers were purchased from TIANGEN, and the reverse primer was complementary to the poly A adapter, provided with the miRcute miRNA qPCR detection kit. The results were normalized to U6 small nuclear RNA.
Statistical analysis
Data were expressed as mean±SEM. The two-tailed Student's t-test was employed to evaluate the difference between two groups, and P<0.05 was considered as significant.
Results
miRNAs regulate the expression of PPM1K genes from different species
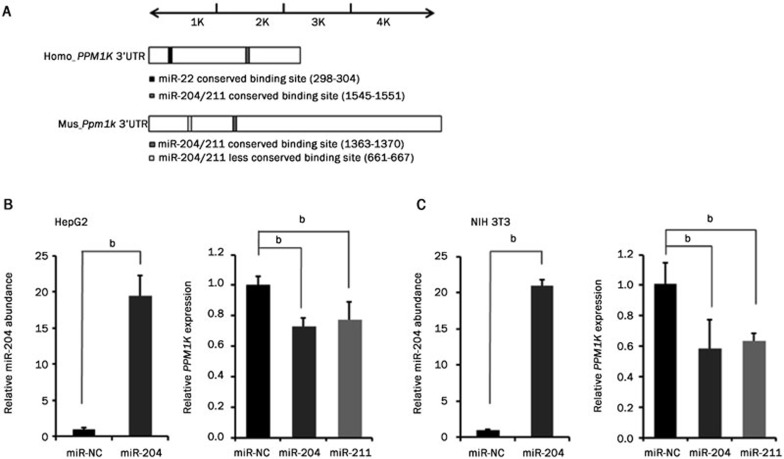
We used TargetScan (online database, http://www.targetscan.org/) to find the binding sites of miRNAs potentially targeting the PPM1K gene via its 3′UTR sequence. The human (2214 base pairs in length) and mouse (4216 base pairs in length) 3′UTR sequences of PPM1K were analyzed. Among the miRNA candidates, miR-204, miR-211 and miR-22 are of particular interest. In addition to a less conserved binding site in mouse, miR-204 and miR-211 have conserved binding sites in the 3′UTR region of both the human and mouse PPM1K genes (Figure 1A). The miR-22 conserved binding site was only detected in the 3′UTR region of the human but not the mouse PPM1K gene (Figure 1A).
Figure 1.
Candidate miRNAs regulate expression of PPM1K gene in human and mouse cell lines. (A) Summary of putative miRNAs targeting human and mouse PPM1K 3′UTR. (B and C) miR-204 abundance and PP2Cm mRNA level were assessed in HepG2 (B) and NIH 3T3 (C) cells after transfection with miRNA mimics. Data are mean±SEM for three individual samples (bP<0.05).
Then, we investigated whether miR-204 and miR-211 regulate PP2Cm expression in cells from different species. The mimics of these miRNAs were transfected into human and mouse cell lines, and their abundance was assessed by quantitative RT-PCR. The abundance of miR-204 and miR-211 increased more than 10-fold upon transfection with the corresponding mimic (Figure 1B and 1C, miR-211 data not shown). In both human hepatocellular liver carcinoma cell line HepG2 and mouse embryonic fibroblast cell line NIH 3T3, the endogenous PPM1K mRNA level was significantly decreased by the expression of miR-204 or miR-211 mimics but not by the non-specific miRNA control (miR-NC) (Figure 1B and 1C). Therefore, miR-204 and miR-211 expression regulates PP2Cm mRNA expression in both human and mouse cells.
miR-204 and miR-211 directly repress PPM1K expression via 3′UTR
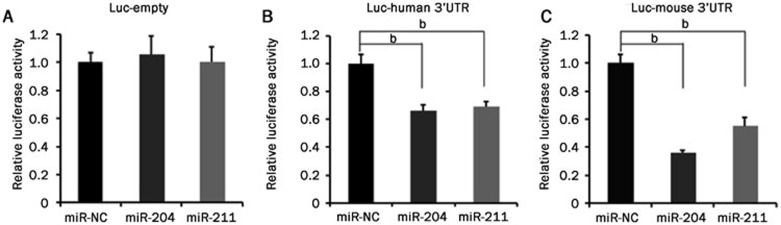
To investigate whether PPM1K 3′UTR is a direct target of miR-204 and miR-211, we cloned mouse Ppm1k 3′UTR (4216 base pairs) and human PPM1K 3′UTR (2214 base pairs) fragments into the reporter vector psiCHECK_2, where Renilla luciferase expression can be regulated by a downstream 3′UTR31, and generated Luc-mouse 3′UTR and Luc-human 3′UTR reporters, respectively. After co-transfecting these reporter vectors with miRNA mimics into cells, the luciferase activity was measured. The results showed that miR-204 and miR-211 significantly inhibited the expression of the luciferase fused with either human or mouse PPM1K 3′UTR but did not affect the activity of luciferase without any 3′UTR (named Luc-empty) (Figure 2). These data suggest that miR-204 and miR-211 target the 3′UTR to suppress human and mouse PPM1K expression.
Figure 2.
miR-204 and miR-211 suppress PPM1K 3′UTR activity. Luciferase assay results from HeLa cells co-transfected with miRNA mimics and luciferase reporter vectors. (A) Luc-empty is the reporter construct without any 3′UTR; (B) Luc-human 3′UTR is the reporter vector containing human PPM1K 3′UTR; (C) Luc-mouse 3′UTR is the reporter vector containing mouse Ppm1k 3′UTR. Data are mean±SEM for three individual samples (bP<0.05).
Validation of miR-204 and miR-211 recognition motifs
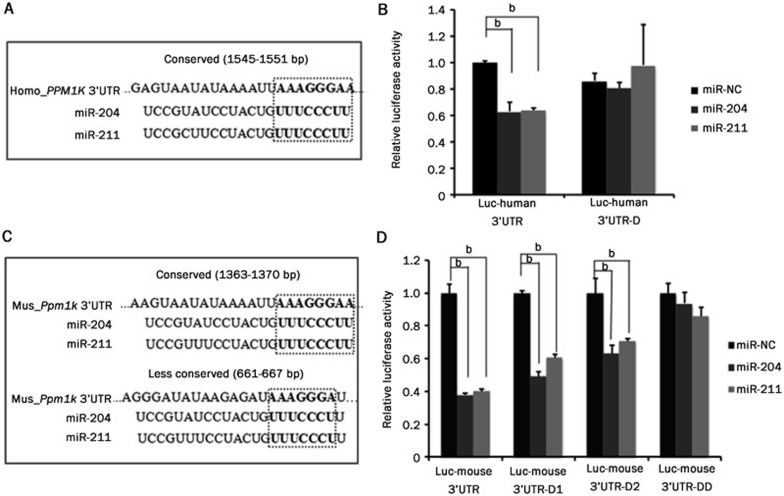
TargetScan predicted numerous putative recognition motifs for miR-204 and miR-211 in the human and mouse PPM1K 3′UTRs. One conserved 8-mer seed match motif was identified in genes from both species, and one less-conserved 7-mer-m8 or 7-mer-1A seed match motif was identified in the mouse gene (Figure 1A). To validate the function of these putative miRNA recognition motifs, we generated mutant reporter constructs with targeted deletion of these motifs. For human PPM1K 3′UTR (Figure 3A), the deletion of the conserved recognition motifs (1545-1551 base pairs) completely abolished the inhibitory effect of miR-204 and miR-211 (Figure 3B). For mouse Ppm1k 3′UTR (Figure 3C), deletion of either the conserved motif (1363-1370 base pairs) or the less-conserved motif (661-667 base pairs) partially abolished the inhibitory effect of miR-204 and miR-211. However, the simultaneous deletion of both sites completely eliminated the effect of miR-204 and miR-211 on the Ppm1k 3′UTR (Figure 3D). These data suggest that the putative recognition motifs within the PPM1K 3′UTR are functional binding sites for miR-204/211 and are required for their inhibitory effect on PP2Cm expression.
Figure 3.
miR-204 and miR-211 directly target recognition motifs in PPM1K 3′UTR. (A) Schematic diagram showing the seed sequence of miR-204 and miR-211 and putative conserved recognition motif in human PPM1K 3′UTR. (B) Luciferase assay results from HeLa cells co-transfected with miRNA mimics and luciferase reporter vectors. Luc-human 3′UTR-D is the mutated reporter vector where the conserved recognition motif was deleted in human_PPM1K 3′UTR. (C) Schematic diagram showing the seed sequences of miR-204 and miR-211, with putative matched conserved and poorly conserved sequences in mouse Ppm1k 3′UTR. (D) Luciferase assay results from HeLa cells. Luc-mouse 3′UTR-D1 is the mouse Ppm1k 3′UTR reporter vector with conserved site deleted. Luc-mouse 3′UTR-D2 is the mouse Ppm1k 3′UTR reporter vector with less-conserved site deleted. Luc-mouse 3′UTR-DD is the mouse Ppm1k 3′UTR reporter vector with both sites deleted. Data are mean±SEM for three individual samples (bP<0.05).
Species-specific regulation of PP2Cm expression by miR-22
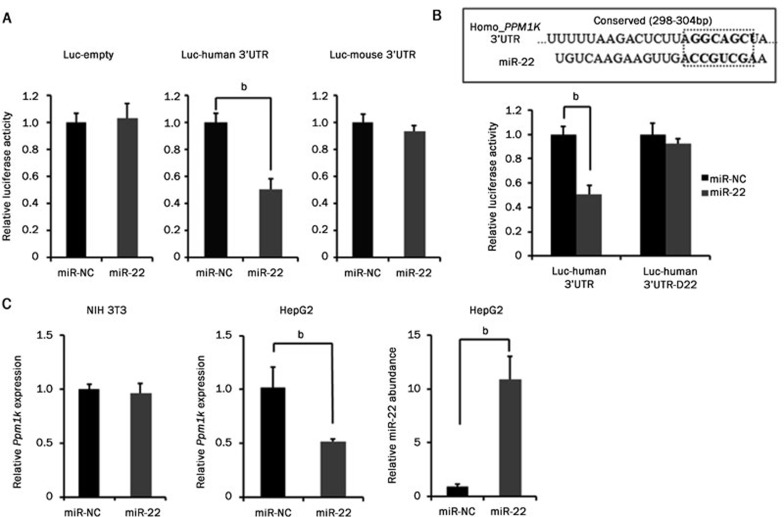
The regulatory function of miR-204/211 on the PPM1K gene was observed in both human and mouse. TargetScan also predicted a putative miR-22 binding motif in human but not mouse PPM1K 3′UTR. Using the 3′UTR luciferase reporter assay, we found that miR-22 inhibited the human PPM1K 3′UTR reporter activity but failed to affect the mouse Ppm1k 3′UTR reporter (Figure 4A). Deletion of the conserved miR-22 binding site completely abolished miR-22 mediated inhibition of the human PPM1K 3′UTR activity (Figure 4B). Furthermore, miR-22 mimic transfection increased the miRNA-22 abundance while decreasing the endogenous PP2Cm mRNA level in human HepG2 cells but not in mouse NIH 3T3 cells (Figure 4C). These data demonstrated that miRNA-22 regulates PP2Cm expression in human but not in mouse.
Figure 4.
miR-22 regulates human but not mouse PPM1K expression. (A) Luciferase assay results from HeLa cells co-transfected with miRNA mimics and luciferase reporter vectors. Luc-empty is the reporter plasmid psiCHECK_2 without any 3′UTR. Luc-human 3′UTR is the reporter vector containing human PPM1K 3′UTR. Luc-mouse 3′UTR is the reporter vector containing mouse Ppm1k 3′UTR. (B) Top, a schematic diagram shows the seed sequence of miR-22 and putative matched conserved sequence in human PPM1K 3′UTR. Bottom, luciferase assay was performed with the reporter vector containing human PPM1K 3′UTR. Luc-human 3′UTR-D22 is the mutated reporter vector with the miR-22 binding site deleted. (C) miR-22 abundance and human PP2Cm mRNA level in HepG2 and mouse PP2Cm mRNA level in NIH 3T3 cells were assessed after transfection with miRNA mimics. Data are mean±SEM for three individual samples (bP<0.05).
Discussion
Our data demonstrated that PP2Cm expression can be regulated by multiple miRNAs via 3′UTR targeting at the transcriptional level. miR-204 and miR-211 play conserved roles across human and mouse, while miR-22 may contribute to PP2Cm regulation only in human but not mouse cells. These results suggest that PP2Cm regulation involves different miRNA networks in different species.
PP2Cm is a key regulator of BCAA catabolism7. In intact animals, BCAA catabolic activity is accomplished through a coordinated process among different organs. In rodents, the transamination step occurs primarily in skeletal muscle, and the subsequent BCKD-mediated catabolic process predominantly occurs in liver. PP2Cm expression is highly enriched in the liver but low in skeletal muscle, consistently with the relative BCAA catabolic activities in different tissues in mice3. The miRNA's regulatory function has been a critical factor in tissue-specific gene expression. Further investigations are warranted to elucidate whether the tissue-specific expression of miR-22/204/211 contributes to the tissue-specific expression of PP2Cm.
The tissue-specific distribution pattern of BCAA catabolic activities is also regulated in a species-dependent manner. Comparing to rodents, where BCAA catabolic activity is primarily restricted to the liver, heart and brain, primate skeletal muscle also consumes a large portion of BCKA32. Considering that PP2Cm is a key regulator of BCAA/BCKA catabolic flux, it is possible that different miRNA networks from different species may contribute to the species-specific patterns of catabolic activities. The human PPM1K gene has a shorter 3′UTR than its mouse counterpart. However, there is no conserved miR-22 binding site detected in the mouse Ppm1k 3′UTR. This difference may represent a case where genetic variation resulted in gaining a new or losing an existing miRNA binding site, leading to different patterns of gene regulation by miRNAs33. Thus, it should be further investigated whether species-specific miRNAs involved in PP2Cm regulation contribute to the species-specific catabolic pattern of BCAA.
Cell death occurs during heart failure and may play a causal role in heart failure34,35,36. Meanwhile, mitochondrial dysfunction has been implicated in cardiomyocyte hypertrophy, ischemia/reperfusion injury and heart failure37,38,39. It has been previously reported that PP2Cm plays an essential role in mitochondrial regulation and cell survival1. The down-regulation of PP2Cm has been linked to cardiac diseases1,3. However, the molecular mechanism of PP2Cm down-regulation in the stressed heart is not known. The current study suggests a mechanism by which miRNA may contribute to stress-induced PP2Cm down-regulation. Further investigations are warranted to elucidate how and whether these different miRNA networks contribute to PP2Cm down-regulation in the diseased heart.
In conclusion, our data showed that miR-204 and miR-211 both negatively regulate human and mouse PP2Cm expression. However, miR-22 repressed only human but not mouse PPM1K 3′UTR activity. The species-specific regulation of PP2Cm via different miRNA networks suggests the complexity of gene regulatory mechanisms involved in BCAA homeostasis and cell survival among different species. On the other hand, miRNA-based therapeutic approaches have been extensively explored in recent years40. Meanwhile, BCAA metabolic defects have been linked with metabolic syndrome, cancer, cardiovascular and hepatic diseases, and cell death has also been implicated in numerous diseases41,42,43. Considering the critical role of PP2Cm in BCAA catabolic and cell death regulation, the identification of miRNAs targeting PP2Cm strongly indicates the potential of developing novel therapeutic strategies to treat common disorders.
Author contribution
Bang-fen PAN, Mei-yi ZHOU, and Hai-peng SUN designed the research; Bang-fen PAN, Chen GAO, Shu-xun REN, and Mei-yi ZHOU performed the research; Bang-fen PAN, Mei-yi ZHOU, and Hai-peng SUN analyzed the data; Yi-bin WANG helped to design the overall study and analyzed the data; and all authors contributed to the manuscript preparation.
Acknowledgments
This work is supported in part by the Ministry of Science and Technology of China Grant 2012BAI02B05, National Institute of Health grants HL108186, HL103205, HL098954, HL080111 (to Yi-bin WANG), the Laubisch Fund (UCLA), the American Heart Association (AHA), the Western States Affiliate Post-doctoral Fellowship Award and AHA Science Development Grant (to Hai-peng SUN), the AHA Established Investigator Award (to Yi-bin WANG), the National Natural Science Foundation of China (30971094 and 81270317), and the Science and Technology Commission of Shanghai Municipality (13ZR1423300, 11410709000 and 12PJ1405800).
References
- 1Lu G, Ren S, Korge P, Choi J, Dong Y, Weiss J, et al. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev 2007; 21: 784–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Oyarzabal A, Martinez-Pardo M, Merinero B, Navarrete R, Desviat LR, Ugarte M, et al. A novel regulatory defect in the branched-chain alpha-keto acid dehydrogenase complex due to a mutation in the PPM1K gene causes a mild variant phenotype of maple syrup urine disease. Hum Mutat 2013; 34: 355–62. [DOI] [PubMed] [Google Scholar]
- 3Zhou M, Lu G, Gao C, Wang Y, Sun H. Tissue-specific and nutrient regulation of the branched-chain alpha-keto acid dehydrogenase phosphatase, protein phosphatase 2Cm (PP2Cm). J Biol Chem 2012; 287: 23397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Lu G, Sun H, Korge P, Koehler CM, Weiss JN, Wang Y. Functional characterization of a mitochondrial Ser/Thr protein phosphatase in cell death regulation. Methods Enzymol 2009; 457: 255–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr 1984; 4: 409–54. [DOI] [PubMed] [Google Scholar]
- 6Harris RA, Joshi M, Jeoung NH. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem Biophys Res Commun 2004; 313: 391–6. [DOI] [PubMed] [Google Scholar]
- 7Lu G, Sun H, She P, Youn JY, Warburton S, Ping P, et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest 2009; 119: 1678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009; 9: 311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Novarino G, El-Fishawy P, Kayserili H, Meguid NA, Scott EM, Schroth J, et al. Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science 2012; 338: 394–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet 2010; 3: 207–14. [DOI] [PubMed] [Google Scholar]
- 11Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–97. [DOI] [PubMed] [Google Scholar]
- 12Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 2009; 11: 228–34. [DOI] [PubMed] [Google Scholar]
- 13Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Ellwanger DC, Buttner FA, Mewes HW, Stumpflen V. The sufficient minimal set of miRNA seed types. Bioinformatics 2011; 27: 1346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Wu YY, Li LJ. MicroRNAs and cancer-associated signal transduction pathways. Yi Chuan 2007; 29: 1419–28. [PubMed] [Google Scholar]
- 16Xu G, Chen J, Jing G, Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat Med 2013; 19: 1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Mikhaylova O, Stratton Y, Hall D, Kellner E, Ehmer B, Drew AF, et al. VHL-regulated MiR-204 suppresses tumor growth through inhibition of LC3B-mediated autophagy in renal clear cell carcinoma. Cancer Cell 2012; 21: 532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011; 208: 535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Kuster DW, Mulders J, Ten Cate FJ, Michels M, Dos Remedios CG, da Costa Martins PA, et al. MicroRNA transcriptome profiling in cardiac tissue of hypertrophic cardiomyopathy patients with MYBPC3 mutations. J Mol Cell Cardiol 2013; 65: 59–66. [DOI] [PubMed] [Google Scholar]
- 20Kuwano Y, Nishida K, Kajita K, Satake Y, Akaike Y, Fujita K, et al. Transformer 2beta and miR-204 regulate apoptosis through competitive binding to 3′UTR of BCL2 mRNA. Cell Death Differ 2015; 22: 815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Chang KW, Liu CJ, Chu TH, Cheng HW, Hung PS, Hu WY, et al. Association between high miR-211 microRNA expression and the poor prognosis of oral carcinoma. J Dent Res 2008; 87: 1063–8. [DOI] [PubMed] [Google Scholar]
- 22Chitnis NS, Pytel D, Bobrovnikova-Marjon E, Pant D, Zheng H, Maas NL, et al. miR-211 is a prosurvival microRNA that regulates chop expression in a PERK-dependent manner. Mol Cell 2012; 48: 353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Sakurai E, Maesawa C, Shibazaki M, Yasuhira S, Oikawa H, Sato M, et al. Downregulation of microRNA-211 is involved in expression of preferentially expressed antigen of melanoma in melanoma cells. Int J Oncol 2011; 39: 665–72. [DOI] [PubMed] [Google Scholar]
- 24Giovannetti E, van der Velde A, Funel N, Vasile E, Perrone V, Leon LG, et al. High-throughput microRNA (miRNAs) arrays unravel the prognostic role of MiR-211 in pancreatic cancer. PLoS One 2012; 7: e 49145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Huang ZP, Chen J, Seok HY, Zhang Z, Kataoka M, Hu X, et al. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ Res 2013; 112: 1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Gurha P, Wang T, Larimore AH, Sassi Y, Abreu-Goodger C, Ramirez MO, et al. microRNA-22 promotes heart failure through coordinate suppression of PPAR/ERR-nuclear hormone receptor transcription. PLoS One 2013; 8: e 75882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Perri R, Nares S, Zhang S, Barros SP, Offenbacher S. MicroRNA modulation in obesity and periodontitis. J Dent Res 2012; 91: 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Zhang H, Wang Q, Zhao Q, Di W. MiR-124 inhibits the migration and invasion of ovarian cancer cells by targeting SphK1. J Ovarian Res 2013; 6: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Sethupathy P, Megraw M, Hatzigeorgiou AG. A guide through present computational approaches for the identification of mammalian microRNA targets. Nat Methods 2006; 3: 881–6. [DOI] [PubMed] [Google Scholar]
- 30Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell 2003; 115: 787–98. [DOI] [PubMed] [Google Scholar]
- 31Zhao Y, Yao Y, Xu H, Lambeth L, Smith LP, Kgosana L, et al. A functional MicroRNA-155 ortholog encoded by the oncogenic Marek's disease virus. J Virol 2009; 83: 489–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Hutson SM, Sweatt AJ, Lanoue KF. Branched-chain [corrected] amino acid metabolism: implications for establishing safe intakes. J Nutr 2005; 135: 1557S–64S. [DOI] [PubMed] [Google Scholar]
- 33Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet 2011; 12: 846–60. [DOI] [PubMed] [Google Scholar]
- 34Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest 2005; 115: 565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Liu LC, Damman K, Lipsic E, Maass AH, Rienstra M, Westenbrink BD. Heart failure highlights in 2012-2013. Eur J Heart Fail 2014; 16: 122–32. [DOI] [PubMed] [Google Scholar]
- 36Mughal W, Kirshenbaum LA. Cell death signalling mechanisms in heart failure. Exp Clin Cardiol 2011; 16: 102–8. [PMC free article] [PubMed] [Google Scholar]
- 37Ong SB, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal 2013; 19: 400–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Gao D, Zhang L, Dhillon R, Hong TT, Shaw RM, Zhu J. Dynasore protects mitochondria and improves cardiac lusitropy in Langendorff perfused mouse heart. PLoS One 2013; 8: e 60967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Marin-Garcia J. Mitochondrial dysfunction in heart failure. J Am Coll Cardiol 2003; 41: 2299. [DOI] [PubMed] [Google Scholar]
- 40Broderick JA, Zamore PD. MicroRNA therapeutics. Gene Ther 2011; 18: 1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Chuang DT, Chuang JL, Wynn RM. Lessons from genetic disorders of branched-chain amino acid metabolism. J Nutr 2006; 136: 243S–9S. [DOI] [PubMed] [Google Scholar]
- 42Sitta A, Ribas GS, Mescka CP, Barschak AG, Wajner M, Vargas CR. Neurological damage in MSUD: the role of oxidative stress. Cell Mol Neurobiol 2014; 34: 157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Huang Y, Zhou M, Sun H, Wang Y. Branched-chain amino acid metabolism in heart disease: an epiphenomenon or a real culprit? Cardiovasc Res 2011; 90: 220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]