Abstract
OBJECTIVES:
To evaluate safety, tolerability, pharmacokinetics, and pharmacodynamics of TAK-438 (vonoprazan, a potassium-competitive acid blocker) in healthy male subjects.
METHODS:
In two phase I, randomized, double-blind, placebo-controlled, single rising-dose studies, healthy male subjects (Japan N=84; UK N=63) received a single TAK-438 dose (1–120 mg in Japan and 1–40 mg in the UK). Assessments included safety, tolerability, pharmacokinetics, and pharmacodynamics (intragastric pH).
RESULTS:
Plasma concentration–time profiles of TAK-438 at all dose levels showed rapid absorption (median Tmax up to 2 h). Estimated mean elimination half-life was up to 9 h. Exposure was slightly greater than dose proportional. No clear difference in TAK-438 pharmacokinetics was observed between Japanese and non-Japanese subjects. Acid suppression was dose dependent and similar in both studies. The 24-h intragastric pH ≥4 holding time ratio with 40 mg TAK-438 was 92% in Japan and 87% in the UK. TAK-438 was well tolerated, with no adverse events reported in Japanese subjects; 10 of 63 UK subjects experienced 12 treatment-emergent adverse events (non-serious). Increases in serum gastrin and pepsinogen I and II concentrations were observed at doses ≥10 mg, but there were no changes in alanine aminotransferase concentrations.
CONCLUSIONS:
Single oral doses of TAK-438 20–120 mg caused rapid, profound, and 24-h suppression of gastric acid secretion in healthy male subjects, regardless of geographical region, and TAK-438 was well tolerated at all doses studied, making it a potential alternative to proton pump inhibitors for the treatment of acid-related disorders.
INTRODUCTION
Gastric acid secretion has a key role in the digestion of protein, as well as in the absorption of several vitamins and minerals, and the prevention of enteric infection.1 However, gastric acid requires tight physiologic regulation and effective mucosal defense mechanisms in order to prevent damage to the esophagus, stomach, and duodenum.1, 2
Acid-related diseases of the upper gastrointestinal tract encompass several conditions with distinctive, but overlapping, pathogenic mechanisms that occur when the normal balance between gastric acid (and pepsin) secretion and gastroduodenal mucosal defense mechanisms becomes disrupted.3 The common factor among these disorders is the excessive exposure of sensitive tissues to gastric acid, and the most common and well-defined disorders are gastroesophageal reflux disease and peptic ulcer disease.3, 4 In gastroesophageal reflux disease, inappropriate reflux of gastric contents into the esophagus occurs, mainly due to dysfunction of the lower esophageal sphincter.4 This is one of the most common gastrointestinal disorders worldwide, with weekly symptoms experienced by between 5% (East Asia) and 20% (North America) of the population.5 In peptic ulcer disease, the gastric or duodenal mucosal defenses become compromised due to various mechanisms, for instance Helicobacter pylori infection or excessive nonsteroidal anti-inflammatory drug (NSAID) use.1, 2 Although eradication of H. pylori has led to a decrease in H. pylori-positive ulcers, this has been offset by greater resistance to antibiotics and an increase in the prevalence of NSAID-induced ulcers and H. pylori-negative/NSAID-negative ulcers.1
Gastric H+,K+-adenosine triphosphatase (ATPase) is the key enzyme responsible for the final step of gastric acid secretion from stimulated parietal cells.6, 7 Upon activation, the process of acid secretion requires recruitment of H+,K+-ATPase from cytoplasmic tubules into the microvilli of the secretory canaliculus of the parietal cell.6, 7 It pumps out H+ at a pH of 0.8 against a large concentration gradient (>106-fold) by ATP-driven exchange of one H+ for one K+.6, 7 The H+,K+-ATPase is able to maintain gastric juice at an acidic normal median fasting pH of 1.7 (range 0.3–2.9) in the stomach, whereas prolonged exposure to even weakly acidic pH levels would be considered pathologic in the esophagus.8, 9
Inhibition of gastric acid secretion remains the cornerstone of treatment for acid-related disorders.10 Current approaches to the treatment involve either preventing parietal cell stimulation using H2 receptor antagonists or inhibiting the gastric H+,K+-ATPase using proton pump inhibitors (PPIs).11 PPIs provide more effective symptom resolution and disease healing than H2 receptor antagonists.11 The current PPIs are prodrugs that require acid activation in the secretory canaliculus and irreversibly inhibit H+,K+-ATPase activity.6 However, agents that inhibit H+,K+-ATPase via new modes of action are in development, most notably the potassium-competitive acid blockers (P-CABs), which are stable and active in an acidic environment and inhibit the gastric H+,K+-ATPase by reversible K+-competitive binding.6
TAK-438, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate, (vonoprazan) is a potent, novel, orally active P-CAB discovered by Takeda Pharmaceutical Company Ltd, Japan and selected as a candidate for development as a treatment for acid-related diseases.12, 13, 14 This water-soluble pyrrole derivative has a significantly different chemical structure than other P-CABs and, importantly, lacks the imidazopyridine ring structure that could be linked to liver enzyme elevation observed in some earlier investigations of P-CABs in man.12, 14, 15 Preclinical data suggest that TAK-438 provides more profound and longer-lasting suppression of gastric acid secretion compared with either PPIs or other P-CABs.16, 17, 18 This appears to be a consequence of the following: (1) high accumulation in gastric glands due to its high pKa (relative to PPIs and other P-CABs) and (2) slow clearance from gastric glands and its slower dissociation from H+,K+-ATPase once bound (relative to other P-CABs).16, 17, 18, 19
Acid-related diseases remain a major healthcare problem and unmet needs persist, despite the improvements in management provided by the PPIs.11, 20 The majority of currently approved PPIs need to be taken before meals, have a slow, cumulative onset of action over several days of dosing, and do not always sufficiently inhibit acid secretion at night.11 At the time of conducting these phase I studies, the preclinical profile of TAK-438 suggested that it has many characteristics that could address these unmet needs.16, 17, 18 Because of its gastric tissue accumulation, slow clearance, and acid independence, it should be possible to take TAK-438 without regard to meal time and it would be expected to provide a rapid onset of action from the first dose, as well as preventing nocturnal acid break-through and reducing acid reflux symptoms.
In addition, PPIs' acid-suppressive efficacy varies due to polymorphisms in the hepatic cytochrome that affect their metabolism, whereas TAK-438 is metabolized mainly by CYP3A4 (and not CYP2C19) and may have less inter-patient variability.11, 21 In this article, we describe the results of two phase I studies investigating the safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising doses of TAK-438 in healthy male subjects in Japan and the UK. These studies provide the first clinical evidence to support the potential of this novel P-CAB as a treatment for acid-related diseases.
METHODS
Study design
Subjects were enrolled in one of two similarly designed phase I, single rising-dose, randomized, double-blind, single-center, placebo-controlled studies to evaluate the single-dose safety, tolerability, pharmacokinetics, and pharmacodynamics of TAK-438 at oral doses of 1, 5, 10, 15 (UK only), 20, 30 (UK only), 40, 80 (Japan only), and 120 mg (Japan only) in healthy male subjects at the Medical Co. LTA Honjo Clinic in Japan (seven cohorts) and at Richmond Pharmacology Ltd, St George's University of London in the UK (seven cohorts). The Japanese study was conducted between 10 September 2007 and 14 December 2007 and the UK study between 21 October 2007 and 28 February 2008.
In the Japanese study, each cohort comprised 12 subjects randomized to TAK-438 (n=9) or placebo (n=3). In both studies, the investigator recruited subjects from a database of healthy volunteers maintained by the investigational site. Each cohort comprised nine subjects randomized to TAK-438 (n=6) or placebo (n=3). Placebo was administered using the same number of tablets required to achieve the TAK-438 dose within each cohort.
The studies were reviewed and approved by their respective ethics committees and were conducted in accordance with the Good Clinical Practice guideline, all applicable local regulations, and the 1996 Declaration of Helsinki. The Japanese ethics committee approved the protocol on 18 August 2007 and an updated protocol on 22 September 2007. The UK ethics committee EC approved the protocol on 14 September 2007, Amendment 001 to the protocol on 20 September 2007, and Amendments 002, 003, and Revised 003 on 28 January 2008. All participants gave written informed consent before their study participation. The studies are registered with http://clinicaltrials.gov: numbers NCT02123927 and NCT02141698.
Treatment
After a 14- (Japan) or 10-h (UK) overnight fast, subjects took the dose with 150 ml water. No food was permitted for 4 h post dose, but 200 ml water was given at 2 and 4 h post dose.
The objective of the single-dose escalation scheme was to escalate to the maximum tolerated dose or to a maximum area under the plasma concentration–time curve (AUC) for TAK-438. The starting dose was 1 mg TAK-438 and the decision to proceed to the next dose in the next cohort was made after blinded review of 24-h safety and pharmacokinetic data from the preceding cohort. The next cohort was dosed only if the preceding dose level had been well tolerated (both studies) and if the predicted mean exposure at the next dose level did not exceed the no-observed-adverse-effect level AUC in dogs (UK criterion only). The different doses were chosen following agreement with the respective regulatory authorities.
Participants
Participants had to be healthy adult Japanese males aged 20–45 years (Japan) or Western non-Japanese males aged 18–45 years (UK), with a body weight ≥50 kg (Japan), and body mass index 18.5–25.0 kg/m2 (Japan) or 18.0–30.0 kg/m2 (UK) at screening. Participants were excluded if they were smokers (UK); had acid-related disorders or a history of any such diseases, including reflux esophagitis, gastric or duodenal ulcer, non-erosive gastroesophageal reflux disease, Barrett's esophagus, and Zollinger–Ellison syndrome (both studies); had previously undergone upper gastrointestinal tract surgery or vagotomy (Japan); or had undergone H. pylori eradication within 6 months before first dosing of TAK-438 (Japan) or had a positive H. pylori test at screening (UK). No prescription and over the counter medications (including PPIs and H2 antagonists) were permitted in the 28 days before the first study dose.
Screening
Participants were initially screened for eligibility using inclusion/exclusion criteria, fasting clinical laboratory tests (hematology, serum chemistry, and urinalysis), medical history (including tobacco, alcohol, and caffeine use), medical examination (including height, body weight, and body mass index), urine drug toxicology and alcohol screening, hepatitis panel, human HIV panel, H. pylori screening using a serum antibody test in Japan or a urea breath test in the UK, vital signs, prior/concomitant medication use, and 12-lead electrocardiogram.
Eligible subjects were sequentially block-randomized to a single dose of TAK-438 or placebo according to the randomization schedule that had been generated by the study Sponsor. All randomization information was stored in a secure area, accessible only by authorized personnel. Investigators and subjects were blinded to each subject's medication and blinding was maintained throughout the studies. The pharmacokinetic analysis data, to be used for dose escalation, was blinded by the bioanalytical laboratory for provision to the study team. The subjects were discharged on day 5 and returned for follow-up on day 15.
Pharmacokinetic measurements
Serial venous blood samples were collected in heparinized tubes for 48 h after dosing (day 1 at pre-dose (0 h) and after 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 24, 30, 36, and 48 h). Plasma was separated by centrifugation at ~1,500 g at 4 °C for 10 min then frozen at –20 °C or lower until analysis.
Total urine collections were made on day 1 at 0–6, 6–12, and 12–24 h post dose, on day 2 at 24–36 and 36–48 h post dose, and on day 5 (Japan only) before discharge. Urine aliquots were frozen at –20 °C or lower until analysis.
Plasma and urine concentrations were determined using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) assay with a run time of 5 min per sample on an AB MDS Sciex API5000 system. The bioanalytical method was confirmed to be reliable over the concentration ranges of 0.1–100 mg/ml for TAK-438 in human plasma and 1–1,000 ng/ml in urine.
The main pharmacokinetics parameters included the maximum observed concentration (Cmax), time to reach Cmax (Tmax), area under the plasma concentration–time curve (AUC) from time 0–24 h (AUC0–24), AUC from time 0 to infinity (AUC0–inf), terminal elimination half-life (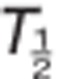 ), and apparent oral clearance (CL/F). The main urine pharmacokinetic parameter for TAK-438 was the fraction of drug excreted per 24 h (%Fe).
), and apparent oral clearance (CL/F). The main urine pharmacokinetic parameter for TAK-438 was the fraction of drug excreted per 24 h (%Fe).
Pharmacodynamic measurements
Intragastric pH was measured continuously for 24 h at baseline (Japan, day −2; UK, day −1), and for 24 (Japan) or 96 h (UK) after study drug dosing (on day 1) using a pH probe (CM-181; Chemical Instruments, Tokyo, Japan in the Japanese study or M3 Symed Glass Catheter; Synetics Medical, Utah, USA in the UK study) inserted into the stomach (Japan) or 10 cm below the lower esophageal sphincter (UK) and its position confirmed by X-ray (Japan), or changes in pressure during deep inspiration (UK). In the Japanese study, intragastric pH was recorded every 10 s from 08:30 to 09:10 of the following day using a one-channel pH meter (101ZG; Chemical Instruments, Tokyo, Japan). In the UK study, intragastric pH was recorded every 6 s using a Flexilog 2020 ambulatory pH monitor (Oakfield Instruments, Oxfordshire, UK).
The primary pharmacodynamic end point was the percentage of total time that the intragastric pH was ≥4 (pH≥4 holding time ratio (HTR)) and ≥5 (pH≥5 HTR) calculated from the intragastric pH in the first 24-h period after dosing. The nighttime pH HTR was defined as the percentage of time pH was ≥4 and pH was ≥5 during the period 12–24 h post dose (Japan) or ~20:00–08:00 (UK).
Safety assessments
Safety assessments were based on the reported incidence of adverse events, medical examinations (including body weight), vital signs, and clinical laboratory tests (hematology, chemistry, and urinalysis).
Spontaneous reports of adverse events were collected throughout the studies from initial screening until follow-up and were assessed for severity and relationship to the study drug. Clinical laboratory tests (serum chemistry including serum alanine aminotransferase, hematology, and urinalysis), medical examinations (including body weight), and vital signs were performed from initial screening to follow-up (day 15, although laboratory tests were not performed on day 15 in the Japanese study).
Triplicate 12-lead electrocardiograms were recorded pre-dose at −1.5 (UK only), −1 (UK only), −0.5 h (UK only), and baseline, post dose at 0.25 (UK only), 0.5, 1, 2, 4, 8, 12, 24, and 48 h (Japan only), on day 5 before discharge, and on day 15 (Japan only).
Serial blood samples for serum gastrin and pepsinogen I and II assay were taken at baseline (0 h), 0.25 (UK only), 0.5, 0.75 (UK only), 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 24, 30, 36, and 48 h after study drug dosing on day 1.
In the Japanese study, blood was also collected at 0.25 h after dosing for evaluation of CYP2C19 genotype.
Statistical analysis
No formal sample size calculation was performed. The planned sample sizes were based on precedent pharmacokinetic studies of similar design.
The pharmacokinetic analysis set comprised all subjects who received TAK-438 and who had sufficient plasma concentration data to calculate at least one pharmacokinetic parameter. Descriptive statistics were used to summarize pharmacokinetic parameters for TAK-438 in plasma and urine. All pharmacokinetic parameters (except Tmax) were log-transformed before analysis.
The pharmacodynamic analysis set comprised all subjects who received TAK-438 on day 1 and who had sufficient intragastric pH data. In the Japanese study, insufficient gastric pH data was defined as an outlier (0.0 or ≥10.0) within any 10-min period or missing pH data at one or more out of 12 time points and the individual subject was excluded from pharmacokinetic analyses. Subjects with outliers or missing pH data at less than one out of 12 time points of the 24- or 12-h period were included in the summary; however, the missing or outlier data were excluded. The average pH value was calculated by subject from the data collected during each monitoring period. The device used in the UK study was very reliable and standard calibration was performed before and after the recordings. If a significant interruption of the pH monitoring occurred, this was indicative of an irreversible mechanical break in the catheter and no data were recorded from this point.
Descriptive statistics were used to summarize pH HTRs, mean pH, and serum concentrations of gastrin and pepsinogen I and II in each treatment group. Primary safety analyses and baseline demographic summaries were performed on the safety analysis set (i.e., all subjects who were enrolled and received a single dose of TAK-438 or placebo on day 1). Safety data were summarized by descriptive statistics and by figures or scatter plots.
A power model with a fixed effect for regional effect (Japanese vs. non-Japanese) was used to investigate dose proportionality and assess potential regional differences.22 An assessment of the lack of fit was determined visually by plotting the data.
All statistical analyses were performed by using the SAS system, version 8.2 or higher (SAS Institute Inc., Cary, NC, USA).
RESULTS
Study population
A total of 84 subjects in the Japanese study (mean age 26±5.4 years; mean body weight 63±6.0 kg, mean body mass index 21±1.6 kg/m2 (range 19–24 kg/m2)) and 63 subjects in the UK study (50 Caucasians, 7 Black or African American, 4 Asians (excluding Japanese), 1 Native Hawaiian or other Pacific Islander, and 1 Multiracial; mean age 26±4.9 years; body weight 78±9.6 kg, mean body mass index 25±2.6 kg/m2 (range 20–30 kg/m2)) received the study drug or placebo and completed their respective study.
The demographic and baseline characteristics of the subjects in both studies are given in Table 1. As expected, subjects in the UK study had higher mean body weight at baseline than those in the Japanese study.
Table 1. Demographic and baseline characteristics of subjects in the Phase I, randomized, double-blind, placebo-controlled Japanese and UK single rising-dose studies in healthy male subjects receiving a single TAK-438 dose (1–120 mg in Japan and 1–40 mg in the UK) (Safety analysis set; Japan N=84, UK N=63).
| Characteristic | Placebo |
TAK-438 |
Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 mg | 5 mg | 10 mg | 15 mg | 20 mg | 30 mg | 40 mg | 80 mg | 120 mg | |||
| Japanese study | |||||||||||
| N | 21 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 84 | ||
| Age (years) | 26±4.6 | 25±4.3 | 26±3.8 | 28±7.7 | 27±5.6 | 25±4.3 | 25±6.4 | 27±7.3 | 26±5.4 | ||
| Height (cm) | 171±5.6 | 173±3.4 | 174±2.6 | 172±5.7 | 172±2.9 | 171±4.6 | 173±5.4 | 172±4.9 | 172±4.6 | ||
| Body weight (kg) | 64±8.6 | 63±4.9 | 63±4.3 | 62±6.1 | 61±2.7 | 64±5.0 | 63±5.9 | 60±5.4 | 63±6.0 | ||
| Body mass index (kg/m2) | 22±2.2 | 21±1.6 | 21±1.3 | 21±1.7 | 21±1.2 | 22±1.6 | 21±1.3 | 20±1.1 | 21±1.6 | ||
| UK study | |||||||||||
| N | 21 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 63 | ||
| Age (years) | 27±5.0 | 27±5.3 | 27±5.4 | 25±3.9 | 26±4.8 | 23±4.6 | 27±6.5 | 26±4.9 | 26±4.9 | ||
| Height (cm) | 177±5.4 | 177±5.0 | 175±6.4 | 182±7.2 | 180±6.2 | 177±6.4 | 173±5.2 | 183±4.6 | 178±6.1 | ||
| Body weight (kg) | 78±7.9 | 80±8.3 | 72±7.5 | 85±13.8 | 73±5.4 | 72±8.3 | 77±11.4 | 85±10.6 | 78±9.6 | ||
| Body mass index (kg/m2) | 25±2.4 | 26±2.6 | 24±1.7 | 26±3.0 | 23±2.6 | 23±2.2 | 26±2.5 | 26±2.9 | 25±2.6 | ||
Data are represented as mean±s.d.
In the Japanese study, the safety, pharmacokinetic, and pharmacodynamic analysis sets comprised 84, 70 (14 exclusions due to hemolytic plasma samples), and 82 subjects (incorrect 24-h pH monitoring in two subjects; one due to a loose connection of the comparison electrode and one due to an abnormal pH elevation during the baseline period), respectively. In the UK study, all subjects were included in the safety, pharmacokinetic, and pharmacodynamic analysis sets.
Pharmacokinetic data
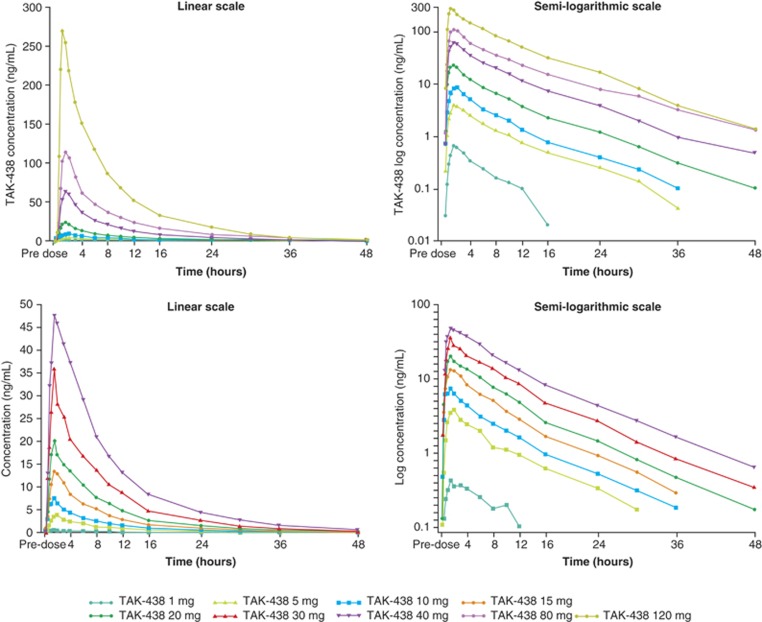
The plasma TAK-438 concentration–time profiles at all dose levels showed rapid absorption (Figure 1). Median Tmax was ≤2 h under fasting conditions and mean elimination 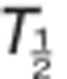 ranged from 5.1 to 8.7 h in the Japanese study and from 7.3 to 9.0 h in the UK study (Figure 1; Table 2).
ranged from 5.1 to 8.7 h in the Japanese study and from 7.3 to 9.0 h in the UK study (Figure 1; Table 2).
Figure 1.
Time course of mean plasma TAK-438 concentrations in the phase I, randomized, double-blind, placebo-controlled, single rising-dose Japanese (top panel) and UK (bottom panel) studies in healthy male subjects receiving a single TAK-438 dose (1–120 mg in Japan and 1–40 mg in the UK; pharmacokinetic analysis set; Japan N=70, UK N=63).
Table 2. Pharmacokinetic parameters of plasma TAK-438 after single rising doses of TAK-438 (1–120 mg in Japan and 1–40 mg in the UK) in the phase I, randomized, double-blind, placebo-controlled Japanese and UK studies in healthy male subjects (Pharmacokinetic analysis set; Japan N=70, UK N=63).
| Parameter | 1 mg | 5 mg | 10 mg | 15 mg | 20 mg | 30 mg | 40 mg | 80 mg | 120 mg |
|---|---|---|---|---|---|---|---|---|---|
| Japanese study | |||||||||
| N | 5 | 9 | 8 | 7 | 9 | 7 | 8 | ||
| Cmax (ng/ml) | 0.7±0.3 | 4.2±1.4 | 9.7±2.1 | 25.0±5.6 | 71.9±23.3 | 129.8±40.6 | 303.8±64.3 | ||
| Tmax (h) | 1.50 (1.50–2.00) | 1.50 (1.00–3.00) | 1.75 (1.00–2.00) | 1.50 (0.75–2.00) | 1.50 (1.00–3.00) | 1.50 (1.00–3.00) | 1.00 (0.75–2.00) | ||
| AUC0–24 (ng·h/ml) | 3.7±1.6 | 28.5±9.4 | 56.5±8.4 | 148.9±35.1 | 433.3±125.8 | 797.3±213.3 | 1828±344.5 | ||
| AUC0–inf (ng·h/ml) | 4.3±1.6 | 31.6±10.4 | 60.8±8.9 | 161.6±39.3 | 474.6±141.0 | 911.3±243.1 | 1985±403.2 | ||
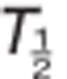 (h) (h) |
5.1±1.1 | 7.6±1.1 | 6.9±1.0 | 6.9±0.8 | 7.1±0.5 | 8.7±1.0 | 6.6±0.7 | ||
| CL/F (l/h) | 287.2±182.4 | 173.6±54.2 | 167.8±26.1 | 130.7±33.3 | 92.8±33.3 | 93.3±25.1 | 62.5±11.9 | ||
| UK study | |||||||||
| N | 6 | 6 | 6 | 6 | 6 | 6 | 6 | ||
| Cmax (ng/ml) | 0.4±0.1 | 3.9±1.9 | 7.9±3.9 | 14.0±4.0 | 23.0±6.4 | 38.6±13.2 | 51.8±17.0 | ||
| Tmax (h) | 1.50 (1.50–3.03) | 2.00 (1.50–2.00) | 1.50 (0.75–4.00) | 1.50 (1.50–2.00) | 1.50 (1.00–3.00) | 1.50 (1.00–1.50) | 1.75 (0.75–3.00) | ||
| AUC0–24 (ng·h/ml) | 3.3±1.5 | 29.0±13.5 | 52.8±19.2 | 99.0±23.7 | 152.9±39.2 | 259.7±94.4 | 418.7±157.9 | ||
| AUC0–inf (ng·h/ml) | 4.6±2.1 | 33.4±15.9 | 59.2±21.8 | 109.6±27.9 | 169.9±49.5 | 289.9±109.4 | 475.2±190.7 | ||
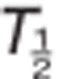 (h) (h) |
7.8±3.0 | 9.0±5.9 | 8.1±1.5 | 7.3±1.1 | 7.8±1.1 | 8.1±1.0 | 8.4±0.9 | ||
| CL/F (l/h) | 245.1±75.9 | 193.2±122.3 | 187.6±65.1 | 143.4±31.0 | 124.3±27.9 | 115.6±39.4 | 98.8±46.6 | ||
AUC, area under the plasma concentration–time curve; AUC0–inf, AUC from time 0 to infinity; AUC0–tau, AUC from time 0 to time tau, where tau equals 24 h; Cmax, maximum observed concentration; CL/F, apparent oral clearance; 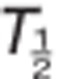 , terminal elimination half-life; Tmax, time to reach Cmax.
, terminal elimination half-life; Tmax, time to reach Cmax.
All data are represented as mean±s.d., except for Tmax values (median plus range).
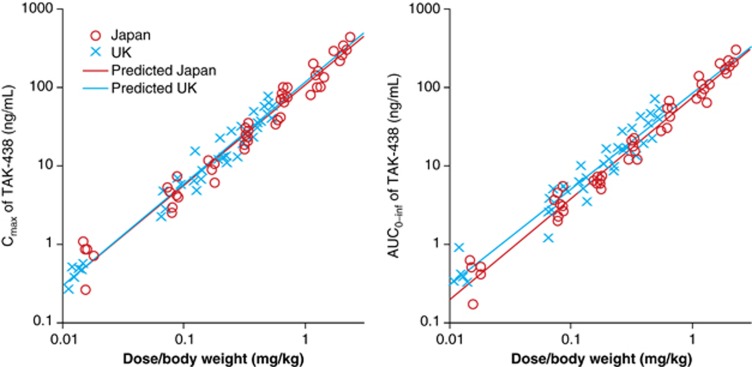
Mean Cmax and AUC0–inf for TAK-438 increased slightly more than dose proportionally. Dose proportionality of Cmax and AUC0–inf was compared between the Japanese and UK subjects using a power model. No differences between Japanese and non-Japanese patients were observed as the two-sided 95% confidence interval of region effects in the slope and y-intercept after logarithmic transformation of the power model included zero in Cmax (−0.083 to 0.116 (slope) and −0.131 to 0.296 (y-intercept) and AUC0–inf (−0.163 to 0.056 (slope) and −0.087 to 0.384 (y-intercept); Figure 2).
Figure 2.
Dose proportionality of Cmax (left) and AUC0–inf (right) for TAK-438 in the phase I, randomized, double-blind, placebo-controlled, Japanese and UK single rising-dose studies in healthy male subjects receiving a single TAK-438 dose (1–120 mg in Japan and 1–40 mg in the UK) (pharmacokinetic analysis set; Japan N=70, UK N=63) evaluated using the power model described by the following equation: ln(PK parameter)=ln(a+β1)+(b+β2) × ln(dose/body weight), where a is the intercept, b is the slope, and β1 or β2 is the regional effect of non-Japanese on the intercept or the slope compared with Japanese. Each symbol shows the observed value for Cmax and AUC. Each line shows the regression line derived from the above power model.
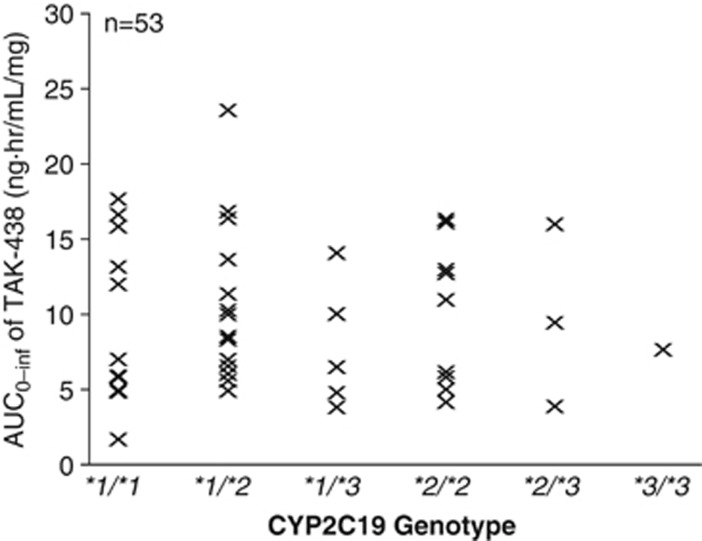
In an exploratory analysis of the Japanese data, the five different CYP2C19 genotypes did not cause any variability in the pharmacokinetic response (dose-normalized AUC0–inf; Figure 3).
Figure 3.
Relationship between CYP2C19 genotype and dose-normalized AUC0–inf of TAK-438 in the phase I, randomized, double-blind, placebo-controlled, Japanese single rising-dose study in healthy male subjects receiving a single TAK-438 dose (1–120 mg).
Urinary excretion of unchanged TAK-438 in the 24 h after dosing was low after all doses: the fraction of dose excreted (%Fe) was 1.9–8.4% in Japan and 1.6–4.7% in the UK.
Pharmacodynamic data
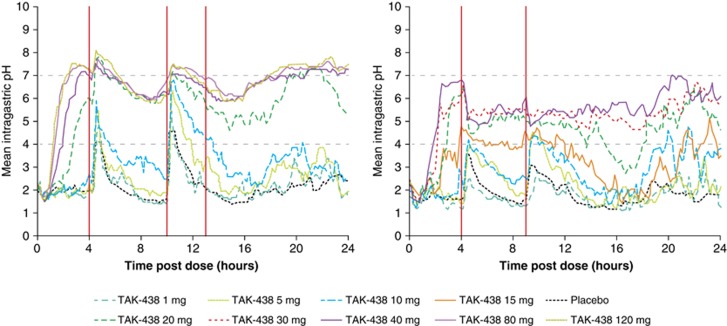
The mean intragastric pH–time parameters after a single dose of TAK-438 showed rapid onset, dose-dependent acid suppression in both studies, with an upward shift in pH compared with placebo (Figure 4).
Figure 4.
Mean intragastric pH–time profiles after a single dose of TAK-438 (1–120 mg in Japan and 1–40 mg in the UK) in the Japanese (left) and UK (right) single rising-dose studies in healthy male subjects (red vertical lines indicate when meals were given) (Pharmacodynamic analysis set; Japan N=82, UK N=63).
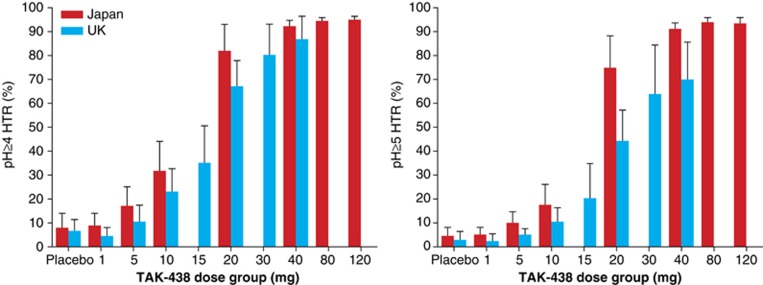
The 24-h pH ≥4 and pH ≥5 HTR increased dose dependently and similarly in both studies, with mean maximum effects after the 120 mg dose in Japan (95% and 93%, respectively) and the 40 mg dose in the UK (87 and 70%, respectively) (Figure 5).
Figure 5.
Dose response for mean pH ≥4 holding time ratio (left) and pH ≥5 holding time ratio (right) in the phase I, randomized, double-blind, placebo-controlled, Japanese and UK single rising-dose studies in healthy male subjects receiving a single TAK-438 dose (1–120 mg in Japan and 1–40 mg in the UK; pharmacodynamic analysis set; Japan N=82, UK N=63).
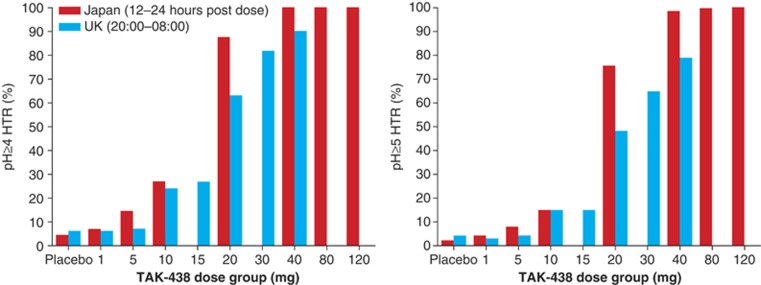
Nighttime acid suppression also increased in a dose-dependent manner. pH ≥4 and pH ≥5 HTR after the 40 mg dose were 100% and 99%, respectively, 12–24 h post dose in the Japanese study and 90 and 79%, respectively, from 20:00–08:00 in the UK study (Figure 6).
Figure 6.
Mean nighttime pH ≥4 holding time ratio (left) and pH ≥5 holding time ratio (right) in the Japanese (12–24 h post dose) and UK (20:00–08:00 h) phase I, randomized, double-blind, placebo-controlled, single rising-dose studies in healthy male subjects receiving a single TAK-438 dose (1–120 mg in Japan and 1–40 mg in the UK; Pharmacodynamic analysis set; Japan N=82, UK N=63).
Safety
TAK-438 was shown to be well tolerated in both studies at all doses studied (up to 120 mg in the Japanese study and up to 40 mg in the UK study). No treatment-emergent adverse events were reported in the Japanese study, whereas 12 treatment-emergent adverse events were reported in 10 of 63 subjects in the UK study (2 events of dizziness (1 with placebo and 1 with TAK-438 1 mg), 2 events of erythema (1 with TAK-438 1 mg and 1 with TAK-438 10 mg), and 1 event each of nasal discomfort (placebo), rhinorrhea (placebo), toothache (TAK-438 1 mg), headache (TAK-438 10 mg), dry skin (TAK-438 10 mg), epistaxis (TAK-438 15 mg), abdominal pain (TAK-438 30 mg), and diarrhea (TAK-438 30 mg)), with no evidence of a dose–response relationship. No serious adverse events were reported in either study.
There were no abnormal changes in vital signs or electrocardiogram. There were also no clinically important changes in any laboratory value, including no obvious increases for alanine aminotransferase or total bilirubin concentration. Serum concentrations of gastrin increased at TAK-438 doses of ≥5 mg in both studies, pepsinogen I increased at doses of ≥5 mg (Japan) and ≥10 mg (UK), and pepsinogen II increased at doses of ≥5 mg (Japan) and ≥10 mg (UK) (data not shown). In both studies, mean serum gastrin concentrations peaked at 6–12 h post dose in the TAK-438 treatment groups, with the Day 1 maximum concentrations reached at 12 h post dose in the 40 mg dose group in the Japanese study (237 pg/ml vs. 85 pg/ml with placebo) and at 10 h post dose in the 20 mg group in the UK study (220 pg/ml vs. 51 pg/ml with placebo).
DISCUSSION
This novel P-CAB, TAK-438, was evaluated for its single rising-dose safety, tolerability, pharmacokinetic, and pharmacodynamic properties in healthy adult males in two phase I, double-blind, placebo-controlled studies in Japan and the UK. The results of both studies were comparable and so are reviewed together in this section.
TAK-438 was safe and well tolerated at all doses tested (1–120 mg), with no adverse events reported in Japanese subjects and only 12 treatment-emergent adverse events in the 10 subjects in the UK study. There were no serious adverse events in either study. Plasma concentrations of TAK-438 increased slightly more than dose proportionally. Saturation of first-pass metabolism may contribute to the non-linearity of the plasma pharmacokinetics. Median Tmax of TAK-438 was ≤2 h and estimated mean terminal elimination half-life was ≤9 h. The combination of the mechanism of action (direct activity on the acid pump) and median plasma half-life of TAK-438 is noteworthy compared with the indirect mechanism of action and very short half-lives of PPIs.
The intragastric pH–time parameters showed dose-dependent acid suppression at doses of 20–120 mg over the entire 24-h period, with a clear dose–response relationship for pH ≥4 and pH ≥5 HTR. The 24-h pH ≥4 HTR was broadly similar for the Japanese and UK subjects, suggesting no substantial differences based on region, although the pH ≥5 HTR was more divergent. Small differences between the studies could be related to the small study population and differences in ethnic demographics and physiology. For example, Asian people typically have a greater response to acid-suppressing drugs, including PPIs.23, 24, 25 Efficacy of PPI treatment in Asian compared with Caucasian populations appears to be influenced by variability in the metabolism of drugs (CYP2C19 polymorphisms), high H. pylori prevalence, a lower parietal cell mass, and lower acid output (even after correcting for body weight, sex, and age).23, 24, 25 This could also be extrapolated to Japanese people.
The 24-h pH HTR reported in our studies (pH ≥4 HTR with TAK-438 40 mg: 92% (Japan) and 87% (UK)) suggests superior acid suppression with TAK-438 than that reported with PPIs.26 It is also worth emphasizing that this high level of acid suppression with TAK-438 occurred after just a single dose, showing a rapid onset. The nighttime pH HTR (pH ≥4 HTR with TAK-438 40 mg: 100% 12–24 h post dose in the Japan study, and 90% from ~20:00–08:00 in the UK study) also indicate potential superiority over PPIs (nighttime pH ≥4 HTR up to 72% depending upon dose and timing.27, 28 This finding is of particular interest for the management of patients with nighttime acid reflux.1
It is generally accepted that suppressing gastric acid secretion enhances healing of damage associated with acid-related disorders, with better healing achieved when intragastric pH >4 is sustained for as long a duration as possible.1, 29, 30 Furthermore, in H. pylori eradication therapy, the role of antisecretory agents is to increase the intragastric pH.31 Therefore, the better acid control with TAK-438 (pH≥5 HTR with 40 mg dose: 91% (Japan) and 70% (UK)) and a faster onset of acid suppression than that seen with PPIs could increase the efficacy (earlier onset of effect) of antibiotics in H. pylori eradication therapy.32, 33, 34, 35, 36
An exploratory analysis of the dose-normalized pharmacokinetic parameters plotted against the CYP2C19 genotype from Japanese data indicated no tendency for CYP2C19 genotype to increase or decrease pharmacokinetic parameters, suggesting that TAK-438 may offer consistently profound and sustained efficacy regardless of CYP2C19 status. This is in contrast to PPIs, which appear to vary in efficacy and in potential for drug–drug interactions depending on patients' CYP2C19 status.37, 38
TAK-438 is estimated to be excreted in the urine within 48 h after study drug administration. The urinary excretion of TAK-438 in the 48-h period after study drug administration indicated a dose-dependent increase for TAK-438 doses of 1–120 mg (Japan) and 1–40 mg (UK) and is estimated to be excreted within 48 h.
In these studies, mean serum gastrin and pepsinogen concentrations were increased above placebo concentrations at most TAK-438 doses. However, mean gastrin levels were within the expected range and increased to similar levels to those observed with PPI therapy (200–400 pg/ml)39, 40, 41 The levels observed for pepsinogen I and II did not indicate corpus atrophy for any subjects at any of the dose levels. Unlike other P-CABs, such as AZD0865, TAK-438 did not increase serum alanine aminotransferase concentrations, probably due to distinct differences in its chemical structure.15
The pharmacokinetic/pharmacodynamic findings after a single dose in these healthy volunteer studies demonstrate a rapid and profound inhibition of gastric acid secretion that was well sustained over a 24-h period and suggest that TAK-438 may prove to be beneficial for the treatment of patients with acid-related disorders. These results support those observed in preclinical animal studies and were predicted based on the compound's high accumulation.16, 17, 18, 19
Nevertheless, findings of the present studies should be considered in light of their practical limitations, including the relatively small study populations of healthy volunteers and minor methodological differences between the Japanese and UK studies, such as different imposed regional dose ranges.
In conclusion, TAK-438 produced rapid, profound, and sustained suppression of gastric acid secretion at single oral doses of 20–120 mg, regardless of CYP2C19 status and region, and was well tolerated in healthy male subjects in two phase I studies. The 24-h intragastric pH HTRs showed dose-dependent acid suppression, with pH ≥4 HTR ≥87% and nighttime pH ≥4 HTR ≥90% following a single dose of 40 mg. TAK-438 may, therefore, represent an efficacious, safe, and well tolerated alternative to PPIs for the treatment of acid-related disorders, particularly in preventing nocturnal acid break-through and reducing acid reflux symptoms.
Study Highlights

Guarantor of the article: Yuuichi Sakurai, MSc.
Specific author contributions: A.N., K.A., H.J., G.K., M.H., and Y.S. were involved in the study concept and design. S.I. and J.T. conducted the studies. Y.S. and H.O. were involved in the statistical analysis. T.Y. was involved with the bioanalytical methodology. Y.S., R.J. and H.J. were involved in the drafting and critical revision of the manuscript. All authors approved the final version of this article, including the authorship list.
Financial support: These studies (TAK-438/CPH-001 and TAK-438_101) were funded in full by Takeda Pharmaceutical Company Ltd, Japan, and Takeda Development Centre Europe Ltd, London, UK. We acknowledge the editorial assistance provided by Hiroaki Itoh of Interface, Kanagawa, Japan and Susan Crawford of Absolute Healthcare Communications, London, UK. Financial support was provided by Takeda Pharmaceutical Company Ltd, Japan and Takeda Pharmaceuticals International, Inc. The results of these studies were previously presented at the 20th United European Gastroenterology Week (UEGW) 2012.
Potential competing interests: K.A. is a paid consultant to Takeda Pharmaceutical Company Ltd. Y.S., A.N., and H.O. are employees of Takeda Pharmaceutical Company Ltd. G.K., M.H., R.J., and H.J. are employees of Takeda Development Centre, Europe Ltd, London, UK. S.I. is an employee of Medical Co. LTA Honjo Clinic (current Sumida Hospital), Tokyo, Japan. J.T. is an employee of Richmond Pharmacology Ltd, London, UK.
References
- Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology 2008; 134: 1842–1860. [DOI] [PubMed] [Google Scholar]
- Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology 2008; 135: 41–60. [DOI] [PubMed] [Google Scholar]
- Modlin IM, Sachs G. Acid Related Diseases: Biology and Treatment 2nd edn. Lippincott Williams & Wilkins: : Philadelphia, USA, 2004. [Google Scholar]
- Boeckxstaens GE, Rohof WO. Pathophysiology of gastroesophageal reflux disease. Gastroenterol Clin N Am 2014; 43: 15–25. [DOI] [PubMed] [Google Scholar]
- Rubenstein JH, Chen JW. Epidemiology of gastroesophageal reflux disease. Gastroenterol Clin N Am 2014; 43: 1–14. [DOI] [PubMed] [Google Scholar]
- Sachs G, Shin JM, Vagin O et al. The gastric H,K ATPase as a drug target: past, present, and future. J Clin Gastroenterol 2007; 41 (Suppl 2): S226–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert ML. Regulation of gastric acid secretion. Johnson LR (ed). Physiology of the Gastrointestinal Tract. 5th edn Oxford: Academic Press: Oxford, UK, 2012; pp. 1281–1309. [Google Scholar]
- Ayazi S, Leers JM, Oezcelik A et al. Measurement of gastric pH in ambulatory esophageal pH monitoring. Surg Endosc 2009; 23: 1968–1973. [DOI] [PubMed] [Google Scholar]
- Carlson DA, Pandolfino JE. Acid and nonacid reflux monitoring. Gastroenterol Clin N Am 2014; 43: 89–104. [DOI] [PubMed] [Google Scholar]
- Shin JM, Vagin O, Munson K et al. Molecular mechanisms in therapy of acid-related diseases. Cell Mol Life Sci 2008; 65: 264–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia A, Kraft WK. Acid peptic diseases: pharmacological approach to treatment. Expert Rev Clin Pharmacol 2009; 2: 295–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa Y, Nishida H, Kurasawa O et al. Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1(pyridin-3-ylsulfonyl)-1H-pyrrol-3-y-l]-N-methylmethanamine fumarate (TAK-438) as a potassium-competitive acid blocker (P-CAB). J Med Chem 2012; 55: 4446–4456. [DOI] [PubMed] [Google Scholar]
- Kondo M, Kawamoto M, Hasuoka A et al. High-throughput screening of potassium-competitive acid blockers. J Biomol Screen 2012; 17: 177–182. [DOI] [PubMed] [Google Scholar]
- Nishida H, Hasuoka A, Arikawa Y et al. Discovery, synthesis, and biological evaluation of novel pyrrole derivatives as highly selective potassium-competitive acid blockers. Bioorg Med Chem 2012; 20: 3925–3938. [DOI] [PubMed] [Google Scholar]
- Dent J, Kahrilas PJ, Hatlebakk J et al. A randomized, comparative trial of a potassium-competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol 2008; 103: 20–26. [DOI] [PubMed] [Google Scholar]
- Hori Y, Matsukawa J, Takeuchi T et al. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther 2011; 337: 797–804. [DOI] [PubMed] [Google Scholar]
- Hori Y, Imanishi A, Matsukawa J et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther 2010; 335: 231–238. [DOI] [PubMed] [Google Scholar]
- Matsukawa J, Hori Y, Nishida H et al. A comparative study on the modes of action of TAK-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol 2011; 81: 1145–1151. [DOI] [PubMed] [Google Scholar]
- Shin JM, Inatomi N, Muson K et al. Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1(pyridin-3-ylsulfonyl)-1 H-pyrrol-3-y-l]-N-methylmethanamine monofumarate (TAK-438). J Pharmacol Exp Ther 2011; 339: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PO, Scheiman JM, Barkun AN. Review article: acid-related disease—what are the unmet clinical needs? Aliment Pharmacol Ther 2006; 23 (Suppl 2): 9–22. [DOI] [PubMed] [Google Scholar]
- Data on file. Takeda Pharmaceutical Company Ltd, Osaka, Japan.
- Gough K, Hutchison M, Keene O et al. Assessment of dose proportionality: report from pharmaceutical industry. Drug Inf J 1995; 29: 1039–1048. [Google Scholar]
- Lam SK. Differences in peptic ulcer between East and West. Baillieres Best Pract Res Clin Gastroenterol 2000; 14: 41–52. [DOI] [PubMed] [Google Scholar]
- Lam SK, Hasan M, Sircus W et al. Comparison of maximal acid output and gastrin response to meals in Chinese and Scottish normal and duodenal ulcer subjects. Gut 1980; 21: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong RW. Differences in peptic ulcer between the East and the West. Gastroenterol Clin N Am 2009; 38: 363–379. [DOI] [PubMed] [Google Scholar]
- Kalaitzakis E, Björnsson E. A review of esomeprazole in the treatment of gastroesophageal reflux disease. Ther Clin Risk Manag 2007; 3: 653–663. [PMC free article] [PubMed] [Google Scholar]
- Morelli G, Chen H, Rossiter G et al. An open-label, parallel, multiple-dose study comparing the pharmacokinetics and gastric acid suppression of rabeprazole extended-release with esomeprazole 40 mg and rabeprazole delayed-release 20 mg in healthy volunteers. Aliment Pharmacol Ther 2011; 33: 845–854. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith C, Röhss K, Bokelund Singh S et al. The effects of dose and timing of esomeprazole administration on 24-h, daytime and night-time acid inhibition in healthy volunteers. Aliment Pharmacol Ther 2010; 32: 1249–1256. [DOI] [PubMed] [Google Scholar]
- Gerson LB, Fass R. A systematic review of the definitions, prevalence, and response to treatment of nocturnal gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2009; 7: 372–378. [DOI] [PubMed] [Google Scholar]
- Orr WC. Review article: sleep-related gastro-oesophageal reflux as a distinct clinical entity. Aliment Pharmacol Ther 2010; 31: 47–56. [DOI] [PubMed] [Google Scholar]
- Sachs G, Shin JM, Munson K et al. Review article: the control of gastric acid and Helicobacter pylori eradication. Aliment Pharmacol Ther 2000; 14: 1383–1401. [DOI] [PubMed] [Google Scholar]
- Fennerty MB, Cutler AF, Go MF et al The treatment of H. pylori: where are we now? http://www.medscape.org/viewarticle/457393 (Accessed 15 April 2014).
- Erah PO, Goddard AF, Barrett DA et al. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother 1997; 39: 5–12. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Furuta T, Shirai N et al. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter 2007; 12: 317–323. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Uotani T, Sahara S et al. Efficacy of tailored Helicobacter pylori eradication treatment based on clarithromycin susceptibility and maintenance of acid secretion. Helicobacter 2014; 19: 312–318. [DOI] [PubMed] [Google Scholar]
- Marcus EA, Inatomi N, Nagami GT et al. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther 2012; 36: 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Shirai N, Sugimoto M et al. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet 2005; 20: 153–167. [DOI] [PubMed] [Google Scholar]
- Chong E, Ensom MHH. Pharmacogenetics of the proton pump inhibitors: a systematic review. Pharmacotherapy 2003; 23: 460–471. [DOI] [PubMed] [Google Scholar]
- Orlando LA, Lenard L, Orlando RC. Chronic hypergastrinemia: causes and consequences. Dig Dis Sci 2007; 52: 2482–2489. [DOI] [PubMed] [Google Scholar]
- Laine L, Ahnen D, McClain C et al. Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther 2000; 14: 651–668. [DOI] [PubMed] [Google Scholar]
- Murugesan SVM, Varro A, Pritchard DM. Review article: strategies to determine whether hypergastrinaemia is due to Zollinger–Ellison syndrome rather than a more common benign cause. Aliment Pharmacol Ther 2009; 29: 1055–1068. [DOI] [PubMed] [Google Scholar]