Abstract
Objectives:
Analysis of volatile organic compounds (VOCs) in the exhaled breath can identify markers for alcoholic and nonalcoholic fatty liver disease. The aim of this pilot study was to investigate the utility of breath VOCs measured by mass spectrometry to diagnose advanced fibrosis in patients with chronic liver disease (CLD).
Methods:
Patients undergoing liver biopsy were recruited. Fibrosis was determined by an experienced pathologist (F0–4) and advanced fibrosis was defined as F3–4. Exhaled breath and plasma samples were collected on the same day of the biopsy. Selective ion flow tube mass spectrometry (SIFT-MS) was used to analyze breath samples. Bonferroni correction was applied to decrease the false discovery rate.
Results:
In all, 61 patients were included with a mean age of 50.7±9.9 years and 57% were male. Twenty patients (33%) had advanced fibrosis (F3–4), 44% had chronic hepatitis C, 30% had nonalcoholic fatty liver disease, and 26% had other CLD. SIFT-MS analysis of exhaled breath revealed that patients with advanced fibrosis had significantly lower values of six compounds compared with those without advanced fibrosis, P value <0.002 for all. Isoprene was found to have the highest accuracy for the prediction of advanced fibrosis with an area under the receiver operating characteristics curve of 0.855 (95% confidence interval: 0.762, 0.948). The median breath isoprene level in patients with F3–4 was 13.5[8.7, 24.7] p.p.b. compared with 40.4[26.2, 54.1] for those with F0–2, P value <0.001. Isoprene is an endogenous VOC that is a byproduct of cholesterol biosynthesis.
Conclusions:
Isoprene is a potential biomarker for advanced fibrosis that deserves further validation.
Introduction
The prevalence of chronic liver disease (CLD) is steadily increasing. It is currently estimated to affect 14.7% of population in the United States,1 accounts for 4 billion dollars spent on hospital admissions every year2 and is the 12th leading cause of death in the country.3 In any cause of CLD, the disease spectrum ranges from no fibrosis to minimal fibrosis to advanced fibrosis and cirrhosis with complications secondary to portal hypertension.
Therefore, it is essential to identify patients with advanced fibrosis and cirrhosis as soon as possible so that appropriate treatment and screening methods can be instituted to prevent, diagnose, and treat complications of end-stage liver disease. Liver biopsy remains the gold standard test to assess the stage of fibrosis but it is an invasive procedure that carries a complication rate of around 5.9%,4 the most worrisome of which is bleeding.5 A number of methods using laboratory markers and imaging techniques (FibroTest, Hepascore, transient elastography)6 have been developed to detect the extent of fibrosis noninvasively; however, the laboratory markers are expensive and the imaging tests require sophisticated equipment, have inter-observer variability, and are often not reliable in obese individuals.
The concept of testing the breath after oral administration of C14 compounds to assess the severity of liver disease was introduced in 1974 (ref. 7) and was based on the simple hypothesis that owing to impaired metabolic activity with advanced fibrosis, there will be less breakdown and hence less exhalation of C14 in the breath. Breath testing gained more importance in the 2000 s with the introduction of more stable C13 compounds and earlier studies reported on the use of breath testing to detect the presence of hepatic dysfunction and also to differentiate between different stages of the fibrosis.8, 9, 10, 11 However, this test requires that the patients ingest a radioactive material and involves a special equipment to measure radioactivity making it an expensive alternative to liver biopsy.
With the rising burden of CLD, there is an increased demand to develop a noninvasive marker of severity of liver disease that will be easy to measure and inexpensive. Measurement of endogenously produced volatile organic compounds (VOCs) in the breath by selective ion flow tube mass spectrometry (SIFT-MS) is an attractive option that will not require the ingestion of any compounds or specific-patient preparation. Our lab has generated data supporting the use of exhaled VOC concentration to identify patients with alcoholic hepatitis12 or nonalcoholic fatty liver disease.13
We performed this pilot study to assess the utility of breath VOCs measured by SIFT-MS to diagnose advanced fibrosis in patients with CLD.
Methods
Study subjects and clinical data
The study protocol was approved by the Cleveland Clinic Institutional Review Board.
Adults between the ages of 18 and 60 undergoing liver biopsy for any cause of CLD at the Cleveland Clinic were recruited. Exhaled breath and plasma samples were collected on the same day of the liver biopsy.
Clinical variables were recorded, which included standard procedures for height, weight, body mass index, and laboratory values of AST, ALT, alkaline phosphatase, bilirubin, serum albumin, prothrombin time/INR, platelet count, serum glucose were measured. An independent pathologist reviewed the liver biopsy slides and determined the stage of fibrosis (F0–F4) based on the Metavir score. Patients were divided into groups of advanced fibrosis (F3–F4) or minimal fibrosis (F0–F2) to compare the difference between the groups.
Exhaled breath and blood sample collection
All exhaled breath samples were collected following an 8-h fast. Study subjects completed a mouth rinse with water before the collection of the breath sample to reduce the contamination from VOCs produced in the mouth. Subjects were prompted to exhale normally to release residual air from the lungs and then inhale to total lung capacity through a disposable mouth filter. The inhaled ambient air was also filtered through an attached N7500-2 acid gas cartridge. The filters were used to prevent viral and bacterial exposure to the subject and to eliminate exogenous VOCs from the inhaled air. The subjects then proceeded to exhale at a rate of 50 ml/s through the mouth filter until the lungs were emptied. The exhaled breath sample was collected into an attached Mylar bag, capped, and analyzed within 4 h. Mylar bags were cleaned by flushing with nitrogen between subjects. Fasting blood samples were obtained from patients in the morning at the time of liver biopsy and were initially processed to serum, then stored frozen at −80 °C.
SIFT-MS analysis
The exhaled breath samples underwent gas analysis using SIFT-MS on a VOICE200 SIFT-MS instrument (Syft Technologies, Christchurch, New Zealand). The SIFT-MS technology and instrument used in this study have previously been described elsewhere by our group and others.14, 15, 16
Mass scans of the product ions generated in the chemical ionization mass spectrum from each reagent ion (H3O+, NO+, and O2+) were obtained in the mass scanning mode. Mass scanning between 14 and 200 a.m.u. was used to identify significant peaks at product ion masses representing unknown breath volatiles relating to liver cirrhosis. More accurate concentration data was obtained by selected ion monitoring of product ions of 21 pre-selected compounds: 2-propanol, acetaldehyde, acetone, acrylonitrile, ammonia, benzene, carbon disulfide, dimethyl sulfide, ethanol, hydrogen sulfide, isoprene, pentane, triethylamine, trimethylamine, 1-decene, 1-heptane, 1-nonene, 1-octane, 3-methyl hexane, (E)-2-nonene, and ethane. These compounds have been previously identified as common constituents of exhaled human breath.15 VOC levels were measured in parts per billion (p.p.b.). To determine the levels of VOCs in the serum, ~1 ml of serum from the samples was extracted and centrifuged for 8 min. Subsequently, 200 ml was extracted and put into a 20-ml headspace vial, and the vial was sealed. The samples were heated to 40 °C to allow the VOCs in the headspace to equilibrate with the samples. Then, 20 ml of headspace gas was removed with a gas syringe and was analyzed with the VOICE200 SIFT-MS instrument to measure VOC levels.
Statistical analysis
Data are presented as mean±s.d. or N (%) after confirming normal distribution. A univariable analysis was done to assess differences between fibrosis groups; analysis of variance was used to compare continuous, the nonparametric Kruskal–Wallis test was used for ordinal factors and Pearson's chi-square tests were used for categorical variables. Multivariable logistic regression analysis was performed to build a model for prediction of advanced fibrosis; all breath compounds were considered for inclusion. Discrimination was used for internal model validation; this measures the ability to rank patients by risk of advanced fibrosis such that patients with a higher predicted risk are more likely to have advanced fibrosis. Discrimination was measured by the area under the receiver operating characteristics curve (AUC). All individual compounds with AUCs of 0.75 or above were further assessed to find the combination of any two that provided the highest AUC. After choosing the final model, the method described by Harrell was used to compute the validation metric with over-fitting bias correction through bootstrap resampling. A thousand bootstrap samples (B=1,000) were drawn from the original data set and a new model with the same model settings was built on each bootstrap resample. Prediction on patients that were not chosen in the resample was calculated. An optimism factor was calculated over the 1,000 new models and the bias-corrected validation metric was obtained by subtracting this optimism value from the AUC directly measured from the original model. Bonferroni correction was applied to decrease the false discovery rate and a P value <0.002 was considered significant. All analyses were performed using SAS (version 9.3, The SAS Institute, Cary, NC) and all figures were constructed using R (version 3.0.3, The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Sixty-one patients were included in the study (20 with F3–F4 and 41 with F0–F2) with baseline characteristics mentioned in Table 1. The mean age was 50.7±9.9 years, 42.6% were females and 75% were Caucasians. The most common etiology of CLD was hepatitis C (44.3%) followed by nonalcoholic fatty liver disease (29.5%). Median AST and ALT levels were 48 [29,81] U/l and 50 [28,80] U/l, respectively. None of the patients had clear evidence of end-stage liver disease or portal hypertension before the liver biopsy with mean albumin of 3.9±0.81 g/dl and mean INR of 1.1±0.24. The mean platelet count was 216.8±55.2 k/μl.
Table 1. Patient characteristics.
| Factor | Summary |
|---|---|
| Total (N=61) | |
| Age | 50.7±9.9 |
| Gender | |
| Male | 35 (57.4%) |
| Female | 26 (42.6%) |
| Caucasian | 39 (75.0%) |
| BMI (kg/m2) | 29.2±5.6 |
| Alcohol | |
| Never | 34% |
| Social | 41% |
| >2 drinks/day | 25% |
| Diabetes | 20.4% |
| HTN | 40.8% |
| Hypertriglyceridemia | 20.4% |
| NAFLD | 18/61 (29.5%) |
| NASH (only for NAFLD patients) | 16/18 (88.9%) |
| HCV | 27/61 (44.3%) |
| ALD | 8/61 (13.1%) |
| Platelet | 216.8±55.2 |
| ALK | 84 [56,105] |
| AST | 48 [29,81] |
| ALT | 50 [28,80] |
| Bilirubin | 0.60 [0.40,1.2] |
| Albumin | 3.9±0.81 |
| INR | 1.1±0.24 |
| Glucose (fasting) | 95 [88,109] |
| Cholesterol | 166 [154,191] |
| Fibrosis stage | |
| 0 | 14 (23.0) |
| 1 | 11 (18.0) |
| 2 | 16 (26.2) |
| 3 | 4 (6.6) |
| 4 | 16 (26.2) |
BMI, body mass index; HCV, hepatitis C virus; HTN, portal hypertension; INR, international normalized ratio; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Values are presented as mean±s.d., median [P25, P75] or N (column %).
Difference in exhaled VOC concentrations in patients with and without advanced fibrosis
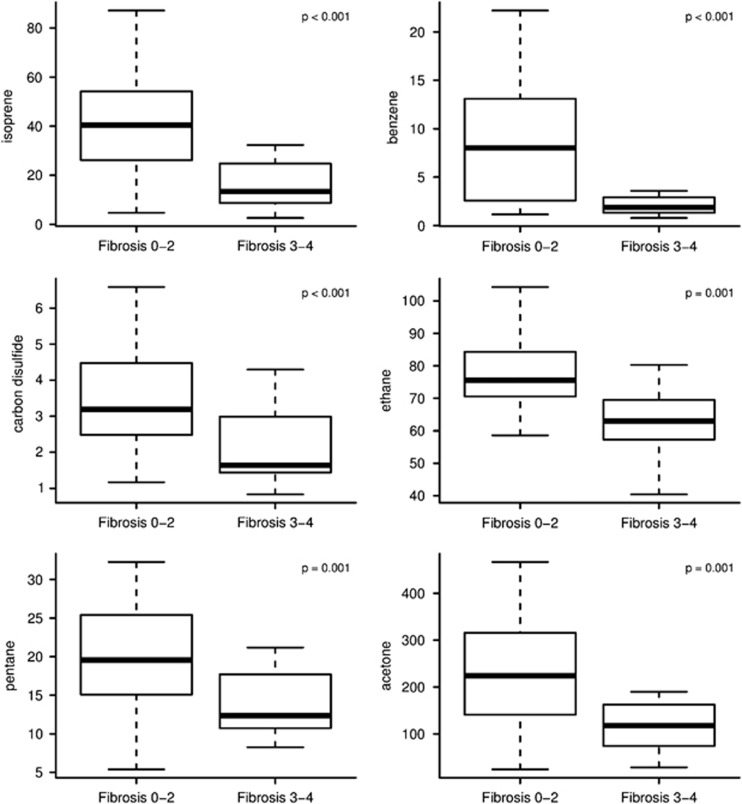
After testing for 21 VOCs in the exhaled breath, six compounds (acetone, benzene, carbon disulfide, isoprene, pentane, and ethane) were shown to have statistically significant concentration difference between the groups of advanced and minimal fibrosis (Table 2). For all the compounds, the levels were lower in the advanced fibrosis group (224.2 p.p.b. vs. 117.8 for acetone, 8.0 vs. 1.9 for benzene, 3.2 vs. 1.6 for carbon disulfide, 40.4 vs. 13.5 for isoprene, 19.5 vs. 12.3 for pentane, and 75.6 vs. 63.0 for ethane, Bonferroni corrected P value <0.002 for all; Figure 1).
Table 2. Breath concentrations of measured volatile organic compounds.
| Factor | Fibrosis 0–2 (N=41) | Fibrosis 3–4 (N=20) | P value |
|---|---|---|---|
| 2-Propanol | 130.7 (93.9, 191.7) | 102.7 (55.1, 124.2) | 0.030 |
| Acetaldehyde | 50.5 (34.1, 77.3) | 33.0 (22.1, 43.5) | 0.004 |
| Acetone | 224.2 (140.8, 315.7) | 117.8 (74.1, 162.4) | 0.001 |
| Acrylonitrile | 0.90 (0.65, 1.09) | 0.63 (0.51, 0.77) | 0.002 |
| Benzene | 8.0 (2.6, 13.1) | 1.9 (1.3, 2.9) | <0.001 |
| Carbon disulfide | 3.2 (2.5, 4.5) | 1.6 (1.4, 3.0) | <0.001 |
| Dimethyl sulfide | 2.8 (1.8, 4.0) | 1.4 (0.76, 2.7) | 0.006 |
| Ethanol | 142.6 (87.1, 219.6) | 114.1 (87.9, 196.4) | 0.40 |
| Isoprene | 40.4 (26.2, 54.1) | 13.5 (8.7, 24.7) | <0.001 |
| Pentane | 19.5 (15.1, 25.4) | 12.3 (10.7, 17.7) | 0.001 |
| 1-Decene | 6.3 (5.3, 8.4) | 5.8 (4.4, 8.1) | 0.30 |
| 1-Heptene | 14.1 (7.8, 23.4) | 9.9 (5.9, 14.5) | 0.038 |
| 1-Nonene | 4.1 (3.3, 5.5) | 3.8 (3.1, 5.4) | 0.70 |
| 1-Octene | 16.2 (12.7, 18.7) | 14.2 (11.3, 17.9) | 0.47 |
| 3-Methyl hexane | 26.8 (16.5, 35.9) | 18.5 (13.1, 29.8) | 0.12 |
| (E)-2-nonene | 2.1 (1.5, 2.7) | 1.7 (1.09, 2.1) | 0.026 |
| Ammonia | 94.3 (74.4, 117.4) | 66.4 (57.5, 83.7) | 0.003 |
| Ethane | 75.6 (70.5, 85.2) | 63.0 (57.3, 69.5) | 0.001 |
| Hydrogen sulfide | 0.50 (0.39, 0.72) | 0.33 (0.26, 0.47) | 0.007 |
| Triethyl amine | 0.82 (0.62, 1.01) | 0.71 (0.58, 0.96) | 0.23 |
| Trimethyl amine | 11.0 (8.1, 13.9) | 6.5 (4.7, 8.4) | 0.003 |
Values are presented as median (P25, P75) with Kruskal–Wallis test.
P<0.002 is considered statistically significant to correct for multiple comparisons.
Bold and italic P values are statistically significant.
Figure 1.
Subjects with advanced fibrosis have lower levels of volatile organic compounds compared with those without advanced fibrosis. The lower boundary of the box-and-whisker plot corresponds to the 25th percentile, the line within the box to the median, and the upper boundary of the box to the 75th percentile. The whiskers extend to the most extreme data point, which is no more than 1.5 times the interquartile range from the box.
Isoprene as a biomarker of advanced fibrosis
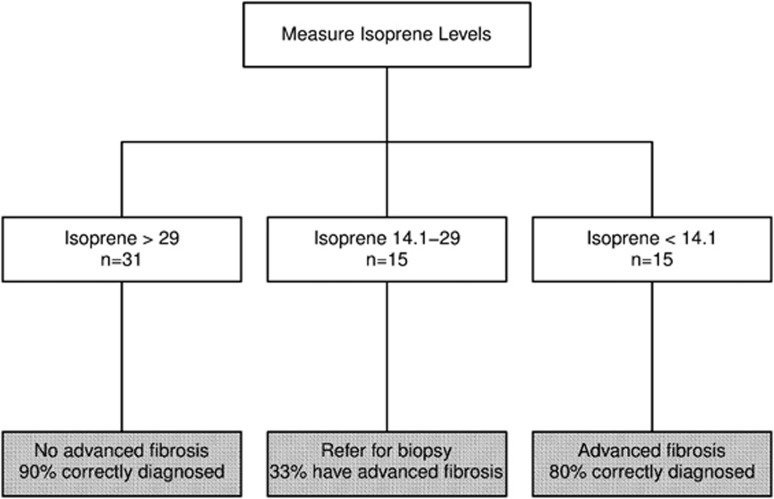
Further analysis of these six compounds revealed that isoprene had the highest AUC for predicting the presence of advanced liver fibrosis on biopsy (0.855 (95% confidence interval:0.762–0.948); Table 3 and Figure 2). The likelihood of having advanced fibrosis decreases by 10% for every one unit increase in isoprene (odds ratio (95% confidence interval): 0.90 (0.85, 0.95); P<0.001). An isoprene level more than or equal to 29 p.p.b. would provide specificity, sensitivity, positive, and negative predictive values of 68, 85, 57, and 90%, respectively, for predicting advanced fibrosis. On the other hand, an isoprene level of less than 14.1 p.p.b. would provide specificity, sensitivity, positive, and negative predictive values of 93, 60, 80, and 83%, respectively. Combining these two cutoff values for exhaled breath isoprene, we propose the diagnostic algorithm presented on Figure 3. A cutoff value of 29 p.p.b. was used to maximize sensitivity and rule out the presence of advanced fibrosis. A cutoff of 14.1 p.p.b. was used to maximize specificity and rule in the presence of advanced fibrosis. Isoprene is an endogenous VOC that is a byproduct of cholesterol biosynthesis, which may explain the lower levels in patients with advanced liver disease. Because of the known association between isoprene and cholesterol biosynthesis, we further investigated the association between exhaled isoprene level and plasma cholesterol levels. There was no significant correlation between isoprene and cholesterol (rho=−0.13 (−0.50, 0.23); P=0.46). Median cholesterol levels were 165 [154, 194.5] mg/dl for F0–2 vs. 172.0 [102, 184] mg/dl for F3–4 (P=0.38). None of our patients were on statins in the previous 3 months before liver biopsy. To confirm the endogenous source of isoprene, we used the same SIFT-MS technology to measure levels of isoprene in the headspace of available serum samples that were obtained on the day of liver biopsy (n=33). We found that patients with advanced fibrosis had a trend toward having lower serum levels of isoprene compared with those without advanced fibrosis (18.0 [14.6, 21.6] p.p.b. vs. 13.2 [11.4, 16.6], P value of 0.052) further validating our findings in the exhaled breath. Combining isoprene with other VOCs did not significantly improve the diagnostic accuracy for predicting the presence of advanced fibrosis as shown in the Supplementary Table.
Table 3. Area under receiver operating curves for different volatile organic compounds to detect advanced fibrosis.
| VOC | AUC (95% CI) |
|---|---|
| Isoprene | 0.855 (0.762, 0.948) |
| Benzene | 0.795 (0.665, 0.925) |
| Ethane | 0.779 (0.638, 0.919) |
| Carbon disulfide | 0.771 (0.634, 0.907) |
| Pentane | 0.761 (0.617, 0.905) |
| Acetone | 0.755 (0.613, 0.896) |
| Acrylonitrile | 0.744 (0.615, 0.873) |
| Trimethyl amine | 0.743 (0.589, 0.896) |
| Ammonia | 0.737 (0.605, 0.868) |
| Acetaldehyde | 0.726 (0.585, 0.866) |
| Dimethyl sulfide | 0.718 (0.567, 0.869) |
| Hydrogen sulfide | 0.716 (0.574, 0.858) |
| Enonene | 0.692 (0.542, 0.843) |
| Heptene | 0.679 (0.525, 0.834) |
| Propanol | 0.672 (0.523, 0.821) |
| Methyl hexane | 0.636 (0.471, 0.802) |
| Triethyl amine | 0.595 (0.441, 0.749) |
| Decene | 0.590 (0.410, 0.769) |
| Octene | 0.563 (0.377, 0.748) |
| Nonene | 0.534 (0.349, 0.718) |
| Ethanol | 0.433 (0.274, 0.591) |
AUC, area under ROC curve; CI, confidence interval; ROC, receiver operating characteristic; VOC, volatile organic compound.
Figure 2.
Isoprene for the diagnosis of advanced fibrosis: receiver operating characteristics curve. Good accuracy of breath isoprene levels in predicting the presence of advanced fibrosis on liver biopsy. The ideal area under the curve is 1.00. AUC, area under ROC curve; CI, confidence interval; ROC, receiver operating characteristic.
Figure 3.
Hypothetical proposed algorithm to rule in or rule out the presence of advanced fibrosis using two different cutoff values for breath isoprene that requires further validation.
Discussion
Our pilot study suggests that the breath concentration of VOCs changes with progression of liver disease and VOC levels can potentially be used as a biomarker to detect advanced fibrosis. Of all the compounds, isoprene can most reliably diagnose advanced fibrosis. Isoprene is a marker of cholesterol biosynthesis and our results suggest that this pathway may be impaired in patients with advanced liver fibrosis and compensated liver disease well before the development of low plasma cholesterol level that is characteristic of patients with end-stage liver disease. By using a cutoff value of >29 p.p.b. for the absence of advanced fibrosis and <14.1 p.p.b. for the presence of advanced fibrosis, we would be able to diagnose advanced fibrosis with high accuracy with only 25% patients falling under the indeterminate category and requiring a liver biopsy thus avoiding a biopsy in a large number of patients.
Traditionally gas chromatography was used to measure VOCs and is still the preferred method if the sample has a large number of VOCs. Because it requires an extensive preparation of the sample and the results take longer to be reported, it is being increasingly replaced by SIFT-MS with which the results are reported in a few minutes and concentrations as low as parts per trillion can be measured.17 SIFT-MS relies on the principle of ionization of gas molecules with H3O+, NO+, and O2+ to produce an ionized product, which is unique for each VOC and measurement of these product ions by a mass spectrometer.16
With liver disease resulting in impairment of metabolic and synthetic functions, two previous studies have shown that the breath concentrations of VOCs in CLD patients differ when compared with healthy individuals.18, 19 In these studies, the comparison was done by using gas chromatography mass spectrometry between patients with cirrhosis and healthy individuals, thus it could not be predicted whether the concentration of VOCs would differ on the basis of the severity of liver fibrosis. Our study has shown that the VOC concentrations measured by SIFT-MS in patients with advanced fibrosis are lower than those with minimal fibrosis, which could be due to subclinical hepatic dysfunction caused by advanced fibrosis.
From the six compounds that had statistically significant concentration differences, isoprene, with an AUC of 0.855 (95% confidence interval: 0.762–0.958) can predict advanced fibrosis most accurately. Isoprene is a reactive aliphatic hydrocarbon that is produced endogenously by humans. It is a major hydrocarbon found in human breath and is considered a byproduct of cholesterol synthesis.20, 21 Statins, by inhibiting HMG-CoA reductase and inhibiting cholesterol synthesis, are shown to lower isoprene concentration.20 Even though in our study the isoprene concentration was lower in the group of patients with advanced fibrosis signifying that cholesterol synthesis pathway is suppressed, the total cholesterol level was the same in both the groups and none of our patients were on statins.
Our study has several limitations including the relatively small sample size of 61 patients and the lack of a validation group. The lack of an external validation group decreases the accuracy of these results and they cannot be generalized till their accuracy has been established in a bigger cohort. Also, given the small study population, we were unable to distinguish whether the VOC concentrations were different among the different etiologies of CLD. Although isoprene had good sensitivity and negative predictive value, the specificity of this test was not very promising suggesting that while it can be accurately used to predict the absence of advanced fibrosis, it is not too reliable to predict the presence of advanced fibrosis. We had no control over the diet or medications these patients were on and their level of physical activity before the collection of breath samples and these factors could have also altered the VOC concentrations. We were unable to determine whether there is any intra-observer variability while measuring isoprene that could preclude its use as a predictive marker of advanced fibrosis. We also did not compare and determine whether breath isoprene was a better marker to predict advanced fibrosis compared with the currently established noninvasive markers that use various laboratory markers. We included only the patients who were referred for a liver biopsy suggesting that the presence of advanced fibrosis was less obvious in these patients. Exclusion of patients with more advanced or end-stage liver disease remains a limitation of our study. Last, this was a cross-sectional study and the samples were not collected to follow up patients over a period of time. Besides isoprene, future prospective studies should be done to re-evaluate the utility of the other VOCs also (acetone, benzene, carbon disulfide, pentane, and ethane) that were also found to be statistically significant in predicting advanced fibrosis.
To conclude, our study shows that breath testing for VOCs (particularly isoprene) is a promising option and can be developed into a noninvasive biomarker for advanced fibrosis. These results are still preliminary and larger scale studies are required for validation and comparison with other known fibrosis scores before these tests can be incorporated into clinical practice. Further research needs to be done on newer methods to measure VOCs more easily. The cost of the test cannot be determined currently because we perform an extensive mass scanning of breath samples but our goal is to ultimately develop small, inexpensive hand-held devices that could measure isoprene concentration and be routinely used by health-care personnel in the office to provide patients with real-time diagnosis of advanced liver disease.
Study Highlights

Guarantor of the article: Naim Alkhouri, MD.
Specific author contributions: Naim Alkhouri: Planning and conducting the study, interpreting the data, and drafting the manuscript. Tavankit Singh: Conducting the study, collecting and interpreting the data, and drafting the manuscript. Eyad Alsabbagh: Planning the study and drafting the manuscript. John Guirguis: Conducting the study, interpreting the data, and drafting the manuscript. Tarek Chami: Planning the study and drafting the manuscript. Ibrahim Hanouneh: Planning the study, interpreting the data, and drafting the manuscript. David Grove: Conducting the study, interpreting the data, and drafting the manuscript. Rocio Lopez: Interpreting the data and drafting the manuscript. Raed Dweik: Planning the study, interpreting the data, and drafting the manuscript.
Financial support: Supported by the ACG Junior Faculty Development Award to N.A. and by the BRCP 08-049 Third Frontier Program grant from the Ohio Department of Development.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Younossi ZM, Stepanova M, Afendy M et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9: 524–530. [DOI] [PubMed] [Google Scholar]
- Volk ML, Tocco RS, Bazick J et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minino AM, Murphy SL, Xu J et al. Deaths: final data for 2008. Natl Vital Stat Rep 2011; 59: 1–126. [PubMed] [Google Scholar]
- Sharma S, Khalili K, Nguyen GC. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol 2014; 20: 16820–16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DC, Caldwell SH, Goodman ZD et al. Liver biopsy. Hepatology 2009; 49: 1017–1044. [DOI] [PubMed] [Google Scholar]
- Scott R, Guha IN. Non-invasive monitoring of liver fibrosis. Br Med Bull 2014; 112: 97–106. [DOI] [PubMed] [Google Scholar]
- Hepner GW, Vesell ES. Assessment of aminopyrine metabolism in man by breath analysis after oral administration of 14C-aminopyrine. Effects of phenobarbital, disulfiram and portal cirrhosis. N Engl J Med 1974; 291: 1384–1388. [DOI] [PubMed] [Google Scholar]
- Giannini EG, Fasoli A, Borro P et al. 13C-galactose breath test and 13C-aminopyrine breath test for the study of liver function in chronic liver disease. Clin Gastroenterol Hepatol 2005; 3: 279–285. [DOI] [PubMed] [Google Scholar]
- Portincasa P, Grattagliano I, Lauterburg BH et al. Liver breath tests non-invasively predict higher stages of non-alcoholic steatohepatitis. Clin Sci (Lond) 2006; 111: 135–143. [DOI] [PubMed] [Google Scholar]
- Park GJ, Wiseman E, George J et al. Non-invasive estimation of liver fibrosis in non-alcoholic fatty liver disease using the 13 C-caffeine breath test. J Gastroenterol Hepatol 2011; 26: 1411–1416. [DOI] [PubMed] [Google Scholar]
- Giannini E, Fasoli A, Chiarbonello B et al. 13C-aminopyrine breath test to evaluate severity of disease in patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther 2002; 16: 717–725. [DOI] [PubMed] [Google Scholar]
- Hanouneh IA, Zein NN, Cikach F et al. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol 2014; 12: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhouri N, Cikach F, Eng K et al. Analysis of breath volatile organic compounds as a noninvasive tool to diagnose nonalcoholic fatty liver disease in children. Eur J Gastroenterol Hepatol 2014; 26: 82–87. [DOI] [PubMed] [Google Scholar]
- Mashir A, Paschke KM, van Duin D et al. Effect of the influenza A (H1N1) live attenuated intranasal vaccine on nitric oxide (FE(NO)) and other volatiles in exhaled breath. J Breath Res 2011; 5: 037107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince BJ, Milligan DB, McEwan MJ. Application of selected ion flow tube mass spectrometry to real-time atmospheric monitoring. Rapid Commun Mass Spectrom 2010; 24: 1763–1769. [DOI] [PubMed] [Google Scholar]
- Smith D, Spanel P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom Rev 2005; 24: 661–700. [DOI] [PubMed] [Google Scholar]
- Langford VS, Graves I, McEwan MJ. Rapid monitoring of volatile organic compounds: a comparison between gas chromatography/mass spectrometry and selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom 2014; 28 p 10–18. [DOI] [PubMed] [Google Scholar]
- Dadamio J, Van den Velde S, Laleman W et al. Breath biomarkers of liver cirrhosis. J Chromatogr B Analyt Technol Biomed Life Sci 2012; 905: 17–22. [DOI] [PubMed] [Google Scholar]
- Van den Velde S, Nevens F, Van Hee P et al. GC-MS analysis of breath odor compounds in liver patients. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 875: 344–348. [DOI] [PubMed] [Google Scholar]
- Stone BG, Besse TJ, Duane WC et al. Effect of regulating cholesterol biosynthesis on breath isoprene excretion in men. Lipids 1993; 28: 705–708. [DOI] [PubMed] [Google Scholar]
- Deneris ES, Stein RA, Mead JF. In vitro biosynthesis of isoprene from mevalonate utilizing a rat liver cytosolic fraction. Biochem Biophys Res Commun 1984; 123: 691–696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.