Abstract
Aim:
To investigate the mechanisms underlying the activation of ATP-sensitive potassium channels (KATP) by iptakalim in cultured rat mesenteric microvascular endothelial cells (MVECs).
Methods:
Whole-cell KATP currents were recorded in MVECs using automated patch clamp devices. Nucleotides (ATP, ADP and UDP) were added to the internal perfusion system, whereas other drugs were added to the cell suspension on NPC-1 borosilicate glass chips.
Results:
Application of iptakalim (10 and 100 μmol/L) significantly increased the whole-cell KATP currents, which were prevented by the specific KATP blocker glibenclamide (1.0 μmol/L). The opening of KATP channels by iptakalim depended upon the intracellular concentrations of ATP or NDPs: iptakalim activated KATP channels when the intracellular ATP or NDPs were at 100 or 1000 μmol/L, and was ineffective when the non-hydrolysable ATP analogue ATPγS (1000 μmol/L) was infused into the cells. In contrast, the KATP opener pinacidil activated KATP channels when the intracellular concentrations of ATP or NDPs ranged from 10 to 5000 μmol/L, and even ATPγS (1000 μmol/L) was infused into the cells.
Conclusion:
Iptakalim activates KATP channels in the endothelial cells of resistance blood vessels with a low metabolic status, and this activation is dependent on both ATP hydrolysis and ATP ligands.
Keywords: ATP-sensitive potassium channels, microvascular endothelial cells, iptakalim, pinacidil, glibenclamide, nucleotides, ATPγS, automated patch clamp
Introduction
ATP-sensitive potassium channels (KATP) link the electrical activity of the cell membrane to cellular metabolism. Because the activation of KATP is crucial for modulating the tone of the resistance vasculature, KATP channel openers (KCOs) have been used as important antihypertensives and have shown therapeutic efficacy1,2,3,4,5.
Iptakalim, a new antihypertensive drug, is a KCO with a unique chemical structure that preferentially relaxes resistance blood vessels without affecting capacitance blood vessels4. Endothelium-denuded arterioles have a very weak maximal iptakalim-mediated dilatatory response4. Therefore, it was reasonable to hypothesize that iptakalim can regulate microvascular tension by activating KATP channels in the endothelium of resistance blood vessels. In this study, KATP channel opening induced by iptakalim was investigated in microvascular endothelial cells (MVECs).
It has been reported that MVECs are sensitive to metabolic status, which is dependent on KATP channel activity. MVEC metabolic disorders have been observed in hypertension, and KATP channels can be activated in MVECs with a low metabolic status caused by elevated shear stress6,7,8,9,10,11. Iptakalim strongly lowers blood pressure in hypertensive rodents and humans but has little effect in normotensive rodents and humans. This selective antihypertensive action has not been observed for pinacidil, which is the most commonly studied KCO4.
A cell internal/external perfusion method was used to investigate the effects of iptakalim on KATP channels and the resultant modulation of metabolic status.
Materials and methods
Cell culture
The Local Ethics Committee on Animal Studies and the Academy of Military Medical Sciences (Beijing, China) approved this protocol. Eighty-eight Sprague–Dawley rats were used in this study (Experimental Animal Center, Academy of Military Medical Science, Beijing, China). MVECs were isolated from the mesentery of eight 6-day-old rats for each cell culture experiment, as previously described in the literature12,13. Briefly, the heart was perfused with phosphate buffered saline (PBS) to flush out the blood cells from the mesenteric vessels after the abdomen was opened. The mesenteric vascular bed was digested with an enzyme solution containing collagenase, papain, dithiothreitol, deoxyribonuclease, and bovine serum albumin for 1.0 h at 37 °C. The cells were then centrifuged and plated on a 60-cm2 culture plate coated with 0.5% gelatin in medium 199 supplemented with 10% fetal bovine serum (FBS), 1.0% heparin, 1.0% endothelial cell growth factor, and antibiotics. After 2.0 h, nonadherent cells were removed, and adherent endothelial cells were further cultured. Approximately 80% confluency was reached after the cells had been cultured for 4–5 d.
Platelet-endothelial cell adhesion molecule (PECAM/CD31) was identified by indirect immunofluorescence using a rabbit anti-rat CD31 antibody as the primary antibody and a fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G as the secondary antibody. More than 95% of the enriched cells cultured from the third passage were CD31+. Only cells from the third to fifth passages were used for the experiment to prevent cellular alterations as a result of additional passages.
Electrophysiology
Optimal results were obtained after splitting the cells every second or third day to avoid confluence. The confluence of MVECs at the time of harvesting was between 50% and 80%. For the automated patch clamp experiment, MVECs were washed twice with PBS (free of Ca2+ and Mg2+) and then dissociated with 2.0 mL of warmed 0.125% trypsin for 5.0 min at 37 °C. The cell suspension was then transferred to a 14-mL tube containing 9.0 mL FBS-containing medium and centrifuged at 100×g for 2.0 min at room temperature. After discarding the supernatant, the cell pellet was resuspended in an external solution at a final density between 1×106 and 5×107 cells/mL. A symmetrical high K+ condition was used to facilitate KATP activation14. The external solution for monitoring KATP activity consisted of the following (in mmol/L): 140.0 KCl, 1.0 CaCl2, 1.0 MgCl2, and 5.0 HEPES (pH 7.4 with KOH). The internal solution contained the following (in mmol/L): 140.0 KCl, 2.0 MgCl2, 5.0 EGTA, and 5.0 HEPES (pH 7.25). Nucleotides were dissolved in the internal solution, which contained 1.4 mmol/L free Mg2+ adjusted by MgCl2. To evaluate the influence of Mg2+ on drug efficacy, Mg2+ in the internal solution was omitted, and 5.0 mmol/L EDTA was added instead of EGTA. Nucleotide-containing solutions were freshly prepared on the day of the experiments. The pH of the solutions was readjusted after adding the nucleotide or drug.
Whole-cell patch clamp recordings were performed using a Port-a-Patch system driven by PatchControl software (Nanion Technologies, Munich, Germany) and a HEKA EPC-10 amplifier. Single-use NPC-1 chips with a resistance of 3.0–5.0 MΩ (Nanion Technologies, Munich, Germany) and a ground reference were used. Whole-cell signals were filtered at 2.0 kHz. Currents were recorded using the PatchMaster and FitMaster acquisition and analysis software (HEKA EleKtronik, Lambrecht, Germany) on a DELL Vostro 220 computer. The holding potential was −10 mV, which is similar to the resting membrane potential under symmetrical high K+ conditions. Currents were elicited by 500-ms depolarizing voltage steps from −100 to +50 mV in 10-mV increments. Whole-cell capacitance and series resistance were adjusted and monitored. A nucleotide-free internal solution was used to form the seal and record baseline currents. Control currents remained unchanged after 30 min (data not shown). In the internal perfusion experiments, stable currents were measured within several minutes of adding intracellular nucleotides to the internal perfusion system, and drugs were added to the cell suspension on the NPC-1 chip at 5.0 min during each recording. The summarized data were measured at −100 mV, and responses to iptakalim and nucleotides were expressed relative to the control currents measured in the external/internal solution without drugs or nucleotides.
Drug and solution
Iptakalim was synthesized by Thadweik Academy of Medicine, Beijing, China. All other chemicals were purchased from Sigma-Aldrich Corporation (St Louis, MO, USA). Glibenclamide and pinacidil were stored as a 10 mmol/L stock solution in dimethyl sulfoxide. Other stock solutions were prepared in distilled water. Stock solutions were frozen at −20 °C. Prior to usage, they were diluted in the internal or external solution to produce the desired concentration.
Statistical analysis
Data are expressed as the mean±standard deviation (SD), and n indicates the number of cells under each experimental condition (each column). Significance was assessed using a two-tailed Student's t-test to compare different groups. The statistical analyses were performed using SPSS 13.0 (SPSS Inc, Chicago, IL, USA), and P<0.05 was considered statistically significant.
Results
The opening effects of iptakalim
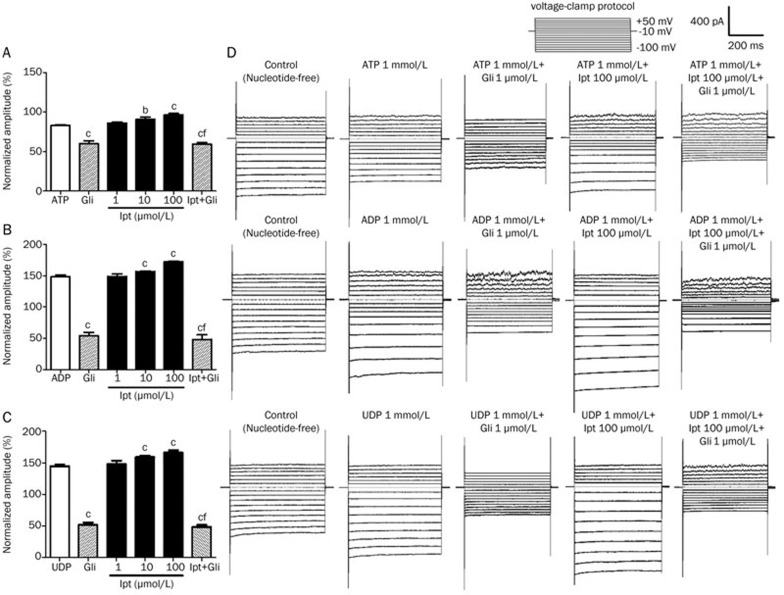
The KATP currents of MVECs were recorded by whole-cell patch clamp with a membrane potential of −100 mV (Figure 1). Whole-cell currents were suppressed by application of 1000 μmol/L intracellular ATP. These currents were enhanced with 1000 μmol/L intracellular ADP or UDP. This result indicates that the toggled channels were in fact KATP channels. In the presence of 1 mmol/L of ATP, ADP, or UDP, 1.0−100 μmol/L iptakalim increases the whole-cell currents. This can be prevented by application of 1.0 μmol/L glibenclamide, a specific KATP blocker. The currents of the control groups, in the presence of only 1000 μmol/L of ATP, ADP, or UDP, also decreased with application of 1.0 μmol/L glibenclamide. The currents decreased by a similar amount to that in groups treated with 100 μmol/L iptakalim, which suggests that the iptakalim-induced currents were completely blocked by 1.0 μmol/L glibenclamide.
Figure 1.
Effects of iptakalim on the KATP currents of microvascular endothelial cells (MVECs). The effects of iptakalim (Ipt) on the KATP currents of MVECs were tested using whole-cell recordings with a membrane potential of −100 mV. Cells were incubated with 1.0–100 μmol/L Ipt, and 1000 μmol/L of a nucleotide (ATP, ADP, or UDP) was given by internal perfusion. The whole-cell currents increased significantly with 10 or 100 μmol/L Ipt in the presence of 1000 μmol/L ATP (A), ADP (B), or UDP (C). The effect was reversed with 1.0 μmol/L glibenclamide (Gli). bP<0.05, cP<0.01 vs ATP, ADP, or UDP group. fP<0.01 vs 100 μmol/L Ipt group (n=8). (D) Typical recordings showed the activation of KATP channels by Ipt.
The modulatory effects of ATP
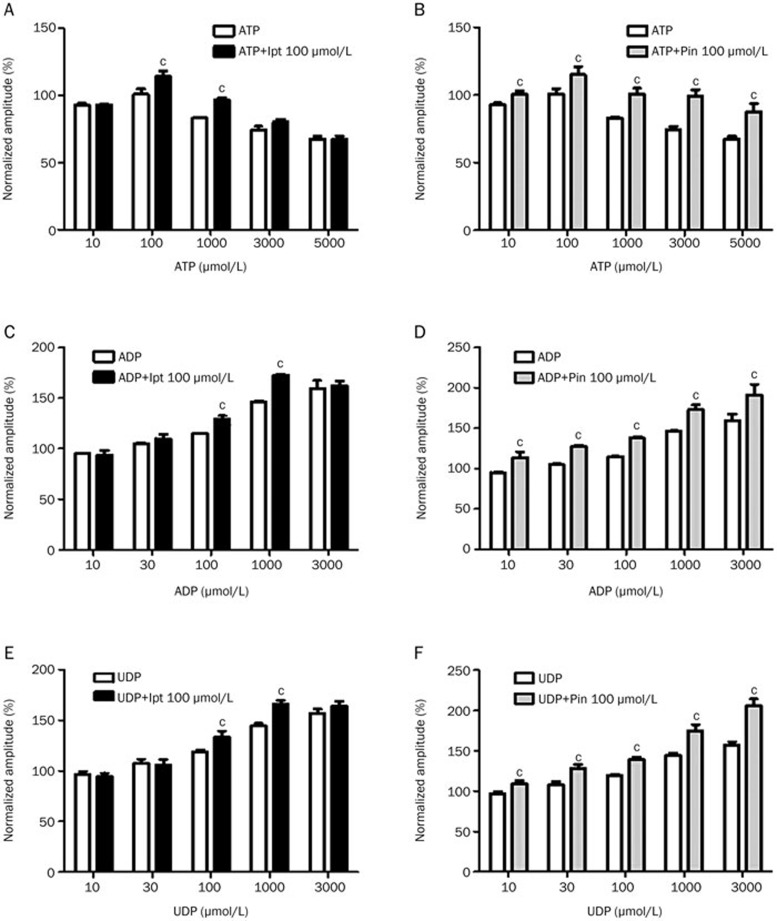
The modulatory effects of intracellular ATP on the channel-opening ability of 100 μmol/L iptakalim or 100 μmol/L pinacidil were investigated. Intracellular ATP was added at 10, 100, 1000, 3000, or 5000 μmol/L, and the resultant current amplitudes were 92.42%±1.48%, 100.67%±3.87%, 83.1%±0.55%, 74.08%±2.69% and 67.3%±2.02% of the control amplitude, respectively. These results suggest that MVEC whole-cell currents can be regulated by intracellular ATP. The channel-opening effects of iptakalim were observed in the presence of 100 or 1000 μmol/L ATP (P<0.01). However, iptakalim was not shown to activate KATP channels in the presence of 10, 3000 or 5000 μmol/L intracellular ATP (Figure 2A). Under the same experimental conditions, the opening effects of pinacidil were observed in the presence of 10 to 5000 μmol/L ATP (Figure 2B, P<0.01).
Figure 2.
Modulation of the KATP opening effects of iptakalim and pinacidil in microvascular endothelial cells (MVECs) by nucleotides. The KATP currents were recorded in MVECs with a membrane potential of −100 mV. Cells were incubated with 100 μmol/L iptakalim (Ipt) or pinacidil (Pin) and nucleotides (ATP, ADP or UDP) at concentrations ranging from 10 to 3000 or 5000 μmol/L. n=8. cP<0.01 vs control.
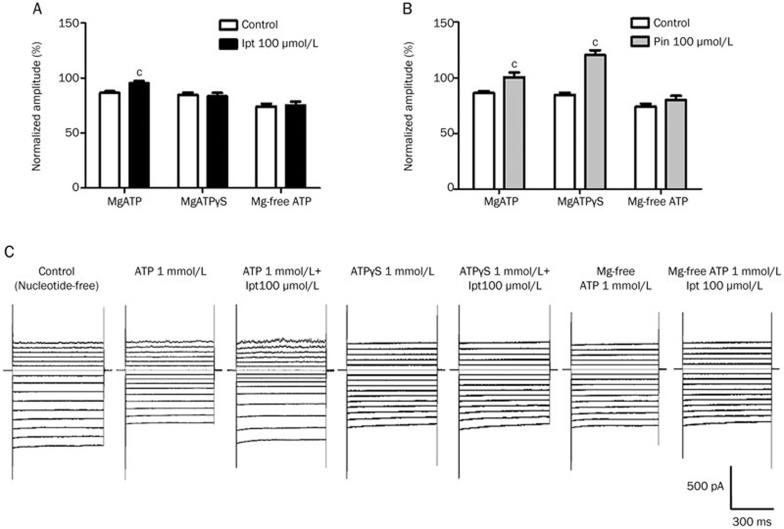
The mechanism by which ATP modulates the KATP opening effects of iptakalim was also investigated. The opening effects of iptakalim and pinacidil were compared in the presence of ATP with Mg2+, ATP without Mg2+, and ATPγS (a nonhydrolysable ATP analogue) with Mg2+. Pinacidil activated KATP in the presence of ATPγS, but iptakalim did not. Both iptakalim and pinacidil were Mg2+ dependent. These results suggest that ATP hydrolysis was necessary for iptakalim to activate KATP channels and that ATP ligands are involved in KATP activation by pinacidil (Figure 3).
Figure 3.
Modulation of the opening effects of iptakalim and pinacidil in microvascular endothelial cells (MVECs) by ATP. The opening effects of 100 μmol/L iptakalim (Ipt, A) or pinacidil (Pin, B) were tested in the presence of MgATP, MgATPγS, or Mg-free ATP (1 mmol/L). The KATP currents were recorded in MVECs with a membrane potential of −100 mV. n=8. cP<0.01 vs control. (C) Typical recordings showed the modulatory effects of ATP on iptakalim-mediated activation.
The modulatory effects of NDPs
As shown in Figure 2C and E, the opening effects of iptakalim were induced by NDPs, such as ADP and UDP, at concentrations of 100 and 1000 μmol/L concentrations but not at 10, 30, or 3000 μmol/L. This was not the case for pinacidil. The channel-opening effects of pinacidil were induced by ADP or UDP at all tested concentrations (10–3000 μmol/L), which is a larger range of activation than that of iptakalim (Figures 2D, F).
Discussion
In our previous research, multiple pharmacological inhibitors were used to explore the signaling pathways underlying the action of iptakalim on mesenteric arterioles. Iptakalim-induced vasodilation was not affected by the K+ channel blockers Ba2+, 5-HD, TEA, and 4-AP, but it was inhibited by glibenclamide (1.0–1000 μmol/L) in a dose-dependent fashion (unpublished data). The electrophysiology method was used to confirm the direct activation of KATP channels in mesenteric endothelium in this study.
The KATP channel activities were tested on MVECs using whole-cell recordings. In recordings using a nucleotide-free internal solution, some portion of KATP was activated. This partially activated state was observed at two stages of the experiment—suppression after the addition of intracellular ATP, and enhancement after the addition of NDPs. Neither iptakalim nor pinacidil had an effect on cells in the partially activated state under nucleotide-free conditions (data not shown); therefore, it was reasonable to speculate that the KATP opening effects of iptakalim and pinacidil are dependent on the presence of intracellular nucleotides. As expected, both drugs caused a significant increase in membrane conductance in the presence of nucleotides. When intracellular nucleotides (ATP, ADP, or UDP) were infused into the MVECs at a concentration of 1000 μmol/L, iptakalim increased whole-cell currents in a dose-dependent fashion. That result was reversed with the addition of 1.0 μmol/L glibenclamide (Figure 1). This finding provides evidence that iptakalim can activate KATP channels in MVECs.
The modulatory effects of intracellular nucleotides were further tested in this study. The required nucleotide concentration for iptakalim was found to be different from that observed for pinacidil. Iptakalim activated KATP channels only at low concentrations of ATP or NDPs (100–1000 μmol/L), but pinacidil was effective at all tested ATP or NDP concentrations, even those <100 μmol/L or >1000 μmol/L (Figure 2). Several studies have shown that a complicated interaction exists between the functional sites of KATP15. ATP and NDPs are major allosteric modulators for KATP channels and affect drug binding differently16,17,18,19,20,21,22. Allosteric regulation by nucleotides makes the KATP opener binding site accessible to iptakalim or pinacidil. Iptakalim attaches to the binding site in a different way than pinacidil does because of differences in chemical structure1, and iptakalim shows a higher selectivity than pinacidil for nucleotide supplements of MVECs.
The differences between iptakalim and pinacidil in terms of the modulatory effects of ATP were further compared in this experiment (Figure 3). Iptakalim's ineffectiveness in the presence of ATPγS suggests that ATP hydrolysis is required to activate KATP channels in MVECs. The opening effects of pinacidil are more potent in the presence of ATPγS than in the presence of ATP (120.45±4.36 vs 100.61±4.17, P<0.05, Figure 3B), which suggests that ATP ligands are required for pinacidil activation. ATP binding alone appears to be sufficient for pinacidil to activate KATP channels; however, for iptakalim, ATP binding with subsequent hydrolysis is necessary.
KATP channel participates in metabolism-related vascular responses23,24. In studies involving endothelial cells, it was shown that one of its main physiological functions is to serve as a metabolic barrier. The physiological concentration of ATP ranges from 3.0 to 5.0 mmol/L in rats. Intracellular NDPs play a critical role in the regulation of cellular energy status25. When MVECs were internally perfused with ATP at physiological or extremely low concentrations, pinacidil, but not iptakalim, affected the KATP channels. This finding indicates that iptakalim might preferentially activate KATP in cells with a specific pathological metabolic status. Endothelial cells and KATP functions are altered by hypertension26,27, and pathological changes in hypertension result in an energy metabolism disorder in endothelial cells6,7,8,9,10. This selectivity of cell energy status by iptakalim might be responsible for its targeted hypertension therapeutic effect. Pinacidil, but not iptakalim, activates KATP in the presence of NDPs at high concentrations (3.0 mmol/L), which indicates that iptakalim might be more suitable for mild-to-moderate hypertension. Pinacidil was shown to be non-selective for nucleotide supplements in our research. It was effective under both physiological and pathological conditions, which might be the underlying cause of its side effects28. Our results offer a new perspective: activation by iptakalim might link functional changes in EC to the perturbation of normal metabolic supplies and the consequent changes in the intracellular concentration of nucleotides. It is reasonable to presume that because of its unique effects, iptakalim might have an advantage in endothelial metabolism-related cardiovascular diseases, such as hypertension and pulmonary hypertension.
Seal formation was particularly difficult in whole-cell recordings of MVECs because of their small size and membrane properties. The internal perfusion method using NPC-1 borosilicate glass chips with a 1.0 μm aperture provided electrophysiological access not only to facilitate seal formation but also to maximize the duration of high-quality recordings. In recent years, studies using patch clamp chips have stressed the advantages of enhanced resolution29. Because of its stable and steady nature, the method can be used successfully in whole-cell recordings of MVECs.
Perspectives
The functions of endothelial KATP in resistance blood vessels can be altered by exposure to elevated shear stress caused by hypertension. The pharmacological characteristics of iptakalim are related to endothelial cell metabolic status, which explains its highly selective antihypertensive activity. The clinical evaluation of iptakalim in hypertension and pulmonary hypertension is ongoing, and iptakalim could become a new generation antihypertensive drug.
Abbreviations
4-AP, 4-aminopyridine; 5-HD, 5-hydroxydecanote; ADP, adenosine diphosphate; ATP, adenosine triphosphate; ATPγS, adenosine 5′-O-(3-thiotriphosphate); EDTA, ethylenediaminetetraacetic acid; EGTA, ethylene glycol tetraacetic acid; FBS, fetal bovine serum; Gli, glibenclamide; Ipt, iptakalim; KATP, ATP-sensitive potassium channel; MVEC, microvascular endothelial cell; PBS, phosphate buffered saline; PECAM/CD31, platelet-endothelial cell adhesion molecule; Pin, pinacidil; TEA, tetraethylammonium; UDP, uridine diphosphate.
Author contribution
Hai WANG designed the study; Su-yang WANG performed the experiments; Su-yang WANG and Wen-yu CUI analyzed the data and wrote the paper.
Acknowledgments
This work was supported by grants from the National Basic Research “973” Program (Grant No 2012CB518200 and JCKY2013000B001) and the State Key Research Project of China (Grant No AWS11J003).
References
- 1Wang H. Cardiovascular ATP-sensitive K+ channels as a new molecular target for development of antihypertensive drugs. Acta Pharmacol Sin 1998; 19: 397–402. [PubMed] [Google Scholar]
- 2Wang H, Zhang YL, Chen YP. Targeting small arteries of hypertensive status with novel ATP-sensitive potassium channel openers. Curr Vasc Pharmacol 2005; 3: 119–24. [DOI] [PubMed] [Google Scholar]
- 3Wang H, Tang Y, Wang L, Long CL, Zhang YL. ATP-sensitive potassium channel openers and 2,3-dimethyl-2-butylamine derivatives. Curr Med Chem 2007; 14: 133–55. [DOI] [PubMed] [Google Scholar]
- 4Pan Z, Huang J, Cui W, Long C, Zhang Y, Wang H. Targeting hypertension with a new adenosine triphosphate-sensitive potassium channel opener iptakalim. J Cardiovasc Pharmacol 2010; 56: 215–28. [DOI] [PubMed] [Google Scholar]
- 5Jackson WF. Ion channels and vascular tone. Hypertension 2000; 35: 173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Barakat AI, Lieu DK, Gojova A. Ion channels in shear stress sensing in vascular endothelium. Molecular Sensors for Cardiovascular Homeostasis. New York: Springer US; 2007. p155–170.
- 7Title LM, Lonn E, Charbonneau F, Fung M, Mather KJ, Verma S, et al. Relationship between brachial artery flow-mediated dilatation, hyperemic shear stress, and the metabolic syndrome. Vascular Med 2008; 13: 263–70. [DOI] [PubMed] [Google Scholar]
- 8Doddaballapur A, Hergenreider E, Houtkooper RH, et al. Shear stress-induced Krúppel-like factor 2 reduces endothelial metabolic activity: interaction between endothelial metabolism and cellular quiescence. Circulation 2012; 21: A 15285. [Google Scholar]
- 9Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 1986; 8: 37–44. [DOI] [PubMed] [Google Scholar]
- 10Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension 1998; 31: 162–9. [DOI] [PubMed] [Google Scholar]
- 11Chatterjee S, Levitan I, Wei Z, Fisher AB. KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation 2006; 13: 633–44. [DOI] [PubMed] [Google Scholar]
- 12Ashley RA, Dubuque SH, Dvorak B, Woodward SS, Williams SK, Kling PJ. Erythropoietin stimulates vasculogenesis in neonatal rat mesenteric microvascular endothelial cells. Pediatr Res 2002; 51: 472–8. [DOI] [PubMed] [Google Scholar]
- 13Snead MD, Papapetropoulos A, Carrier GO, Catravas JD. Isolation and culture of endothelial cells from mesenteric vascular bed. Methods Cell Sci 1996; 17: 257–62. [Google Scholar]
- 14Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 1983; 305: 147–8. [DOI] [PubMed] [Google Scholar]
- 15Findlay I. Interactive regulation of the ATP-sensitive potassium channel of cardiac muscle. J Cardiovasc Pharmacol 1994; 24 (Suppl 4): S6. [PubMed] [Google Scholar]
- 16Zhu QL, He HM, Xiao WB, Wang H. Modulation by nucleotides of binding sites for [3H] glibenclamide in rat aorta and cardiac ventricular membranes. J Cardiovasc Pharmacol 2001; 37: 522–31. [DOI] [PubMed] [Google Scholar]
- 17Shen WK, Tung RT, Kurachi Y. Activation of the cardiac ATP-sensitive K+ channel by ER-001533, a newly synthesized vasorelaxant. Circ Res 1992; 70: 1054. [DOI] [PubMed] [Google Scholar]
- 18Terzic A, Jahangir A, Kurachi Y. HOE-234, a second generation K+ channel opener, antagonizes the ATP-dependent gating of cardiac ATP-sensitive K+ channels. J Pharmacol Exp Ther 1994; 268: 818. [PubMed] [Google Scholar]
- 19Terzic A, Findlay I, Hosoya Y, Kurachi Y. Dualistic behavior of ATP-sensitive K+ channels towards intracellular nucleoside diphosphates. Neuron 1994; 12: 1049. [DOI] [PubMed] [Google Scholar]
- 20Thuringer D, Cavero I, Coraboeuf E. Time-dependent fading of the activation of KATP channels, induced by aprikalim activated by lemakalim and nucleotides, in excised membrane patches from cardiac myocytes. Br J Pharmacol 1995; 115: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Shen WK, Tung RT, Machulda MM, Kurachi Y. Essential role of nucleotide diphosphates in nicorandil-mediated activation of cardiac ATP-sensitive K+ channel. A comparison with pinacidil and lemakalim. Circ Res 1991; 69: 1152. [DOI] [PubMed] [Google Scholar]
- 22Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 1997; 77: 1165. [DOI] [PubMed] [Google Scholar]
- 23Nichols CG, lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol 1991; 261: H1675. [DOI] [PubMed] [Google Scholar]
- 24Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Günther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science 1990; 247: 1341–3. [DOI] [PubMed] [Google Scholar]
- 25Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem 1994; 140: 1–22. [DOI] [PubMed] [Google Scholar]
- 26Chatterjee S, Al-Mehdi AB, Levitan I, Stevens T, Fisher AB. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol 2003; 285: C959–967. [DOI] [PubMed] [Google Scholar]
- 27Ghosh M, Hanna ST, Wang R, McNeil JR. Altered vascular reactivity and KATP channel currents in vascular smooth muscle cells from deoxycorticosterone acetate (DOCA)-salt hypertensive rats. J Cardiovasc Pharmacol 2004; 44: 525–31. [DOI] [PubMed] [Google Scholar]
- 28Goldberg MR, Sushak CS, Rockhold FW, Thompson WL. Vasodilator monotherapy in the treatment of hypertension: comparative efficacy and safety of pinacidil, a potassium channel opener, and prazosin. Clin Pharmacol Ther 1988; 44: 78–92. [DOI] [PubMed] [Google Scholar]
- 29Sigworth FJ, Klemic KG. Microchip technology in ion-channel research. IEEE Trans Nanobiosci 2005; 4: 121–7. [DOI] [PubMed] [Google Scholar]