Abstract
OBJECTIVES:
Decreased magnetization transfer ratio (MTR) in the brain characterizes cerebral edema (CE) in patients with liver cirrhosis, but the role of treatment on its reversibility has not been studied in patients who have minimal hepatic encephalopathy (MHE). This study was carried to evaluate the reversibility of CE with lactulose and rifaximin treatment in patients with MHE and role of ammonia, pro-inflammatory interleukins (IL-1, IL-6) and tumor necrosis factor (TNF)-α in its pathogenesis.
METHODS:
Twenty-three patients with cirrhosis (14 with MHE, 9 without MHE (NMHE)) and 6 healthy controls underwent ammonia, IL-1, IL-6, TNF-α estimation, and MTR in frontal white matter (FWM), parietal white matter (PWM), internal capsule (IC), and basal ganglia (BG).
RESULTS:
Ammonia was significantly higher in the cirrhosis group compared with controls and in MHE compared with the NMHE group. Ammonia correlated positively with IL-1 and IL-6. MTRs in FWM, PWM, IC, and BG were significantly lower in the MHE group compared with controls and in PWM, IC, and BG compared with the NMHE group. MHE patients showed significant MTR increase in FWM, PWM, and IC with treatment. IL-6 and ammonia had significant negative and significant positive psychometric hepatic encephalopathy score (PHES) correlation with MTR in various regions.
CONCLUSIONS:
This study, for the first time, demonstrated the reversibility of low-grade CE with treatment in patients with MHE. Negative correlation between ammonia, IL-6 levels, and MTR and positive correlation between PHES and MTR in MHE patients suggests the role of inflammation and ammonia in the genesis of low-grade CE.
INTRODUCTION
Spectrum of Neurocognitive Impairment in Cirrhosis spans the range from normal cognitive function to minimal hepatic encephalopathy (MHE) to overt hepatic encephalopathy (OHE).1, 2 Cerebral edema is associated with HE both in patients with acute liver failure and with cirrhosis. However, in acute liver failure, cerebral edema develops rapidly and does not allow the system to compensate to counteract the osmotic imbalance induced by rapid intra-astrocytic accumulation of glutamine, while in cirrhosis there is sufficient time for effective compensation and stabilization of osmolyte shift.3 Intracranial hypertension develops in 80% of patients with acute liver failure and leads to 25% mortality, while intracranial hypertension is rarely observed in patients with cirrhosis, even though cerebral edema may be present.4
Although MHE is seen in 30–80% of patients with cirrhosis of liver, OHE occurs in approximately 30–45% of patients with cirrhosis.2, 5 Both MHE and OHE are considered to be fully reversible with treatment.6 The burden and impact of MHE is significant. MHE impairs daily functioning and health-related quality of life, predicts the development of OHE, and is associated with poor survival,6, 7, 8 impaired driving skills, increased number of traffic violations and even crashes.9 Psychometric hepatic encephalopathy score (PHES) is a reliable tool for the diagnosis of MHE, has been validated, and has a high sensitivity (96%) and specificity (100%).8, 10 However, these tests do not reveal the underlying pathophysiology.
Brain magnetic resonance imaging (MRI) reveals characteristic findings in HE and may complement neuropsychological (NP) examination. Classic MRI abnormalities include high signal intensity in the globus pallidus on T1-weighted images, likely a reflection of increased tissue concentrations of manganese. An elevated glutamine/glutamate peak coupled with decreased myo-inositol and choline signals on proton MR spectroscopy (MRS) represents disturbances in cell-volume homeostasis secondary to brain hyperammonemia. Recent data have demonstrated that low-grade cerebral edema can be detected with several MR techniques such as magnetization transfer ratio (MTR) measurements, fast fluid-attenuated inversion recovery (FLAIR) sequences, diffusion-weighted images (DWI), and diffusion tensor imaging (DTI). All these MRI abnormalities, which return to normal with restoration of liver function, probably reflect the presence of mild diffuse cerebral edema, which seems to have an essential role in the pathogenesis of HE in cirrhosis.11 MTR is relatively simpler and useful technique to detect low-grade cerebral edema in HE, which may not be visible by using standard MRI and is mainly based on the interaction between protons in a relatively free environment (bulk water) and those in which motion is restricted (immobile water).
Although the evidence base supporting a pivotal role of ammonia is robust, in everyday clinical practice a consistent correlation between the concentration of ammonia in the blood and the manifest symptom HE is not observed. More recently, the synergistic role of inflammation and infection in modulating the cerebral effects of ammonia has shown to be important.12, 13 It has been recognized that infection impairs brain function both in the presence and absence of liver disease. Thus it could be postulated that, in the presence of ammonia, the brain is sensitized to a systemic inflammatory stimulus and is able to elicit an inflammatory response involving both pro-inflammatory and neurotransmitter pathways.12, 13
Studies of MTR in MHE are limited and its correlations with ammonia and inflammatory mediators have not been studied thus far. This study was undertaken to assess the presence of low-grade cerebral edema as assessed by MTR in patients with MHE and its reversibility following treatment of MHE. To understand the pathophysiology of cerebral edema in MHE, we also assessed the correlation of inflammatory markers such as interleukin (IL)-1, IL-6 and tumor necrosis factor alpha (TNF-α) and ammonia with cerebral edema.
METHODS
The Ethics Committee of Postgraduate Institute of Medical Education and Research (PGIMER), a tertiary level health-care center in Chandigarh, India approved the study (Endst number 1Trg-PG-2012/5053, dated 19 April 2014). Each subject gave written informed consent before being included in the study. The guidelines laid down by Indian Council of Medical Research (1994) and Helsinki declarations (modified in 1989) were adhered to in all patients in the study.
Patients
All patients diagnosed to have cirrhosis of liver attending the outpatient liver clinic as well as those admitted under Hepatology services, PGIMER, Chandigarh were candidates for enrollment. The diagnosis of cirrhosis was based on clinical, biochemical, and ultrasonographic or liver histological data. Exclusion criteria were OHE; alcohol intake during the past 6 weeks; history of gastrointestinal bleed in the past 6 weeks; active ongoing sepsis; history of porto-systemic shunt surgery or transjugular intrahepatic portosystemic shunt; significant co-morbid illness such as heart, respiratory, or renal failure, any neurological diseases such as Alzheimer's disease, Parkinson's disease, non-hepatic metabolic encephalopathies and stroke; patients on psychoactive drugs such as antidepressants or sedatives; those who restarted alcohol consumption during follow-up; serum creatinine >1.5 mg; electrolyte imbalance; hepatocellular carcinoma; contra-indications to MRI; inability to perform PHES; and unable to give informed consent. Etiology of cirrhosis of liver was determined as described previously.6
Six age- and sex-matched healthy volunteers were included in the study for comparison.
Clinical and laboratory assessment
All included patients underwent a thorough general physical and systemic examination, including complete neurological examination to exclude the presence of any illness which can affect the neurological status. Laboratory investigations included a complete hemogram, serum electrolytes, renal and liver function tests, and complete coagulogram. The severity of cirrhosis was determined by Child–Pugh's and model for end-stage liver disease scores. Ultrasound of abdomen and upper gastrointestinal endoscopy was performed in all patients. Triphasic contrast enhanced computed tomography of the abdomen was carried out in all patients; one patient demonstrated spontaneous leno-renal portosystemic shunt. Evidence for systemic inflammatory response syndrome (SIRS) was assessed in all patients at enrollment into the study.
NP assessment
The West Haven grade=0, a mini mental state examination score ≥25 and Clinical Hepatic Encephalopathy Staging Scale=0 determined grade 0 HE in patients with cirrhosis.1, 14, 15 NP assessment was done by PHES. PHES has been validated for the assessment of MHE in an Indian population and can be carried out in 15–20 min.8 The clinical significance of the PHES has also been evaluated in a large number of healthy volunteers and patients with MHE.8, 16 PHES ≤−5 was considered to be diagnostic of MHE.8
Neuroimaging
Whole-brain conventional MRI, including T2, FLAIR, and DWI in axial planes and T1 MPRAGE (magnetization-prepared rapid gradient echo) or SPGR (spoiled gradient recalled acquisition in steady state), was performed on a 3.0 Tesla MR scanner (Verio, Siemens Medical Solutions, Erlangen, Germany or GE Healthcare, Waukesha, WI). Subsequently MTR was performed. The MR findings were analyzed by two observers (C.K.A. and N. Kalra) blinded to the clinical and biochemical characteristics and levels of inflammatory mediators. The total examination time was approximately 20 min in each subject. T2-weighted images (repetition time (TR)=9000 ms; echo-time (TE)=94 ms; matrix size=320 × 320; field of view (FOV)=220 × 220 mm2; slice thickness=4.0 mm; interslice gap=0.0 mm; turbo factor=5; flip angle (FA)=158°) were acquired in the axial plane covering whole brain. High-resolution T1-weighted images were collected using a SPGR or MPRAGE sequence (TR=1800 ms; TE=3.87 ms; inversion time=900 ms; FA=9° matrix size=256 × 100; FOV=230 × 230 mm; slice thickness=1 mm and transverse imaging plane). Magnetization transfer imaging study was performed in the transverse plane before and after application of a proton saturation pulse. MTR values were quantified as a percentage of signal loss according to the following equation: MTR=100(S0−SS)/S0 in which S0 is the mean signal intensity for a particular region without the saturation pulse and SS is the mean signal intensity for the same region with saturation pulse. The mean MTRs were determined in regions of interest (ROIs) manually drawn by one examiner (C.K.A.), placed in the right and left frontal white matter (FWM); right and left parietal white matter (PWM); right and left internal capsule (IC) (average of anterior limb, genu, and posterior limb of IC on each side); and right and left basal ganglia (BG). MTR values were reported as a mean of right and left ROIs. ROI size was kept constant for all regions.
Blood samples analysis
Blood samples from a peripheral vein were collected at baseline and after 8 weeks of the follow-up. Blood sample analysis for ammonia and cytokines were performed as described by us previously.17 Ammonia estimation was carried out immediately using the Ammonia Checker II (Daiichi Kagaku, Kyoto, Japan) using finger prick blood. Cytokines (TNF-α, IL-1β, and IL-6) were measured in plasma derived from patients with cirrhosis using a specific Enzyme-linked Immunosorbent Assay Kits (RayBiotech,Norcross, GA) according to the manufacturer's protocol. The plate was read at 450 nm. Absorbance was converted to pg/ml using a standard curve prepared with recombinant human TNF-α, IL-1β, and IL-6.
Study design
Patients who met eligibility criteria were included in the study and underwent NP evaluation by PHES. PHES ≤−5 was diagnostic of MHE.8 They were divided into two groups, i.e., MHE and NMHE based upon the results of PHES. Patients in each group were followed for 8 weeks. In the MHE group, patients received 30–60 ml of lactulose in 2 or 3 divided doses so that patient passed 2–3 semisoft stools per day and rifaximin 550 mg twice daily for 8 weeks. Clinical and standard laboratory assessment, ammonia, inflammatory mediators (IL-1, IL-6 and TNF-α), PHES, and MRI (T1, T2W images, and MTR) were analyzed in MHE and NMHE patients at baseline and at 8 weeks. Control subjects were also investigated in a similar manner at baseline.
Statistical analysis
Data were expressed as mean (95% confidence interval) for normally distributed data and median for skewed data. One-way analysis of variance and post-hoc analysis was carried out to assess the trend of baseline parameters between various groups and to compute statistical significance between them. Categorical variables were compared using Chi square and Fisher exact tests. Continuous variables were compared using Mann–Whitney U-test for unpaired data and Wilcoxon signed-rank test for paired data. Spearman's rank correlation was used to explore the association between PHES scores, ammonia, inflammatory markers, and MTR indices. The probability level of <0.05 was set for statistical significance. SPSS 17 (SPSS, Chicago, IL) was used for statistical computations.
RESULTS
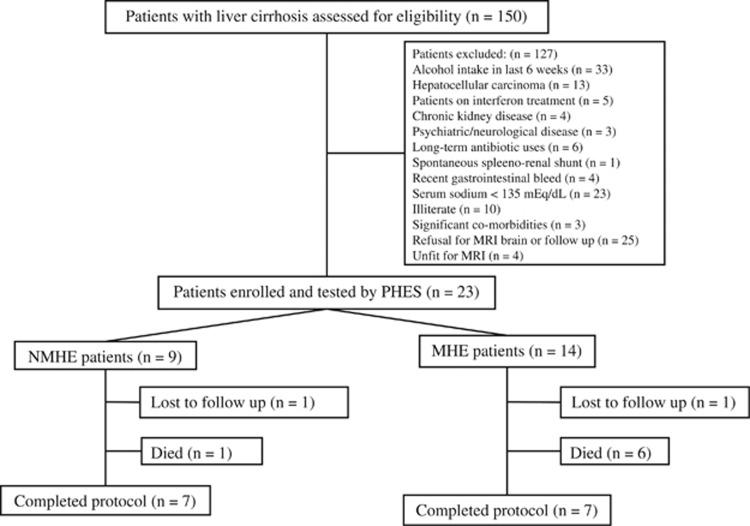
Between April 2012 and June 2013, 150 patients diagnosed to have cirrhosis of liver attending the outpatient liver clinic as well as those admitted under Hepatology services, PGIMER, Chandigarh were screened. Twenty-three patients (15.3%) who met the eligibility criteria were included in the study. Reasons that 127 patients (84.7%) were excluded from the study were: history of alcohol intake in the past 6 weeks (33 patients), hepatocellular carcinoma (13 patients), chronic kidney disease (4 patients), psychiatric/neurological disease (3 patients), need for long-term antibiotic use (6 patients), interferon treatment for hepatitis C virus (5 patients), spontaneous spleno-renal shunt (1patient), recent gastrointestinal bleed <6 weeks (4 patients), serum sodium <135 mEq/dl (23 patients), illiterate (10 patients), significant co-morbidities (3 patients), refusal to give consent for MRI brain or follow-up (25 patients), and unfit for MRI (4 patients). Several patients were excluded for more than one reason. After exclusion, 23 patients included in the study were screened with PHES: 14 (60.9%) patients were found to have MHE and 9 (39.1%) constituted the NMHE group. Patients found to have MHE were treated with rifaximin and lactulose as per protocol. During a follow-up of 8 weeks, 1 patient was lost to follow-up and 1 died (owing to spontaneous bacterial peritonitis resulting in sepsis and renal failure) in the NMHE group, while 1 patient was lost to follow-up and 6 died (1 owing to refractory gastrointestinal bleed, 2 owing to development of overt HE followed by sepsis and 3 owing to community-acquired infections resulting in sepsis and multi-organ failure) in the MHE group. Rest of the patients, 7 in each group, completed the study. Figure 1 shows flow of participants into the study. The clinical and demographic characteristics of patients enrolled in the study are shown in Table 1. There was no statistically significant difference between patients completing and those not completing the study (Supplementary Table S1).
Figure 1.
Flow of the patients into the study. MHE, minimal hepatic encephalopathy; MRI, magnetic imaging resonance; NMHE, no minimal hepatic encephalopathy; PHES, psychometric hepatic encephalopathy score.
Table 1. Clinical and demographic characteristics of patients.
| Parameters | Controls | NMHE | MHE | P value |
|---|---|---|---|---|
| N | 6 | 9 | 14 | |
| Age ( in years) | 47.5 (31.75 to 55.25) | 47.00 (40.00 to 56.00) | 49.50 (34.75 to 55.00) | 0.855 |
| Sex (male: female) | 5:1 | 8:1 | 11:3 | 0.814 |
| Etiology | ||||
| Alcohol | — | 4 (44.4%) | 9 (64.3%) | 0.417 |
| HBV | — | 1 (11.1%) | 1 (7.1%) | 1.000 |
| Alcohol+HBV | 0 (0%) | 1 (7.1%) | 1.000 | |
| HCV | — | 2 (22.2%) | 1 (7.1%) | 0.538 |
| NASH | — | 1 (11.1%) | 1 (7.1%) | 1.000 |
| Others | — | 1 (11.1%) | 1 (7.1%) | 1.000 |
| Previous gastrointestinal bleed (%) | — | 1 (11.1) | 3 (21.4) | 0.448 |
| Previous decompensation | — | 7 (77.8) | 13 (92.9) | 0.295 |
| Previous HE | — | 1 (11.1) | 5 (35.7) | 0.190 |
| Diabetes mellitus | — | 2 (22.2) | 3 (21.4) | 0.964 |
| Hypertension | — | 2 (22.2) | 1 (7.1) | 0.295 |
| Creatinine (mg/dl) | — | 0.90 (0.75 to 1.18) | 0.95 (0.90 to 1.12) | 0.689 |
| Na+ (mEq/l) | — | 138.0 (136.0 to 141.0) | 136.0 (135.0 to 141.5) | 0.754 |
| CTP score | — | 10.0 (7.0 to 10.5) | 10.5 (9.0 to 11.2) | 0.197 |
| Child class A/B/C (%) | — | 1/3/5 (11.1/33.3/55.6) | 0/4/10 (0/28.5/71.5) | 0.405 |
| MELD score | — | 12.00 (11 to 25) | 18.50 (16.7 to 22.5) | 0.230 |
| PHES score | 2.0 (1.7 to 3.7) | −1.0 (−3.0 to 0.0) | −7.5 (−9.0 to −6.0) | <0.001 |
CTP, Child–Turcotte–Pugh; HBV, hepatitis B virus; HCV, hepatitis C virus; HE, hepatic encephalopathy; MELD model of end-stage liver disease; MHE, minimal hepatic encephalopathy; NASH, non-alcoholic steatohepatitis; NMHE, no minimal hepatic encephalopathy; PHES, psychometric hepatic encephalopathy score. Data are expressed as median (25th–75th percentile range) or number (%).
Ammonia and inflammatory markers
Ammonia was significantly higher in the NMHE and MHE groups as compared with controls and was significantly higher in MHE when compared with NMHE (Table 2). Among inflammatory markers, IL-1 and IL-6 were significantly elevated in MHE patients when compared with controls. There was no significant difference in TNF-α level between any study groups. SIRS was present in 50% of patients of MHE and was not seen in controls or the NMHE group (Table 2).
Table 2. Baseline arterial ammonia and IL-1, IL-6, and TNF-α in the study population.
| Parameters | Controls (a) | NMHE (b) | MHE (c) | P value a vs. b | P value b vs. c | P value a vs. c |
|---|---|---|---|---|---|---|
| N | 6a | 9 | 14a | |||
| SIRS | 0% | 0% | 7 (50%) | — | 0.019 | 0.051 |
| Arterial ammonia (μg/dl) | 37.50 (30.2–41.5) | 51.00 (48.0–82.5) | 98.0 (85.2–117.5) | 0.024 | 0.003 | <0.001 |
| IL-1 (pg/ml) | 2.56 (2.08–16.29) | 23.45 (13.79–42.25) | 28.46 (17.49–49.90) | 0.053 | 0.382 | 0.027 |
| IL-6 (pg/ml) | 31.47 (29.07–98.23) | 63.65 (39.34–65.05) | 69.03 (51.94–558.85) | 0.147 | 0.382 | 0.045 |
| TNF-α (pg/ml) | 2153.19 (1492.59–2568.58) | 1985.24 (954.04–2354.72) | 1682.93 (1174.61–2264.03) | 0.463 | 0.776 | 0.461 |
IL-1, interleukin 1; MHE, minimal hepatic encephalopathy; NMHE, no minimal hepatic encephalopathy; SIRS, systemic inflammatory response syndrome; TNF-α, tumor necrosis factor alpha. Data are expressed as median (25th–75th percentile range) or numbers (%).
IL-1, IL-6, and TNF-α values are not available in 1 patient in the control group and 2 patients in the MHE group.
In the cirrhosis group, PHES correlated negatively with ammonia levels (r=−0.502, P=0.015). Ammonia also had a significantly positive correlation with IL-1 and IL-6 in patients with cirrhosis (r=0.566, P=0.009 and r=0.473, P=0.030, respectively).
Table 3 shows changes in ammonia and inflammatory mediators in the NMHE and MHE group at baseline and 8 weeks. Ammonia and inflammatory markers did not show any significant change in the NMHE and MHE group although IL-6 levels showed a trend in improvement during follow-up. PHES scores significantly improved in the MHE group after treatment but did not show any significant change on follow-up in the NMHE group.
Table 3. Arterial ammonia, inflammatory markers and PHES in the NMHE and MHE groups at baseline and after 8 weeks.
| Parameters |
NMHE |
MHE |
||||
|---|---|---|---|---|---|---|
| Baseline | 8 weeks | P value | Baseline | 8 weeks | P value | |
| N | 7 | 7 | 7 | 7 | ||
| Arterial ammonia (μg/dl) | 51.0 (48.0 to 72.5) | 54.0 (44.0 to 65.0) | 0.735 | 93.0 (74.7 to 102.5) | 48.0 (42.0 to 106.0) | 0.176 |
| IL-1 (pg/ml) | 15.93 (12.48 to 39.33) | 28.47 (16.66 to 50.92) | 0.866 | 23.81 (12.89 to 45.31) | 20.52 (16.76 to 23.45) | 0.249 |
| IL-6 (pg/ml) | 62.96 (39.00 to 65.71) | 32.15 (29.42 to 60.91) | 0.128 | 62.02 (35.58 to 156.00) | 40.03 (21.88 to 71.18) | 0.063 |
| TNF-α (pg/ml) | 1,985.24 (957.40 to 2,464.45) | 2,182.30 (1,644.86 to 2,193.49) | 0.866 | 1,685.17 (1,174.61 to 2,264.03) | 1,597.84 (1,252.99 to 1,774.74) | 0.917 |
| PHES | −1.0 (−2.0 to 0.0) | 0.0 (−3.0 to 0.0) | 0.564 | −6.5 (−8.7 to −6.0) | −4.0 (−6.0 to −3.0) | 0.044 |
IL-1, interleukin 1; MHE, minimal hepatic encephalopathy; NMHE, no minimal hepatic encephalopathy; PHES, psychometric hepatic encephalopathy score; TNF-α, tumor necrosis factor alpha. Data are expressed in median (25th–75th percentile range).
MRI evaluation
Upon brain MRI, diffuse atrophy was seen in 47.9% of patients with liver cirrhosis. T1 sequence showed bilateral basal ganglion hyperintensities in 60.8% of patients with cirrhosis and were more common in the MHE group (78.6%) than in the NMHE group (33.3%) (P=0.077) although not statistically significant. T2 white matter hyperintensities were seen in 21.7% of patients with cirrhosis and were similar between patients with and without MHE (28.6% and 11.1%, respectively, P=0.611).
MTR values in FWM, PWM, IC, and BG were significantly lower in the MHE group when compared with controls (P=0.002, 0.002, 0.003 and 0.019, respectively). When the MHE group was compared with the NMHE group, MTR in all regions were significantly lower except in FWM (FWM, P=0.105; PWM, P=0.001; IC, P=0.013; and BG, P=0.031; Table 4).
Table 4. Magnetization transfer ratio findings at baseline in the study population.
| Parameters | Controls (a) | NMHE (b) | MHE (c) | P value a vs. b | P value b vs. c | P value a vs. c |
|---|---|---|---|---|---|---|
| N | 6 | 8 | 13 | |||
| FWM | 25.789 (24.071–26.835) | 23.313 (21.330–29.824) | 21.743 (19.342–23.095) | 0.245 | 0.105 | 0.002 |
| PWM | 25.227 (24.290–27.465) | 23.987 (23.657–29.498) | 21.687 (18.667–23.053) | 0.573 | 0.001 | 0.002 |
| IC | 26.383 (23.387–28.011) | 24.410 (20.946–25.998) | 20.776 (18.738–21.706) | 0.345 | 0.013 | 0.003 |
| BG | 20.353 (18.367–22.708) | 20.590 (16.330–23.634) | 18.124 (13.316–19.135) | 1.000 | 0.031 | 0.019 |
BG, basal ganglia; FWM, frontal white matter; IC, internal capsule; MHE, minimal hepatic encephalopathy; NMHE, no minimal hepatic encephalopathy; PWM, parietal white matter. Data expressed in median (25th–75th percentile range). In one patient each in the NMHE and MHE groups, magnetization transfer ratio study was suboptimal and hence was excluded.
In the MHE group at 8 weeks follow-up, there were significantly increased MTR values in all the brain regions studied except BG (FWM (P=0.028), PWM (P=0.043), and IC (P=0.028)), demonstrating improvement in cerebral edema. The 8-week MTR values were statistically not different from those of controls in all regions of the brain studied, suggesting that MHE patients improved to the level of controls with treatment. In the NMHE group, there were no significant changes in MTR values between baseline and follow-up (Table 5).
Table 5. Magnetization transfer ratio in the NMHE and MHE groups at baseline and 8 weeks.
| Control |
NMHE |
MHE |
||||||
|---|---|---|---|---|---|---|---|---|
| (a) | Baseline (b) | 8 weeks (c) | P value (b vs. c) | Baseline (d) | 8 weeks (e) | P value (d vs. e) | P value (a vs. e) | |
| N | 6 | 7 | 7 | 7 | 7 | |||
| FWM | 25.789 (24.071–26.835) | 23.100 (20.686–31.992) | 23.165 (22.973–25.402) | 0.398 | 21.110 (20.740–22.736) | 24.720 (20.874–28.203) | 0.028 | 0.612 |
| PWM | 25.227 (24.290–27.465) | 23.890 (23.600–30.937) | 23.529 (21.014–24.353) | 0.063 | 21.687 (20.690–23.470 | 24.459 (21.909–25.760) | 0.043 | 0.248 |
| IC | 26.383 (23.387–28.011) | 24.550 (20.286–26.450) | 23.649 (19.642–25.540) | 0.499 | 20.776 (20.180–22.190) | 24.290 (21.233–27.931) | 0.028 | 0.381 |
| BG | 20.353 (18.367–22.708) | 20.967 (16.040–23.652) | 18.159 (16.900–19.354) | 0.237 | 18.124 (14.020–18.629) | 17.990 (15.072–23.831) | 0.398 | 0.368 |
BG, basal ganglia; FWM, frontal white matter; IC, internal capsule; MHE, minimal hepatic encephalopathy; NMHE, no minimal hepatic encephalopathy; PWM, parietal white matter. Data expressed in median (25th–75th percentile range).
Correlations of ammonia, inflammatory markers and PHES scores with MTR
IL-6 levels had significant negative correlations with MTR values in PWM and IC (r=−0.608, P=0.004 and r=−0.454, P=0.044, respectively) in patients with liver cirrhosis. Ammonia correlated negatively with MTR values of PWM only (r=−0.445, P=0.043). PHES correlated positively with MTR values in PWM (r=0.589, P=0.005) and IC (r=0.533, P=0.013). However, there was no significant correlation between changes in MTR values and changes in PHES, ammonia, or inflammatory markers levels after treatment in MHE patients (Supplementary Table S2).
DISCUSSION
This study demonstrated the presence of low-grade cerebral edema in patients with cirrhosis and MHE, which is reversible after the treatment with lactulose and rifaximin. We also demonstrated that there is a significant correlation between ammonia levels, inflammatory markers, and MTR at baseline in patients of cirrhosis with MHE. PHES also correlated with ammonia and MTR values. These finding suggests that inflammation and ammonia have a role to play in the genesis of low-grade cerebral edema in patients with MHE with a resultant cognitive decline.
Studies have shown that serum ammonia levels correlate with the severity of HE, with the highest ammonia levels seen in patients with grade 3/4 HE compared with grade 1/2 HE.18 Arterial ammonia levels are significantly higher in patients with MHE than those without MHE and controls.19 Our study also demonstrated that ammonia levels were significantly higher in the MHE than in the NMHE and control groups. However, Shawcross et al.20 demonstrated that a consistent correlation between ammonia levels and severity of HE is not always observed and other factors such as inflammation may be involved in the pathogenesis of HE. They showed that inflammatory markers such as white cell count, C-reactive protein levels and IL-6 were significantly higher in those with MHE than in those without MHE.21 Montoliu et al.22 also demonstrated that IL-6 concentration was significantly higher in the MHE group compared with the NMHE group and in the NMHE group compared with controls with a good correlation of IL-6 with PHES. Jain et al.19 showed that levels of inflammatory cytokines and arterial ammonia decreased significantly in MHE patients when treated with lactulose for 3 months. Fifty percent of our patients with MHE had SIRS in the absence of active infection compared with none in the NMHE and control groups. Also, markers of inflammation such as IL-1 and IL-6 levels were significantly higher in the MHE group compared with controls. A positive correlation between ammonia, IL-1, and IL-6 levels in patients of cirrhosis with or without MHE and a negative correlation between ammonia levels with composite PHES shows that with increasing levels of ammonia there is increasing level of inflammation and progressive deterioration of NP functions. These data together suggest a synergism of ammonia and inflammation in the pathogenesis of MHE and confirms previous observations.23 Treatment with rifaximin and lactulose for 8 weeks in MHE patients resulted in reversal of cerebral edema coupled with improvement in PHES and a trend towards reduction in ammonia, IL-1, and IL-6 levels. In a recent study of 30 patients with cirrhosis and MHE, Jain et al.19 found significant decrease in arterial ammonia and inflammatory mediators after 12 weeks of lactulose therapy.
However, while both MTR and PHES improved with treatment in MHE group, and ammonia, inflammatory markers, and PHES correlated with MTR at baseline, we could not demonstrate a correlation between changes in MTR values and changes in PHES, ammonia, or inflammatory markers to conclusively demonstrate the pathogenic role of neuroinflammation and cerebral edema in MHE. We believe this could be a type 2 error due to small number of MHE patients completing the study, and a larger sample size would be needed to demonstrate these correlations. Similarly, lack of significant improvement in ammonia and inflammatory markers with treatment in the MHE group also could be due to the small number of patients completing treatment.
Bilateral BG hyperintensities were common (60.8%) in patients with cirrhosis in our study and were more common in the MHE group (78.6%) than in the NMHE (33.3%) group although not statistically significant. In an earlier study from our center, it was found in 80% patients with cirrhosis.24 T1 hyperintensity is likely due to increased concentration of manganese in the central nervous system with a preferential deposition in the globus pallidus. Pallidus hyperintensity occurs secondary to portal-systemic shunting, as it is also seen in patients with portal-systemic collaterals secondary to portal thrombosis and normal liver function.25 Diffuse atrophy of the brain on MRI was seen in 47.5% patients of cirrhosis in our study. In a study by Thuluvath et al.26 it was seen in 45% of patients with cirrhosis and was significantly more common in alcoholics. Most of the patients in our study had alcoholic cirrhosis. The mechanism is not known, but it appears to be related to chronic porto-systemic shunting and ammonia exposure.
In early HE, early phases of astrocytic changes characterized by cytoplasmic hypertrophy, increased mitochondria, dense endoplasmic reticulum, and increased water content are seen. Increased water content decreases the amount of magnetization transfer by diluting the number of structural protons in tissues, which may explain the reduced MTR values.27 Rovira et al.28 showed that MTR values of normal appearing white matter in patients with liver cirrhosis and MHE were significantly lower than those in controls. There was a trend towards lower MTR values in the patients with MHE compared with the NMHE group.28 A study using voxel-based approach in MTR demonstrated that, compared with controls, patients with cirrhosis without HE displayed significantly decreased MTR values in the basal ganglia and in the hemispheric white matter. The group with MHE had a significant MTR decrease in hemispheric white matter, deep gray matter, brainstem, and cerebellum while the group with OHE showed an MTR decrease in the entire brain compared with controls, suggesting that as grade of edema increases, more areas of brain get involved. However, the authors did not compare MTR between MHE and NMHE group.29 We demonstrated that MTR in all regions of the brain were significantly lower in MHE patients than controls. To best of our knowledge, we are the first to demonstrate significantly lower MTR in PWM, IC, and BG regions of brain in the MHE compared with the NMHE group. This is indicative of a low-grade cerebral edema seen in patients with MHE and not with NMHE, demonstrating the anatomical basis of MHE to complement the proposed pathophysiological role of neuroinflammation in MHE. MTR values along with MRS abnormalities and NP tests tend to improve after liver transplantation.30 We demonstrated that after treatment of MHE patients with rifaximin and lactulose for 8 weeks MTR values improved to levels similar to that of controls, suggesting significant improvement in cerebral edema and establishing the reversible nature of MHE at the anatomical level. IL-6 and ammonia levels had significant negative correlation with MTR only in cortical areas, suggesting that with reduction in inflammation (IL-6) and ammonia there is a reduction in cerebral edema, and vice versa, in cortical white matter. Although studies have correlated ammonia and inflammatory mediators with MRS and DWI/DTI, there is no previous study correlating these parameters with MTR values.19, 31
We also demonstrated the clinical counterpart of improved MTR in the form of significant positive correlation of PHES with MTR. To the best of our knowledge, there is no study that correlated PHES or other neurophysiological tests with MTR values to substantiate our findings. However, there are few studies to show that cognitive domains as tested by various neurophysiological methods are selectively affected in MHE and correlate with MRS and DTI matrices.19, 32 The clinical significance of this study lies in that MTR may be a completely objective quantitative tool while PHES and other NP testing systems have an element of subjectivity to them. Thus MTR may be used for the diagnosis and follow-up of early neurological changes in patients with cirrhosis in future studies on the treatment of patients with cirrhosis with MHE.
As this study did not have a no-treatment arm in the MHE group, we cannot say for certain that the improvement in various parameters were due to the treatment or occurred spontaneously. However, this study did not aim to assess the efficacy of treatment in MHE but to demonstrate the pathology and reversibility of MHE at the anatomical and physiological level. We also did not study patients with OHE to see whether similar reversible MRI changes are also seen in them as part of this study. The importance of MTR changes in OHE could be the subject of further studies.
In conclusion, this study has demonstrated that MTR detects low-grade cerebral edema in MHE patients, which correlates with ammonia and inflammatory markers. Cerebral edema is reversible after treatment. MTR may be used as an objective surrogate end point in future trials assessing the efficacy of treatment of MHE.
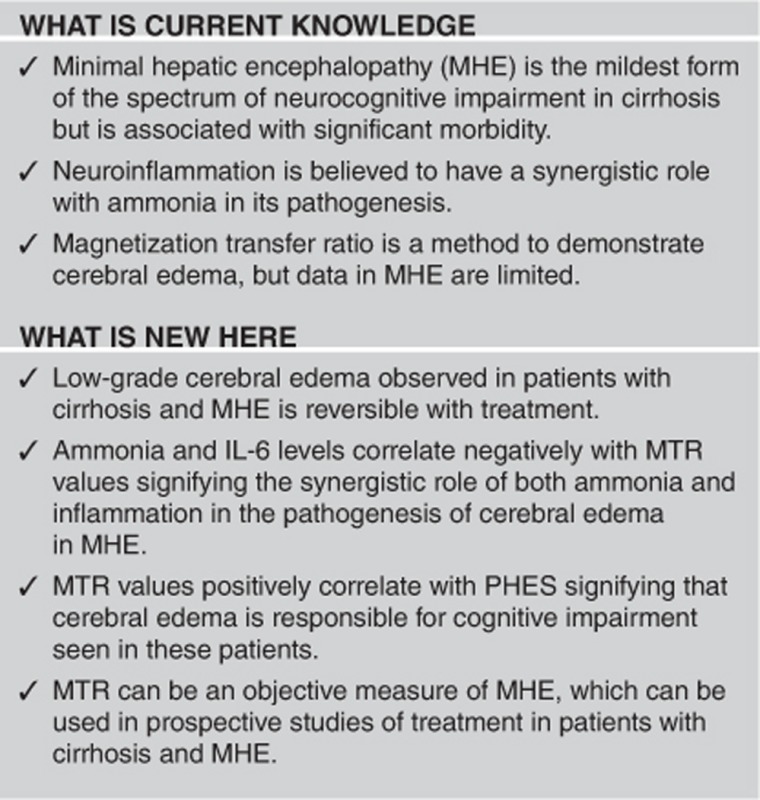
Study Highlights
Acknowledgments
Dr Dhiman presented this work at the Annual meeting of American College of Gastroenterology at Philadelphia, USA in October 2014 (Am J Gastroenterol 2014; 109:S156).
Guarantor of the article: Radha K. Dhiman, MD, DM, FAMS, FACG.
Specific author contributions: R.K.D. conceived the idea for the study, wrote the protocol, performed analyses, interpreted data, and prepared the manuscript. R.R. wrote the protocol, performed analyses, interpreted data, and prepared the manuscript. C.K.A., N. Kalra and N. Khandelwal performed MRI, interpreted data, and assisted in preparing the manuscript. S.A. assisted in data analyses, interpreting data, and in preparing the manuscript. A.D. and Y.C. were involved in patient care management and assisted in the interpretation of the data and in preparing the manuscript.
Financial support: None.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Ferenci P, Lockwood A, Mullen K et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002; 35: 716–721. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology 2009; 50: 2014–2021. [DOI] [PubMed] [Google Scholar]
- Kale RA, Gupta RK, Saraswat VA et al. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology 2006; 43: 698–706. [DOI] [PubMed] [Google Scholar]
- Bosoi CR, Rose CF. Brain edema in acute liver failure and chronic liver disease: similarities and differences. Neurochem Int 2013; 62: 446–457. [DOI] [PubMed] [Google Scholar]
- Dhiman RK, Saraswat VA, Sharma BK et al. Minimal hepatic encephalopathy: consensus statement of a working party of the Indian National Association for Study of the Liver. J Gastroenterol Hepatol 2010; 25: 1029–1041. [DOI] [PubMed] [Google Scholar]
- Prasad S, Dhiman RK, Duseja A et al. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007; 45: 549–559. [DOI] [PubMed] [Google Scholar]
- Amodio P, Del Piccolo F, Marchetti P et al. Clinical features and survivial of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology 1999; 29: 1662–1667. [DOI] [PubMed] [Google Scholar]
- Dhiman RK, Kurmi R, Thumburu KK et al. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci 2010; 55: 2381–2390. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Saeian K, Schubert CM et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 2009; 50: 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn K, Ennen JC, Schomerus H et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol 2001; 34: 768–773. [DOI] [PubMed] [Google Scholar]
- Chavarria L, Cordoba J. Magnetic resonance imaging and spectroscopy in hepatic encephalopathy. J Clin Exp Hepatol 2013; 5: S69–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge DR, Tranaha EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol 2014; 5: S7–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology 2011; 53: 1372–1376. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry 1983; 40: 812. [DOI] [PubMed] [Google Scholar]
- Ortiz M, Cordoba J, Doval E et al. Development of a clinical hepatic encephalopathy staging scale. Aliment Pharmacol Ther 2007; 26: 859–867. [DOI] [PubMed] [Google Scholar]
- Dhiman RK, Saraswat VA, Verma M et al. Figure connection test: a universal test for assessment of mental state. J Gastroenterol Hepatol 1995; 10: 14–23. [DOI] [PubMed] [Google Scholar]
- Dhiman RK, Rana B, Agrawal S et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology 147: 1327–1337. e3. [DOI] [PubMed] [Google Scholar]
- Ong JP, Aggarwal A, Krieger D et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med 2003; 114: 188–193. [DOI] [PubMed] [Google Scholar]
- Jain L, Sharma BC, Srivastava S et al. Serum endotoxin, inflammatory mediators, and magnetic resonance spectroscopy before and after treatment in patients with minimal hepatic encephalopathy. J Gastroenterol Hepatol 2013; 28: 1187–1193. [DOI] [PubMed] [Google Scholar]
- Shawcross DL, Sharifi Y, Canavan JB et al. Infection and systemic inflammation, not ammonia, are associated with grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol 2011; 54: 640–649. [DOI] [PubMed] [Google Scholar]
- Shawcross DL, Wright G, Olde Damink SW et al. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis 2007; 22: 125–138. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Piedrafita B, Serra MA et al. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J Clin Gastroenterol 2009; 43: 272–279. [DOI] [PubMed] [Google Scholar]
- Butterworth RF. The liver-brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol 2013; 10: 522–528. [DOI] [PubMed] [Google Scholar]
- Das K, Singh P, Chawla Y et al. Magnetic resonance imaging of brain in patients with cirrhotic and non-cirrhotic portal hypertension. Dig Dis Sci 2008; 53: 2793–2798. [DOI] [PubMed] [Google Scholar]
- Cordoba J, Sanpedro F, Alonso J et al. 1H magnetic resonance in the study of hepatic encephalopathy in humans. Metab Brain Dis 2002; 17: 415–429. [DOI] [PubMed] [Google Scholar]
- Thuluvath PJ, Edwin D, Yue NC et al. Increased signals seen in globus pallidus in T1-weighted magnetic resonance imaging in cirrhotics are not suggestive of chronic hepatic encephalopathy. Hepatology 1995; 21: 440–442. [PubMed] [Google Scholar]
- Iwasa M, Kinosada Y, Nakatsuka A et al. Magnetization transfer contrast of various regions of the brain in liver cirrhosis. AJNR Am J Neuroradiol 1999; 20: 652–654. [PMC free article] [PubMed] [Google Scholar]
- Rovira A, Grive E, Pedraza S et al. Magnetization transfer ratio values and proton MR spectroscopy of normal-appearing cerebral white matter in patients with liver cirrhosis. AJNR Am J Neuroradiol 2001; 22: 1137–1142. [PMC free article] [PubMed] [Google Scholar]
- Miese FR, Wittsack HJ, Kircheis G et al. Voxel-based analyses of magnetization transfer imaging of the brain in hepatic encephalopathy. World J Gastroenterol 2009; 15: 5157–5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba J, Alonso J, Rovira A et al. The development of low-grade cerebral edema in cirrhosis is supported by the evolution of (1)H-magnetic resonance abnormalities after liver transplantation. J Hepatol 2001; 35: 598–604. [DOI] [PubMed] [Google Scholar]
- Sugimoto R, Iwasa M, Maeda M et al. Value of the apparent diffusion coefficient for quantification of low-grade hepatic encephalopathy. Am J Gastroenterol 2008; 103: 1413–1420. [DOI] [PubMed] [Google Scholar]
- Weissenborn K, Ahl B, Fischer-Wasels D et al. Correlations between magnetic resonance spectroscopy alterations and cerebral ammonia and glucose metabolism in cirrhotic patients with and without hepatic encephalopathy. Gut 2007; 56: 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.