Abstract
Aim:
Platycodin D, the main saponin isolated from Chinese herb Platycodonis Radix, exhibits anticancer activities against various cancer cell lines. Here we evaluated its anticancer action against human hepatocellular carcinoma cells in vitro and in vivo, and elucidated the relationship between platycodin D-induced apoptosis and autophagy.
Methods:
The viability of human hepatocellular carcinoma BEL-7402 cells was evaluated with MTT assay, and the apoptosis was examined using Annexin V/PI and Hoechst 33342 staining assays. Monodansylcadaverine (MDC) staining was used to label autophagic vacuoles. The proteins were detected using Western blot analysis. For studying its anticancer action in vivo, platycodin D (5 and 10 mg· kg−1·d−1) was intraperitoneally injected to BEL-7402-bearing mice for 21 days.
Results:
Platycodin D (5–40 μmol/L) inhibited the cell proliferation in vitro with IC50 values of 37.70±3.99, 24.30±2.30 and 19.70±2.36 μmol/L at 24, 48 and 72 h, respectively. Platycodin D (5–20 μmol/L) dose-dependently increased BEL-7402 cell apoptosis, increased the Bax/Bcl-2 ratio and the levels of cleaved PARP and cleaved caspase-3, and decreased the level of Bcl-2. Furthermore, platycodin D (5–20 μmol/L) induced autophagy in BEL-7402 cells, as evidenced by formation of cytoplasmic vacuoles, increased amounts of LC3-II, and increased numbers of MDC-positive cells. Pretreatment with the autophagy inhibitor chloroquine (5 μmol/L) or BAF (50 nmol/L) significantly enhanced platycodin D-induced proliferation inhibition and apoptosis. Moreover, platycodin D (20 μmol/L) activated the ERK and JNK pathways in BEL-7402 cells, and simultaneous blockage of the two pathways effectively suppressed platycodin D-induced autophagy and enhanced platycodin D-induced apoptosis. In BEL-7402-bearing mice, platycodin D (10 mg·kg−1•d−1) significantly reduced relative tumor volume with decreased body weight.
Conclusion:
Platycodin D not only inhibits the proliferation of BEL-7402 cells but also suppresses BEL-7402 xenograft tumor growth. Platycodin D-induced cell proliferation inhibition and apoptosis are amplified by co-treatment with autophagy inhibitors
Keywords: platycodin D, human hepatocellular carcinoma, BEL-7402 cells, apoptosis, autophagy, ERK, JNK, U0126, SP600125, chloroquine, BAF
Introduction
Platycodon grandiflorum (Jacq.) A. DC, commonly known as the balloon flower, is widely distributed in Northeast Asia. Platycodonis radix is the two- or three-year-old root of Platycodon grandiflorum (Jacq.) A. DC, with a long history of use as a dietary source and a folk remedy for pulmonary diseases and respiratory system disorders in Korea, Japan and China1. Platycodin D (PD) (Figure 1A) is one of the main saponins extracted from Platycodonis radix, and it possesses immune-stimulatory2, anti-inflammatory3,4, anti-nociceptive5, anti-obesity5,6, and anti-atherogenic7 activities. In particular, PD exhibits excellent anticancer effects against various cancer cell lines mainly by inhibiting cell proliferation, inducing cell cycle arrest and promoting apoptosis8,9,10,11,12,13,14. PD-induced G2/M phase cycle arrest may be regulated by suppressing spindle microtubule dynamics in leukemia U937, THP-1, and K562 cells11. PD-mediated apoptosis may be related to the activation of caspase 3 and the induction of reactive oxygen species12. In our previous studies, PD inhibited cell proliferation and induced apoptosis via the induction of poly ADP-ribose polymerase (PARP) cleavage, the up-regulation of Bax and the down-regulation of survivin in hepatocellular carcinoma cells15. In addition, PD also triggered autophagy in a broad spectrum of cell lines including breast cancer, lung cancer, and hepatocellular carcinoma cells16.
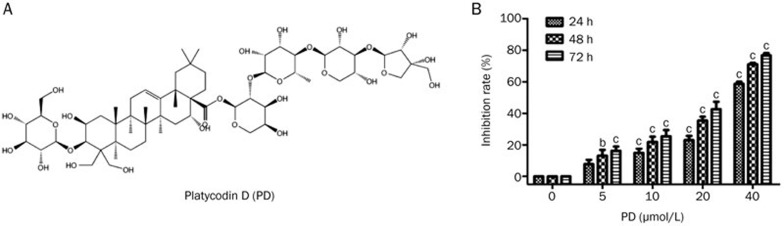
Figure 1.
PD inhibits the proliferation of hepatocellular carcinoma BEL-7402 cells. (A) The chemical structure of PD. (B) Cells were treated with different concentrations of PD for 24, 48, and 72 h, and cell proliferation inhibition was detected by the MTT assay. Statistical significance was analyzed using one-way analysis of variance using Graph Pad Prism (Demo, Version 5) with bP<0.05, cP<0.01 vs control.
As a major intracellular degradation mechanism, autophagy is a highly conserved process that degrades intracellular material including proteins and even organelles in response to cellular stresses17,18. A growing body of evidence demonstrates that autophagy is implicated in human carcinogenesis and is considered a “double-edged sword” for cancer treatment19,20. The cytotoxic and apoptotic effects of PD are enhanced with co-treatment of PD and autophagy inhibitors, such as chloroquine (CQ) or bafilomycin A1 (BAF), in HepG2 cells16.
This study evaluated the anticancer potential of PD both in vitro and in vivo, and it further validated the role and mechanism of autophagy triggered by PD to provide more evidence to support PD as an alternative therapy for hepatocellular carcinoma.
Materials and methods
Reagents
PD was purchased from Best-Reagent (Chengdu, China). PD was diluted using dimethyl sulfoxide (DMSO) to make a stock concentration of 40 mmol/L. 3-[4,5-Dimethyl-2-thiazolyl]-2,5-diphenyltetrazolium bromide (MTT), DMSO, CQ, BAF, phenylmethanesulfonyl fluoride, monodansylcadaverine (MDC), and Hoechst 33342 were obtained from Sigma (St Louis, MO, USA). The protease and phosphatase inhibitor cocktail was bought from Thermo Fisher Scientific Inc (Waltham, MA, USA). Radio immunoprecipitation assay buffer (RIPA buffer), SP600125 and U0126 were purchased from Beyotime (Haimen, China). The primary antibodies against microtubule-associated protein 1 light chain 3 (LC3), Bcl-2, Bax, PARP, extracellular signal-regulated kinase (ERK), p-ERK (Thr202/Tyr204), c-Jun N-terminal kinase (JNK), p-c-Jun (Ser73) and GAPDH as well as the anti-rabbit IgG HRP-conjugated secondary antibody were all purchased from Cell Signaling Technology (Beverly, MA, USA). Lipofectamine® 2000 Reagent was purchased from Invitrogen (Carlsbad, CA, USA). ERK1 siRNA (sense, 5′-ACA CGC AGU UGC AGU ACA UTT-3′ antisense, 5′-AUG UAC UGC AAC UGC GUG UTT-3′), ERK2 siRNA (sense, 5′-GUG CUC UGC UUA UGA UAA UTT-3′ antisense 5′-AUU AUC AUA AGC AGA GCA CTT-3′), JNK1 siRNA (sense, 5′-GCA GAA GCA AGC GUG ACA ATT-3′ antisense, 5′-UUG UCA CGC UUG CUU CUG CTT-3′), JNK2 siRNA (sense, 5′-GCU AAC UUA UGU CAG GUU ATT-3′ antisense 5′-UAA CCU GAC AUA AGU UAG CTT-3′), and negative control (NC) siRNA (sense, 5′-UUC UCC GAA CGU GUC ACG UTT-3′ antisense, 5′-ACG UGA CAC GUU CGG AGA ATT-3′) were purchased from Shanghai GenePharma (Shanghai, China).
Cell culture
Human hepatocellular carcinoma BEL-7402 cells were obtained as a gift from Prof Jian DING (Shanghai Institute of Materia Medica, Shanghai, China). BEL-7402 cells were cultured in RPMI 1640 medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, Grand Island, NY, USA). The cells were maintained at 37 °C in a humidified incubator with an atmosphere containing 5% CO2. Exponentially growing cells were used in the experiments.
Animals
Female athymic nude mice (BALB/cA; aged 4 to 5 weeks and weight of 17±2 g) were obtained from the Shanghai Institute of Materia Medica and then farmed in sterile cages under laminar airflow hoods in a specific pathogen-free room with a 12 h light and 12 h dark schedule. The BALB/cA mice were fed autoclaved chow and water ad libitum. All experiments were performed according to the institutional ethical guidelines on animal care and approved by the Institute Animal Care and Use Committee at Shanghai Institute of Materia Medica.
MTT assay
BEL-7402 cells were plated in 96-well plates at a density of 7000 cells per well for 24 h. The cells were treated with different concentrations of compounds diluted in RPMI-1640 medium containing 0.5% FBS for indicated time periods. The supernatant was then removed and RPMI-1640 medium containing MTT solution (1 mg/mL) was added to the wells (100 μL per well). The cells were maintained in a humidified environment for 4 h. Cell proliferation was determined by addition of 100 μL of DMSO and shaking for 10 min in the dark to solubilize formazan. The absorbance at 570 nm was recorded using a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Observation of morphological changes
BEL-7402 cells were plated in 96-well plates at a density of 7000 cells per well. After treatment with PD diluted in medium containing 0.5% FBS for 24 h, the cellular morphology was observed with an Axiovert 200 inverted microscope (Zeiss, Oberkochen, Germany).
Western blot analysis
BEL-7402 cells were lysed in RIPA lysis buffer containing PMSF and protease inhibitor cocktail. After incubation for 20 min on ice, the lysis buffer containing cellular contents was centrifuged at 4 °C and 12 500 r/min for 20 min. The cell supernatants containing the proteins were obtained and quantified using a BCA™ protein assay kit (Pierce, Rockford, IL, USA). Approximately 50 μg of total protein was subjected to SDS-PAGE, transferred onto polyvinylidene fluoride membranes, and then blocked with 5% nonfat milk in TBST (20 mmol/L Tris, 500 mmol/L NaCl, and 0.1% Tween-20) at room temperature for 2 h with continuous rocking. The membranes were probed with specific primary antibodies overnight at 4 °C. The membranes were then washed with TBST three times for 15 min each and incubated with anti-rabbit IgG HRP-conjugated secondary antibody in TBST at room temperature for 1 h. Specific protein bands were visualized using an ECL advanced Western blot detection kit (GE Healthcare, Uppsala, Sweden).
MDC staining
BEL-7402 cells were seeded into a 96-well black culture plate and maintained in the incubator. After adhesion, the cells were treated with different concentrations of PD diluted in medium containing 0.5% FBS. After a 24 h incubation, the cells were labeled with MDC (50 μmol/L) for 10 min at 37 °C and washed three times with phosphate-buffered saline (PBS). Cells were then imaged and analyzed by an In Cell Analyzer 2000 System (GE Healthcare, Uppsala, Sweden).
Annexin V/PI staining
The Annexin V-FITC/PI apoptosis detection kit was used to detect the apoptotic cells according to the manufacturer's instructions. BEL-7402 cells were seeded into a 12-well black culture plate at a density of 20000 cells per well and maintained in the incubator for 24 h. Cells were then treated with different concentrations of PD diluted in medium containing 0.5% FBS. After a 24 h incubation, cells were harvested, washed twice with cold PBS and re-suspended in 100 μL of binding buffer. After a 15 min incubation in the dark at room temperature, 5 μL of Annexin V-FITC and 5 μL of PI were added to the cells, and the cells were then analyzed by a flow cytometer (BD FACS Canto™, BD Biosciences, San Jose, USA).
Hoechst 33342 staining
BEL-7402 cells were seeded into 96-well plates at a density of 7000 cells per well and maintained in the incubator for 24 h. Cells were then treated with different concentrations of PD for 24 h. After incubation, the medium was removed, and cells were fixed with 4% paraformaldehyde for 20 min. Hoechst 33342 at a final concentration of 1 μg/mL was then added, and cells were incubated for 10 min at 37 °C in the dark. Apoptotic morphological changes of the cell nucleus were then observed and analyzed by an In Cell Analyzer 2000 System (GE Healthcare, Uppsala, Sweden).
Gene silencing experiments
BEL-7402 cells were seeded in a 6-well plate the day before transfection at 50% confluency. Lipofectamine 2000 Reagent was used to transfect cells with siRNA. Cells were transfected with the indicated siRNAs (10 nmol/L) for 30 h according to the manufacturer's protocol and then treated with PD (20 μmol/L) for an additional 24 h. The protein levels of JNK, ERK and LC3 were measured by Western blot analysis.
In vivo BEL-7402 xenograft tumors
Human hepatocellular carcinoma BEL-7402 cells were subcutaneously injected into female BALB/cA nude mice aged 4 to 5 weeks. The subcutaneously transplanted tumors (volume of 1.5 mm3) were cut out and implanted into BALB/cA nude mice after one passage in nude mice. Thirty mice with a mean tumor volume of 180 mm3 were randomly divided into four experimental groups, as follows: solvent control group (12), MMC group (6), 10 mg/kg PD group (6) and 5 mg/kg PD group (6). MMC was iv administered through the tail vein weekly on the first day, and PD was intraperitoneally administered once daily for 21 d. Mice in the solvent control group were treated with phosphate-buffered saline for comparison at the same time. Tumors were measured individually twice per week. Tumor volumes were calculated according to the following formula: length×width×width×0.5. The tumor volumes were presented as follows: RTV=tumor volume (day after initial treatment, Vt)/tumor volume (day of initial treatment, V0). Body weights of the animals were measured on the days of initial injection and twice per week until autopsy.
Statistical analysis
Data were expressed as the mean±SD. Statistical significance was analyzed by analysis of variance (ANOVA) using Graph Pad Prism in Demo, Version 5 (GraphPad Software, La Jolla, CA, USA). P<0.05 was considered to be statistically significant.
Results
PD inhibits BEL-7402 cell proliferation in vitro
Previous studies have shown that PD exhibits anticancer properties in vitro8,9,10,11,12,13,14. This study used hepatocellular carcinoma BEL-7402 cells to detect the antiproliferative effect of PD. BEL-7402 cells were treated with various concentrations of PD for 24, 48, and 72 h, and the inhibition rate was then measured using the MTT assay. As shown in Figure 1B, PD inhibited BEL-7402 cell proliferation in vitro in concentration- and time-dependent manners with IC50 values of 37.70±3.99, 24.30±2.30, and 19.70±2.36 μmol/L at 24, 48, and 72 h, respectively. Treatment with 20 and 40 μmol/L PD for 24 h resulted in a cell proliferation inhibition rate of 22.93%±4.76% and 58.01%±2.82%, respectively.
PD retards the growth of BEL-7402 xenograft tumors in BALB/cA nude mice in vivo
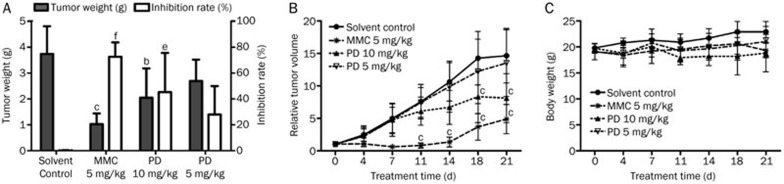
We next evaluated the in vivo effect of PD on BEL-7402 xenograft tumor growth. BALB/cA nude mice were subcutaneously injected with BEL-7402 cells and intraperitoneally administered with 10 mg/kg or 5 mg/kg PD for 21 d. The intravenous 5 mg/kg mitomycin C (MMC) group was used as a positive control. As shown in the Figure 2A, a significant decrease (P<0.05) of the tumor weights was detected in mice of the 10 mg/kg PD group compared with the solvent control group. The relative tumor volume in the 10 mg/kg PD-treated group was much lower than that in the solvent control group. After the 21-d treatment, the relative tumor volumes in the 10 mg/kg PD-treated and 5 mg/kg PD-treated groups were 8.10±3.78 and 13.50±5.12, respectively, and the relative tumor volume in the solvent control group was 14.65±4.2 (Figure 2B). In addition to the reduction of body weight after 10 mg/kg PD treatment (Figure 2C), two mice died during the 10 and 5 mg/kg PD treatments, respectively, suggesting that intraperitoneally administered PD had toxic effects in BEL-7402 xenograft mice.
Figure 2.
PD retards the growth of BEL-7402 xenograft tumors in vivo. After the injection of BEL-7402 cells, mice were treated with different concentrations of PD. The tumor masses taken from tumor-bearing mice were weighed, and the inhibition rate was evaluated (A). The relative tumor volume (B) and body weight (C) were measured. bP<0.05, cP<0.01; eP<0.05, fP<0.01 vs control.
PD induces apoptosis in BEL-7402 cells
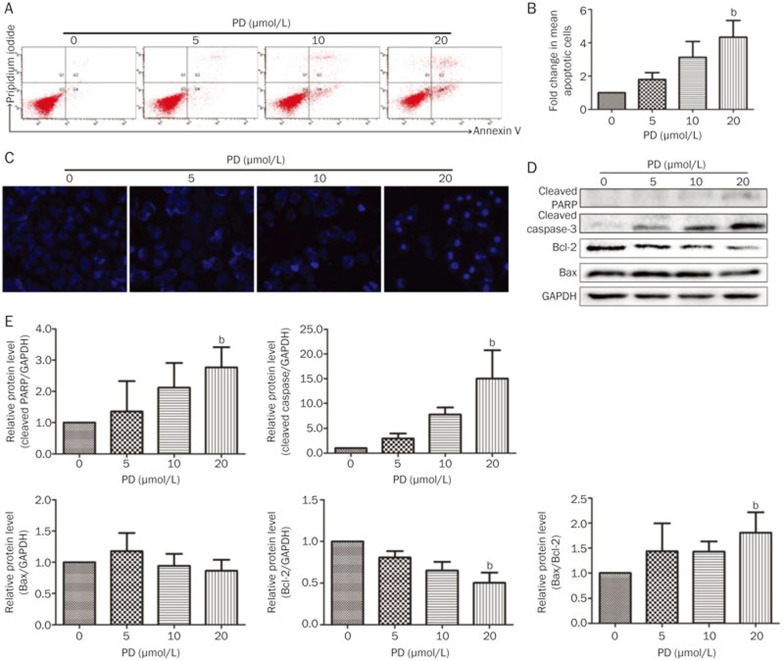
In our previous study, PD induced apoptosis in hepatocellular carcinoma HepG2 cells by inducing the protein expression of cleaved PARP and Bax as well as by inhibiting survivin protein expression15. PD also enhances the apoptotic effect of doxorubicin in breast cancer MCF-7 and MDA-MB-231 cells14. To investigate whether PD promotes BEL-7402 cell apoptosis, several apoptotic assays were employed. Firstly, cell death was measured in PD-treated BEL-7402 cells using an Annexin V/PI staining assay. As shown in Figures 3A and 3B, apoptotic cells increased in a concentration-dependent manner after PD treatment for 24 h. A significant increase (P<0.05) in the mean number of apoptotic cells was observed after treatment with 20 μmol/L PD with a fold change of 4.34±1.73. Secondly, morphological alteration of BEL-7402 cells after 24 h PD treatment was revealed by Hoechst 33342 staining. As shown in Figure 3C, no apoptotic nuclei were observed in the control group. In contrast, 5 μmol/L PD treatment for 24 h slightly altered BEL-7402 cell morphology, and cell nuclear condensation developed in a concentration-dependent manner (Figure 3C). The majority of the 20 μmol/L PD-exposed cells was shriveled and exhibited typical apoptotic morphology characterized by nuclear condensation and DNA fragmentation (Figure 3C). We further detected the levels of several proteins related to apoptosis. After PD treatment, the antiapoptotic protein Bcl-2 was significantly down-regulated, while the Bax/Bcl-2 ratio and the levels of cleaved PARP and caspase-3 were significantly up-regulated (Figures 3D and 3E), indicating that PD induces apoptosis in BEL-7402 cells.
Figure 3.
PD induces apoptosis in BEL-7402 cells. BEL-7402 cells were treated with different concentrations of PD for 24 h. (A) Apoptotic cells were detected by Annexin V/PI staining using flow cytometry. (B) Quantitation of the results obtained from (A). (C) The apoptotic cells were evaluated with Hoechst 33342 staining and imaged using the In Cell Analyzer 2000 System. (D) The expression of apoptosis-related proteins, including cleaved PARP, cleaved caspase-3, Bcl-2, and Bax, were analyzed by Western blot analysis. (E) Quantitation of the results obtained from (D). bP<0.05, cP<0.01 vs control.
PD triggers autophagy in BEL-7402 cells
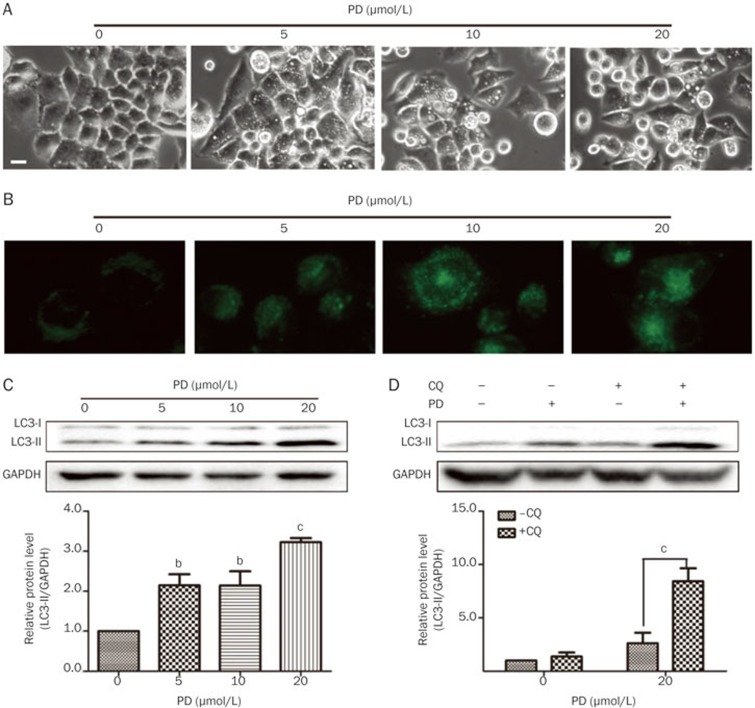
Morphological features of cytoplasmic vacuole accumulation are often observed in cells undergoing autophagy16,21,22. We have demonstrated that PD triggers autophagic characteristics, such as cytoplasmic vacuole accumulation, in many cell lines16. In this study, PD also induced the formation of cytoplasmic vacuoles in BEL-7402 cells as observed under an Axiovert 200 inverted microscope (Figure 4A). To confirm if autophagy is triggered in PD-treated BEL-7402 cells, we performed several experiments. MDC, which can accumulate in acidic vesicular organelles, was used to detect autophagic activity. The cells were treated with different concentrations of PD for 24 h and then labeled with 50 μmol/L MDC. The number of MDC-positive cells increased in a concentration-dependent manner in PD-treated cells after a 24 h incubation. The levels of LC3, a hallmark of autophagy, were also determined by Western blot analysis. As shown in Figure 4C, a concentration-dependent increase in LC3-II was detectable in BEL-7402 cells after a 24 h PD treatment. CQ has been widely used as late-stage autophagy inhibitor, and CQ leads to an increase of LC3-II levels due to the accumulation of undigested autophagosomes23,24,25. Western blot analysis revealed that LC3-II further accumulated in cells co-treated with CQ and PD compared to cells treated with CQ or PD alone (Figure 4D). Taken together, our results indicated that PD triggers autophagy in BEL-7402 cells.
Figure 4.
PD triggers autophagy in BEL-7402 cells. BEL-7402 cells were treated with different concentrations of PD for 24 h. (A) The cell morphology was directly observed under an Axiovert 200 inverted microscope. Bar: 20 μm. (B) The autophagic vacuoles were labeled with MDC by incubating cells with 0.05 mmol/L MDC. (C) The protein levels of LC3 were determined by Western blot analysis. (D) The BEL-7402 cells were pretreated with 5 μmol/L CQ for 1 h followed by 20 μmol/L PD treatment for an additional 24 h, and the protein levels of LC3 were determined by Western blot analysis. cP<0.01 vs -CQ.
Inhibition of autophagy enhances PD-induced proliferation inhibition and apoptosis in BEL-7402 cells
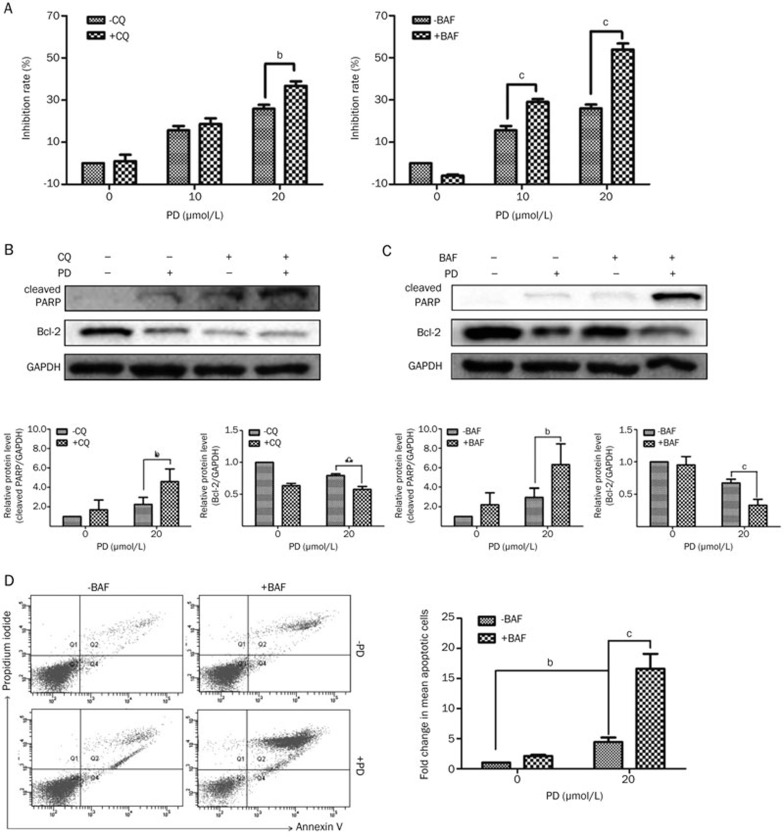
The role of autophagy in cancer therapy is complex17,26. To investigate the role of autophagy induced by PD, BEL-7402 cells were pretreated with CQ or BAF for 1 h before PD treatment. As shown in Figure 5A, after pretreatment with CQ or BAF, the PD-induced proliferation inhibition was significantly enhanced. The proliferation inhibition rate increased from 24.39%±2.8% (20 μmol/L PD only) to 36.74%±3.79% (20 μmol/L PD+CQ) and 51.00%±0.03% (20 μmol/L PD+BAF). Consistent with the results from the MTT assay, the Western blot analysis showed that pretreatment with CQ or BAF significantly up-regulated the protein level of cleaved PARP and down-regulated the protein level of Bcl-2 (Figures 5B and 5C). Moreover, as shown in Figure 5D, BAF-mediated increase in apoptosis was also confirmed by Annexin V/PI staining during PD treatment. Significant increases (P<0.01) in apoptotic cells were obtained in cells treated with both PD and BAF compared to cells treated with only PD. Therefore, these data suggested that the inhibition of autophagy does not decrease but instead enhances the PD-induced proliferation inhibition and apoptosis.
Figure 5.
Inhibition of autophagy enhances PD-mediated cell death in BEL-7402 cells. BEL-7402 cells were pretreated with 10 μmol/L CQ or 50 nmol/L BAF for 1 h followed by treatment with different concentrations of PD for an additional 24 h. (A) Cell viability was measured using MTT assay. (B and C) The protein levels of cleaved PARP and Bcl-2 were measured by Western blot analysis. (D) Apoptotic cells were detected by Annexin V/PI staining using flow cytometry. bP<0.05, cP<0.01.
The ERK and JNK pathways may cooperatively regulate PD-triggered autophagy in BEL-7402 cells
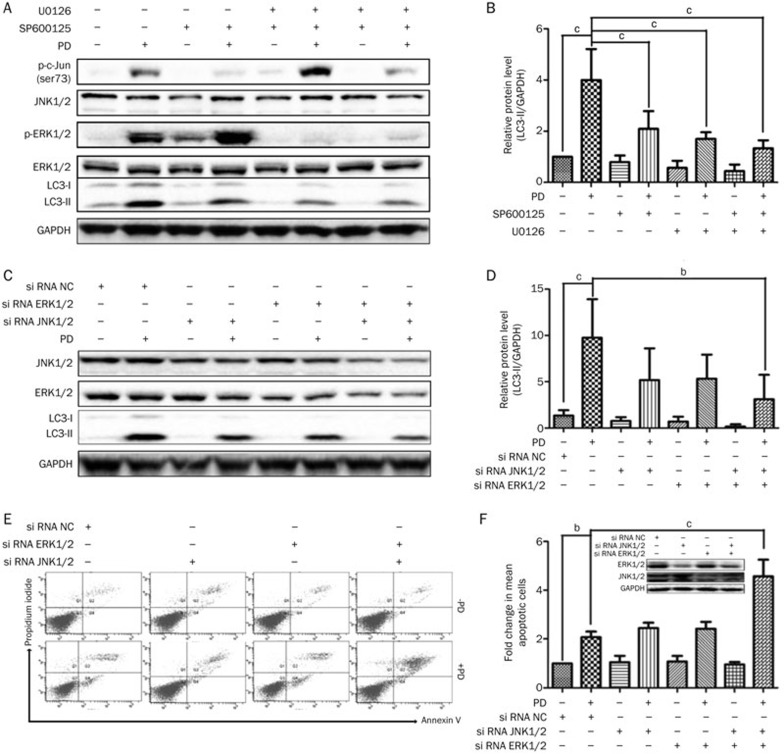
The ERK pathway is a key mediator of autophagy in cancer treatment27. PD mediates autophagy through activation of the ERK pathway in HepG2 cells16. Therefore, we determined whether the ERK pathway is also involved in PD-induced autophagy in BEL-7402 cells. As expected, PD treatment resulted in the up-regulation of p-ERK without any obvious change in total ERK. The decreased ERK phosphorylation induced by U0126 (a widely used inhibitor of MEK28) led to a confined suppression of LC3-II (Figure 6A and 6B), indicating that the ERK pathway might limitedly regulate PD-triggered autophagy in BEL-7402 cells. To further determine the mechanism of autophagy in PD-treated BEL-7402 cells, the JNK signaling pathway, which is a potential autophagy regulation pathway29, was evaluated by Western blot analysis. As shown in Figures 6A and 6B, PD treatment increased c-Jun phosphorylation at Ser73, which is the site regulated by JNK30. Pretreatment with SP600125, an inhibitor of JNK31, blocked the phosphorylation of c-Jun and resulted in a slight reversal of LC3-II up-regulation. Blocking the ERK and JNK pathways by co-pretreatment of SP600126 and U0126 effectively suppressed PD-induced LC3-II expression. These observations were further supported by blocking both ERK (by targeting ERK1 and ERK2) and JNK (by targeting JNK1 and JNK2) with corresponding siRNAs (neither ERK nor JNK alone), which was confirmed by Western blot analysis, to effectively suppress PD-induced autophagy (Figures 6C and 6D) and to promote PD-induced apoptosis (Figures 6E and 6F). Taken together, these data demonstrated that the activation of ERK and JNK pathways may work together to mediate PD-triggered autophagy in BEL-7402 cells.
Figure 6.
ERK and JNK pathways may cooperatively regulate PD-triggered autophagy in BEL-7402 cells. (A and B) BEL-7402 cells pretreated with 10 μmol/L SP600125 and/or 20 μmol/L U0126 for 1 h followed by 20 μmol/L PD treatment for an additional 24 h. The protein levels of p-c-Jun (Ser73), JNK, ERK, p-ERK (Thr202/Tyr204) and LC3 were measured by Western blot analysis. (C–F) BEL-7402 cells were transfected with the indicated siRNAs (10 nmol/L) for 30 h and then treated with PD (20 μmol/L) for an additional 24 h. (C and D) The protein levels of JNK, ERK and LC3 were measured by Western blot analysis. (E and F) Apoptotic cells were detected by Annexin V/PI staining using flow cytometry. bP<0.05, cP<0.01.
Discussion
Natural products are a favorable resource for anticancer drug discovery32,33,34,35,36. As the principal active component in Platycodonis Radix, PD has been confirmed to have potential anticancer effects by inhibiting proliferation, inducing apoptosis, inducing cell cycle arrest, and blocking metastasis9,10,13,14,15,37. This study provided evidence that PD inhibits cell proliferation of human hepatocellular carcinoma BEL-7402 cells. Furthermore, this work showing that PD has anticancer activity in vivo against BEL-7402 xenograft-bearing mice. However, a decrease in body weight was observed during 10 mg/kg PD treatment (Figure 2C). In addition, two mice died in the 10 mg/kg and 5 mg/kg PD-treated groups during the 21 d period, revealing that the intraperitoneal treatment with PD had toxic effects in BEL-7402 xenograft mice. More recently, oral administration of PD (50, 100, and 200 mg/kg) has been reported to reduce the tumor volumes in lung cancer H520 cell-bearing nude mice37. Thus, different administration methods of PD should be taken into consideration. It is tempting to speculate that oral treatment, which is the traditional method of Platycodonis Radix application, might be a preferable mode for PD treatment in future trials. Nevertheless, it has been reported that intraperitoneal treatment with 5 mg/kg PD also significant inhibits tumor growth in breast cancer MDA-MB-231 cell-bearing nude mice without any meaningful decrease in body weight10. Therefore, disparate inhibition effects on tumor growth and side effects of PD are obtained with different administration methods in various tumor types.
Apoptosis and autophagy are two main types of programmed cell death38. We previously confirmed that apoptosis and autophagy play important roles in PD-mediated cancer cell death15. In the current study, an increased number of apoptotic cells induced by PD in BEL-7402 cells was observed using an Annexin V/PI staining assay (Figure 3A and 3B) and a Hoechst 33342 staining assay (Figure 3C). The levels of cleaved PARP, cleaved caspase-3 and Bax/Bcl-2 ratio were up-regulated after PD treatment, while Bcl-2 was decreased after PD treatment (Figures 3D and 3E), further verifying that PD induces apoptosis in various cancer cell types. Moreover, our data confirmed that PD triggered autophagy in BEL-7402 cells with visible autophagic vacuoles (Figure 4A), increased MDC-positive cells (Figure 4B), and enhanced levels of LC3-II (Figures 4C and 4D). Until now, accumulating results from various cancer cell lines (human breast cancer MCF-7 and MDA-MB-231 cells; human lung cancer A549 and 95D cells; human hepatocellular carcinoma HepG2, Hep3B and BEL-7402 cells; and the non-cancer human L-O2 cells and rat myocardium H9c2 cells) have shown that PD triggers autophagy, indicating that this is not a cell type-specific but instead a universal phenomenon.
Various anticancer agents, such as berberine, oridonin, paclitaxel, glycyrrhetinic acid and Mono-Pt, have been reported to be autophagy regulators39,40,41. For example, glycyrrhetinic acid, one of the triterpene saponins isolated from licorice, induces autophagy as a defense mechanism against apoptosis in hepatocellular carcinoma cells40, while Mono-Pt, a novel monofunctional platinum (II) complex, induces apoptosis-independent autophagic cell death in human ovarian carcinoma cells41. Using the CQ and BAF autophagy inhibitors, the role of autophagy in PD-induced cell death was further investigated. The PD-induced proliferative inhibition and apoptotic effects were enhanced by either CQ or BAF co-treatment (Figure 5), suggesting that autophagy may serve as a critical defensive mechanism in PD-treated BEL-7402 cells.
For a better understanding of PD-triggered autophagy, the effects of PD on signaling pathways were further defined. The promotion of autophagy via Akt/mTOR pathway inhibition is the classical regulatory pathway of autophagy42. In our previous study and in the preliminary experiment, the Akt/mTOR pathway was not essential for PD-triggered autophagy in HepG2 cells16 and BEL-7402 cells (data not shown). The ERK pathway, which was activated and partially regulated autophagy in PD-treated HepG2 cells16, is expected to mediate autophagy in response to PD treatment in BEL-7402 cells. JNK is also known as a stress-activated protein kinase (SAPK) of the MAPK family, and it is initially activated in response to a variety of stress signals43. Increasing evidence shows that JNK has been implicated in many cellular events including apoptosis and autophagy29. PD treatment led to activation of the ERK and JNK pathways in BEL-7402 cells. Blockage of either of these pathways using respective kinase inhibitors (U0126 and SP600125) or the corresponding siRNAs limitedly abated PD-triggered autophagy. PD-triggered autophagy was only effectively reversed when the ERK and JNK signals were simultaneously abolished. Additionally, PD-mediated apoptosis was amplified when both ERK and JNK pathways were blocked (Figure 6C and 6F). Thus, ERK and JNK have distinct but cooperative roles in the regulation of PD-mediated autophagy. Our recent data also clearly showed that these two pathways might influence each other by a positive feedback when using the respective kinase inhibitors (Figure 6A), which was consistent with a previous study44. Nevertheless, the relationship between these two signaling pathways involved in the autophagy process is still unclear and needs further clarification.
Overall, these data clearly demonstrated that PD not only inhibits cell proliferation of BEL-7402 cells but also suppresses BEL-7402 xenograft tumor growth. The PD-induced cell proliferation inhibition and apoptosis are further amplified by co-treatment with autophagy inhibitors and PD. Our results also established a novel mechanism in which the ERK and JNK pathways may cooperatively mediate PD-triggered autophagy.
Author contribution
Ting LI and Xiao-huang XU performed the majority of experiments; Zheng-hai TANG, Ya-fang WANG, Chung-hang LEUNG, Dik-lung MA, Xiu-ping CHEN, and Yi-tao WANG analyzed the data as well as wrote and revised the manuscript; Ting LI, Xiao-huang XU, Yi CHEN, and Jin-jian LU designed the study.
Acknowledgments
This work was supported by the Macao Science and Technology Development Fund (070/2013/A), the Research Fund of University of Macau (MRG008-LJJ2014-ICMS MYRG2015-00091-ICMS-QRCM, and MYRG2015-00101-ICMS-QRCM), and the State Key Laboratory of Drug Research (SIMM1403KF-11).
References
- 1Park DI, Lee JH, Moon SK, Kim CH, Lee YT, Cheong J, et al. Induction of apoptosis and inhibition of telomerase activity by aqueous extract from Platycodon grandiflorum in human lung carcinoma cells. Pharmacol Res 2005; 51: 437–43. [DOI] [PubMed] [Google Scholar]
- 2Xie Y, Deng W, Sun H, Li D. Platycodin D2 is a potential less hemolytic saponin adjuvant eliciting Th1 and Th2 immune responses. Int Immunopharmacol 2008; 8: 1143–50. [DOI] [PubMed] [Google Scholar]
- 3Ahn KS, Noh EJ, Zhao HL, Jung SH, Kang SS, Kim YS. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-kappaB activation in RAW 264.7 cells. Life Sci 2005; 76: 2315–28. [DOI] [PubMed] [Google Scholar]
- 4Kim YP, Lee EB, Kim SY, Li D, Ban HS, Lim SS, et al. Inhibition of prostaglandin E2 production by platycodin D isolated from the root of Platycodon grandiflorum. Planta Med 2001; 67: 362–4. [DOI] [PubMed] [Google Scholar]
- 5Choi SS, Han EJ, Lee TH, Lee JK, Han KJ, Lee HK, et al. Antinociceptive mechanisms of platycodin D administered intracerebroventricularly in the mouse. Planta Med 2002; 68: 794–8. [DOI] [PubMed] [Google Scholar]
- 6Lee H, Kang R, Kim YS, Chung SI, Yoon Y. Platycodin D inhibits adipogenesis of 3T3-L1 cells by modulating Kruppel-like factor 2 and peroxisome proliferator-activated receptor gamma. Phytother Res 2010; 24: S161–7. [DOI] [PubMed] [Google Scholar]
- 7Wu JT, Yang GW, Zhu WX, Wen WJ, Zhang FM, Yuan JD, et al. Anti-atherosclerotic activity of platycodin D derived from roots of Platycodon grandiflorum in human endothelial cells. Biol Pharm Bull 2012; 35: 1216–21. [DOI] [PubMed] [Google Scholar]
- 8Ahn KS, Hahn BS, Kwack KB, Lee EB, Kim YS. Platycodin D-induced apoptosis through nuclear factor-kappa B activation in immortalized keratinocytes. Eur J Pharmacol 2006; 537: 1–11. [DOI] [PubMed] [Google Scholar]
- 9Chun J, Joo EJ, Kang M, Kim YS. Platycodin D induces anoikis and caspase-mediated apoptosis via p38 MAPK in AGS human gastric cancer cells. J Cell Biochem 2013; 114: 456–70. [DOI] [PubMed] [Google Scholar]
- 10Chun J, Kim YS. Platycodin D inhibits migration, invasion, and growth of MDA-MB-231 human breast cancer cells via suppression of EGFR-mediated Ala and MAPK pathways. Chem-Biol Interact 2013; 205: 212–21. [DOI] [PubMed] [Google Scholar]
- 11Kim MO, Moon DO, Choi YH, Lee JD, Kim ND, Kim GY. Platycodin D induces mitotic arrest in vitro, leading to endoreduplication, inhibition of proliferation and apoptosis in leukemia cells. Int J Cancer 2008; 122: 2674–81. [DOI] [PubMed] [Google Scholar]
- 12Kim MO, Moon DO, Choi YH, Shin DY, Kang HS, Choi BT, et al. Platycodin D induces apoptosis and decreases telomerase activity in human leukemia cells. Cancer Lett 2008; 261: 98–107. [DOI] [PubMed] [Google Scholar]
- 13Qin H, Du X, Zhang Y, Wang R. Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, induces G2/M arrest and apoptosis in human hepatoma HepG2 cells by modulating the PI3K/Akt pathway. Tumour Biol 2014; 35: 1267–74. [DOI] [PubMed] [Google Scholar]
- 14Tang ZH, Li T, Gao HW, Sun W, Chen XP, Wang YT, et al. Platycodin D from Platycodonis Radix enhances the anti-proliferative effects of doxorubicin on breast cancer MCF-7 and MDA-MB-231 cells. Chin Med 2014; 9: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Li T, Xu WS, Wu GS, Chen XP, Wang YT, Lu JJ. Platycodin D induces apoptosis, and iInhibits adhesion, migration and invasion in HepG2 hepatocellular carcinoma cells. Asian Pac J Cancer P 2014; 15: 1745–9. [DOI] [PubMed] [Google Scholar]
- 16Li T, Tang ZH, Xu WS, Wu GS, Wang YF, Chang LL, et al. Platycodin D triggers autophagy through activation of extracellular signal-regulated kinase in hepatocellular carcinoma HepG2 cells. Eur J Pharmacol 2015; 749: 81–8. [DOI] [PubMed] [Google Scholar]
- 17Pardo R, Lo Re A, Archange C, Ropolo A, Papademetrio DL, Gonzalez CD, et al. Gemcitabine induces the VMP1-mediated autophagy pathway to promote apoptotic death in human pancreatic cancer cells. Pancreatology 2010; 10: 19–26. [DOI] [PubMed] [Google Scholar]
- 18Levine B. Cell biology: autophagy and cancer. Nature 2007; 446: 745–7. [DOI] [PubMed] [Google Scholar]
- 19Chen P, Cescon M, Bonaldo P. Autophagy-mediated regulation of macrophages and its applications for cancer. Autophagy 2014; 10: 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 2012; 12: 401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Menon MB, Kotlyarov A, Gaestel M. SB202190-induced cell type-specific vacuole formation and defective autophagy do not depend on p38 MAP kinase inhibition. PLoS One 2011; 6: e 23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Kitanaka C, Kuchino Y. Caspase-independent programmed cell death with necrotic morphology. Cell Death Differ 1999; 6: 508–15. [DOI] [PubMed] [Google Scholar]
- 23Yoon YH, Cho KS, Hwang JJ, Lee SJ, Choi JA, Koh JY. Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Invest Ophthalmol Vis Sci 2010; 51: 6030–7. [DOI] [PubMed] [Google Scholar]
- 24Amaravadi RK, Yu DB, Thomas-Tikhonenko A, Thompson CB. Chloroquine inhibits autophagy, enhances p53-dependent apoptosis, and delays tumor recurrence in a mouse model of B cell lymphoma. Blood 2005; 106: 681–9.15817677 [Google Scholar]
- 25Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy 2007; 3: 542–5. [DOI] [PubMed] [Google Scholar]
- 26Shi Y, Han JJ, Tennakoon JB, Mehta FF, Merchant FA, Burns AR, et al. Androgens promote prostate cancer cell growth through induction of autophagy. Mol Endocrinol 2013; 27: 280–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Shinojima N, Yokoyama T, Kondo Y, Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy 2007; 3: 635–7. [DOI] [PubMed] [Google Scholar]
- 28Duncia JV, Santella JB, Higley CA, Pitts WJ, Wityak J, Frietze WE, et al. MEK inhibitors: The chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett 1998; 8: 2839–44. [DOI] [PubMed] [Google Scholar]
- 29Sui XB, Kong N, Ye L, Han WD, Zhou JC, Zhang Q, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett 2014; 344: 174–9. [DOI] [PubMed] [Google Scholar]
- 30Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000; 103: 239–52. [DOI] [PubMed] [Google Scholar]
- 31Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A 2001; 98: 13681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Huang M, Lu JJ, Huang MQ, Bao JL, Chen XP, Wang YT. Terpenoids: natural products for cancer therapy. Expert Opin Investig Drugs 2012; 21: 1801–18. [DOI] [PubMed] [Google Scholar]
- 33Lu JJ, Bao JL, Chen XP, Huang M, Wang YT. Alkaloids isolated from natural herbs as the anticancer agents. Evid Based Complement Alternat Med 2012; 2012: 485042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Lu JJ, Bao JL, Wu GS, Xu WS, Huang MQ, Chen XP, et al. Quinones derived from plant secondary metabolites as anti-cancer agents. Anticancer Agents Med Chem 2013; 13: 456–63. [PubMed] [Google Scholar]
- 35Chen X, Bao J, Guo J, Ding Q, Lu J, Huang M, et al. Biological activities and potential molecular targets of cucurbitacins: a focus on cancer. Anticancer drugs 2012; 23: 777–87. [DOI] [PubMed] [Google Scholar]
- 36Lu JJ, Dang YY, Huang M, Xu WS, Chen XP, Wang YT. Anti-cancer properties of terpenoids isolated from Rhizoma Curcumae--a review. J Ethnopharmacol 2012; 143: 406–11. [DOI] [PubMed] [Google Scholar]
- 37Park JC, Lee YJ, Choi HY, Shin YK, Kim JD, Ku SK. In vivo and in vitro antitumor effects of platycodin D, a saponin purified from platycodi radix on the H520 lung cancer cell. Evid Based Complement Alternat Med 2014; 2014: 478653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, et al. Apoptosis and cancer: mutations within caspase genes. J Med Genet 2009; 46: 497–510. [DOI] [PubMed] [Google Scholar]
- 39Ding Q, Bao J, Zhao W, Hu Y, Lu J, Chen X. Natural autophagy regulators in cancer therapy: a review. Phytochem Rev 2014: 1–18.
- 40Tang ZH, Li T, Chang LL, Zhu H, Tong YG, Chen XP, et al. Glycyrrhetinic Acid triggers a protective autophagy by activation of extracellular regulated protein kinases in hepatocellular carcinoma cells. J Agric Food Chem 2014; 62: 11910–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Guo WJ, Zhang YM, Zhang L, Huang B, Tao FF, Chen W, et al. Novel monofunctional platinum (II) complex Mono-Pt induces apoptosis-independent autophagic cell death in human ovarian carcinoma cells distinct from cisplatin. Autophagy 2013; 9: 996–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 2010; 465: 942–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298: 1911–2. [DOI] [PubMed] [Google Scholar]
- 44He W, Wang Q, Srinivasan B, Xu J, Padilla MT, Li Z, et al. A JNK-mediated autophagy pathway that triggers c-IAP degradation and necroptosis for anticancer chemotherapy. Oncogene 2014; 33: 3004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]