Abstract
Objectives:
The tumor necrosis factor superfamily member 14, LIGHT (homologous to lymphotoxin, exhibits inducible expression, and competes with HSV glycoprotein D for herpes virus entry mediator (HVEM), a receptor expressed by T lymphocytes), has been involved in various autoimmune disorders and has been shown to influence hepatic lipid metabolism. We hypothesized that LIGHT could also have a pathogenic role in nonalcoholic fatty liver disease (NAFLD).
Methods:
Serum levels of LIGHT in NAFLD patients and control subjects, as well as LIGHT and interleukin (IL)-8 released from Huh7 (human hepatoma cell line) hepatocytes, were determined by enzyme-linked immunosorbent assay. The mRNA expression of LIGHT in the liver tissue and mRNA levels of LIGHT and IL-8 in Huh7 hepatocytes were assessed by real-time quantitative reverse transcription-PCR.
Results:
(i) Serum levels of LIGHT were significantly elevated in NAFLD patients (n=66) as compared with healthy controls (n=16), with no differences between simple steatosis (n=34) and nonalcoholic steatohepatitis (NASH) (n=32). (ii) Within the liver, NAFLD patients (n=14) had significantly increased mRNA levels of the two LIGHT receptors, herpes virus entry mediator and lymphotoxin β receptor (LTβR), as compared with controls (n=7), with no difference between simple steatosis (n=8) and NASH (n=6). (iii) LIGHT markedly increased the release of IL-8 in Huh7 hepatocytes in a time- and dose-dependent manner. (iv) The reactive oxygen species (ROS) H2O2 (hydrogen peroxide) enhanced the LIGHT-mediated release of IL-8 in Huh7 hepatocytes.
Conclusion:
We show increased levels of LIGHT and its two membrane-bound receptors in NAFLD, potentially promoting hepatic inflammation through ROS interaction. Our findings should encourage further studies on the role of LIGHT in NAFLD development and progression.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease, with an estimated prevalence of about 20%.1 NAFLD ranges from simple fat deposition to nonalcoholic steatohepatitis (NASH), characterized by steatosis, inflammation, and progressive fibrosis, ultimately leading to cirrhosis and end-stage liver disease.1, 2 NAFLD is frequently associated with obesity, dyslipidemia, and insulin resistance, disorders that constitute metabolic syndrome.3 Although enhanced oxidative stress, inflammation, and metabolic disturbances, potentially representing interacting mechanisms, have been implicated in the pathogenesis of NAFLD, the mechanisms that underlie the initiation and progression of this disorder are still not clear.4, 5, 6
LIGHT (homologous to lymphotoxin, exhibits inducible expression, and competes with HSV glycoprotein D for herpes virus entry mediator (HVEM), a receptor expressed by T lymphocytes) is a 29 kDa type 2 transmembrane protein belonging to the tumor necrosis factor (TNF) superfamiliy (TNFSF).7 LIGHT/TNFSF14 is primarily expressed on T cells and dendritic cells, but has also been found on platelets, monocytes, and granulocytes, being involved in innate and adaptive immune responses, as well as in the regulation of cell survival and proliferation.7, 8 LIGHT binds to the HVEM (HVEM/TNFRSF14), and is also a shared ligand with membrane-bound lymphotoxin αβ for LTβR/TNFRSF3.7 Studies in animal models and some clinical studies indicate that LIGHT may be crucial for the development of various autoimmune and inflammatory disorders,9, 10 and has also been implicated in the pathogenesis of atherosclerosis.11, 12 In addition, LIGHT has been found to promote hepatic inflammation in two independent experimental hepatitis models (i.e., hepatitis induced by concanavalin A and Listeria monocytogenes),13 and has been reported to modulate lipid homeostasis through interaction with LTβR, at least partly through inhibition of hepatic lipase expression in hepatocytes.14
Based on its role in inflammatory disorders such as atherosclerosis, we hypothesized a possible role for LIGHT in NAFLD, as similar pathogenic interactions between inflammation and lipid metabolism are involved. In the present study, this hypothesis was tested through various experimental approaches including in vivo studies in NAFLD patients as well as in vitro studies in hepatocytes.
Methods
Patients and controls
The study population is part of a published cohort,15 and all patients with available serum samples for cytokine analyses were included in this study (66 patients with biopsy-verified NAFLD; 34 with simple steatosis and 32 with NASH) (Table 1). In NASH, the grade of inflammation was mild (n=24) or moderate to severe (n=8). Fibrosis was observed in 30 patients (stage 1, n=21; stage 2, n=5; stage 3, n=3; and stage 4, n=1), including 5 patients without necroinflammation. Type 2 diabetes mellitus and the metabolic syndrome (by the Adult Treatment Panel III definition)16 were present in 26% and 71% of the cases, respectively. No other liver diseases (e.g., chronic hepatitis B or C virus infection, hemochromatosis, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, or malignancy) were present. None of the patients had ingested any known hepatotoxic medications or herbal products, and the average alcohol consumption was <24 g per day in all subjects. For comparison, serum samples were also obtained from 16 age-, sex-, and ethnicity-matched healthy controls, all with serum levels of alanine aminotransferase (ALT) within the normal range (Table 1). None of the controls took any medication regularly, the alcohol consumption was <24 g per day and the body mass index (BMI) was <27 kg/m2 in all individuals.
Table 1. Anthropometric data and serum ALT for healthy controls, patients with simple steatosis, and patients with NASH.
| Controls (n=16) | Simple steatosis (n=34) | NASH (n=32) | P value | |
|---|---|---|---|---|
| Age (years), mean (s.d.) | 42.1 (10.2) | 44.3 (12.6) | 44.9 (12.6) | 0.748 |
| Sex, male/female | 8/8 | 19/15 | 18/14 | 0.908 |
| Body mass index (kg/m2), mean (s.d.) | 23.0 (2.4) | 29.5 (3.7) | 31.6 (4.7) | <0.001 |
| S-ALT (U/l), median (interquartile range) | 22 (14) | 73 (62) | 98 (56) | <0.001 |
ALT, alanine aminotransferase; NASH, nonalcoholic steatohepatitis; S-ALT, serum ALT.
P values represent comparisons between all three groups.
In the study of hepatic expression of LIGHT/HVEM/LTβR, we included another 14 patients (age: 48.1 (±9.7) years; BMI: 29.8 (±2.1) kg/m2; ALT 82 (37–273) U/l) with biopsy-verified simple steatosis (n=8) or NASH (n=6). Seven patients who underwent hepatic resection for liver metastasis from colorectal cancer (n=6) or carcinoid (n=1) served as controls (age: 62.7 (±7.5) years; BMI: 26.6 (±3.3) kg/m2; ALT: 22 (14–36) U/l). In the controls, the liver specimens were carefully evaluated for cancer cell infiltration both macroscopically and microscopically. However, although the control specimens showed few infiltrating lymphocytes, we cannot totally exclude that these specimens contain tumor-infiltrating T cells with low expression of LIGHT.17 Written informed consent for participation in the studies was obtained from all individuals. The regional Ethical Committee approved the studies, which were conducted according to the Declaration of Helsinki. Serum samples for the study were collected and stored as described previously.15
Liver biopsy and histological examinations
All biopsies were obtained using a biopsy gun and were snap frozen in liquid nitrogen and thereafter stored at −80 °C (for the study of hepatic gene expression) or fixed in formalin. Slides were routinely stained with hematoxylin/eosin, Masson trichrome or AFOG, Gomori and Perls' staining for iron, and examined by two liver pathologists blinded for clinical data. Histological examinations showed normal liver tissue for the all controls. Steatosis was defined when fat droplets were present in >5% of hepatocytes. NASH was defined when either ballooning and lobular inflammation or typical lobular fibrosis was present in addition to steatosis. Fibrosis was staged as follows: 0=no fibrosis, 1=pericellular fibrosis or isolated portal fibrosis, 2=combined pericellular and portal fibrosis, 3=bridging fibrosis, and 4=cirrhosis.
Hepatocyte cultures
Huh7 (human hepatoma cell line), obtained from the Health Science Research Resources Bank (JCRB0403; Osaka, Japan), were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich, St Louis, MO), supplemented with 10% heat-inactivated fetal calf serum, to 70–85% confluence, and then stimulated with different concentrations (1–100 ng/ml) of recombinant LIGHT (R&D Systems, Minneapolis, MN). In a separate set of experiments, the cells were incubated with LIGHT with and without different concentration of H2O2 (hydrogen peroxide; Sigma-Aldrich). In these coculture experiments, we used 300 ng/ml LIGHT to ensure a proper and significant response of LIGHT. After incubation, cell-free supernatants and cell pellets were harvested and stored at −80 °C.
Real-time quantitative RT-PCR
Total RNA was isolated from frozen liver specimens using Trizol reagent (Life Technologies, Invitrogen, San Diego, CA) and Huh7 cells using RNeasy mini columns (Qiagen, Hilden, Germany), DNase treated, and stored at −80 °C. cDNA was synthesized using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Primers for LIGHT, HVEM, and LTβR were designed using the Primer Express software, version 2 (Applied Biosystems). Primer sequences could be provided upon request. Quantification of mRNA was performed using the ABI Prism 7000 (Applied Biosystems). Gene expression of the housekeeping genes GAPDH and β-actin were used for normalization. SYBR Green assays were performed with the qPCR Master Mix for Power SYBR Green I (Eurogentech, Seraing, Belgium).
Measurement of cytokines by enzyme immunoassay
Concentrations of LIGHT, interleukin-8 (IL-8), IL-6, monocyte chemoattractant protein-1/CCL2, and TNFα were measured by enzyme immunoassays obtained from R&D Systems. The intra- and intercoefficient of variation were <10% for all assays.
Statistical analyses
This study was designed to analyze the difference in serum or plasma levels of cytokines or more specific LIGHT between NAFLD patients and healthy controls. We were looking for rather large differences (>50% changes from reference group), and found that 50 NAFLD patients and 15 healthy controls would have >90% power to detect a difference between means of 1.5 with an α of 0.05 (two-tailed). Data were analyzed using SPSS 14.0 (SPSS, Chicago, IL) and GraphPad Prism 5 (La Jolla, CA, USA). T-test or Mann–Whitney U-test were used for univariate comparisons between groups as appropriate. Correlations were calculated by the Spearman rank test. Multiple linear regression analyses were performed to explore the effects of NAFLD and NASH on serum levels of LIGHT after adjustment for age, sex, and BMI. Probability values (two-sided) were considered significant at P<0.05.
Results
Serum levels of LIGHT in NAFLD patients and healthy controls
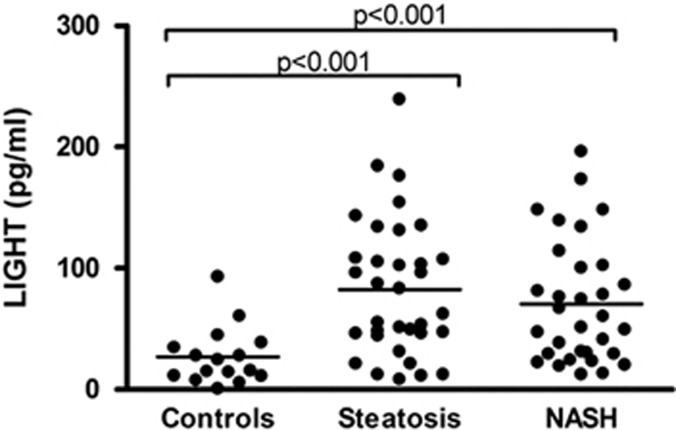
Patients with NAFLD (n=66) had significantly raised serum levels of LIGHT as compared with healthy controls (n=16) (mean (s.d.): 79.1 (53.49) vs. 26.4 (24.6) pg/ml, P<0.001), with no significant difference between simple steatosis (n=34) and NASH (n=32) (mean (s.d.): 85.3 (55.6) vs. 70.4 (50.8) pg/ml, P=0.26), simple steatosis, and NASH, respectively) (Figure 1). After adjustment for sex, BMI, and age, the diagnosis of NAFLD was still a significant predictor of LIGHT (P<0.001; Table 2). Moreover, within the NAFLD goup, there was no difference in LIGHT levels between those who met (n=47) and those who did not meet (n=19) the criteria for metabolic syndrome (mean (s.d.): 79.1 (55.1) vs. 75.5 (50.5) pg/ml, P=0.80). Also, there was no difference in serum levels of LIGHT when comparing those with and without fibrosis (data not shown).
Figure 1.
Levels of LIGHT (homologous to lymphotoxin, exhibits inducible expression, and competes with HSV glycoprotein D for herpes virus entry mediator, a receptor expressed by T lymphocytes) in patients and controls. Serum levels of LIGHT were assessed by enzyme immunoassay, in healthy control subjects (n=16), patients with simple steatosis (n=34), and patients with nonalcoholic steatohepatitis (NASH) (n=32). Horizontal lines indicate median values.
Table 2. NAFLD is an independent predictor of elevated serum levels of LIGHT after adjustment for sex, BMI, and age (adj.R2=0.135).
| Dependent variable | Explanatory variable | β | 95% CI for β | P value |
|---|---|---|---|---|
| LIGHT | NAFLD present | 64.9 | 30.2, 100.0 | <0.001 |
| Sex (ref.=male) | 14.5 | −8.7, 37.7 | 0.218 | |
| BMI (per kg/m2) | −1.5 | −4.3, 1.2 | 0.277 | |
| Age (per year) | −0.4 | −1.4, 0.6 | 0.408 |
BMI, body mass index; CI, confidence interval; LIGHT, homologous to lymphotoxin, exhibits inducible expression, and competes with HSV glycoprotein D for herpes virus entry mediator, a receptor expressed by T lymphocytes; NAFLD, nonalcoholic fatty liver disease.
Serum LIGHT levels are estimated to be 64.9 pg/ml higher in NAFLD patients as compared with controls according to the model, which explains 13.5% of the variability of serum LIGHT levels (adj. R2=0.135). β Values show the degree of association for each parameter with NAFLD.
There are a few studies on increased LIGHT levels in obesity associated with elevated levels of triglycerides and impaired glycemic control.18, 19 In the present study, however, we found no correlation between LIGHT levels and BMI in either patients (r=−0.13, P=0.30) or controls (r=−0.16, P=0.56). Moreover, within the NAFLD group, there was no difference in LIGHT levels between those with BMI above (n=34) and below 30 kg/m2 (n=32) (mean (s.d.): 72.2 (49.4) vs. 84.4 (57.8) pg/ml, P=0.36, above and below 30 kg/m2, respectively).
Hepatic gene expression of LIGHT and its receptors in NAFLD patients and controls
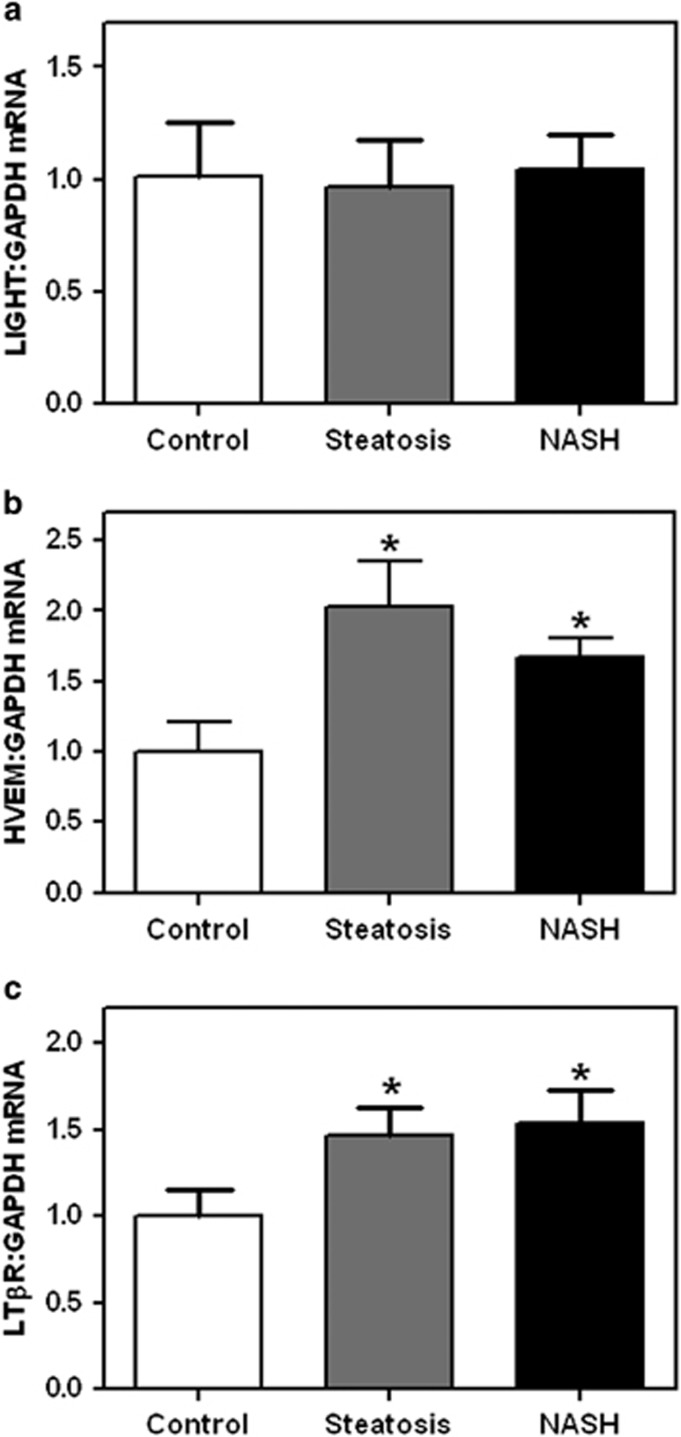
To further characterize the regulation of LIGHT in NAFLD, we examined the mRNA levels of LIGHT and its corresponding receptors, HVEM and LTβR, in liver biopsies from 14 patients with NAFLD (eight with simple steatosis and six with NASH) and seven controls. As shown in Figure 2, mRNA levels of HVEM and LTβR were both significantly enhanced in NAFLD with no differences between simple steatosis and NASH. In contrast, LIGHT gene expression did not differ between patients and controls (Figure 2).
Figure 2.
Levels of LIGHT (homologous to lymphotoxin, exhibits inducible expression, and competes with HSV glycoprotein D for herpes virus entry mediator (HVEM), a receptor expressed by T lymphocytes), HVEM, and lymphotoxin β receptor (LTβR) in liver biopsies from patients and controls. mRNA levels of (a) LIGHT and its corresponding receptors, (b) HVEM and (c) LTβR, in liver biopsies from control subjects (n=7), patients with simple steatosis (n=8), and patients with nonalcoholic steatohepatitis (NASH) (n=6). mRNA levels were quantified by real-time reverse transcription-PCR (RT-PCR), in relation to the control gene GAPDH (glyceraldehyde 3-phosphate dehydrogenase). Data are given as mean±s.e.m. *P<0.05 vs. control subjects.
LIGHT enhances IL-8 release in Huh7 hepatocytes
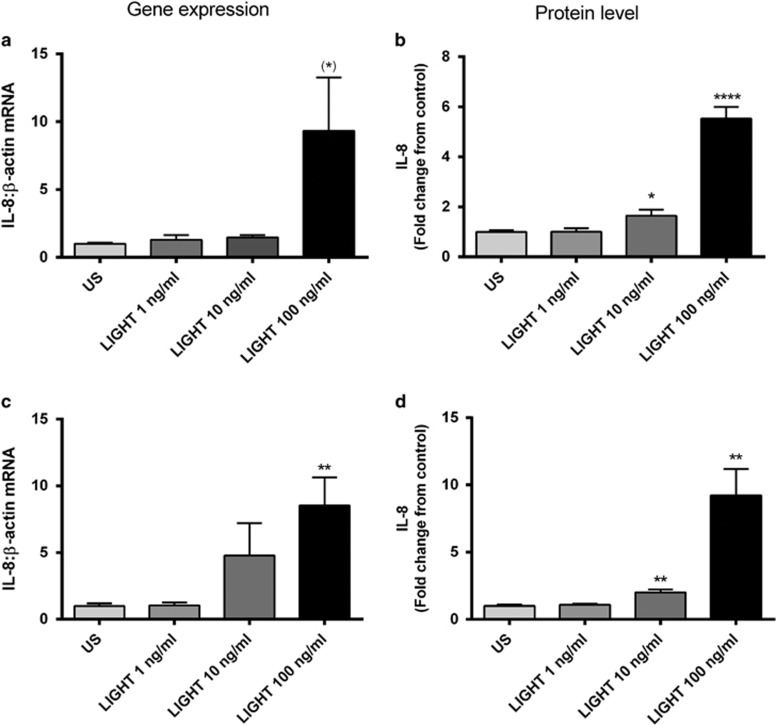
Hepatic inflammation is an important feature of NAFLD-related disease. To elucidate any potential pathogenic role of LIGHT in NAFLD, we next examined the ability of LIGHT to promote the production and release of prototypical inflammatory cytokines in Huh7 hepatocytes. Whereas LIGHT had no effect on the release of IL-6, TNFα, and monocyte chemoattractant protein-1, it markedly enhanced the IL-8 response in a time- and dose-dependent manner (Figure 3) both on protein and mRNA level.
Figure 3.
Impact of LIGHT (homologous to lymphotoxin, exhibits inducible expression, and competes with HSV glycoprotein D for herpes virus entry mediator (HVEM), a receptor expressed by T lymphocytes) on the expression of interleukin-8 (IL-8) in Huh7 (human hepatoma cell line) hepatocytes. Effect of recombinant human LIGHT (ng/ml) on the expression of IL-8 in Huh7 hepatocytes after (a and b) 2 h and (c and d) 8 h stimulation. Left panels show mRNA levels of IL-8 from cell lysates, as assessed by real-time reverse transcription-PCR (RT-PCR), in relation to the control gene β-actin, and right panels show protein levels of IL-8 measured in the supernatant from cells by enzyme immunoassay. Data are given as mean±s.e.m., n=6. *P=0.06, *P<0.05, **P<0.01, and ****P<0.0001 vs. US (unstimulated).
H2O2 augments the LIGHT-mediated release of IL-8 in Huh7 hepatocytes
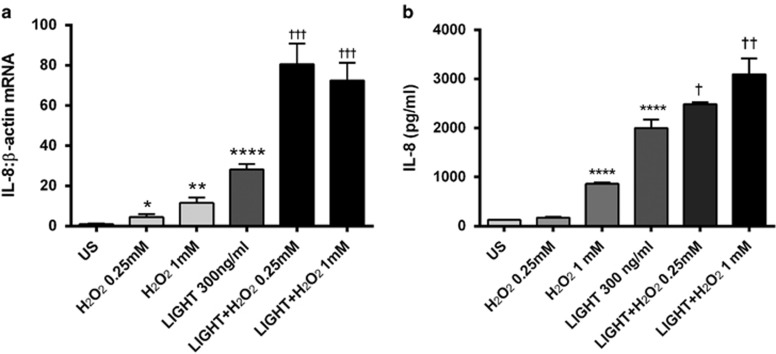
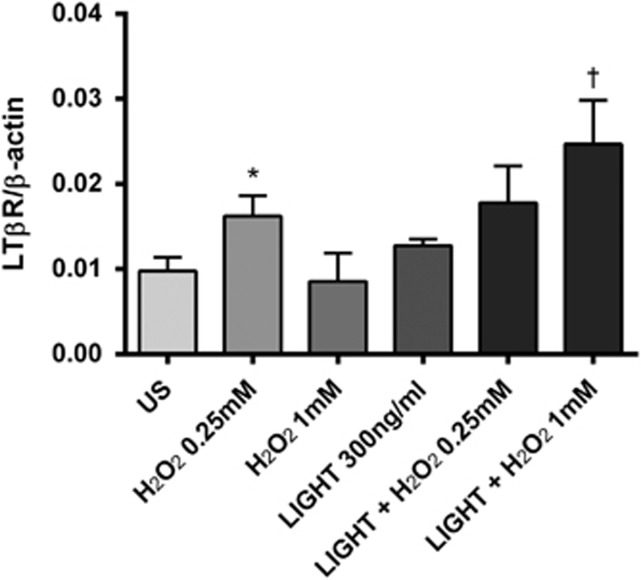
Increased oxidative stress has been implicated in the pathogenesis of NAFLD.4 As shown in Figure 4, H2O2 increased the release of IL-8 from Huh7 cells, and notably, it also enhanced the stimulating effect of LIGHT on IL-8 release in these cells. Similar effects or even stronger effects were seen at the mRNA levels (Figure 4). LIGHT interact with HVEM and LTβR, and while HVEM in general showed very low expression in these cells, LTβR was clearly present and H2O2 enhanced the expression of LTβR, in particular when combined with LIGHT stimulation (Figure 5).
Figure 4.
Impact of LIGHT (homologous to lymphotoxin, exhibits inducible expression, and competes with HSV glycoprotein D for herpes virus entry mediator (HVEM), a receptor expressed by T lymphocytes) on the expression of interleukin-8 (IL-8) in Huh7 (human hepatoma cell line) hepatocytes in the presence of H2O2. Huh7 hepatocytes were stimulated with recombinant human LIGHT (300 ng/ml) with or without coincubation with two different concentrations of H2O2. The panels show mRNA levels in cell lysates (a), as assessed by real-time reverse transcription-PCR (RT-PCR), in relation to the control gene β-actin, and (b) protein levels in cell supernatants, as assessed by enzyme immunoassay, of IL-8 after 5 and 18 h stimulation, respectively. Data are given as mean±s.e.m., n=8. *P<0.05, **P<0.01, and ****P<0.0001 vs. US (unstimulated). †P<0.05, ††P<0.01, and †††P<0.001 vs. LIGHT when given alone.
Figure 5.
Effect of LIGHT (homologous to lymphotoxin, exhibits inducible expression, and competes with HSV glycoprotein D for herpes virus entry mediator (HVEM), a receptor expressed by T lymphocytes) on the regulation of lymphotoxin β receptor (LTβR) in Huh7 (human hepatoma cell line) hepatocytes in the presence of H2O2. mRNA levels of LTβR, as assessed by real-time reverse transcription-PCR (RT-PCR) in relation to the control gene β-actin, were analyzed in cell lysates after 5 h stimulation with 300 ng/ml recombinant human LIGHT. Data are given as mean±s.e.m., n=8. *P<0.05 vs. US (unstimulated); †P<0.05 vs. LIGHT when given alone.
Discussion
In the present study, we show that NAFLD patients are characterized by increased serum levels of the TNFSF member LIGHT as well as enhanced hepatic expression of its receptors, HVEM and LTβR, with no significant differences between simple steatosis and NASH. Our in vitro findings in Huh7 hepatocytes suggest that LIGHT induces an increased release of the inflammatory chemokine IL-8 in these cells, with enhancing effects when coincubated with H2O2. Our findings suggest that LIGHT-mediated inflammation could be involved in NAFLD pathogenesis, potentially involving interaction with enhanced oxidative stress. It could be argued that in contrast to serum levels, LIGHT levels were not increased in the liver from NAFLD patients. However, the hepatic expression of the LIGHT receptors were significantly upregulated in NAFLD, indicating a possibility for LIGHT-mediated pathology in NAFLD even if LIGHT itself is not upregulated within the liver.
Several inflammatory cytokines have been implicated in the pathogenesis of NAFLD including TNF-related molecules such as TNF/TNFSF2,20 Fas ligand (TNFSF6),21 TNF-related apoptosis inducing ligand (TNFSF10),22 and osteoprotegerin (TNFRSF11b).23 Experimental studies have previously shown that LIGHT could promote hepatic inflammation and metabolic disturbances within the liver.13, 14 This cytokine has also been linked to the pathogenesis of various inflammatory and autoimmune disorders such as inflammatory bowel disease, nephritis, and rheumatoid arthritis.10, 24, 25, 26 However, to the best of our knowledge, this is the first report of increased serum levels of LIGHT, as well as increased hepatic expression of its receptors, HVEM and LTβR, in patients with NAFLD. Celik et al.27 have reported elevated LIGHT levels in patients with hepatitis C virus infection and in patients with rheumatoid arthritis, and we have previously shown elevated LIGHT levels in patients with coronary artery disease.28 However, although raised LIGHT levels are not specific for NAFLD, LIGHT could still have a pathogenic role in this disorder. In fact, a common feature of several inflammatory cytokines is that they are elevated and have a pathogenic role in a magnitude of disorders with inflammation as a common feature.
Unfortunately, we have no data on the cellular source of LIGHT in fatty liver. In general, LIGHT is strongly expressed by activated T cells, and is also found in granulocytes, immature dendritic cells, and platelets.7, 8 In the liver, LIGHT+ T cells have been shown to inhibit hepatic lipase,14 and hepatic NK1.1+ T cells have been shown to produce LIGHT during experimental hepatitis.13 However, as soluble LIGHT may be rapidly cleaved from its membrane-bound form, mediating LIGHT-driven inflammation, the cellular source of LIGHT can be difficult to prove. Nonetheless, it is not inconceivable that the cellular source of LIGHT in NAFLD may be infiltrating T cells.
Whereas several LIGHT effects seems to be mediated by its membrane-bound form, in particular on T cells (e.g., metabolic effects within the liver),7, 10, 14 soluble LIGHT has been found to promote hepatic inflammation.13 In the present study, we show that soluble LIGHT is a potent inducer of IL-8 production and release in Huh7 hepatocytes. Moreover, in line with studies in experimental hepatitis,13 the LIGHT-mediated inflammatory response in Huh7 hepatocytes seemed to be mediated through LTβR, as HVEM was expressed at very low levels in these cells. Thus, while HVEM is prominently expressed in T cells, LTβR is expressed in stromal cells or non-lymphoid hematopoietic cells including hepatocytes.7, 13 Yet, although HVEM was scarcely expressed in Huh7 hepatocytes, we cannot exclude an inflammatory interaction between LIGHT and HVEM during hepatic inflammation through T- cell-related mechanisms. However, even in experimental hepatitis, abundant with T cells and NK-T cells, the LIGHT-mediated hepatic inflammation seems primarily to be mediated through LTβR.13
Enhanced oxidative stress has been implicated to have an important role in the development and progression of NAFLD.4 In the present study, we show that the reactive oxygen species H2O2, in addition to promote IL-8 release in itself, enhanced the LIGHT-induced production and release of IL-8. IL-8 is a potent inflammatory cytokine that could promote generation of reactive oxygen species in leukocytes,29 and has also been linked to development of NAFLD.30 If IL-8 is operating in vivo within the liver, the interaction between LIGHT, H2O2, and IL-8 could potentially be part of a pathogenic loop during NAFLD progression. Moreover, although there was no difference between the expression of LIGHT and LTβR between simple steatosis and NASH, H2O2 level is presumable higher in NASH, potentially resulting in increased LIGHT-mediated effects. On the other hand, LIGHT has also been shown to promote liver regeneration31 and to counteract the TNF-induced apoptosis in hepatocytes,32 and the role of LIGHT in NAFLD is far from clear.
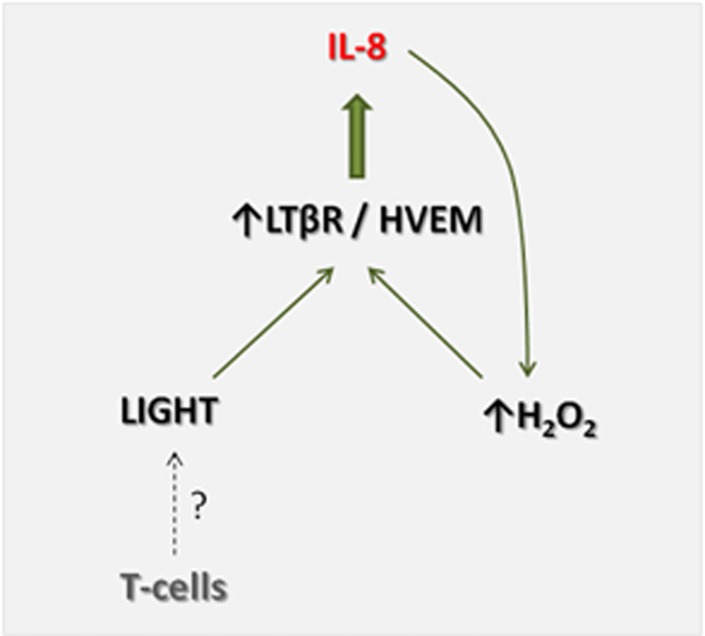
The present study has some limitations such as relatively low number of patients in particular in the analyses of LIGHT expression in the liver, and the number of patients with simple steatosis and NASH could have been too low to detect differences between these two NAFLD subgroups. In addition, the control samples for liver specimens were not ideal and we lack data on the cellular source of LIGHT. However, we suggest that our findings indicate that LIGHT-mediated inflammation could be operating in NAFLD, involving interactions between IL-8 and oxidative stress, potentially representing a pathogenic loop in the deleveopment and progression of NAFLD (Figure 6). Our findings should encourage further studies on the role of LIGHT in NAFLD development and progression.
Figure 6.
Postulated mechanism of LIGHT (homologous to lymphotoxin, exhibits inducible expression, and competes with HSV glycoprotein D for herpes virus entry mediator (HVEM), a receptor expressed by T lymphocytes) in nonalcoholic fatty liver disease (NAFLD). Within the liver, LIGHT, potentially produced by infiltrating T cells, binds to its receptors, lymphotoxin β receptor (LTβR) and HVEM, and is found to be upregulated in NAFLD, leading to induction and release of IL-8. IL-8 could promote H2O2 production, which in turn will increase LIGHT-mediated IL-8 release through upregulation of its receptors, creating a pathogenic loop that may contribute to the development and progression of NAFLD.
Study Highlights
Acknowledgments
We thank Ellen Lund Sagen for excellent technical assistance.
Guarantor of the article: Kari Otterdal, MSc, PhD.
Author contributions: K.O., contributions to the conception and design of study, performing the in vitro experiments, performing analyses and interpretation of data, drafting the manuscript; JWH/IPG/ZK, contributions to the conception and design of study, collection of patient and control material; AY/SH/FMS, performing analyses and interpretation of data; TBD, culturing the hepatocytes, performing analyses and interpretation of data; JKD/BH, contributions to the conception and design of study, involved in interpretation of data; PA, contributions to the conception and design of study, drafting the manuscript. All the authors have read and approved the manuscript.
Financial support: The Norwegian Research Council, University of Oslo, the Southern and Eastern Norway Regional Health Authority and Inven2.
Potential competing interests: None.
References
- Björnsson E, Angulo P. Non-alcoholic fatty liver disease. Scand J Gastroenterol 2007; 42: 1023–1030. [DOI] [PubMed] [Google Scholar]
- Pais R, Charlotte F, Fedchuk L et al. LIDO Study Group. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol 2013; 59: 550–556. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Bugianesi E, Forlani G et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003; 37: 917–923. [DOI] [PubMed] [Google Scholar]
- Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol 2013; 58: 395–398. [DOI] [PubMed] [Google Scholar]
- Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lesions from genetically engineered mice. J Clin Invest 2008; 118: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology 2012; 143: 1158–1172. [DOI] [PubMed] [Google Scholar]
- Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev 2004; 202: 49–66. [DOI] [PubMed] [Google Scholar]
- Otterdal K, Smith C, Øie E et al. Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood 2006; 108: 928–935. [DOI] [PubMed] [Google Scholar]
- Lin WW, Hsieh SL. Decoy receptor 3: a pleiotropic immunomodulator and biomarker for inflammatory disease, autoimmune disease and cancer. Biochem Pharmacol 2011; 81: 838–847. [DOI] [PubMed] [Google Scholar]
- Ware CF. Targeting the LIGHT-HVEM pathway. Adv Exp Med Biol 2009; 647: 146–155. [DOI] [PubMed] [Google Scholar]
- Sandberg WJ, Halvorsen B, Yndestad A et al. Inflammatory interaction between LIGHT and proteinase-activated receptor-2 in endothelial cells. Potential role in atherogenesis. Circ Res 2009; 104: 60–68. [DOI] [PubMed] [Google Scholar]
- Lee WH, Kim SH, Lee Y et al. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol 2001; 21: 2004–2010. [DOI] [PubMed] [Google Scholar]
- Anand S, Yoshimura K, Choi IH et al. Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. J Clin Invest 2006; 116: 1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Wang Y, Tumanov AV et al. Lymphotoxin beta receptor-dependent control of lipid homeostasis. Science 2007; 316: 285–288. [DOI] [PubMed] [Google Scholar]
- Haukeland JW, Damås JK, Konopski Z et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol 2006; 44: 1167–1174. [DOI] [PubMed] [Google Scholar]
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- Qin JZ, Upadhyay V, Prabhakar B, Maker AV. Shedding LIGHT (TNFSF14) on the tumor microenvironment of colorectal cancer liver metastases. J Transl Med 2013; 11: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassols J, Moreno JM, Ortega F, Ricart W, Fernandez-Real JM. Characterization of herpes virus entry mediator as a factor linked to obesity. Obesity 2010; 18: 239–246. [DOI] [PubMed] [Google Scholar]
- Bassols J, Moreno-Navarrete JM, Ortega F, Ricart W, Fernandez-Real JM. LIGHT is associated with hypertriglyceridemia in obese subjects and increased cytokine secretion from cultured human adipocytes. Int J Obes 2010; 34: 146–156. [DOI] [PubMed] [Google Scholar]
- Tokushige K, Takakura M, Tsuschiya-Matsushita N et al. Influence of TNF gene polymrphism in Japanese patients with NASH and simple steatosis. J Hepatol 2007; 46: 1104–1110. [DOI] [PubMed] [Google Scholar]
- Tamimi TI, Elgouhari HM, Alkhouri N et al. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J Hepatol 2011; 54: 1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsova P, Ibrahim SH, Bronk SF et al. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS One 2013; 22: e70599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz Y, Yonal O, Kurt R et al. Serum levels of osteoprotegrin in the spectrum of nonalcoholic fatty liver disease. Scand J Clin Lab Invest 2010; 70: 541–546. [DOI] [PubMed] [Google Scholar]
- Krause P, Zahner SP, Kim G et al. The tumor necrosis factor family member TNFSF14 (LIGHT) is required for resolution of intestinal inflammation in mice. Gastroenterology 2014; 146: 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Anders RA, Wu Q et al. Dysregulated LIGHT expression on T cells mediates intestinal inflammation and contributes to IgA nephropathy. J Clin Invest 2004; 113: 826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierer M, Brentano F, Rethage J et al. The TNF superfamily member LIGHT contributes to survival and activation of synovial fibroblasts in rheumatoid arthritis. Rheumatology 2007; 46: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Celik S, Shankar V, Richter A et al. Proinflammatory and prothrombotic effects on human vascular endothelial cells of immune-cell-derived LIGHT. Eur J Med Res 2009;14:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz H, Sandberg W, Damås JK et al. Enhanced plasma levels of LIGHT in unstable angina. Possible pathogenic role in foam cell formation and thrombosis. Circulation 2005;112:2121–2129. [DOI] [PubMed] [Google Scholar]
- Guichard C, Pedruzzi E, Dewas C et al. Interleukin-8 induced priminmg of neutrophil oxidative burst requeires sequential recruitment of NADPH oxidase components into lipid rafts. J Biol Chem 2005; 280: 37021–37032. [DOI] [PubMed] [Google Scholar]
- Jarrar MH, Baranova A, Collantes R et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2008; 27: 412–421. [DOI] [PubMed] [Google Scholar]
- Anders RA, Subudhi SK, Wang J et al. Contribution of the lymphotoxin beta receptor to liver regeneration. J Immunol 2005; 175: 1295–1300. [DOI] [PubMed] [Google Scholar]
- Matsui H, Hikichi Y, Tsuji I et al. LIGHT, a member of the tumor necrosis ligandsuperfamily, prevents tumor necrosis factor-α-mediated human primary hepatocyte apoptosis, but not Fas-mediated apoptosis. J Biol Chem 2002; 277: 50054–50061. [DOI] [PubMed] [Google Scholar]