Abstract
Objectives:
In clinical trials, treatment with the glucagon-like peptide 2 analog teduglutide was associated with improved fluid and nutrient absorption and increased intestinal villus height and crypt depth in patients with short bowel syndrome (SBS). Plasma citrulline, an amino acid produced by enterocytes, is considered a measure of enterocyte mass. This analysis assessed changes in plasma citrulline levels in patients with SBS in 2 phase III clinical studies of teduglutide.
Methods:
Both teduglutide studies (0.05 or 0.10 mg/kg/day in CL0600-004 and 0.05 mg/kg/day in CL0600-020) were phase III, 24-week, double-blind, and placebo controlled. Plasma citrulline levels were analyzed and validated by liquid chromatography coupled to tandem mass spectrometry.
Results:
In both the CL0600-004 and CL0600-020 studies, change in mean plasma citrulline concentrations at Week 24 vs. baseline was significantly greater with teduglutide compared with placebo (10.9 (0.05-mg/kg/day dose) and 15.7 (0.10-mg/kg/day dose) vs. 2.0 μmol/L and 20.6 vs. 0.7 μmol/L, respectively, for each study (P≤0.0001 for each comparison with placebo)). Teduglutide treatment was associated with reductions from baseline in PS (parenteral support) volume requirements; however, a significant correlation between PS reduction and increase in plasma citrulline at Week 24 was observed in only one out of the three teduglutide treatment groups.
Conclusions:
In 2 phase III studies, patients receiving teduglutide had significant increases in plasma citrulline at Week 24 compared with patients receiving placebo. Increases in plasma citrulline concentrations likely reflect enterocyte mass expansion, but no clear correlation was detected between change in plasma citrulline and change in weekly PS volume.
Introduction
Many patients with short bowel syndrome (SBS) lack the intestinal absorptive capacity necessary to meet nutritional and hydration requirements with an oral diet; these patients require parenteral support (PS: parenteral nutrition and/or intravenous fluids) as compensatory treatment for malabsorption.1 Intestinal adaptation, which begins immediately following bowel resection, is the innate process whereby morphologic and functional changes occur in the remnant intestines, leading to an increase in absorption.2 This adaptation, which is stimulated by luminal nutrition and hyperphagia, is sufficient to permit a return to enteral autonomy in some patients.2, 3 The majority of intestinal adaptation in adults is generally thought to occur during the first 1–2 years following resection,2 but no objective, clinically practical markers have established the time course or extent of adaptation in humans.
Multiple factors are thought to mediate intestinal adaptation, including glucagon-like peptide 2 (GLP-2), an intestinotrophic peptide released by enteroendocrine L cells in response to nutrient stimulation.2 Teduglutide (Gattex, NPS Pharmaceuticals, Bedminster, NJ, USA) is a recombinant human GLP-2 analog indicated for the treatment of SBS in adult patients who are dependent on PS. Teduglutide promotes expansion of normal intestinal epithelium and augments enterocyte mass as demonstrated by increased villus height and crypt depth.4 In phases II and III clinical studies, teduglutide treatment has been associated with increased absorption, as evidenced by a significant decrease in weekly PS requirements, while maintaining fluid and nutrition status. Of the total of 134 patients treated with teduglutide 0.05 mg/kg/day, 16 patients have successfully achieved independence from PS in the phase III studies and their long-term extensions.5
Plasma citrulline, an amino acid produced predominantly by enterocytes, is considered a measure of enterocyte mass in patients with SBS.6 Several studies have documented decreased plasma citrulline levels among patients with SBS compared with healthy controls and have shown a strong positive association between plasma citrulline and small bowel length.7, 8, 9, 10, 11 Additionally, plasma citrulline may correlate with the extent of intestinal adaptation in patients with SBS. In one report, among 268 patients with SBS followed up on for a median of 4.4 years after final digestive circuit modification, higher plasma citrulline levels were significantly correlated with PS independence.12 In another study, the conversion of an oral bolus of alanine-glutamine into citrulline was significantly enhanced in patients with SBS who were ≥24 months after intestinal resection compared with patients who were earlier in their post-operative course.13
Here we evaluated changes in plasma citrulline levels with teduglutide treatment in 2 phase III clinical trials conducted in patients with SBS.
Methods
Study design
Two phase III, double-blind, randomized, parallel-group, controlled, multicenter studies of teduglutide were conducted in patients with SBS: CL0600-004 (ClinicalTrials.gov identifier NCT00081458) and CL0600-020 (STEPS; ClinicalTrials.gov identifier NCT00798967).14, 15 Details of trial designs and methodologies have been described previously.14, 15 Briefly, patients with SBS who required PS at least three times per week for ≥12 months were eligible for the studies. Before treatment initiation, patients underwent 0–8 weeks of PS optimization and 4–8 weeks of PS stabilization. Patients were then randomized 2:2:1 to treatment with teduglutide 0.05 mg/kg/day (the approved dose for clinical use), teduglutide 0.10 mg/kg/day, or placebo in study CL0600-004 and randomized 1:1 to teduglutide 0.05 mg/kg/day or placebo in study CL0600-020 for 24 weeks. Patients, study center personnel, the sponsor, and all persons associated with the monitoring or data management for the clinical study were blinded to the treatment assignments. Teduglutide and placebo were identical in appearance.
The primary efficacy end point for the CL0600-004 study was a graded response score criterion that accounted for both the intensity and duration of response at Week 24. For the CL0600-020 study, the primary efficacy end point was the percentage of patients who achieved a 20–100% reduction in PS volume from baseline at Week 20 and maintained that response at Week 24. Change in plasma citrulline levels from baseline was an exploratory end point in each of these studies. Analysis data sets included the data available; no imputation or last-observation-carried-forward methods were applied. Data from patients who withdrew early from the study or who did not have data available were not included in Week 24 assessments.
Citrulline measurements
Plasma citrulline levels were assessed at baseline and Weeks 4, 8, 16, and 24 in both studies. In addition, citrulline levels were assessed at Weeks 12 and 20 in the CL0600-004 study. Plasma samples were maintained at –70 °C until analysis. The analyte, L-citrulline, and internal standard D4 L-citrulline were extracted from 0.05 ml of human plasma by protein precipitation. Extracts were separated by reverse-phase chromatography on a Primesep 100 column (SIELC Technologies, Prospect Heights, IL, USA) using an isocratic gradient system of 0.2% formic acid in water and acetonitrile. Compounds were detected and quantified by tandem mass spectrometry in positive ion mode on an API 3000 mass spectrometer equipped with a TurboIonSpray interface (AB Sciex, Framingham, MA, USA). A generalized linear model was used for statistical analysis.
Results
In the CL0600-004 study (May 2004 to November 2007), 35 patients were randomized to teduglutide 0.05 mg/kg/day, 32 to teduglutide 0.10 mg/kg/day, and 16 to placebo.15 Of these, 27 patients in the teduglutide 0.05-mg/kg/day arm, 29 patients in the 0.10-mg/kg/day arm, and 16 patients in the placebo arm had citrulline data available at Week 24 and were included in the analyzed data set. In the CL0600-020 study (November 2008 to January 2011), 43 patients were randomized to teduglutide 0.05 mg/kg/day and 43 patients to placebo.14 Thirty-nine patients in each arm had citrulline data available at Week 24 and were included in the analyzed data set. Baseline demographics and characteristics of patients in both studies are reported by treatment group in Table 1. Within each study, there were no statistically significant differences between treatment groups in any of the demographic and baseline characteristics.
Table 1. Baseline patient and disease characteristics.
|
Study CL0600-004 |
Study CL0600-020 |
||||
|---|---|---|---|---|---|
| Placebo (n=16) | Teduglutide 0.05 mg/kg/day (n=35) | Teduglutide 0.10 mg/kg/day (n=32) | Placebo (n=43) | Teduglutide 0.05 mg/kg/day (n=43) | |
| Mean (s.d.) age, years | 49.4 (15.1) | 47.1 (14.2) | 50.3 (14.0) | 49.7 (15.6) | 50.9 (12.6) |
| Mean (s.d.) weight, kg | 61.3 (9.7) | 59.0 (8.4) | 59.6 (10.0) | 61.7 (12.6) | 62.7 (11.4)a |
| Sex, n (%) | |||||
| Women | 9 (56) | 18 (51) | 19 (59) | 24 (56) | 22 (51) |
| Race, n (%) | |||||
| Black | 1 (6) | 3 (9) | 2 (6) | 1 (2) | 0 |
| White | 15 (94) | 32 (91) | 30 (94) | 41 (95) | 42 (98) |
| Other | 0 | 0 | 1 (2) | 1 (2) | |
| Reason for major intestinal resection, n (%) | |||||
| Crohn's disease | 7 (44) | 10 (29) | 13 (41) | 8 (19) | 10 (23) |
| Vascular disease | 3 (19) | 14 (40) | 8 (25) | 16 (37) | 13 (30) |
| Injury | 1 (6) | 3 (9) | 2 (6) | 4 (9) | 4 (9) |
| Volvulus | 2 (13) | 5 (14) | 4 (13) | 6 (14) | 3 (7) |
| Cancer | 0 | 0 | 0 | 2 (5) | 1 (2) |
| Other | 3 (19) | 3 (9) | 5 (16) | 7 (16) | 12 (28) |
| Stoma, n (%) | 5 (31) | 10 (29) | 14 (44) | 17 (40) | 21 (49) |
| Jejunostomy | 4 (80) | 6 (60) | 4 (29) | 5 (29) | 11 (52) |
| Ileostomy | 1 (20) | 2 (20) | 7 (50) | 9 (53) | 6 (29) |
| Colostomy | 0 | 2 (20) | 3 (21) | 1 (6) | 4 (19) |
| Other | 0 | 0 | 0 | 2 (12) | 0 |
| Mean (s.d.) remnant small bowel length,f cm | 77.3 (52.9) | 58.3 (43.6) | 68.1 (43.1) | 68.7 (63.9) | 84.4 (64.6) |
| Presence of distal/terminal ileum, n (%) | 3 (19) | 6 (18)b | 8 (25) | 14 (33) | 10 (24) |
| Colon-in-continuity, n (%) | 11 (69) | 26 (74) | 19 (59) | 23 (53) | 26 (60) |
| Mean (s.d.) percentage of colon remaining | 67 (22)c | 71 (23)d | 69 (24) | 70 (27)e | 56 (20)e |
n=42.
n=34.
n=11.
n=26.
n=25.
Includes only patients with known residual small intestine length (n=15 for 004 placebo arm; n=31 for 004 teduglutide 0.05-mg/kg/day arm; n=27 for 004 teduglutide 0.10-mg/kg/day arm; n=40 for each arm of study 020).
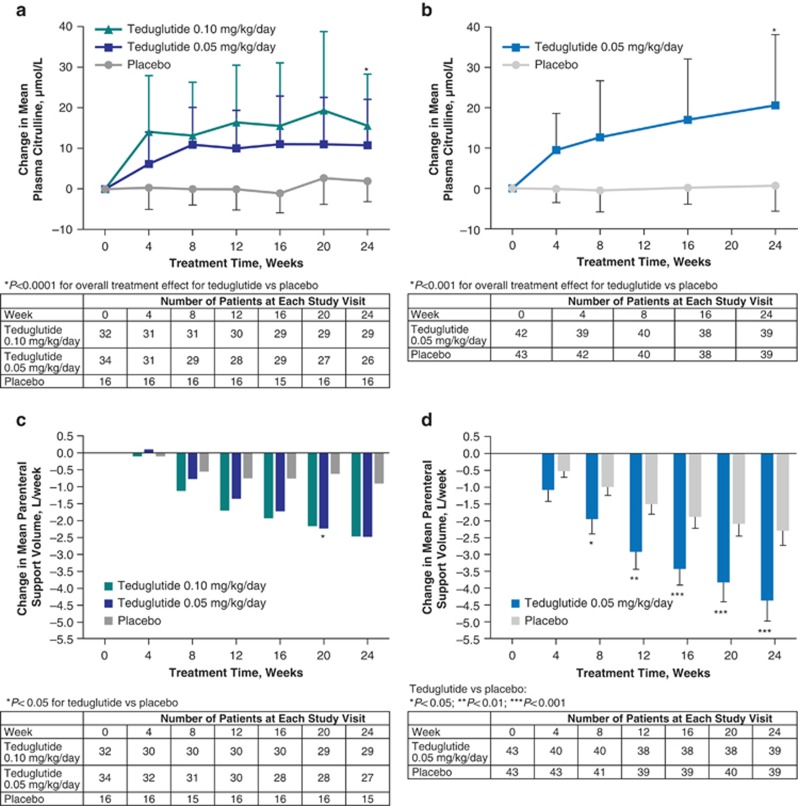
In both studies, increases in mean plasma citrulline at Week 24 vs. baseline were significantly greater in patients receiving teduglutide compared with those receiving placebo (Table 2). In the CL0600-004 study, teduglutide-treated patients experienced a 67% (0.05-mg/kg/day dose; n=26) and 113% (0.10-mg/kg/day dose) increase in mean plasma citrulline vs. an 8% increase for placebo-treated patients (P<0.0001 for each teduglutide dose compared with placebo). In the CL0600-020 study, mean plasma citrulline levels increased by 111% in the teduglutide group but increased by only 13% in the placebo group (P<0.0001). At Week 24, mean (s.d.) absolute citrulline levels among teduglutide-treated patients reached 29.5 (16.2) μmol/L (0.05-mg/kg/day dose) and 32.1 (15.4) μmol/L (0.10-mg/kg/day dose) in the CL0600-004 study and 37.9 (24.8) μmol/L in the CL0600-020 study (Table 2).
Table 2. Mean (s.d.) plasma citrulline levels at baseline and Week 24 in 2 phase III, placebo-controlled trials.
|
Study CL0600-004 |
Study CL0600-020 |
||||||
|---|---|---|---|---|---|---|---|
| Placebo (n=16) | Teduglutide 0.05 mg/kg/day (n=35) | Teduglutide 0.10 mg/kg/day (n=32) | P value | Placebo (n=43) | Teduglutide 0.05 mg/kg/day (n=43) | P value | |
| Baseline, μmol/L | 22.2 (10.6) | 18.0 (10.3)a | 16.6 (8.3) | — | 17.5 (9.0) | 18.4 (9.5)b | — |
| Week 24, μmol/L | 24.2 (13.6) | 29.5 (16.2)c | 32.1 (15.4)d | — | 17.2 (9.1)e | 37.9 (24.8)e | — |
| Change at Week 24 from baseline, μmol/L | 1.9 (5.0) | 10.9 (11.3)f | 15.7 (12.7)d | <0.0001 | 0.7 (6.3)e | 20.6 (17.5)e | <0.0001 |
| Change at Week 24 from baseline, % | 7.9 (20.5) | 66.7 (66.9)f | 113.1 (84.0)d | <0.0001 | 13.3 (55.8)e | 110.5 (66.2)e | <0.0001 |
n=34.
n=42.
n=27.
n=29.
n=39.
n=26.
Increases from baseline in mean plasma citrulline levels occurred early in the course of teduglutide treatment and were observed as soon as Week 4 (the first time point evaluated; Figure 1a). In both studies, mean plasma citrulline levels increased most rapidly during the first 8 weeks of teduglutide treatment. In the CL0600-004 study, mean (s.d.) plasma citrulline levels increased from baseline values of 18.0 (10.3) μmol/L with teduglutide 0.05 mg/kg/day (n=34) and 16.6 (8.3) μmol/L with teduglutide 0.10 mg/kg/day, to 29.4 (13.7) μmol/L (n=30) and 29.7 (16.2) μmol/L (n=31), respectively, at Week 8. In the CL0600-020 study, mean (s.d.) plasma citrulline levels increased from a baseline value of 18.4 (9.5) μmol/L (n=42) to 30.5 (20.1) μmol/L at Week 8 (n=40) with teduglutide 0.05 mg/kg/day. Between Weeks 8 and 24, mean plasma citrulline levels were maintained with teduglutide in study CL0600-004 (29.5 (16.2) and 32.1 (15.4) μmol/L at Week 24 with teduglutide 0.05 and 0.10 mg/kg/day, respectively) and increased more gradually with teduglutide 0.05 mg/kg/day in study CL0600-020 (37.9 (24.8) μmol/L at Week 24).
Figure 1.
Change in mean (s.d.) plasma citrulline concentration (a and b) and change in mean weekly parenteral support volume (c and d; error bars in d represent SE) in placebo-controlled, 24-week studies CL0600-004 (a and c) and CL0600-020 (b and d).
In the same studies, teduglutide treatment was also associated with decreases in PS requirements (Figure 1c). In the CL0600-004 study, patients receiving either dose of teduglutide experienced a 2.5-L/week reduction in PS volume at Week 24 compared with baseline (P≤0.001) vs. a 0.9-L/week reduction for patients receiving placebo (n=15) (P≤0.05; P=0.08 for comparisons between each teduglutide dose and placebo).15 In the CL0600-020 study, teduglutide-treated patients had a significantly greater reduction in PS volume from baseline compared with placebo-treated patients (–4.4 and –2.3 L/week, respectively; P<0.001 for between-group comparison).14 The correlation between change in plasma citrulline and change in PS volume was analyzed in study patients who received teduglutide. In the 0.05-mg/kg/day teduglutide arm of the CL0600-004 study, PS volume requirements decreased significantly with increasing plasma citrulline levels (r=–0.57; P=0.002; n=26). In contrast, in the 0.10-mg/kg/day teduglutide arm of the CL0600-004 study, and in the 0.05-mg/kg/day teduglutide arm of the CL0600-020 study, no correlation was found between change in plasma citrulline and change in PS volume requirements for patients receiving teduglutide (study CL0600-004: r=0.22; P=0.25; study CL0600-020: r=–0.23; P=0.16).
Discussion
In two placebo-controlled phase III studies, patients receiving teduglutide showed significant increases in mean plasma citrulline levels at Week 24 relative to baseline, whereas patients who received placebo had minimal changes. After 24 weeks of teduglutide treatment in patients with SBS, mean plasma citrulline levels were 29.5 and 32.1 μmol/L at the 0.05-mg/kg/day dose and the 0.10-mg/kg/day dose, respectively, in the CL0600-004 study and 37.9 μmol/L in the CL0600-020 study. These values are within the range observed for healthy controls from Western countries (~20–60 μmol/L; reported means, 26–40 μmol/L).7, 16, 17, 18, 19, 20 In a long-term follow-up study of patients who had undergone digestive circuit modification, citrulline levels >30 μmol/l adequately differentiated healthy controls from patients with SBS.7 Therefore, the increases in plasma citrulline observed in the studies reported here may be clinically relevant and may signify a greater ability of patients receiving teduglutide to attain enteral autonomy.
Increases in plasma citrulline levels occurred rapidly and were detected as early as 4 weeks following initiation of teduglutide therapy (the first time point evaluated). Citrulline levels did not increase indefinitely throughout the treatment period but reached a plateau or increased only modestly between 8 and 24 weeks of treatment. Interestingly, citrulline levels appear to decrease following discontinuation of teduglutide treatment. In a 28-week extension of the CL0600-004 study, mean plasma citrulline decreased by 20% (0.05 mg/kg/day) and 32% (0.10 mg/kg/day) within 4 weeks of teduglutide withdrawal, although final citrulline levels in both dose groups were still above baseline levels.21 These data suggest that uncontrolled intestinal growth does not occur throughout teduglutide treatment. Rather, the enterocyte mass expands most rapidly during the first 8 weeks of therapy and then achieves a steady state. Furthermore, decreases in citrulline concentrations following teduglutide discontinuation after short-term treatment (≤6 months) suggest that the effects on enterocyte mass may be at least partially reversible.
Multiple studies have confirmed the correlation between plasma citrulline levels and bowel length, suggesting that citrulline is a marker of enterocyte mass.7, 8, 9, 10, 11 However, the question of whether changes in plasma citrulline reflect functional changes in intestinal absorptive capacity remains unresolved. Three previous studies have demonstrated positive correlations between plasma citrulline levels and absorption of nutrients, including fat, protein, and xylose, in adult patients with SBS.7, 10, 11 Furthermore, in a longitudinal study of 36 hospitalized pediatric patients dependent on PS, plasma citrulline levels increased over a 3-week study period in the subset of patients without bowel losses who received some enteral nutrition and who were expected to regain full bowel activity.22 These data suggest that citrulline levels may function as a biomarker for intestinal absorptive capacity in these patients.23 In contrast, two prior studies found no significant association between plasma citrulline levels and intestinal nutrient or energy absorption in patients with SBS.9, 18 Thus, the changes in plasma citrulline reported here likely reflect increased enterocyte mass but may be less well correlated to changes in intestinal absorptive capacity.
No clear association was detected between change in plasma citrulline levels and change in specific weekly PS volume. Although decreases in PS requirements were significantly correlated with increases in plasma citrulline levels at Week 24 with teduglutide 0.05 mg/kg/day in study CL0600-004, no correlation was observed with teduglutide 0.10 mg/kg/day, or with teduglutide 0.05 mg/kg/day in study CL0600-020. Evaluations of citrulline levels were exploratory end points in these studies, which were not designed to detect correlations between changes in citrulline and other study end points and may not have been sufficiently powered to do so. Interestingly, a potential temporal relationship between plasma citrulline levels and weekly PS volume with teduglutide may be inferred from the figure. Mean plasma citrulline levels rose most rapidly during the first 8 weeks of teduglutide treatment in study CL0600-004 (Figure 1a) and CL0600-020 (Figure 1b), possibly indicating an increase in enterocyte mass during this period. In contrast, the majority of the PS volume reductions were achieved between Weeks 8 and 24 (Figure 1c), likely reflecting enhanced intestinal absorption following an initial teduglutide-mediated mucosal expansion. Nonetheless, further clinical evidence is required to determine whether citrulline may function as a biomarker for adaptive changes induced by teduglutide.
Alternative methods of assessing citrulline may provide further information regarding enterocyte function. Whereas most studies, including this one, have evaluated fasting plasma citrulline levels, Peters et al.13 monitored the conversion of an oral bolus of alanine-glutamine into citrulline. Using this technique, they showed that patients with SBS who had undergone final digestive circuit modification ≥24 months before, and who presumably had achieved most of their post-resection adaptive potential, generated greater amounts of citrulline than did patients who had undergone resection more recently and were still in the adaptive phase.13
Rapid, clinically relevant increases in plasma citrulline concentrations following teduglutide treatment likely reflect expansion of enterocyte mass. These data, combined with the increases in villus height and crypt depth associated with teduglutide therapy,4 suggest a mechanistic basis for the observed enhancements in absorption among SBS patients treated with teduglutide.14, 15, 24 In addition, increases in citrulline may indicate enhanced enterocyte function, improvements in intestinal absorptive capacity, and a greater probability of achieving enteral autonomy, although currently available data are not sufficient to draw firm conclusions at this time. Additional studies evaluating citrulline levels in real-world practice are needed to further establish its usefulness as a predictive biomarker of intestinal function.
Study Highlights
Footnotes
Guarantor of the article: Douglas L. Seidner, MD, AGAF, FACG, CNSC.
Specific author contributions: All authors assisted in drafting and/or revising the manuscript and approved the final version of the manuscript. All authors contributed to planning and/or conducting the study, and collecting and/or interpreting data. D.L.S. and F.J. were investigators of the studies.
Financial support: This study was funded by NPS Pharmaceuticals. Writing support was provided by Heather Heerssen, PhD, of Complete Healthcare Communications, and was funded by NPS Pharmaceuticals.
Potential competing interests: D.L.S. was an investigator for teduglutide clinical studies sponsored by NPS Pharmaceuticals, and has received funding for research; he is the medical director for Vanderbilt-Walgreens Home Infusion and Respiratory Services and is a consultant to Walgreens Home Infusion. F.J. was an investigator for teduglutide clinical studies sponsored by NPS Pharmaceuticals. N.N.Y. is an employee of NPS Pharmaceuticals.
References
- O'Keefe SJ, Buchman AL, Fishbein TM et al. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol 2006; 4: 6–10. [DOI] [PubMed] [Google Scholar]
- Tappenden KA. Intestinal adaptation following resection. JPEN J Parenter Enteral Nutr 2014; 38: 23S–31S. [DOI] [PubMed] [Google Scholar]
- Crenn P, Morin MC, Joly F et al. Net digestive absorption and adaptive hyperphagia in adult short bowel patients. Gut 2004; 53: 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappenden KA, Edelman J, Joelsson B. Teduglutide enhances structural adaptation of the small intestinal mucosa in patients with short bowel syndrome. J Clin Gastroenterol 2013; 47: 602–607. [DOI] [PubMed] [Google Scholar]
- Jeppesen PB, Boullata J, Ziegler ZR et al. Independence from parenteral support achieved with teduglutide treatment in patients with intestinal failure associated with short bowel syndrome. Clin Nutr 2014; 33: S68–S69. [Google Scholar]
- Lutgens L, Lambin P. Biomarkers for radiation-induced small bowel epithelial damage: an emerging role for plasma citrulline. World J Gastroenterol 2007; 13: 3033–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenn P, Coudray-Lucas C, Thuillier F et al. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 2000; 119: 1496–1505. [DOI] [PubMed] [Google Scholar]
- Santarpia L, Catanzano F, Ruoppolo M et al. Citrulline blood levels as indicators of residual intestinal absorption in patients with short bowel syndrome. Ann Nutr Metab 2008; 53: 137–142. [DOI] [PubMed] [Google Scholar]
- Luo M, Fernandez-Estivariz C, Manatunga AK et al. Are plasma citrulline and glutamine biomarkers of intestinal absorptive function in patients with short bowel syndrome? JPEN J Parenter Enteral Nutr 2007; 31: 1–7. [DOI] [PubMed] [Google Scholar]
- Papadia C, Sherwood RA, Kalantzis C et al. Plasma citrulline concentration: a reliable marker of small bowel absorptive capacity independent of intestinal inflammation. Am J Gastroenterol 2007; 102: 1474–1482. [DOI] [PubMed] [Google Scholar]
- Jianfeng G, Weiming Z, Ning L et al. Serum citrulline is a simple quantitative marker for small intestinal enterocytes mass and absorption function in short bowel patients. J Surg Res 2005; 127: 177–182. [DOI] [PubMed] [Google Scholar]
- Amiot A, Messing B, Corcos O et al. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin Nutr 2013; 32: 368–374. [DOI] [PubMed] [Google Scholar]
- Peters JH, Wierdsma NJ, Teerlink T et al. The citrulline generation test: proposal for a new enterocyte function test. Aliment Pharmacol Ther 2008; 27: 1300–1310. [DOI] [PubMed] [Google Scholar]
- Jeppesen PB, Pertkiewicz M, Messing B et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 2012; 143: 1473–1481. [DOI] [PubMed] [Google Scholar]
- Jeppesen PB, Gilroy R, Pertkiewicz M et al. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 2011; 60: 902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol AT, Tayek JA. A decrease in glucose production is associated with an increase in plasma citrulline response to oral arginine in normal volunteers. Metabolism 2003; 52: 1512–1516. [DOI] [PubMed] [Google Scholar]
- Crenn P, Vahedi K, Lavergne-Slove A et al. Plasma citrulline: a marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 2003; 124: 1210–1219. [DOI] [PubMed] [Google Scholar]
- Peters JH, Wierdsma NJ, Teerlink T et al. Poor diagnostic accuracy of a single fasting plasma citrulline concentration to assess intestinal energy absorption capacity. Am J Gastroenterol 2007; 102: 2814–2819. [DOI] [PubMed] [Google Scholar]
- Pita AM, Wakabayashi Y, Fernandez-Bustos MA et al. Plasma urea-cycle-related amino acids, ammonium levels, and urinary orotic acid excretion in short-bowel patients managed with an oral diet. Clin Nutr 2003; 22: 93–98. [DOI] [PubMed] [Google Scholar]
- Rabier D, Kamoun P. Metabolism of citrulline in man. Amino Acids 1995; 9: 299–316. [DOI] [PubMed] [Google Scholar]
- O'Keefe SJ, Jeppesen PB, Gilroy R et al. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol 2013; 11: 815–823. [DOI] [PubMed] [Google Scholar]
- Stultz JS, Tillman EM, Helms RA. Plasma citrulline concentration as a biomarker for bowel loss and adaptation in hospitalized pediatric patients requiring parenteral nutrition. Nutr Clin Pract 2011; 26: 681–687. [DOI] [PubMed] [Google Scholar]
- Papadia C, Di Sabatino A, Corazza GR et al. Diagnosing small bowel malabsorption: a review. Intern Emerg Med 2014; 9: 3–8. [DOI] [PubMed] [Google Scholar]
- Jeppesen PB, Sanguinetti EL, Buchman A et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 2005; 54: 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]