Abstract
Aim:
Xuezhikang (XZK), an extract of red yeast rice, has been widely used in traditional Chinese medicine to treat cardiovascular disease. Three fractions F1, F2 and F3 (primarily containing isoflavones, monacolins or phytosterols, respectively) are extracted from Xuezhikang capsules. In this study we evaluated the lipid-lowering effects of these fractions and explored the potential mechanisms of actions.
Methods:
Mice treated with a high-fat diet (HFD) were orally adminis¬tered lovastatin (10 mg·kg−1·d−1), XZK (1200 mg·kg−1·d−1), F1 (27.5 mg·kg−1·d−1), F2 (11.3 mg·kg−1·d−1) or F3 (35 mg·kg−1·d−1) for 10 weeks. Lipids were measured using commercial enzymatic kits, and the mRNA and protein levels of genes involved in cholesterol and bile acid homeostasis were evaluated using qRT-PCR and Western blot analysis, respectively.
Results:
XZK increased the fecal excretion of lipids and bile acids, reduced serum TC, TG and LDL-C levels by 40%, 55% and 46%, respectively, and increased serum HDL-C by 31%. Administration of F1 repressed serum TC and TG by 24% and 52%, respectively, and elevated hepatic synthesis of CYP7A1. It also increased hepatic elimination of bile acids in the fecal excretions by 79% through upregulating BSEP and downregulating NTCP. Administration of F3 decreased serum TC, TG and LDL-C levels by 33%, 29% and 39%, respectively, and increased serum HDL-C by 28%, sig¬nificantly reduced intestinal absorption of cholesterol by inhibiting the transcription of NPC1L1, and elevated excretion of TC, FC and CE by 96%, 72% and 101%, respectively. Administration of F2 showed pharmacological effects similar to those of lovastatin.
Conclusion:
Isoflavones and phytosterols in XZK exert cholesterol-lowering effects in HFD mice through mechanisms that differ from those of lovastatin. Isoflavones and phytosterols act in a complimentary manner: through enhancing the elimination of bile acids and reducing intestinal cholesterol absorption, respectively.
Keywords: hyperlipidemia, cholesterol-lowering medication, red yeast rice, Xuezhikang, lovastatin, isoflavones, phytosterols, cholesterol, bile acids, traditional Chinese medicine
Introduction
Despite efforts to fight cardiovascular disease (CVD), it remains the leading cause of morbidity and mortality in China and abroad. Hyperlipidemia is considered to be the major contributing factor to CVD1,2. Diet, exercise and medication are seen as the key factors that regulate the serum lipid profile. Several types of cholesterol-lowering medications, including statins, are available on the market and are taken alone or in combination with other medications. However, reported increases in the clinical side effects of these medications have made further investigations into complementary or alternative therapies necessary. The use of functional foods, for example, has attracted a great deal of interest as a possible alternative therapy for lowering plasma cholesterol.
Red yeast rice (RYR), a fermented rice generally produced using a specific strain of red yeast called Monascus purpureus, has been used as a food supplement to improve blood circulation and decrease levels of serum cholesterol3,4,5,6,7,8,9,10. Monacolin K, also known as lovastatin, is the conserved, active component of RYR11,12,13. In addition to Monacolin K, RYR also contains other monacolins and pigments, unsaturated fatty acids, plant sterols, amino acids, isoflavones, alkaloids and trace elements13,14,15,16,17. Interestingly, RYR, which contains approximately 2–7.5 mg of lovastatin, lowers cholesterol with an efficacy equivalent to 20 mg of administered lovastatin7,18,19,20,21. This data suggests that other components in RYR may exert an additive and/or synergistic pharmacological effect in combination with lovastatin17.
Xuezhikang, an extract of red yeast rice, has been widely used in traditional Chinese medicine as a functional food to treat patients with cardiovascular disease for more than two thousand years. Today, Xuezhikang is recommended for the treatment of dyslipidemia22,23. Clinical benefits of Xuezhikang use have also been documented in coronary heart disease (CHD) patients who received medication combined with Xuezhikang to treat dyslipidemia in randomized controlled trials24,25. Clinical trials have also shown that Xuezhikang has anti-inflammatory activity26 and improves endothelial function27.
The majority of cholesterol in the body is synthesized in the liver. The molecule HMG-CoAR is known to carry out the rate-limiting step for this catalysis reaction18. Intestinal absorption of exogenous cholesterol, mediated by NPC1L1, may be another source21. When the cholesterol level in an organism becomes too high, excess cholesterol is actively taken up into the liver by SR-BI28,29, a receptor for HDL. Cholesterol is then converted into bile acid in the liver by CYP7A130,31,32. Together, BSEP33,34 and NTCP35,36 mediate the biliary excretion and reabsorption of bile acids.
Although there have been several studies reporting on the efficacy and mechanisms of RYR, few have provided information regarding whether other components in RYR might have additive pharmacological effects. In addition, the specific mechanisms engaged by RYR to lower cholesterol, either through an influence on cholesterol or bile acid homeostasis, remains unclear, as does the contribution of other components involved in such mechanisms. To address this question, Xuezhikang was examined in the present study. Three fractions enriched in isoflavones, monacolins or phytosterols were isolated according to their different polarities. A hypercholesterolemic mouse model was then used to explore their hypolipidemic activity and potential mechanisms of action.
Materials and methods
Extraction of XZK
XZK powder was provided by WBL Peking University Biotech Co, Beijing, China. Crude XZK powder (100.0 g) was extracted twice with 95% ethanol (1000 mL each). After reflux, the pooled solution was filtered and concentrated to give a primary extract weight of 6.7 g.
The primary extract was dissolved in 25% ethanol (30 times volume) to form the sample solution. Wet, pretreated resin (bed volume (BV) 1600 mL) was packed in the glass column. The adsorption process was conducted by loading the sample solution onto the resin overnight. The adsorbate-laden column was then eluted with an ethanol–water gradient solution at a flow rate of 25 mL/90 s. The gradient elution was carried out as follows: first, the adsorbate-laden column was washed with distilled water (5 BV) and eluted with ethanol–water of different ratios (30/70, 50/50, 70/30, 90/10, 95/5, v/v) and with different volumes (6, 8, 8, 8, 8 BV), successively. After identification by HPLC (Shimadzu, Kyoto, Japan), three fractions containing isoflavones (F1), monacolins (F2) and sterols (F3) were obtained, with a yield of 34%, 14% and 44%, respectively, relative to the primary extract.
Diets
Diets were purchased from TROPHIC Animal Feed High-tech Co, Ltd, Nantong, China. The control diet (Control) was prepared by mixing the following ingredients (g/kg diet): casein, 140; corn starch, 465.69; maltodextrin, 155; sucrose, 100; soybean oil, 40; cellulose, 59; mineral mix, 35; vitamin mix, 10; L-cystine, 1.8; choline bitartrate, 2.5; and TBHQ, 0.008. The high fat diet (HFD) was as follows (g/kg diet): casein, 198.21; maltodextrin, 219.99; sucrose, 90.63; soybean oil, 34.04; lard, 306.37; cellulose, 70.79; mineral mix, 49.55; vitamin mix, 14.16; L-cystine, 2.55; choline bitartrate, 3.54; TBHQ, 0.068; and cholesterol, 10.
Animals and experiments
Six-week-old specific-pathogen-free (SPF) male C57BL/6 mice were purchased from the Animal Center of Nanjing Medical University. Research on animals was approved by the Animal Experimental Ethical Committee of China Pharmaceutical University. All mice were housed at 25 °C in a 12-h light-dark cycle. Mice were fed a control diet for 1 week before the experiment. Fifty-six (18±1 g) male mice were randomly divided into seven groups (n=8): control group (Control), high-fat diet group (HFD), HFD+lovastatin (Lv, 10 mg/kg), HFD+XZK (1200 mg/kg, equivalent to 10 mg/kg of lovastatin), HFD+Fraction 1 (F1, 27.5 mg/kg), HFD+Fraction 2 (F2, 11.3 mg/kg), and HFD+Fraction 3 (F3, 35.0 mg/kg). Mice were treated according to their group assignment for the following 10 weeks. Mice were provided feed and tap water ad libitum. Food intake was measured and body weight was recorded once per week.
Food was removed 12 h before the end of the experiment. Mice were then sacrificed, and serum/tissue samples were collected. Blood was obtained from the retinal vein and was centrifuged at 5000×g for 10 min at 4 °C after coagulation. Serum was collected for determination of lipids and lipoproteins. The liver and intestine were immediately removed, rinsed with saline solution, weighed and stored in liquid nitrogen until used to measure lipids, or for assays for gene or protein expression. One part of the liver was carefully removed, rinsed several times with saline solution to eliminate any blood, and subsequently immersed in 10% formalin stock solution for histological analysis. Fecal pellets were collected during the last week immediately prior to the end of the experiment and stored at −80 °C until analysis.
Determination of lipids in serum, liver and feces
Serum lipid levels were determined using commercial enzymatic kits (Biosino Bio-Technology & Science Inc, Beijing, China) for markers including total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). TC, free cholesterol (FC) and cholesterol ester (CE) concentrations in liver and fecal samples were determined using commercial kits (Applygen Technologies Inc, Beijing, China).
Determination of bile acid pool in liver and feces, fecal bile acids composition
To measure bile acid pool size, total bile acids were extracted from liver and feces and quantified using a commercial assay kit (Jiancheng Bioengineering Institute, Nanjing, China).
To determine the bile acid composition in fecal pellets, samples were extracted and measured with an LC-MS/MS API4000 and an Agilent ZORBAX SB-Aq column (2.1×150 mm, 3.5 μm), as has been previously described37.
Histology of the liver
Liver tissue was collected to investigate changes in histology. After fixation in 10% formalin, tissue was embedded in paraffin and a small section was subjected to hematoxylin and eosin (H&E) staining. For hepatic lipid staining, frozen liver tissues were cryosectioned, briefly fixed in 10% formalin, and stained with Oil Red O.
Real-time PCR analyses
Total RNA was extracted from liver using a TRIzol plus RNA purification kit (Invitrogen, Life Technologies, USA) according to the manufacturer's instruction. First strand cDNA was synthesized from RNA using a PrimeScript™ 1st Strand cDNA Synthesis Kit (TaKaRa Bio Inc, Japan). mRNA levels were determined by quantitative reverse transcription (qRT)-PCR using SYBR Premix Ex Taq™ (TaKaRa Bio Inc, Japan) in a Bio-Rad real-time PCR machine. Initial denaturation at 95 °C for 5 min was followed with 40 cycles of 94 °C for 15 s, 60 °C for 15 s, and 68 °C for 30 s. The sequences of the primers used for this study are shown in Table S1. Results were normalized to GAPDH expression.
Western blot analysis
Total proteins from the liver and ileum were extracted according to a previously described method38. Membrane and cytoplasm proteins were electrophoresed by 8% SDS-PAGE and then transferred to a 0.22 μm polyvinylidene difluoride membrane. After blocking in 5% (w/v) nonfat milk, membranes were incubated with anti-SR-BI antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-HMG-CoAR antibody (Upstate USA, Lake Placid, NY, USA), anti-CYP7A1 (Santa Cruz Biotechnology), anti-CYP27A1 or anti-GAPDH antibody (Santa Cruz Biotechnology, Santa Cruz, USA) overnight at 4 °C. After a wash step, blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG for 1 h at room temperature. After washing in TBS-T, signals were detected using an ECL enhanced chemiluminescence agent (Millipore Corporation, USA). Densitometry was quantified using BioRad Quantity One software. The SR-BI, CYP7A1 and CYP27A1 abundance values were normalized to GAPDH.
Statistical analysis
Data were expressed as the mean±standard deviation (SD). Statistical significance of differences was evaluated using Student's t-test and significance was set at P<0.05.
Results
Body weight and food intake
The body weight and food intake (Table S2) of mice in the HFD group were significantly higher than those of the Control group (P<0.05). Lv had no effect on body weight and food intake of mice compared to the HFD group.
XZK repressed the HFD-induced elevation of body weight (P<0.01) without affecting food intake compared with HFD group (P>0.05).
F1 and F3 showed similar effects to the HFD+XZK group.
F2 had no effect on body weight and food intake compared with the HFD group.
Effects of XZK on serum lipids and atherogenic indices
Significant increases were observed in the HFD group in serum TC (by 84%) and LDL-C (by 95%), compared to the Control group (P<0.05, Figure 1). There was a slight, but not significant, increase in serum HDL-C and TG in the HFD group (P>0.05). The ratio of LDL to HDL-C was clearly increased by 1.9-fold and the HDL-C to TC ratio was significantly decreased (P<0.01) in the HFD group compared to the Control group (Table S3).
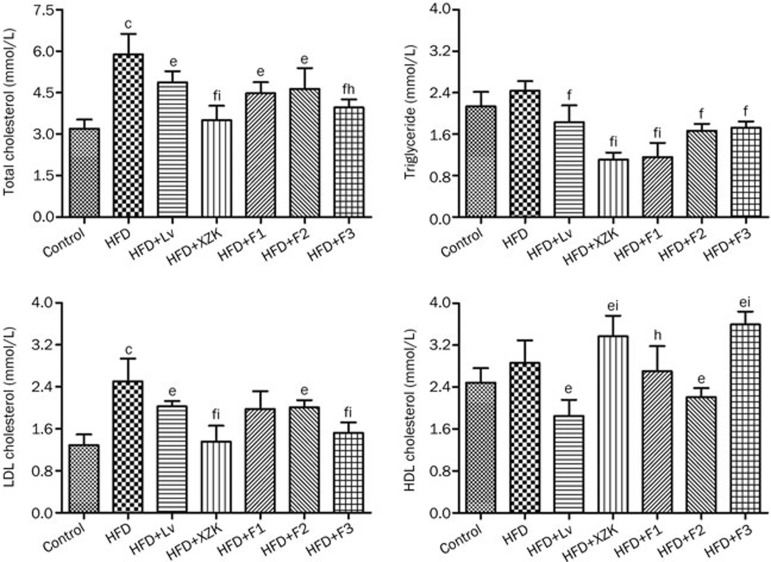
Figure 1.
Quantification of serum cholesterol (total, LDL, HDL) and triglyceride in high fat diet-treated C57BL/6 male mice after oral administration of lovastatin (Lv) 10 mg/kg, Xuezhikang (XZK) 1200 mg/kg, F1 (isoflavones) 27.5 mg/kg, F2 (monacolins) 11.3 mg/kg, or F3 (sterols) 35.0 mg/kg for 10 weeks, respectively. Mean±SD. n=8. cP<0.01 vs Control; eP<0.05, fP<0.01 vs HFD. hP<0.05, iP< 0.01 vs HFD+Lv. HFD, high fat diet.
Lv strongly reduced serum TG and HDL-C levels by 25% and 33%, respectively, and modestly reduced serum TC and LDL-C levels by 22% and 19%, respectively, compared to the HFD group (Figure 1). As expected, the LDL-C/HDL-C ratio was significantly improved following exposure to Lv (P<0.05, Table S3).
XZK strongly inhibited the increase in TC, TG, LDL-C levels induced by HFD by 40%, 55% and 46%, respectively, compared to the HFD-only group (Figure 1). XZK also significantly increased serum levels of HDL-C by 31%. XZK dramatically lowered the LDL-C/HDL-C and TG/HDL-C ratios (P<0.05, Table S3) and increased the HDL-C/TC ratio (P<0.01).
F1 significantly repressed TC and TG levels by 24% and 52%, respectively, in comparison to the HFD group (Figure 1). F1 also lowered the LDL-C/HDL-C and TG/HDL-C ratios (P<0.05, Table S3).
Effects on serum levels of TC, LDL-C, TG and HDL-C in the HFD+F2 group were similar to those observed in the HFD+Lv group.
F3 not only significantly decreased serum levels of TC, TG and LDL-C by 33%, 29% and 39%, respectively; it also increased serum HDL-C levels by 28% (Figure 1) and significantly lowered the LDL-C/HDL-C and TG/HDL-C ratios (P<0.05, Table S3). An increased HDL-C/TC ratio was observed in the HFD+F3 groups (P<0.01).
Hepatic lipids and histopathology
The HFD treatment resulted in significant liver weight gain (P<0.01, Table S2) and greater lipid accumulation compared with the Control group (Figure 2). HFD significantly increased serum levels of hepatic TC, FC and CE by 116%, 83% and 167%, respectively, compared to the Control group (Figure 3A).
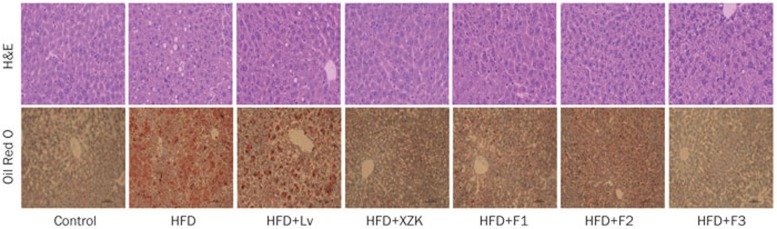
Figure 2.
H&E staining and Oil Red O staining of liver sections from high fat diet-treated C57BL/6 male mice after oral administration of lovastatin (Lv) 10 mg/kg, Xuezhikang (XZK) 1200 mg/kg, F1 (isoflavones) 27.5 mg/kg, F2 (monacolins) 11.3 mg/kg, or F3 (sterols) 35.0 mg/kg for 10 weeks, respectively (200×).
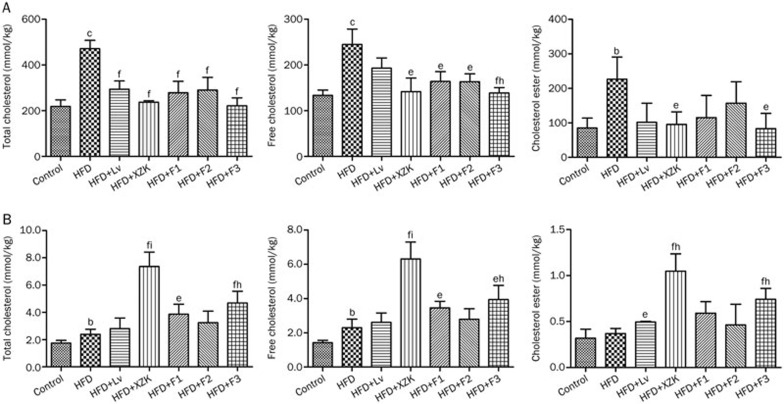
Figure 3.
Hepatic and fecal lipid concentrations in high fat diet-treated C57BL/6 male mice after oral administration of lovastatin (Lv) 10 mg/kg, Xuezhikang (XZK) 1200 mg/kg, F1 (isoflavones) 27.5 mg/kg, F2 (monacolins) 11.3 mg/kg, or F3 (sterols) 35.0 mg/kg for 10 weeks, respectively. (A) Hepatic lipids; (B) Fecal lipids. Mean±SD. n=8. bP<0.05, cP<0.01 vs Control; eP<0.05, fP<0.01 vs HFD; hP<0.05, iP<0.01 vs HFD+Lv. HFD, high fat diet.
Compared with the HFD group, the Lv group exhibited significantly decreased liver weight (P<0.01, Table S2). Lipid accumulation was improved slightly in livers from HFD+LV mice (Figure 2). Lv caused a remarkable decrease in TC hepatic levels (by 38%) and a slight reduction in FC and CE hepatic levels (Figure 3A).
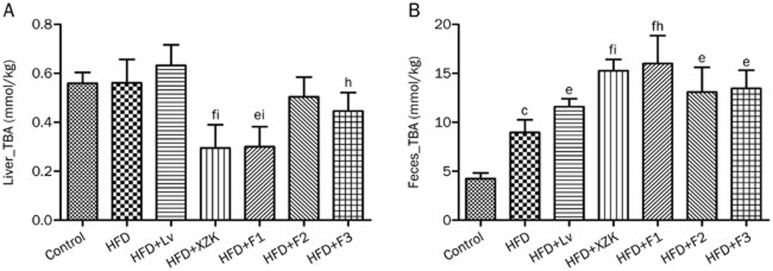
XZK repressed the HFD-induced increase in liver weight (P<0.01, Table S2). Livers collected from the HFD+XZK group appeared normal (Figure 2). XZK significantly reduced HFD-induced increases in TC, FC and CE serum levels by 50%, 42% and 58%, respectively (Figure 3A). XZK also lowered the accumulation of total bile acid in the liver by 47% compared to the HFD group (Figure 4A).
Figure 4.
Hepatic and fecal total bile acid (TBA) concentrations in high fat diet-treated C57BL/6 male mice after oral administration of lovastatin (Lv) 10 mg/kg, Xuezhikang (XZK) 1200 mg/kg, F1 (isoflavones) 27.5 mg/kg, F2 (monacolins) 11.3 mg/kg, or F3 (sterols) 35.0 mg/kg for 10 weeks, respectively. Mean±SD. n=8. bP<0.05, cP<0.01 vs Control. eP<0.05, fP<0.01 vs HFD. hP<0.05, iP<0.01 vs HFD+Lv. HFD: high fat diet.
F1 also lowered liver weight (P<0.01, Table S2) and lipid accumulation (Figure 2) compared to the HFD group. HFD+F1 decreased the hepatic bile acid pool by 46% (Figure 4A).
Lipid accumulation was decreased in the HFD+F2 group compared to the HFD group (Figure 2). F2 affected hepatic lipids and the bile acid pool in a manner similar to Lv (Figure 3A and 4A).
F3 decreased liver weight (P<0.01, Table S2) and lipid accumulation (Figure 2) compared to the HFD group. HFD-induced elevated TC, FC and CE levels were lowered by 53%, 43% and 63%, respectively, in the HFD+F3 group (Figure 3A).
Excretion of lipids and BA into feces
HFD significantly elevated the fecal excretion of TC and FC by 37% and 61%, respectively, compared to the Control group (Figure 3B). The fecal bile acid pool increased 1.1-fold in the HFD group (Figure 4B). The HFD group showed increases in all types of bile acid excretion in feces compared to the Control group (Figure S1).
Mice receiving Lv displayed an increased excretion of total bile acids in feces of 30% compared with the HFD group (Figure 4B).
The fecal excretion of TC, FC and CE (Figure 3B) were significantly increased by 207%, 175% and 183%, respectively, in the HFD+XZK group. XZK also increased the fecal excretion of total bile acids by 71% compared to the HFD group (Figure 4B). Exposure to XZK resulted in a remarkable increase in fecal excretion of all types of bile acids compared to the HFD group (Figure S1).
Mice receiving F1 displayed an increase in excretion of total bile acids into the feces of 79% (Figure 4B). Administration of F1 lead to increased fecal excretion of all types of bile acids, compared to the HFD group (Figure S1). F2 had an effect similar to that observed in the HFD+Lv group on changes in fecal lipids. F3 increased TC, FC and CE excretion into feces by 96%, 72%, and 101%, respectively (Figure 3B).
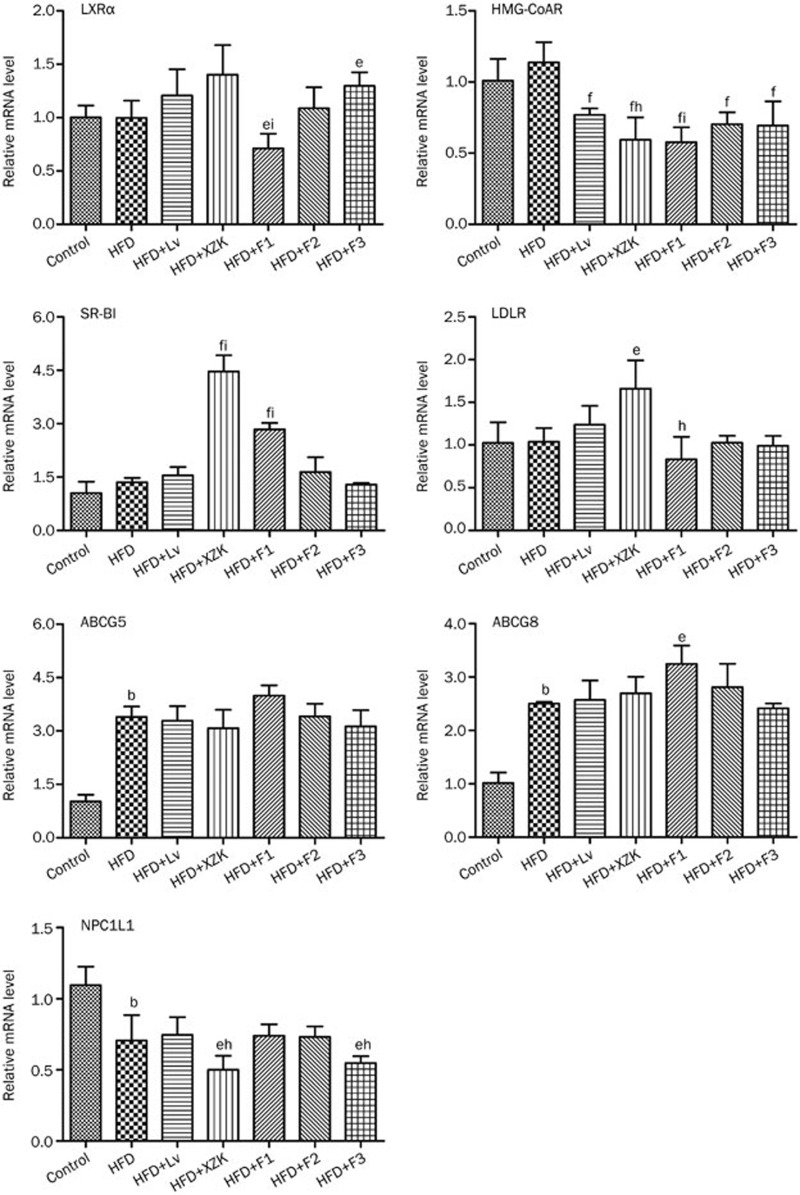
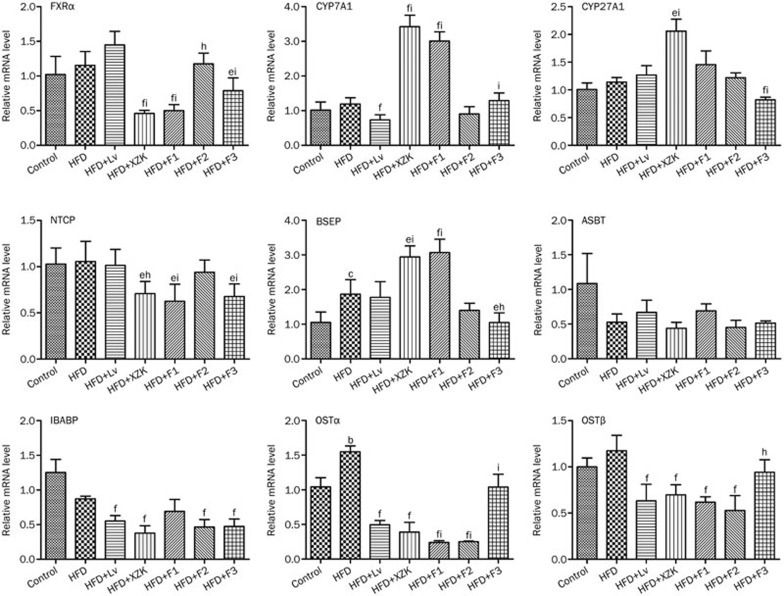
Transcriptional and translational regulation of the enzymes involved in homeostasis of cholesterol and bile acids
Up-regulated hepatic ABCG5 and ABCG8 (P<0.05) and down-regulated intestinal NPC1L1 (Figure 5, P<0.05) were observed in the HFD group, compared to the Control group. HFD did not affect the hepatic mRNA levels of CYP7A1 and CYP27A1 (P>0.05), but it robustly increased BSEP (P<0.01) and intestinal OSTα (P<0.05). Compared to the HFD group, HMG-CoAR and intestinal OSTα/β were significantly down-regulated in the Lv group (Figure 6, P<0.01).
Figure 5.
mRNA expression of genes related to cholesterol synthesis and transport in high fat diet-treated C57BL/6 male mice after oral administration of lovastatin (Lv) 10 mg/kg, Xuezhikang (XZK) 1200 mg/kg, F1 (isoflavones) 27.5 mg/kg, F2 (monacolins) 11.3 mg/kg, or F3 (sterols) 35.0 mg/kg for 10 weeks, respectively. Mean±SD. n=8. bP<0.05, cP<0.01 vs Control. eP<0.05, fP<0.01 vs HFD. hP<0.05, iP<0.01 vs HFD+Lv. HFD: high fat diet.
Figure 6.
mRNA expression of genes related to bile acid synthesis and transport in high fat diet-treated C57BL/6 male mice after oral administration of lovastatin (Lv) 10 mg/kg, Xuezhikang (XZK) 1200 mg/kg, F1 (isoflavones) 27.5 mg/kg, F2 (monacolins) 11.3 mg/kg, or F3 (sterols) 35.0 mg/kg for 10 weeks, respectively. Mean±SD. n=8. bP<0.05, cP<0.01 vs Control. eP<0.05, fP<0.01 vs HFD. hP<0.05, iP<0.01 vs HFD+Lv. HFD: high fat diet.
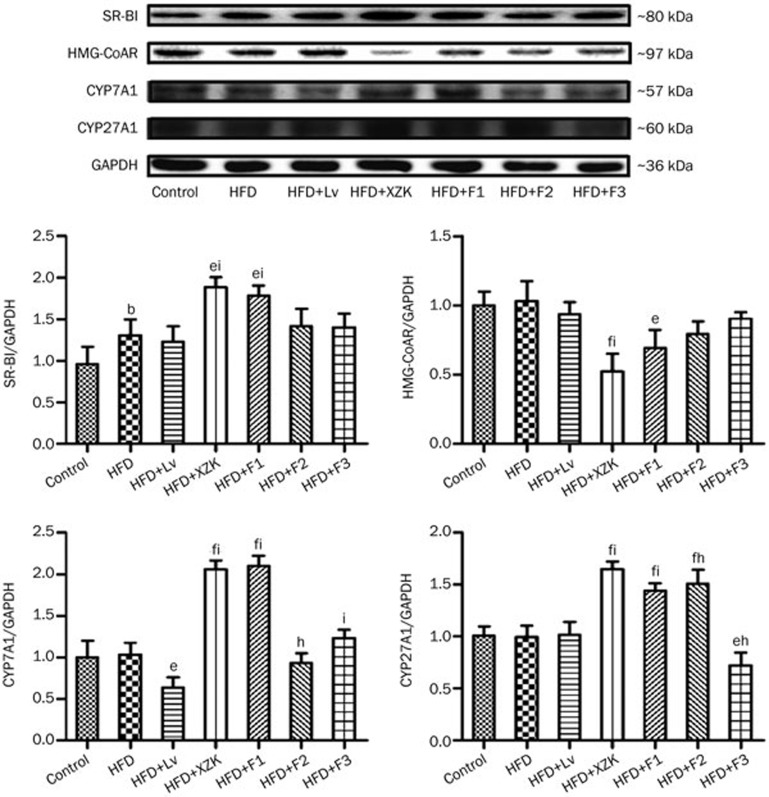
HMG-CoAR was significantly down-regulated and hepatic SR-BI was markedly up-regulated in the XZK group compared to the HFD group (Figure 5, P<0.01). Protein levels followed a similar trend (Figure 7). HFD+XZK markedly suppressed intestinal NPC1 and up-regulated hepatic LDL-R (P<0.05). The mRNA levels of hepatic FXRα, NTCP and intestinal OSTα/β were lower, while those of hepatic CYP7A1, CYP27A1 and BSEP were higher, in the HFD+XZK group (Figure 6, P<0.05).
Figure 7.
Hepatic protein expression related to homeostasis of cholesterol and bile acid metabolism in high fat diet-treated C57BL/6 male mice after oral administration of lovastatin (Lv) 10 mg/kg, Xuezhikang (XZK) 1200 mg/kg, F1 (isoflavones) 27.5 mg/kg, F2 (monacolins) 11.3 mg/kg, or F3 (sterols) 35.0 mg/kg for 10 weeks, respectively. Mean±SD. n=8. bP<0.05, cP<0.01 vs Control. eP<0.05, fP<0.01 vs HFD. hP<0.05, iP<0.01 vs HFD+Lv. HFD: high fat diet.
SR-BI and CYP7A1 were significantly increased in F1 compared to HFD (P<0.01), and their protein levels followed similar trends (Figure 5 and 7). HFD + F1-treated mice had higher BSEP mRNA levels (P<0.01). F1 strongly suppressed the mRNA levels of intestinal OSTα/β (P<0.01) and hepatic NTCP (Figure 6, P<0.05).
F2 significantly lowered the mRNA (P<0.01) and protein level of HMG-CoAR compared to the HFD group. It also strongly suppressed the mRNA levels of intestinal OSTα/β (Figure 5 and 7, P<0.01).
F3 significantly decreased the HMG-CoAR mRNA level (Figure 5, P<0.01), and the protein level followed a similar trend (Figure 7). mRNA levels of intestinal NPC1L1 and hepatic NTCP were markedly suppressed by F3 (Figure 5, P<0.05).
Discussion
XZK, an extract of RYR, has been shown to be effective in reducing dyslipidemia both clinically and pre-clinically. In this study, an HFD-fed mouse model was used to confirm the anti-hyperlipidemic effect of XZK. The results indicated that XZK treatment lowered serum levels of TC, TG and LDL-C by 41%, 55% and 46%, respectively, and increased HDL-C by 31% in serum (Figure 1). HDL-C has been described as the “good” cholesterol; it mediates reverse cholesterol transport (RCT) from the periphery to the liver39,40. Lv, used as a positive control, lowered serum TG and HDL-C levels by 25% and 33%, respectively, while modestly reducing serum TC and LDL-C levels by 17% and 19%, respectively. These results show that XZK has a greater effect on the improvement of dyslipidemia than a lovastatin equivalent (Table 1), which is consistent with previous research7.
Table 1. Level of lipids and related genes altered by three fractions from Xuzhikang (F1, F2, F3) compared with lovastatin.
| Biochemical index | Xuezhikang | F1 (Isoflavones) | F2 (Monacolins) | F3 (Sterols) |
|---|---|---|---|---|
| Serum lipids | ||||
| Total cholesterol (TC) | ↓↓ | – | – | ↓ |
| Triglyceride (TG) | ↓↓ | ↓↓ | – | – |
| LDL-C | ↓↓ | – | – | ↓↓ |
| HDL-C | ↑↑ | ↑ | – | ↑↑ |
| Hepatic lipids | ||||
| Total cholesterol (TC) | – | – | – | – |
| Free cholesterol (FC) | – | – | – | ↓ |
| Cholesterol esters (CE) | – | – | – | – |
| Total bile acids (TBA) | ↓↓ | ↓↓ | – | ↓ |
| Fecal lipids | ||||
| Total cholesterol (TC) | ↑↑ | – | – | ↑ |
| Free cholesterol (FC) | ↑↑ | – | – | ↑ |
| Cholesterol ester (CE) | ↑ | – | – | ↑ |
| Total bile acids (TBA) | ↑↑ | ↑ | – | – |
| Molecular mechanism | ||||
| Cholesterol pathway related genes | ||||
| HMG-CoAR | ↓ | ↓↓ | – | – |
| SR-BI | ↑↑ | ↑↑ | – | – |
| NPC1L1 | ↓ | – | – | ↓ |
| Bile acid pathway related genes | ||||
| CYP7A1 | ↑↑ | ↑↑ | – | ↑↑ |
| NTCP | ↓ | ↓↓ | – | ↓↓ |
| BSEP | ↑↑ | ↑↑ | – | ↓ |
↑↑, induced (P<0.01); ↓, repressed (P<0.05); ↓↓, repressed (P<0.01); –, without significant difference.
In the present study, the source of the improved pharmacological function of XZK relative to lovastatin was further explored. The fraction enriched in monacolins (F2) induced similar effects to Lv. The fraction enriched in isoflavones (F1) significantly repressed the TG serum level by 52% compared to the HFD group. F3, which was enriched in phytosterols, not only significantly decreased TC, TG and LDL-C levels by 33%, 30% and 32%, respectively; it also increased the serum HDL-C level by 29%. The pharmacological effects of XZK and XZK-derived fractions were compared with Lv, with results shown in Table 1. F1 contributed to the serum TG-lowering effect of XZK. F3 contributed to the TC- and LDL-C-lowering effects of XZK. Importantly, F3 contributed to the HDL-C-increasing effect of XZK.
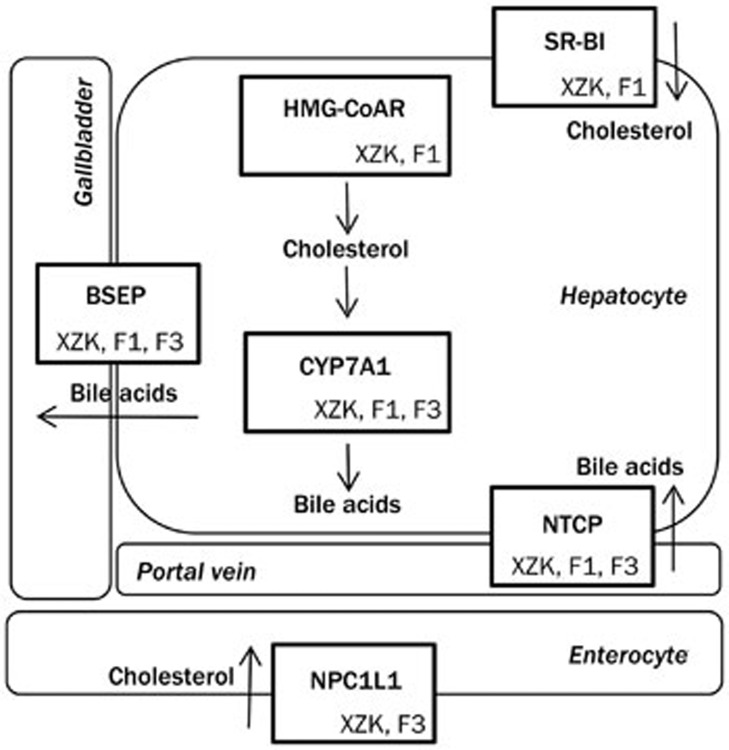
The mechanism of XZK action and the contribution of each of the three fractions to lowering cholesterol levels were further explored (Figures 5,6,7). XZK and F1 increased the reverse transport of excess cholesterol from the periphery to the liver by up-regulating hepatic SR-BI. XZK and F3 reduced intestinal absorption of exogenous cholesterol from foods by significantly inhibiting the transcriptional level of NPC1L1. XZK and F1 increased levels of bile acid synthase (CYP7A1) leading to increased conversion of cholesterol to bile acids. XZK and F1 augmented BA biliary secretion by increasing hepatic BSEP and repressing BA reabsorption by reducing NTCP. These results collectively demonstrate that XZK regulates cholesterol-bile acids homeostasis, reduces lipid absorption, and increases lipid excretion, thereby contributing to lower serum and hepatic lipid concentrations. F1 decreased the hepatic concentration and increased the fecal concentration of BA. F3 decreased hepatic lipids, while simultaneously increasing the excretion of fecal lipids. A similar effect by plant sterols has also been reported41,42,43,44,45. Our results reveal that F1 and F3 contribute to the cholesterol-lowering effect of XZK mainly by regulating bile acid and cholesterol homeostasis, respectively (Figure 8).
Figure 8.
Mechanisms of the lipid-lowering effects of Xuezhikang (XZK) and F1 (isoflavones), F2 (monacolins), or F3 (sterols).
In conclusion, the present study demonstrates that isoflavones and phytosterols in XZK have cholesterol-lowering effects through mechanisms that are different than those induced by Lv (Table 1). F1 and F3 contributed to the effect of XZK on dyslipidemia through regulation of bile acid and cholesterol homeostasis, respectively. Our results provide a basis for the development of these two substances as new functional foods.
Author contribution
Dong FENG contributed to the design and performance of experiments; Guang-ji WANG and Ji-ye AA contributed to the development of project and experimental design; Rui-bin SUN completed the analysis of lipids and bile acids; Bing-chen OU-YANG and Lan YAO performed Western blot analysis and qRT-PCR; Jian ZHANG performed the extraction of Xuezhikang; Jian-guo SUN, Fang ZHOU and Jing-wei ZHANG participated in the data analysis and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The study was financially supported by the National Natural Science Foundation of China (81373481), Jiangsu Provincial Promotion Foundation for the Key Lab of Drug Metabolism and Pharmacokinetics (Grant No BM2012012) and the Key Technology Projects of China “Creation of New Drugs” (No 2015ZX09501001).
Footnotes
Supplementary information is available at Acta Pharmacologica Sinica's website.
Supplementary Information
Fecal BA composition in C57BL/6 male mice.
List of primers used to amplify mRNA by real-time PCR
Body weight, Food intake and Liver weight in C57BL/6 male mice.
Changes of plasma atherogenic indices in C57BL/6 male mice.
References
- 1Chen ZY, Ma KY, Liang YT, Peng C, Zuo YY. Role and classification of cholesterol-lowering functional foods. J Funct Foods 2011; 3: 61–9. [Google Scholar]
- 2Gropper SS, Smith LJ, Groff LJ. Advanced nutrition and human metabolism. 7th ed. Ohio (USA): Peter Marshall, Wadsworth; 2005.
- 3Ma JY, Li YG, Ye Q, Li J, Hua YJ, Ju DJ. Constituents of red yeast rice, a traditional Chinese food and medicine. J Agr Food Chem 2000; 48: 5220–5. [DOI] [PubMed] [Google Scholar]
- 4Wei W, Li CL, Wang YY, Su HD, Zhu JS, Kritchevsky D. Hypolipidemic and anti-atherogenic effects of long-term Cholestin (Monascus purpureus-fermented rice, red yeast rice) in cholesterol fed rabbits. J Nutr Biochem 2003; 14: 314–8. [DOI] [PubMed] [Google Scholar]
- 5Lee CL, Tsai TY, Wang JJ, Pan TM. In vivo hypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl Microbiol Biot 2006; 70: 533–40. [DOI] [PubMed] [Google Scholar]
- 6Lin CC, Li TC, Lai MM. Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia. Eur J Endocrinol 2005; 153: 679–86. [DOI] [PubMed] [Google Scholar]
- 7Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VLW. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr 1999; 69: 231–6. [DOI] [PubMed] [Google Scholar]
- 8Li CL, Zhu Y, Wang YY, Zhu JS, Chang J, Kritchevsky D. Monascus purpureus-fermented rice (red yeast rice): a natural food product that lowers blood cholesterol in animal models of hypercholesterolemi. Nutr Res 1998; 71–81.
- 9Wang JJ, Pan TM, Shieh MJ, Hsu CC. Effect of red mold rice supplements on serum and meat cholesterol levels of broilers chicken. Appl Microbiol Biot 2006; 71: 812–8. [DOI] [PubMed] [Google Scholar]
- 10Liu JP, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fonnebo V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials. Chin Med 2006; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Grieco A, Miele L, Pompili M, Biolato M, Vecchio FM, Grattagliano I, et al. Acute hepatitis caused by a natural lipid-lowering product: When “alternative” medicine is no“alternative”at all. J Hepatol 2009; 50: 1273–7. [DOI] [PubMed] [Google Scholar]
- 12Klimek M, Wang S, Ogunkanmi A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. J Clin Pharm Ther 2009; 34: 313–27. [PMC free article] [PubMed] [Google Scholar]
- 13Heber D, Lembertas A, Lu QY, Bowerman S, Go VLW. An analysis of nine proprietary Chinese red yeast rice dietary supplements: implications of variability in chemical profile and contents. J Altern Complem Med 2001; 7: 133–9. [DOI] [PubMed] [Google Scholar]
- 14Manzoni M, Rollini M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl Microbiol Biot 2002; 58: 555–64. [DOI] [PubMed] [Google Scholar]
- 15Trimarco B, Benvenuti C, Rozza F, Cimmino CS, Giudice R, Crispo S. Clinical evidence of efficacy of red yeast rice and berberine in a large controlled study versus diet. Med J Nutrition Metab 2011; 4: 133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Shang QH, Liu ZL, Chen KJ, Xu H, Liu JP. A systematic review of Xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid-Based Compl Alt 2012; 2012: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Ma KY, Zhang ZS, Zhao SX, Chang Q, Wong YM, Yeung SYV, et al. Red yeast rice increases excretion of bile acids in hamsters. Biomed Environ Sci 2009; 22: 269–77. [DOI] [PubMed] [Google Scholar]
- 18Becker DJ, Gordon RY, Halbert SC, French B, Morris PB, Rader DJ. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med 2009; 150: 830. [DOI] [PubMed] [Google Scholar]
- 19Bartley GE, Yokoyama W, Young SA, Anderson WHK, Hung SC, Albers DR, et al. Hypocholesterolemic effects of hydroxypropyl methylcellulose are mediated by altered gene expression in hepatic bile and cholesterol pathways of male hamsters. J Nutr 2010; 140: 1255–60. [DOI] [PubMed] [Google Scholar]
- 20Gordon RY, Cooperman T, Obermeyer W, Becker DJ. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware!. Arch Intern Med 2010; 170: 1722–7. [DOI] [PubMed] [Google Scholar]
- 21Kidambi S, Patel SB. Cholesterol and non-cholesterol sterol transporters: ABCG5, ABCG8 and NPC1L1: a review. Xenobiotica 2008; 38: 1119–39. [DOI] [PubMed] [Google Scholar]
- 22Liu ZL, Liu JP, Zhang AL, Wu Q, Ruan Y, Lewith G, et al. Chinese herbal medicines for hypercholesterolemia. Cochrane Database of Systematic Reviews 2011, no. 7, Article ID CD008305. [DOI] [PMC free article] [PubMed]
- 23Joint Commission on China Adult Dyslipidemia Prevention Guildeline. China adult dyslipidemia prevention guildeline. Chin J Cardiol 2007; 35: 390–419. [Google Scholar]
- 24Dai XH, Zhuo XZ, Xue XY, Yang WM, Yu XQ, Zhou YX. Xuezhikang capsule for unstable angina pectoris. Tradit Chin Drug Res Clin Pharmacol 1999; 10: 202–4. [Google Scholar]
- 25Wang WH, Zhang H, Yu YL, Ge ZC, Xue C, Zhang PY. Effect of Xuezhikang for patients with acute coronary syndrom complicated with different serum lipid levels. Chin J Integr Med 2004; 24: 1073–6. [PubMed] [Google Scholar]
- 26Li JJ, Hu SS, Fang CH, Hui RT, Miao LF, Yang YJ, et al. Effects of xuezhikang, an extract of cholestin, on lipid profile and C-reactive protein: a short-term time course study in patients with stable angina. Clin Chim Acta 2005; 352: 217–24. [DOI] [PubMed] [Google Scholar]
- 27Lu ZL, Kou WR, Du BM, Wu YF, Zhao SP, Brusco OA, et al. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am J Cardiol 2008; 101: 1689–93. [DOI] [PubMed] [Google Scholar]
- 28Fuchs M, Ivandic B, Muller O, Schalla C, Scheibner J, Bartsch P, et al. Biliary cholesterol hypersecretion in gallstone-susceptible mice is associated with hepatic up-regulation of the high-density lipoprotein receptor SRBI. Hepatology 2001; 33: 1451–9. [DOI] [PubMed] [Google Scholar]
- 29Wiersma H, Gatti A, Nijstad N, Elferink RPJO, Kuipers F, Tietge UJF. Scavenger receptor class B type I mediates biliary cholesterol secretion independent of ATP-binding cassette transporter g5/g8 in mice. Hepatology 2009; 50: 1263–72. [DOI] [PubMed] [Google Scholar]
- 30Mitro N, Godio C, Fabiani ED, Scotti E, Galmozzi A, Gilardi F, et al. Insights in the regulation of cholesterol 7α-hydroxylase gene reveal a target for modulating bile acid synthesis. Hepatology 2007; 46: 885–97. [DOI] [PubMed] [Google Scholar]
- 31Wooton-Kee CR, Coy DJ, Athippozhy AT, Zhao TY, Jones BR, Vore M. Mechanisms for increased expression of cholesterol 7α-hydroxylase (Cyp7a1) in lactating rats. Hepatology 2010; 51: 277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Li TG, Matozel M, Boehme S, Kong B, Nilsson LM, Guo G, et al. Overexpression of cholesterol 7alpha-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 2011; 53: 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Morgan R E, Trauner M, van Staden CJ, Lee PH, Ramachandran B, Eschenberg M, et al. Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci 2010; 118: 485–500. [DOI] [PubMed] [Google Scholar]
- 34Pauli-Magnus C, Meier PJ. Hepatocellular transporters and cholestasis. J Clin Gastroenterol 2005; 39: S103–10. [DOI] [PubMed] [Google Scholar]
- 35Greupink R, Dillen L, Monshouwer M, Huisman MT, Russel FGM. Interaction of fluvastatin with the liver-specific Na+-dependent taurocholate cotransporting polypeptide (NTCP). Eur J Pharm Sci 2011; 44: 487–96. [DOI] [PubMed] [Google Scholar]
- 36Arrese M, Trauner M. Molecular aspects of bile formation and cholestasis. Trends Mol Med 2003; 9: 558–64. [DOI] [PubMed] [Google Scholar]
- 37Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 2007; 48: 2664–72. [DOI] [PubMed] [Google Scholar]
- 38Jiao R, Zhang ZS, Yu HJ, Huang Y, Chen ZY. Hypocholesterolemic activity of grape seed proanthocyanidin is mediated by enhancement of bile acid excretion and up-regulation of CYP7A1. J Nutr Biochem 2010; 21: 1134–9. [DOI] [PubMed] [Google Scholar]
- 39Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA 2007; 298: 786–98. [DOI] [PubMed] [Google Scholar]
- 40Assmann G, Gotto AM. HDL cholesterol and protective factors in atherosclerosis. Circulation 2004; 109: 8–14. [DOI] [PubMed] [Google Scholar]
- 41Amundsen AL, Ose L, Nenseter MS, Ntanios FY. Plant sterol ester–enriched spread lowers plasma total and LDL cholesterol in children with familial hypercholesterolemia. Am J Clin Nutr 2002; 76: 338–44. [DOI] [PubMed] [Google Scholar]
- 42Pollak OJ. Reduction of blood cholesterol in man. Circulation 1953; 7: 702–6. [DOI] [PubMed] [Google Scholar]
- 43QuIlez J, GarcIa-Lorda P, Salas-Salvadó J. Potential uses and benefits of phytosterols in diet: present situation and future directions. Clin Nutr 2003; 22: 343–51. [DOI] [PubMed] [Google Scholar]
- 44Temme EHM, Hoydonck PGAV, Schouten EG, Kesteloot H. Effects of a plant sterol-enriched spread on serum lipids and lipoproteins in mildly hypercholesterolaemic subjects. Acta Cardiol 2002; 57: 111–5. [DOI] [PubMed] [Google Scholar]
- 45López-Juárez A, Howard J, Ullom K, Howard L, Grande A, Pardo A. Gsx2 controls region-specific activation of neural stem cells and injury-induced neurogenesis in the adult subventricular zone. Genes Dev 2013; 27: 1272–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fecal BA composition in C57BL/6 male mice.
List of primers used to amplify mRNA by real-time PCR
Body weight, Food intake and Liver weight in C57BL/6 male mice.
Changes of plasma atherogenic indices in C57BL/6 male mice.