Abstract
There is significant research interest in developing and validating novel pancreatic cyst-fluid biomarkers given the increasing recognition of the prevalence of pancreatic cysts and their associated malignant potential. Although current international consensus guidelines are helpful, they fail to diagnose with certainty the cyst type and the level of epithelial dysplasia. They also fall short in predicting the future likelihood of malignant transformation. A systematic review was performed with the objective of summarizing cyst-fluid-based biomarkers that have been published in the medical literature over the past 10 years and characterizing the current quality of evidence. Our review demonstrates that there is an increasing interest in this topic with several different and innovative approaches including DNA, RNA, proteomic, and metabolomics profiling. Further techniques to improve upon cytological yield have also been studied. Besides identifying potentially useful clinical biomarkers, these empiric approaches have provided further insight into their pathogenesis. The level of evidence for the vast majority of these studies, however, is limited to retrospective early validation studies. The path forward will be to select out the most promising biomarkers and develop multicenter consortiums capable of capturing adequate sample sizes with appropriate study designs.
Introduction
Pancreatic cystic lesions are more frequently being identified with routine cross-sectional imaging. Although the exact prevalence in the general population is unknown, the best estimate is ~2% for adults over the age of 40.1, 2 The incidence increases with age with ~10% of patients over 80 having an incidental pancreatic cyst. The most commonly observed cyst types include intraductal papillary mucinous neoplasms (IPMN), mucinous cystic neoplasms (MCN), serous cystic neoplasms (SCN), and pseudocysts (PC).3 MCNs and IPMNs harbor malignant potential and therefore require an accurate diagnosis with appropriate management. SCNs and PCs have no malignant potential and are subsequently managed differently.
International consensus guidelines were first developed in 2006 and recently updated in 2012 to standardize clinical management.4, 5 These guidelines utilize the available evidence of clinical and imaging characteristics to recommend surgery or surveillance. In the 2006 criteria, surgery was recommended for all clinically suspected MCNs and main duct IPMNs. For cysts suspected of being a branch-duct IPMN, surgery was recommended if they had any of the following: (1) an intracystic mural nodule; (2) an associated dilated main duct; (3) a cyst size >3 cm; (4) associated clinical symptoms; or (5) positive cytology by endoscopic ultrasound (EUS) guided fine needle aspiration (FNA). Although this algorithm demonstrated 100% sensitivity in identifying cysts with malignancy it had very-low specificity (23–31%) leading to many operations of questionable benefit.6, 7, 8 The revised 2012 guidelines intended to increase the specificity without compromising sensitivity for malignancy. Criteria to proceed directly to surgery were narrowed to those with (1) obstructive jaundice, (2) an enhancing solid component associated with the cyst, and (3) a main duct dilated >10 mm. These differences are highlighted in Table 1. For those with worrisome features, but none of the above high-risk stigmata, EUS is recommended to triage patients for surgery or surveillance.
Table 1. Changes in imaging and clinical criteria for recommending surgery between the original consensus guidelines (a.k.a. Sendai criteria, 2006) and revised consensus guidelines (a.k.a. Fukuoka criteria, 2012).
| Sendai criteria (2006) | Fukuoka criteria (2012) |
|---|---|
| Size>3 cm | Size cutoff abandoned |
| Presence of mural nodule | Presence of enhanced mural nodule |
| Dilated main duct>6 mm | Dilated main duct>10 mm |
| Symptomatic | Obstructive jaundice |
| Positive cytology | Unchanged |
Although these guidelines are helpful, they exist because there is currently no accurate and reliable method to obtain a definitive diagnosis. The preoperative diagnosis for cyst type is incorrect in 20–30% of cases.9, 10 Imaging modalities including computed tomography and magnetic resonance imaging are also suboptimal with poor interobserver variability.11, 12 Among the imaging modalities, EUS is considered the most sensitive in delineating malignant characteristics such as mural nodules.13, 14, 15, 16, 17, 18, 19, 20, 21 With EUS, FNA of the cyst can be performed for cytology. Because of the low amount of epithelial cells in the cyst fluid however, cytology has had a limited diagnostic yield with a pooled sensitivity of 63% and specificity of 88%.22 Beyond cytology, the tumor markers such as carcinoembryonic antigen (CEA) have been studied in the cyst fluid and found to differentiate mucinous from non-mucinous cysts with a diagnostic accuracy of 80%.23
The accuracy of CEA to define mucinous cysts is not high enough to be widely accepted as a routine diagnostic test. Specifically, a low value does not rule out a mucinous cyst and often surveillance is still required.23, 24, 25 The most recent consensus guideline recommends CEA be used in limited settings “in whom additional information will have an impact on the surgical decision making.”14 Given this limit in diagnostic accuracy with current imaging modalities, there remains a growing research interest in discovering and validating novel cyst-fluid biomarkers that may improve upon the performance of CEA. The types of biomarkers available for analysis have been expanding rapidly due to the development of innovative technologies that enable profiling of various bioparticles in the cyst fluid.
In this review, we attempt to comprehensively describe and categorize the published pancreatic cyst-fluid biomarkers. In doing so, we describe the current level of evidence for each biomarker in the context of a proposed framework for further validation to bring it into clinical practice.26
Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Eligible articles were identified by search of the MEDLINE database for the past 10 years (1 February 2005 to 1 February 2015). The following keywords were used: “pancrea*” AND “cyst*” AND “biomarker”, “pancrea*” AND “cyst*” AND “diagnosis”, “intraductal papillary mucinous neoplasm” AND (“RNA”, OR “DNA”, OR “protein” OR “proteomics” OR “cytokine” OR “metabolomics”), “mucinous cystic neoplasm” AND (“RNA”, OR “DNA”, OR “protein” OR “proteomics” OR “cytokine” OR “metabolomics”), “serous cystadenoma” AND (“RNA”, OR “DNA”, OR “protein” OR “proteomics” OR “cytokine” OR “metabolomics”) and “pseudocyst” AND (“RNA”, OR “DNA”, OR “protein” OR “proteomics” OR “cytokine” OR “metabolomics”).
Reported biomarkers based on immunohistochemistry or those not obtained directly from the pancreatic cyst by FNA were excluded (this includes studies of duodenal fluid). Studies that used cyst fluid obtained at surgery were included as there does not appear to be a difference with cyst fluid obtained during EUS.27 Studies only in English using human samples were included. Only studies that used histology as the gold standard were included. Review articles were excluded. As CEA is already used in current practice, further validation studies of CEA were excluded. The references of retrieved relevant reviews and articles were reviewed to identify other potential eligible studies. Both authors reviewed eligible studies from the search results. Data abstraction included the following variables: publication year, study design, sample size, distribution of cyst types, presence of histologic correlation, primary comparison (e.g., mucinous vs. non-mucinous, cancer vs. non-cancer, and serous cystadenoma vs. non-serous cystadenoma), area under the receiver operator curve (ROC), sensitivity, specificity, and diagnostic accuracy.
Results
A total of 2,840 articles were screened. Of these, 203 articles were further assessed for eligibility, and 41 studies were included in this review. Reasons for exclusion include: CEA validation studies (n=21), Immunohistochemistry only studies (n=45), pancreatic juice studies (n=13), review articles (n=12), and not meeting other inclusion/exclusion criteria (n=71).
DNA-based biomarkers
One of the first DNA-based biomarker studies in cyst-fluid was of KRAS mutations, which are present in over 90% of pancreatic adenocarcinomas and represent the most frequent and earliest genetic alterations toward cancer.28 Khalid et al.29 first described a potential difference in cyst-fluid expression using 36 samples that included 11 malignant cysts and 15 premalignant cysts. Besides studying KRAS mutations, DNA quantity, quality, number and sequence of mutations were also studied. Several subsequent studies followed using these set of DNA parameters (Table 1).30, 31, 32, 33, 34, 35, 36, 37 In sum, these studies showed the presence of a KRAS mutation to be highly specific (80–100%) but limited in sensitivity (33–86%) for diagnosing mucinous cysts (IPMN or MCN). More importantly, the majority of these studies did not find KRAS to be useful for differentiating malignant from premalignant mucinous cysts. However, in combination with other DNA parameters such as allelic loss, a few studies demonstrated decent sensitivity and specificity for diagnosing malignant cysts. Its routine use, consequently, remains controversial and is not widely accepted.
In search of other relevant mutations that might provide insight into the pathogenesis of IPMNs, Wu et al.38 performed an exploratory DNA analysis of cyst fluid from 19 patients with IPMNs. They identified recurrent mutations in codon 201 of GNAS (guanine nucleotide-binding protein G subunit alpha isoforms short) that is highly specific for IPMNs. Using a larger cohort of 147 cyst-fluid samples, they observed that 61% of IPMNs had GNAS mutations compared to 0% in SCNs and MCNs. Subsequent studies have since been performed on cyst fluid that validate these earlier findings (Table 2).39, 40 Although these studies were relatively smaller in sample size, they reproduced the observation that GNAS mutations were found only in IPMNs, (but not all IPMNs) and that the presence of either a GNAS or KRAS mutation was highly sensitive for IPMNs. The increasing use of next-generation sequencing technology may further elucidate other clinically useful DNA mutations to aid in the diagnosis and management of premalignant and malignant IPMNs and MCNs.41 Specifically, DNA mutations that are specific for high-risk cystic lesions (i.e., high-grade dysplasia and/or cancer) are yet to be identified.
Table 2. Summary of DNA-based cyst-fluid biomarkers.
| Year | First author | Biomarker | Sample size | Sample distribution | Phase | Observations |
|---|---|---|---|---|---|---|
| 2005 | Khalid et al.29 | KRAS mutation DNA quantity, quality, no. of mutations, and sequence of mutations | 36 | 15 Premalignant 11 Malignant | 2 | Difference observed with DNA quantity, quality, no. of mutations, and presence of KRAS mutation plus by LOH |
| 2006 | Schoedel et al.30 | KRAS mutation, LOH | 16 | IPMN: 4 HGD/Cancer vs. 12 (LGD/MGD) | 2 | KRAS mutation plus LOH present 50% of HGD/cancers vs. 8% in (LGD/MGD) premalignant IPMNs |
| 2009 | Khalid et al.31 | KRAS mutation, DNA quantity, allelic loss, mutational amplitude, and sequence of mutations | 113 | 40 Malignant 48 Premalignant 25 Benign | 2 | KRAS mutation had good specificity (96%) but limited sensitivity (45%) for mucinous cysts. KRAS mutations followed by allelic loss had limited sensitivity (37%) but good specificity (96%) for diagnosis of malignancy. Mean allelic loss amplitude (MALA) had the best diagnostic performance for distinguishing malignancy cysts. |
| 2009 | Sawhney et al.32 | KRAS mutation, DNA quantity, allelic loss | 19 | 17 Mucinous 2 Non-mucinous | 2 | DNA quantity: sensitivity 76% and specificity 100% for diagnosing mucinous cyst. |
| 2009 | Shen et al.33 | DNA Pathfinder TG | 35 | 6 Malignant 15 Pre-malignant, 14 Benign | 2 | Malignancy: sensitivity 83%, specificity 100% mucinous: sensitivity 86% specificity 93% |
| 2009 | Sreenarasimhaiah et al.34 | DNA Pathfinder TG | 20 | 2 Malignant 7 Pre-malignant 11 Benign | 2 | Mucinous: sensitivity 33%, specificity 92% |
| 2011 | Wu et al.38 | GNAS and KRAS mutations | 147 | 84 IPMN 21 MCN 42 SCA | 2 | GNAS mutations: 61% of IPMNs, 0% SCAs, 0% MCNs, KRAS mutations: 82% of IPMNs, 0% in SCAs and 33% MCNs |
| 2012 | Talar-Wojnarowski et al.35 | KRAS mutation | 56 | 17 Mucinous 39 Benign non-mucinous | 2 | Mucinous: sensitivity 65%, specificity 97% |
| 2013 | Siddiqui et al.39 | GNAS and KRAS mutations | 25 | 9 IPMN 16 non-IPMN | 2 | KRAS mutation (+) 60–70% Mucinous Cysts GNAS mutation (+) 44% IPMNs, 0% other mucinous cysts. All IPMNs had either KRAS or GNAS. |
| 2013 | Al-Haddad et al.37 | KRAS mutation | 48 | 38 Mucinous 10 Non-mucinous | 2 | Mucinous: sensitivity 50%, specificity 80% |
| 2013 | Nikifovora et al.36 | KRAS mutation | 142 | 12 Malignant 85 Pre-malignant 45 Benign non-mucinous | 2 | Mucinous: sensitivity 54%, specificity 100% |
| 2014 | Amato et al.41 | Next-generation sequencing | 10 | 10 IPMNs | 1 | 7 Out of 10 cyst-fluid samples provided adequate DNA for deep sequencing |
| 2014 | Singhi et al.40 | GNAS and KRAS mutations | 91 | 50 IPMNs, 16 MCNs, 25 Non-mucinous | 2 | Mucinous: GNAS or KRAS mutation sensitivity 65%, specificity 100% IPMNs: GNAS or KRAS mutation sensitivity 84%, specificity 98%. |
EUS, endoscopic ultrasound; FNA, fine needle aspiration; IPMN, intraductal papillary mucinous neoplasms; GNAS, guanine nucleotide-binding protein G subunit alpha isoforms short; LOH, loss of heterozygosity; MALA, mean allelic loss amplitudeic neoplasms; PC, pseudocysts; SCN, serous cystic neoplasms.
RNA-based biomarkers
There were five studies investigating micro-RNA (miRNA) biomarkers from cyst fluid (Table 3).42, 43, 44, 45, 46 Compared with DNA-based studies, this research studies have all been recently published in the last 4 years. While, the majority of these studies were primarily exploratory in nature without an a priori hypothesis, the fundamental basis for investigating miRNA stems from earlier observations that they can be involved in tumor initiation and progression.47 Two of the studies identified miRNA 21 to have clinical utility. Ryu et al.44 showed that miRNA 21 could differentiate mucinous cysts from non-mucinous cysts. Farrell et al.43 also observed that miRNA 21 could differentiate premalignant mucinous cysts from malignant cysts. Other studies identified a panel of miRNAs, and miRNA 216 and 217 as potentially useful for diagnosing surgically recommended cysts.42, 46 These studies suggest that miRNA bear significant potential to become useful clinical tests with further validation.
Table 3. Summary of RNA-based cyst-fluid biomarkers for cyst-fluid analysis.
| Year | First author | Biomarker | Sample size | Sample distribution | Phase | Observations |
|---|---|---|---|---|---|---|
| 2011 | Ryu et al.44 | Micro-RNA 21 | 40 | 24 Mucinous 16 Non-mucinous | 1 | Micro-RNA 21 had highest ROC for mucinous cysts: sensitivity 76%, specificity 80% |
| 2011 | Carrarra et al.45 | Mucin RNA expression | 19 | IPMNs | 1 | No unique significant pattern of RNA expression of mucin proteins identified among IPMNs. |
| 2012 | Matthaei et al.42 | Micro-RNA profile | 65 | 30 IPMNs 35 Non-mucinous | 1 | 37 candidate micro-RNA candidates could distinguish high-grade IPMNs. A panel of 9 micro-RNA candidates could differentiate surgically recommended lesions with sensitivity 89%, specificity 100% |
| 2013 | Farrell et al.43 | 6 micro-RNA panel | 38 | 4 Malignant 18 Pre-malignant 16 Benign non-mucinous | 1/2 | Mi-R21 had increasing levels from benign to premalignant to malignant |
| 2014 | Wang et al.46 | Micro-RNA profile | 17 | 3 Malignant 8 High Risk 6 Low Risk | 1 | Micro-RNA- 216 and 217 were differentially expressed between low grade, high grade, and cancer. |
IPMN, intraductal papillary mucinous neoplasms; ROC, region of interest.
Exploratory proteomic approaches
Like differential expressions observed in DNA and RNA profiles observed between cancer and normal tissue, differential protein expression may be logical focus for diagnostic test development. Several exploratory studies looking for differential protein expression in pancreatic cyst fluid have been performed (Table 4).48, 49, 50, 51, 52, 53, 54, 55 Like the RNA-based cyst studies, these did not have an a priori target and used technical variations of mass spectrometry. The sample sizes were generally small ranging from 8 to 59 samples, and the majority of them reported select protein groups that were differentially expressed in clinically relevant cyst types. For example, Allen et al.49 used a commercially available luminex assay and found a cluster of 14 proteins that differentiated SCNs from IPMNs with 92% accuracy. Although these empirical studies provide indirect insight into the pathogenesis of the various relevant cyst types and may identify candidate targets for further study, translation of these immediate findings into an easily usable diagnostic or predictive test remains a challenge.
Table 4. Summary of proteomic approaches for cyst-fluid analysis.
| Year | First author | Biomarker | Sample size | Sample distribution | Phase | Observations |
|---|---|---|---|---|---|---|
| 2007 | Scarlett et al.48 | SELDI-TOF MS | 10 | 3 Cancer 7 Non-cancer | 1 | 12 Non-specified protein peaks were differentially expressed between the two groups |
| 2009 | Allen et al.49 | Luminex Assay | 59 | 32 BD-IPMN 12 MCN 15 SCN | 1 | A cluster of 14 proteins differentiated SCA from IPMN in 92% of patients |
| 2009 | Ke et al.50 | MALDI-TOF MS 2D gel electrophoresis GeLC/MS/MS | 20 | 5 With histology | 1 | Found potential candidate biomarkers in homologs of amylase, mucin, CEACAM, and S100 |
| 2011 | Cuoghi et al.51 | SDS-PAGE LC-MS/MS | 8 | 2 MCN 2 SCN 2 PNET 1 IPMN 1 PC | 1 | Found unique protein expressions among different cyst types |
| 2012 | Corcos et al.52 | SELDI-TOF MS | 43 | 21 LGD IPMN 22 HGD IPMNs | 1 | 5 Non-specified protein profiles had ROC of 0.88 |
| 2012 | Lee et al.53 | 89—Cytokine Panel | 10 | 5 BD-IPMNs 5 Pseudocysts | 1 | GM-CSF and HGF were differentially present in inflammatory cysts |
| 2012 | Mann et al.54 | MALDI-TOF MS LC/MS | 20 | 13 Mucinous 7 Non-mucinous | 1 | Overexpressed glycoproteins differentially noted in mucinous cysts. |
| 2014 | Gbormittah et al.55 | HP-MLAC SDS-PAGE LC/MS | 20 | 10 Mucinous 10 Non-mucinous | 1 | Panel of proteins of interest were differentially expressed |
BD, branch duct; CEACAM, carcinoembryonic antigen-related cell adhesion molecule; GM-CSF, granulocyte macrophage colony stimulating factor; HGD, high grade dysplasia; HGF, hepatocyte growth factor; IPMN, intraductal papillary mucinous neoplasms; LC-MS/MS, liquid chromatography tandem mass spectrometry; LGD, low grade dysplasia; MALDI-TOF MS, matrix assisted lasor desorption/ionization time of flight mass spectrometry; MCN, mucinous cystic neoplasms; PC, pseudocysts; ROC, region of interest; SCA, serous cystadenoma; SCN, serous cystic neoplasms; SDS-PAGE, sodium dodecyl sulfate - polyacrylamide gel electrophoresis; SELDI-TOF MS, surface enhanced lasor desorption/ionization time of flight mass spectrometry; PNET, pancreatic neuroendocrine tumor.
Targeted protein-based biomarkers
Twelve studies were identified that reported on a specific protein or group of proteins in an exploratory or validation study for being potentially useful as a cyst-fluid biomarker (Table 5).56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67 Not surprisingly, a commonly studied protein group in cyst-fluid biomarkers is the secreted mucin proteins. Glycan variants on certain mucin proteins such as MUC5AC, have been described to be particularly sensitive (87–89%) and specific (80–100%) for diagnosing mucinous cysts.57, 66 Maker et al.65 found certain mucin proteins (MUC2 and MUC4) could differentiate dysplasia status in IPMNs. Morris-Stiff et al.64 studied general mucin expression by direct staining of the cyst fluid and observed a sensitivity of 80% and specificity of 40% for diagnosing mucinous cysts. Cao et al.66 also studied glycan alterations on mucin proteins and found that a three-marker panel could differentiate mucinous from non-mucinous cysts with high diagnostic accuracy. Of the different subtypes of mucin proteins, aberrant MUC1 has been strongly associated with pancreatic cancer. Jabbar et al.59 targeted MUC1 and observed it to diagnose malignant cysts with a sensitivity of 87.5% and specificity of 92.3% in a sample of 29 cysts.
Table 5. Summary of protein-based biomarkers for cyst-fluid analysis.
| Year | First author | Biomarker | Sample size | Sample distribution | Phase | Observations |
|---|---|---|---|---|---|---|
| 2008 | Schmidt et al.56 | Prostaglandin E2 | 58 | 29 IPMN 11 MCN 12 PDAC 6 Non-Mucinous | 2 | Prostaglandin E2 was elevated in IPMNs compared with MCNs |
| 2010 | Haab et al.57 | Glycosylation Variants | 53 | 32 Mucinous 21 Non-mucinous | 2 | Glycan variant MUC5AC sensitivity 78% specificity 80% for mucinous cysts |
| 2010 | Morris-Stiff et al.64 | Mucin | 128 | 86 Mucinous 42 Non-mucinous | 2 | Mucinous cysts: sensitivity 80% specificity 40%, when combined with CEA, sensitivity 83% specificity 65% |
| 2011 | Maker et al.65 | MUC1, MUC2, MUC4, MUC5AC | 40 | 21 LGD IPMN 19 HGD/Cancer IPMN | 2 | MUC 2 & 4 elevated in HGD IPMN compared with LGD IPMN. Intestinal type had increased expression of MUC2 |
| 2011 | Doyle et al.60 | TGF-alpha | 46 | 26 IPMN 9 MCN 6 SCN 5 PC | 1 | Only IPMN cysts had any levels above 95 pg/ml |
| 2011 | Maker et al.61 | Cytokine Panel Assay | 40 | 21 LGD IPMN 19 HGD/Cancer IPMN | 1 | IL—1beta had high diagnostic accuracy (ROC 0.92) for HGD/Cancer IPMNs compared with LGD IPMNs |
| 2012 | Tun et al.58 | Amphiregulin | 33 | 12 Malignant 15 Mucinous 6 Benign non-mucinous | 1 | Level>300 pg/ml sensitivity 83%, specificity 73% for malignancy (ROC 0.76) |
| 2013 | Cao et al.66 | MUC5AC-WGA, MUC5AC-BGH, Endorepellin-WGA | 44 | 27 Mucinous 17 Non-mucinous | 1 | Mucinous: sensitivity 89% specificity 100% |
| 2013 | Raty et al.67 | SPINK1 | 61 | 33 Mucinous 28 Non-mucinous | 2 | Surgically recommended lesions (MCN and main duct IPMN) vs. SCA or branch-duct IPMN=ROC 0.94 |
| 2014 | Jabbar et al.59 | MUC1 | 29 | 16 Malignant 13 Non-malignant | 2 | Malignancy: sensitivity 87.5% and specificity 92.3% |
| 2014 | Das et al.62 | mAb Das-1 | 38 | 27 IPMN 11 Non-mucinous | 1 | High-Risk IPMNs: Sensitivity 89% specificity 100% (High risk=IPMN-gastric with HGD, any IPMN-intestinal, and IPMN-pancreatico-biliary, any IPMN-oncocytic, and invasive IPMN.) |
| 2014 | Yip-Schneider et al.63 | VEGF | 87 | 17 SCN 70 Non-SCN | 2 | VEGF-A had an ROC of >0.99 for SCNs. With a cutoff set at 8,500 pg/ml, VEGF-A provides 100% sensitivity and 97% specificity as a biomarker for benign SCN lesions. VEGF-C had a similar diagnostic performance |
CEA, carcinoembryonic antigen; IPMN, intraductal papillary mucinous neoplasms; MCN, mucinous cystic neoplasms; PDAC, pancreatic ductal adenocarcinoma; ROC, region of interest; SCN, serous cystic neoplasms; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
Among the other studies, there are a few that appear to be promising and worth further validation primarily because of their potential to directly impact surgical decision-making. Maker et al.61 looked at cytokine expression differences and observed that IL-1B, an important pro-inflammatory mediator, was significantly higher in IPMNs with high-grade dysplasia and malignancy than low grade IPMNs (ROC 0.92). Amphiregulin, a secreted epidermal growth factor receptor ligand that is overexpressed in pancreatic cancer, has also been shown to differentiate high-grade dysplastic and cancerous cysts with an ROC of 0.76.58, 68 Raty et al.67 observed that cyst-fluid SPINK1 levels in pancreatic cysts could differentiate surgically recommended lesions (MCN, and main/mixed IPMNs) with an ROC of 0.94. More recently, Das et al.62 reported on the clinical utility of a monoclonal antibody reactive against a colonic phenotype of epithelium in pancreatic cysts. Monoclonal antibody Das-1 could differentiate high-risk IPMNs that might require surgery from low-risk IPMNs. This particular analysis was unique in that definition of high-risk incorporated IPMN histological subtype. The majority of these biomarkers focuses on identifying which lesions are high risk and necessitate immediate surgery. In contrast, Yip-Schneider et al.63 showed that cyst-fluid VEGF could diagnose SCNs with an ROC>0.99. Such a biomarker would remain valuable as these cysts do not need require surgery or need to be followed.
Metabolomics
If DNA profiling explains “what can happen”, RNA profiling explains “what appears to be happening”, and protein profiling explains “what makes it happen”, then metabolite profiling explains “what has happened and is happening”.69 To date, there is one study that profiles metabolites in the cyst fluid (Table 6). Park et al. used a semi-targeted method and identified four metabolites that were differentially expressed in mucinous cysts compared to non-mucinous cysts. With mass spectrometry, two were identified as glucose and kynurenine. The latter is a tryptophan metabolite that has been associated with cancer development.70 With a sample size of 45 cysts, both glucose (ROC 0.92) and kynurenine (0.94) demonstrated high diagnostic accuracies for mucinous cysts.71
Table 6. Summary of metabolomic and modified cytology techniques for cyst-fluid analysis.
| Year | First author | Biomarker | Sample size | Sample distribution | Phase | Observations |
|---|---|---|---|---|---|---|
| 2008 | Pitman et al.73 | AECs | 18 | 6 Malignant 12 Non-malignant | 2 | 5/6 (83%) malignant cysts+AEC vs. 4/12 (33%) non-malignant cysts+AEC |
| 2010 | Pitman et al.74 | AECs | 112 | 39 Malignant 73 Pre-malignant | 2 | Malignancy: AEC sensitivity 72%, specificity 85% |
| 2013 | Park et al.71 | Metabolomics: glucose and kynurenine | 45 | 31 Mucinous 14 Non-mucinous | 1 | Mucinous cysts: glucose ROC 0.92 and kynurenine ROC 0.94) |
AEC, atypical epithilial cell; ROC, region of interest.
Cytology revisited
One of the major impetuses for identifying novel cyst-fluid biomarkers stems from the poor diagnostic accuracy of cyst-fluid cytology. For the diagnosis of high-grade dysplasia or cancer, the specificity is high, while the sensitivity is low making it of limited value for surgical decision-making.72 This has been attributed to the scant quantity and quality of aspirated cells from the cyst cavity. To make cytological-based analysis more clinically useful, Pitman et al. devised a modified cytological system that included a category of atypical epithelial cells (AEC). AECs were defined by the presence of a single or cluster of cells with a relatively increased nuclear to cytoplasmic ratio and enlarged irregular nuclei. With this new classification system, they showed that in a sample of 18 patients, 83% (5 out of 6) of those with high-grade dysplasia or higher had the presence of AECS compared to 33% (4 out of 12) that did not.73 Using a larger sample cohort of 112 patients with mucinous cysts (39 of which were malignant), AEC sensitivity was 72% and specificity was 85% for diagnosing malignant cysts (Table 6).74 While this is significantly improved from the standard cytological classification system, its generalizability remains a significant barrier as substantial experience is required to achieve fair agreement among pathologists.75
Discussion
Our systemic review identified a substantial number of promising and novel cyst-fluid based biomarkers. From 2005 to 2015, the number of publications by time period demonstrates a growth in interest. From 2005 to 2009, there were 11 studies within our inclusion and exclusion criteria. In the latter half, there were 30 studies. This does not include numerous studies that were based on immunohistochemistry expression. The vast majority of the studies included in this review were single center retrospective studies and required histology as the gold standard for comparison. This latter part includes a selection bias that may limit the generalizability of these studies. Specifically, these sample cohorts select for a higher proportion of high-risk cystic lesions, which will likely impact the diagnostic performance characteristics in routine clinical settings when the majority of lesions tested will be low-risk cysts.
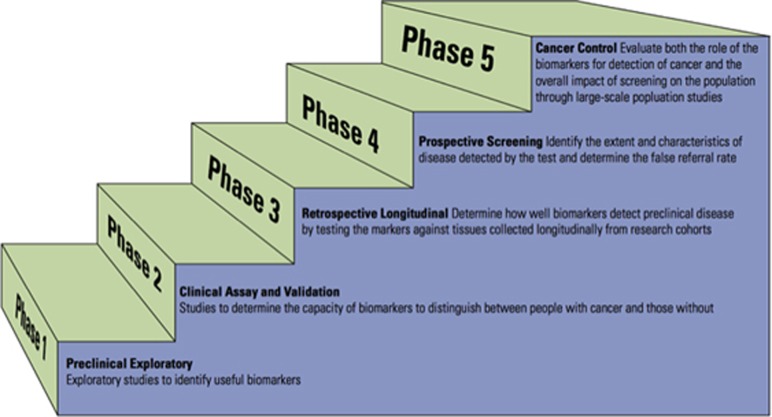
A framework for advancing biomarkers from the laboratory to the bedside has been proposed.26 This proposed pathway for the discovery and validation of cancer biomarkers has five phases: preclinical exploratory (phase 1), clinical assay and validation (phase 2), retrospective longitudinal (phase 3), prospective screening (phase 4), and cancer control (phase 5). (Figure 1) Preclinical exploratory studies typically compare tumor tissue from nontumor tissue to look for difference in tumor markers or gene expression levels. A clinical assay uses non-invasive methods to obtain specimens from cases as well as control subjects or subjects with benign growths to estimate the sensitivity and specificity to evaluate if biomarkers can be used for screening purposes. The third phase is a retrospective longitudinal study with aims to understand as a function of time before clinical diagnosis, the capacity of the biomarker to detect preclinical disease and identify the criteria for a positive screening test. The fourth phase is a prospective study where the diagnostic test is applied to individuals, with a standardized protocol for definitive treatment (i.e., surgery) to those testing positive. The final phase is a population-based cancer control study, which investigates whether testing reduces the burden of cancer on the population. Not all phases are required to bring biomarkers to clinical use but at least successful performance in a phase 3 study is required for validation.
Figure 1.
Graphic of the five proposed phases for diagnostic test discovery and validation as defined by Pepe et al.26
In the context of this framework, the studies described in this review are in phases 1 or 2 (listed in Tables 2, 3, 4, 5, 6). The next steps for new cyst-fluid biomarkers to enter clinical practice will be to complete phase 2 validation studies for those that have only undergone phase 1 and begin planning a phase 3 studies of the most promising biomarkers. Phase 3 studies will be based on repositories from centers that have already been banking pancreatic cyst fluid. These samples will be collected only by EUS that in the future (but not immediately) will have undergone surgery. The goal will be to assess the biomarker's capacity to predict disease and will be assessed retrospectively. Sample sizes with adequate power will need to be collated and will require a multicenter collaboration. With proper design and application, retrospective longitudinal studies can allow for rapid clinical uptake of novel biomarkers. Successful phase 3 studies should be enough to enable implementation into clinical practice. These studies should include minimally acceptable true- and false- positive rates for testing defined by community consensus. Based on what is known of the indolent nature for most of these premalignant cysts, phase 4 prospective studies, while important, will require a very large sample with longitudinal follow-up that may not be practical or feasible to define as the threshold prior to introduction into clinical practice.
In conclusion, there are several promising cyst-fluid biomarkers in the literature that warrant a concerted coordinated multicenter effort. Among those discussed, the most promising include KRAS (V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog), GNAS, miRNA 21, and glucose to differentiate non-mucinous from mucinous cyst types. MUC1, amphiregulin, IL-1B, SPINK1, mAb Das-1, and miRNA 21 should be further studied to differentiate low-risk from high-risk cyst types (those harboring high-grade dysplasia, or invasive cancer). VEGF should be further validated for its ability to diagnose serous cystic neoplasms. Phase 3 retrospective longitudinal study designs represent the next rational step forward. As this will require significant pooling of resources, careful consideration in the planning stages should be made to allow the most number of potential biomarkers to be studied, both alone and in combination with each other. This will also ensure that we reach our shared goal of bringing new diagnostic tests and improving the management of patients with pancreatic cysts.
Guarantor of the article: Walter G. Park, MD, MS.
Specific author contributions: Walter G. Park: project concept, data collection, synthesis, manuscript preparation and editing; N. Thiruvengadam: data collection, synthesis and manuscript preparation.
Financial support: WGP is supported by an American College of Gastroenterology Junior Faculty Development Award.
Potential competing interests: None.
References
- Laffan TA, Horton KM, Klein AP et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 2008; 191: 802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong K, Nio CY, Hermans JJ et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol 2010; 8: 806–811. [DOI] [PubMed] [Google Scholar]
- Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007; 102: 2339–2349. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fernandez-del Castillo C, Adsay V et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12: 183–197. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Chari S, Adsay V et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006; 6: 17–32. [DOI] [PubMed] [Google Scholar]
- Pelaez-Luna M, Chari ST, Smyrk TC et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol 2007; 102: 1759–1764. [DOI] [PubMed] [Google Scholar]
- Rodriguez JR, Salvia R, Crippa S et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology 2007; 133: 72–79 quiz 309-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang RS, Weinberg B, Dawson DW et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol 2008; 6: 815–819 quiz 719. [DOI] [PubMed] [Google Scholar]
- Correa-Gallego C, Ferrone CR, Thayer SP et al. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology 2010;10:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CS, Russ AJ, Loeffler AG et al. Preoperative classification of pancreatic cystic neoplasms: the clinical significance of diagnostic inaccuracy. Ann Surg Oncol 2013; 20: 3112–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MJ, Buchanan AS, Neal CP et al. Imaging of indeterminate pancreatic cystic lesions: a systematic review. Pancreatology 2013;13:436–442. [DOI] [PubMed] [Google Scholar]
- de Jong K, Nio CY, Mearadji B et al. Disappointing interobserver agreement among radiologists for a classifying diagnosis of pancreatic cysts using magnetic resonance imaging. Pancreas 2012; 41: 278–282. [DOI] [PubMed] [Google Scholar]
- Ohno E, Hirooka Y, Itoh A et al. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg 2009; 249: 628–634. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fernandez-Del Castillo C, Adsay V et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12: 183–197. [DOI] [PubMed] [Google Scholar]
- Sedlack R, Affi A, Vazquez-Sequeiros E et al. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc 2002;56:543–547. [DOI] [PubMed] [Google Scholar]
- Pais SA, Schmidt CM, DeWitt J et al. Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: correlation with surgical histopathology. Clin Gastroenterol Hepatol 2007;5:489–495. [DOI] [PubMed] [Google Scholar]
- Koito K, Namieno T, Nagakawa T et al. Solitary cystic tumor of the pancreas: EUS-pathologic correlation. Gastrointest Endosc 1997;45:268–276. [DOI] [PubMed] [Google Scholar]
- Hernandez LV, Mishra G, Forsmark C et al. Role of endoscopic ultrasound (EUS) and EUS-guided fine needle aspiration in the diagnosis and treatment of cystic lesions of the pancreas. Pancreas 2002; 25: 222–228. [DOI] [PubMed] [Google Scholar]
- Frossard JL, Amouyal P, Amouyal G et al. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am J Gastroenterol 2003; 98: 1516–1524. [DOI] [PubMed] [Google Scholar]
- Ahmad NA, Kochman ML, Lewis JD et al. Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas? Am J Gastroenterol 2001;96:3295–3300. [DOI] [PubMed] [Google Scholar]
- Gerke H, Jaffe TA, Mitchell RM et al. Endoscopic ultrasound and computer tomography are inaccurate methods of classifying cystic pancreatic lesions. Dig Liver Dis 2006; 38: 39–44. [DOI] [PubMed] [Google Scholar]
- Thosani N, Thosani S, Qiao W et al. Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Dig Dis Sci 2010;55:2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge WR, Lewandrowski K, Lee-Lewandrowski E et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004; 126: 1330–1336. [DOI] [PubMed] [Google Scholar]
- Park WG, Mascarenhas R, Palaez-Luna M et al. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas 2011; 40: 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizginer S, Turner BG, Bilge AR et al. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas 2011;40:1024–1028. [DOI] [PubMed] [Google Scholar]
- Pepe MS, Etzioni R, Feng Z et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 2001; 93: 1054–1061. [DOI] [PubMed] [Google Scholar]
- Partyka K, McDonald M, Maupin KA et al. Comparison of surgical and endoscopic sample collection for pancreatic cyst fluid biomarker identification. J Proteome Res 2012; 11: 2904–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser S, Schnieke A, Schneider G et al. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer 2014;111:817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid A, McGrath KM, Zahid M et al. The role of pancreatic cyst fluid molecular analysis in predicting cyst pathology. Clin Gastroenterol Hepatol 2005; 3: 967–973. [DOI] [PubMed] [Google Scholar]
- Schoedel KE, Finkelstein SD, Ohori NP. K-Ras and microsatellite marker analysis of fine-needle aspirates from intraductal papillary mucinous neoplasms of the pancreas. Diagn Cytopathol 2006; 34: 605–608. [DOI] [PubMed] [Google Scholar]
- Khalid A, Zahid M, Finkelstein SD et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc 2009; 69: 1095–1102. [DOI] [PubMed] [Google Scholar]
- Sawhney MS, Devarajan S, O'Farrel P et al. Comparison of carcinoembryonic antigen and molecular analysis in pancreatic cyst fluid. Gastrointest Endosc 2009; 69: 1106–1110. [DOI] [PubMed] [Google Scholar]
- Shen J, Brugge WR, Dimaio CJ et al. Molecular analysis of pancreatic cyst fluid: a comparative analysis with current practice of diagnosis. Cancer 2009;117:217–227. [DOI] [PubMed] [Google Scholar]
- Sreenarasimhaiah J, Lara LF, Jazrawi SF et al. A comparative analysis of pancreas cyst fluid CEA and histology with DNA mutational analysis in the detection of mucin producing or malignant cysts. JOP 2009;10:163–168. [PubMed] [Google Scholar]
- Talar-Wojnarowska R, Pazurek M, Durko L et al. A comparative analysis of K-ras mutation and carcinoembryonic antigen in pancreatic cyst fluid. Pancreatology 2012; 12: 417–420. [DOI] [PubMed] [Google Scholar]
- Nikiforova MN, Khalid A, Fasanella KE et al. Integration of KRAS testing in the diagnosis of pancreatic cystic lesions: a clinical experience of 618 pancreatic cysts. Mod Pathol 2013; 26: 1478–1487. [DOI] [PubMed] [Google Scholar]
- Al-Haddad M, DeWitt J, Sherman S et al. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc 2014; 79: 79–87. [DOI] [PubMed] [Google Scholar]
- Wu J, Matthaei H, Maitra A et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011; 3: 92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui AA, Kowalski TE, Kedika R et al. EUS-guided pancreatic fluid aspiration for DNA analysis of KRAS and GNAS mutations for the evaluation of pancreatic cystic neoplasia: a pilot study. Gastrointest Endosc 2013; 77: 669–670. [DOI] [PubMed] [Google Scholar]
- Singhi AD, Nikiforova MN, Fasanella KE et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res 2014; 20: 4381–4389. [DOI] [PubMed] [Google Scholar]
- Amato E, Molin MD, Mafficini A et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol 2014; 233: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaei H, Wylie D, Lloyd MB et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res 2012; 18: 4713–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell JJ, Toste P, Wu N et al. Endoscopically acquired pancreatic cyst fluid microRNA 21 and 221 are associated with invasive cancer. Am J Gastroenterol 2013; 108: 1352–1359. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Matthaei H, Dal Molin M et al. Elevated microRNA miR-21 Levels in Pancreatic Cyst Fluid Are Predictive of Mucinous Precursor Lesions of Ductal Adenocarcinoma. Pancreatology 2011; 11: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara S, Cangi MG, Arcidiacono PG et al. Mucin expression pattern in pancreatic diseases: findings from EUS-guided fine-needle aspiration biopsies. Am J Gastroenterol 2011; 106: 1359–1363. [DOI] [PubMed] [Google Scholar]
- Wang J, Paris PL, Chen J et al. Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions. Cancer Lett 2015; 356: 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Gusev Y, Jiang J et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer 2007; 120: 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett CJ, Samra JS, Xue A et al. Classification of pancreatic cystic lesions using SELDI-TOF mass spectrometry. ANZ J Surg 2007;77:648–653. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Qin LX, Tang L et al. Pancreatic cyst fluid protein expression profiling for discriminating between serous cystadenoma and intraductal papillary mucinous neoplasm. Ann Surg 2009;250:754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke E, Patel BB, Liu T et al. Proteomic analyses of pancreatic cyst fluids. Pancreas 2009; 38: e33–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuoghi A, Farina A, Z'Graggen K et al. Role of proteomics to differentiate between benign and potentially malignant pancreatic cysts. J Proteome Res 2011; 10: 2664–2670. [DOI] [PubMed] [Google Scholar]
- Corcos O, Couvelard A, Dargere D et al. Proteomic assessment of markers for malignancy in the mucus of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2012; 41: 169–174. [DOI] [PubMed] [Google Scholar]
- Lee LS, Banks PA, Bellizzi AM et al. Inflammatory protein profiling of pancreatic cyst fluid using EUS-FNA in tandem with cytokine microarray differentiates between branch duct IPMN and inflammatory cysts. J Immunol Methods 2012; 382: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann BF, Goetz JA, House MG et al. Glycomic and proteomic profiling of pancreatic cyst fluids identifies hyperfucosylated lactosamines on the N-linked glycans of overexpressed glycoproteins. Mol Cell Proteomics 2012;11:M111.015792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbormittah FO, Haab BB, Partyka K et al. Characterization of glycoproteins in pancreatic cyst fluid using a high-performance multiple lectin affinity chromatography platform. J Proteome Res 2014;13:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CM, Yip-Schneider MT, Ralstin MC et al. PGE(2) in pancreatic cyst fluid helps differentiate IPMN from MCN and predict IPMN dysplasia. J Gastrointest Surg 2008; 12: 243–249. [DOI] [PubMed] [Google Scholar]
- Haab BB, Porter A, Yue T et al. Glycosylation variants of mucins and CEACAMs as candidate biomarkers for the diagnosis of pancreatic cystic neoplasms. Ann Surg 2010; 251: 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun MT, Pai RK, Kwok S et al. Diagnostic accuracy of cyst fluid amphiregulin in pancreatic cysts. BMC Gastroenterol 2012; 12: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbar KS, Verbeke C, Hyltander AG et al. Proteomic mucin profiling for the identification of cystic precursors of pancreatic cancer. J Natl Cancer Inst 2014;106:djt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle CJ, Agaram NP, Yip-Schneider MT et al. Transforming growth factor alpha levels in pancreatic fluid. Pancreas 2011;40:260–264. [DOI] [PubMed] [Google Scholar]
- Maker AV, Katabi N, Qin LX et al. Cyst fluid interleukin-1beta (IL1beta) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res 2011; 17: 1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KK, Xiao H, Geng X et al. mAb Das-1 is specific for high-risk and malignant intraductal papillary mucinous neoplasm (IPMN). Gut 2014; 63: 1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip-Schneider MT, Wu H, Dumas RP et al. Vascular endothelial growth factor, a novel and highly accurate pancreatic fluid biomarker for serous pancreatic cysts. J Am Coll Surg 2014; 218: 608–617. [DOI] [PubMed] [Google Scholar]
- Morris-Stiff G, Lentz G, Chalikonda S et al. Pancreatic cyst aspiration analysis for cystic neoplasms: mucin or carcinoembryonic antigen—which is better? Surgery 2010; 148: 638–644 discussion 44-45. [DOI] [PubMed] [Google Scholar]
- Maker AV, Katabi N, Gonen M et al. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol 2011; 18: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Maupin K, Curnutte B et al. Specific glycoforms of MUC5AC and endorepellin accurately distinguish mucinous from nonmucinous pancreatic cysts. Mol Cell Proteomics 2013; 12: 2724–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raty S, Sand J, Laukkarinen J et al. Cyst fluid SPINK1 may help to differentiate benign and potentially malignant cystic pancreatic lesions. Pancreatology 2013; 13: 530–533. [DOI] [PubMed] [Google Scholar]
- Lemoine NR, Hughes CM, Barton CM et al. The epidermal growth factor receptor in human pancreatic cancer. J Pathol 1992; 166: 7–12. [DOI] [PubMed] [Google Scholar]
- Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev 2007; 26: 51–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 2011; 10: 671–684. [DOI] [PubMed] [Google Scholar]
- Park WG, Wu M, Bowen R et al. Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: glucose and kynurenine. Gastrointest Endosc 2013; 78: 295–302 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maker AV, Lee LS, Raut CP et al. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol 2008;15:3187–3192. [DOI] [PubMed] [Google Scholar]
- Pitman MB, Michaels PJ, Deshpande V et al. Cytological and cyst fluid analysis of small (< or =3 cm) branch duct intraductal papillary mucinous neoplasms adds value to patient management decisions. Pancreatology 2008;8:277–284. [DOI] [PubMed] [Google Scholar]
- Pitman MB, Genevay M, Yaeger K et al. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than "positive" cytology. Cancer Cytopathol 2010; 118: 434–440. [DOI] [PubMed] [Google Scholar]
- Pitman MB, Centeno BA, Genevay M et al. Grading epithelial atypia in endoscopic ultrasound-guided fine-needle aspiration of intraductal papillary mucinous neoplasms: an international interobserver concordance study. Cancer Cytopathol 2013;121:729–736. [DOI] [PubMed] [Google Scholar]