Abstract
OBJECTIVES:
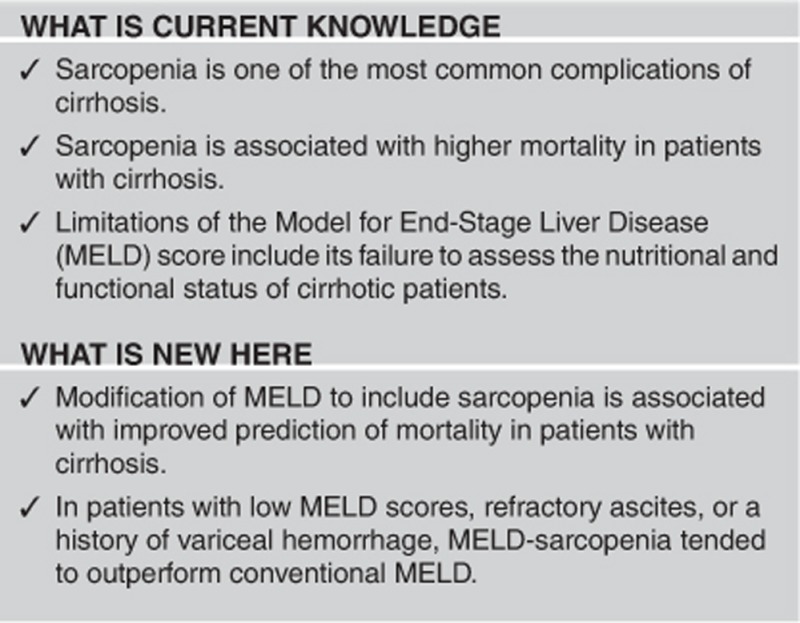
Limitations of the Model for End-Stage Liver Disease (MELD) score include its failure to assess the nutritional and functional status of cirrhotic patients. Our objectives were to evaluate the impact of sarcopenia in cirrhosis and whether the inclusion of muscularity assessment within MELD could improve the prediction of mortality in patients with cirrhosis.
METHODS:
We included 669 cirrhotic patients who were consecutively evaluated for liver transplantation. Skeletal muscle index at the third lumbar vertebra (L3 SMI) was measured by computed tomography, and sarcopenia was defined using previously published gender and body mass index–specific cutoffs. Using Cox proportional hazards regression, a novel MELD-sarcopenia score was derived.
RESULTS:
Sarcopenia was present in 298 patients (45%); sarcopenic patients had shorter median survival than non-sarcopenic patients (20±3 vs. 95±24 months, P<0.001). By Cox regression analysis adjusted for age, gender, and hepatocellular carcinoma, both MELD (hazard ratio (HR) 1.08, 95% confidence interval (CI) 1.06–1.10, P<0.001), and the L3 SMI (HR 0.97, 95% CI 0.96–0.99, P<0.001) were associated with mortality. Overall, the c-statistics for 3-month mortality were 0.82 (95% CI 0.78–0.87) for MELD and 0.85 (95% CI 0.81–0.88) for MELD-sarcopenia (P=0.1). Corresponding figures for 1-year mortality were 0.73 (95% CI 0.69–0.77) and 0.77 (95% CI 0.73–0.80), respectively (P=0.03). The c-statistics for 3-month mortality in patients with MELD<15 (0.85 vs. 0.69, P=0.02) and refractory ascites (0.74 vs. 0.71, P=0.01) were significantly higher for MELD-sarcopenia compared with MELD.
CONCLUSIONS:
Modification of MELD to include sarcopenia is associated with improved prediction of mortality in patients with cirrhosis, primarily in patients with low MELD scores. External validation of this prognostic index in larger cohorts of cirrhotic patients is warranted.
INTRODUCTION
In most liver transplant centers worldwide, the Model for End-Stage Liver Disease (MELD) score has replaced the Child–Pugh score for the prioritization of organ allocation.1 MELD has the advantage over the Child–Pugh score of being based on objective parameters (international normalized ratio (INR) and serum bilirubin and creatinine) rather than on subjective evaluation of the severity of clinical findings (ascites and hepatic encephalopathy).
Since implementation of the MELD score, there have been reports of reductions in the number of patients listed for liver transplantation, waiting time for transplantation, and deaths on the waiting list.2, 3 Despite the irrefutable benefits of MELD, limitations of this score have been recognized and attempts are ongoing to improve it.4, 5 One of the major limitations of MELD is that it does not include an assessment of the nutritional and functional status of patients.
Sarcopenia is defined as a muscle mass two s.ds. below the healthy young adult mean.6 Although sarcopenia is associated with aging, it can also be present as a result of chronic diseases and malignancy,7 and it ultimately leads to decreased functional capacity and higher risk of mortality, including among patients with cirrhosis.8, 9, 10, 11, 12 Despite the important role that sarcopenia has in the prognosis of patients with cirrhosis, it is frequently overlooked, in part because nutritional assessment can be a difficult task in these patients due to fluid retention and/or overweight.13, 14
In this study, our objectives were to evaluate the impact of sarcopenia among cirrhotic patients evaluated for liver transplantation and to determine whether the inclusion of muscularity assessment (i.e. sarcopenia) within MELD could improve the prediction of mortality in these patients.
METHODS
Study population
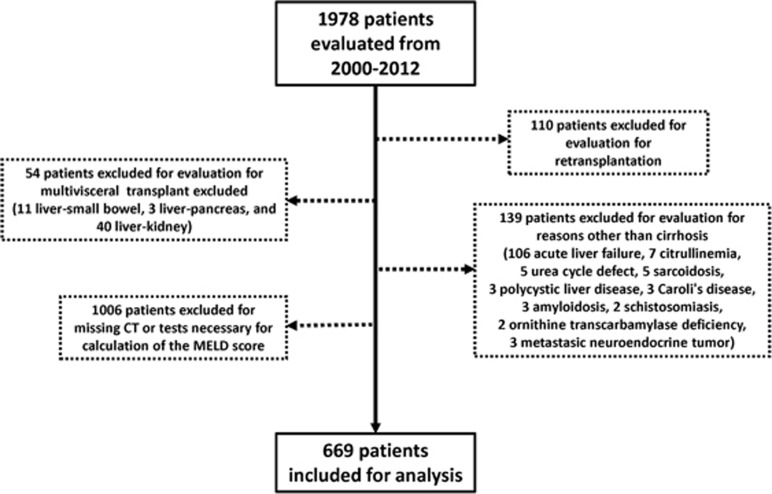
Six hundred and sixty-nine adult patients with cirrhosis who were consecutively evaluated for liver transplantation at the University of Alberta Hospital (Edmonton, AB, Canada) between January 2000 and December 2012 were evaluated. During this period, we evaluated 1978 patients and we excluded 1309 patients who were evaluated for liver transplant for reasons other than cirrhosis, retransplantation, multiple organ transplantation, and those with missing computed tomography (CT) or tests necessary for calculation of the MELD score (Figure 1).
Figure 1.
Flowchart of patients excluded/included for the muscularity assessment analyses in this cohort. CT, computed tomography; MELD, Model for End-Stage Liver Disease.
As a significant number of patients were not eligible for inclusion, we briefly analyzed patients included (n=669) and excluded (n=1309) from this study. We did not find significant differences in relation to age (57±0.5 vs. 58±0.5 years, P=0.4), gender (males, 68% vs. 65%, P=0.5), etiology of cirrhosis (hepatitis C virus 40% vs. 39%, P=0.2), and overall survival time (mean survival, 67±4 vs. 69±2 months, P=0.2) between these two groups.
Clinical and laboratory assessments
Data obtained from medical records included gender, age at the time of the CT, body weight, height, etiology of cirrhosis, liver biochemistries, serum albumin, serum creatinine, INR, and the Child–Pugh and MELD scores. Clinical, laboratory, and radiological data used for the analysis and to calculate the MELD and Child–Pugh scores were obtained within 1 week of the date of the CT used to quantify muscle indices (see below).
Ascites was evaluated clinically and with ultrasound or CT and defined as absent in patients not using diuretics and with no previous record of ascites in the electronic chart in the preceding year. Refractory ascites was defined as the absence of response to sodium restriction and diuretic treatment or complications of diuretic therapy and need for intermittent large volume paracentesis or transjugular portosystemic shunts.
Hepatic encephalopathy was evaluated clinically at the time of the assessment and using the electronic records and defined as absent in patients not using specific treatment (i.e., lactulose, rifaximin) and with no prior episodes of hepatic encephalopathy in the preceding year. Patients with previous episodes of hepatic encephalopathy were classified according to the West Haven criteria (grades I–IV), and severe hepatic encephalopathy was defined as the occurrence of episodes of grades III–IV. The presence of esophageal varices was evaluated with upper endoscopy and defined as present or absent, and a history of variceal bleeding episodes before the CT was also recorded.
Muscularity assessment and sarcopenia evaluation
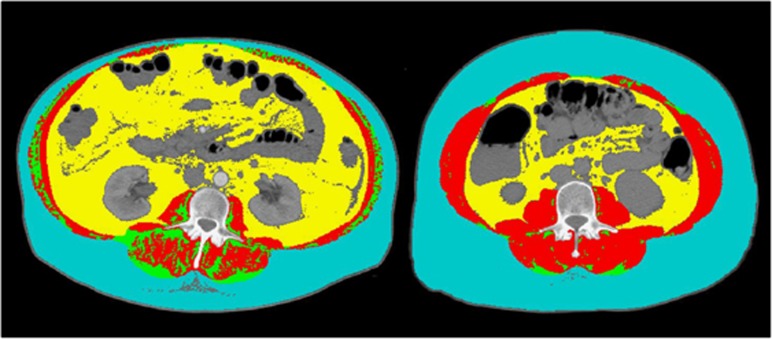
CT scans used for muscularity analysis were performed as part of the routine liver transplantation assessment. A transverse CT image from L3 was assessed from each scan. Images were analyzed with the SliceOmatic V4.3 software (Tomovision, Montreal, QC Canada), which enables specific tissue demarcation using previously reported Hounsfield unit (HU) thresholds.15 Skeletal muscle was identified and quantified using HU thresholds of −29 to +150. Muscles in the L3 region encompass psoas, erector spinae, quadratus lumborum, transversus abdominus, external and internal obliques, and rectus abdominus. The following HU thresholds were used for adipose tissues: −190 to −30 for subcutaneous and intermuscular adipose tissue,16 and −150 to −50 for visceral adipose tissue.17 Using these specific HU thresholds, measurements of the skeletal muscle index are not influenced by the presence of ascites, overweight, or obesity in patients with cirrhosis. Cross-sectional areas (cm2) were automatically computed by summing tissue pixels and multiplying by pixel surface area. All CT images were analyzed by two trained observers (C.B., N.E.) who demonstrated an intraobserver coefficient of variation of approximately 1%, and the sarcopenia assessment was blinded to MELD score. Cross-sectional area of muscle and adipose tissue was normalized for stature (cm2/m2) as reported elsewhere18 and this value is referred to as the L3 skeletal muscle index (L3 SMI). Sarcopenia was defined according to a CT-based study in patients with solid tumors using optimal stratification, a statistical method similar to receiver operating characteristic curve analysis that links specific threshold values for L3 SMI to an outcome (i.e., death) (L3 SMI: ≤41 cm2/m2 for women and ≤53 cm2/m2 for men with body mass index (BMI) ≥25 kg/m2 and ≤43 cm2/m2 in all patients with BMI<25 kg/m2.19 Representative images of two female cirrhotic patients with and without sarcopenia are illustrated in Figure 2.
Figure 2.
Comparison of two female patients with cirrhosis. Abdominal computed tomography images taken at third lumbar vertebra. Red color indicates skeletal muscle, green color indicates intermuscular adipose tissue, yellow color indicates visceral adipose tissue, and teal indicates subcutaneous adipose tissue. The patient at the left has severe sarcopenia (lumbar skeletal index, 35 cm2/m2) and the patient at the right is not sarcopenic (lumbar skeletal index, 54 cm2/m2).
Statistical analyses
Data are presented as the mean±s.e. or frequencies and percentages. Non-normally distributed variables were compared with Mann–Whitney test, as appropriate. Survival was calculated using the Kaplan–Meier method and between-groups comparisons were made using the log-rank (Mantel–Cox) statistic. Patients were followed from the date of the CT performed for muscularity assessment (time zero) until the date of death, liver transplantation, or the last clinic visit. Prognostic factors for overall mortality were analyzed by univariate Cox proportional hazards regression analyses. Variables of interest plus variables with a P-value <0.05 in univariate analysis were included in multivariate Cox regression models. In the first model, we included MELD, sarcopenia, albumin, sodium, and the presence of ascites, while excluding creatinine, INR, bilirubin, L3 SMI, and the Child–Pugh score to avoid colinearity with the aforementioned variables. In the second model, we included the L3 SMI instead of the dichotomous variable, sarcopenia. Biochemical parameters such as serum bilirubin, creatinine, sodium, and albumin were included as continuous variables, whereas clinical variables such as gender, sarcopenia, ascites, and hepatic encephalopathy were analyzed as categorical variables. Interaction terms including MELD with sarcopenia or L3 SMI were not statistically significant (data not shown).
Using Cox proportional hazards regression, a novel MELD-sarcopenia score was derived for the prediction of overall mortality according to the formula: MELD-sarcopenia=MELD+(beta[sarcopenia]/beta[MELD]) × sarcopenia. An analogous model, referred to as MELD-L3 SMI, was also constructed that included L3 SMI as a continuous variable rather than sarcopenia as a dichotomous variable (see Appendix for formulae). Discrimination, which refers to the ability of a model to correctly distinguish between two outcomes (i.e., death or survival at 3, 6, and 12 months and overall), was assessed using the concordance statistic (c-statistic) with a modification for survival data. The c-statistic from the Cox model is conceptually analogous to the area under a receiver operating characteristic curve estimated for logistic models. P-values for comparisons of c-statistics were calculated using the group jackknife method.5 These analyses were conducted overall and in patient subgroups restricted to those with refractory ascites, severe hepatic encephalopathy, and a history of variceal hemorrhage. We also performed subgroup analyses in patients with MELD<15 and ≥15 as we hypothesized that MELD-sarcopenia would have improved discrimination in patients with apparently milder liver disease in whom MELD may not accurately capture the risk of death (e.g., in malnourished patients). We also performed a competing risk analysis with transplantation as a competing risk, and the results were similar (data not shown).
RESULTS
Clinical and biochemical features of patients with cirrhosis
Among 669 patients who met the inclusion criteria, 454 were male (68%) and 291 patients had concomitant hepatocellular carcinoma (43%). The etiology of cirrhosis was hepatitis C virus in 265 patients (40%), alcohol in 152 (23%), non-alcoholic steatohepatitis/cryptogenic in 152 (23%), autoimmune liver disease in 51 (8%), and hepatitis B virus in 43 (6%).
Two hundred and ninety-eight patients (45%) had sarcopenia. Patients with sarcopenia were more frequently males (P<0.001), had lower body weight (P=0.001), BMI (P<0.001), and by definition, L3 SMI (P<0.001), compared with non-sarcopenic individuals (Table 1). Moreover, sarcopenic patients had a higher frequency of ascites (P=0.001), refractory ascites (P<0.001), hepatic encephalopathy (P<0.001), severe hepatic encephalopathy (P=0.02), and a history of variceal hemorrhage (P<0.001) than non-sarcopenic patients. Finally, sarcopenic patients had higher serum levels of creatinine (P=0.001) and bilirubin (P=0.008), INR (P=0.03), MELD (P=0.001), and Child–Pugh scores (P<0.001) compared with non-sarcopenic patients (Table 1). Characteristics between listed and non-listed patients with cirrhosis are shown in Supplementary Table.
Table 1. Features associated with sarcopenia in patients with cirrhosis.
| Features | All patients (n=669) | No sarcopenia (n=371) | Sarcopenia (n=298) | P-value |
|---|---|---|---|---|
| Age (years) | 57±0.5 | 57±0.5 | 57±1 | 0.3 |
| Gender (M:F) | 454:215 | 218:153 | 262:62 | <0.001 |
| Weight (kg) | 79±1 | 82±1 | 77±1 | 0.001 |
| BMI (kg/m2) | 27±0.5 | 29±0.5 | 25±0.5 | <0.001 |
| L3 SMI (cm2/m2) | 50±0.5 | 56±1 | 43±0.5 | <0.001 |
| Ascites | 481 (72) | 248 (67) | 233 (78) | 0.001 |
| Refractory ascites | 219 (33) | 83 (22) | 136 (46) | <0.001 |
| Hepatic encephalopathy | 251 (38) | 106 (29) | 145 (49) | <0.001 |
| Severe hepatic encephalopathy | 59 (9) | 24 (7) | 35 (12) | 0.02 |
| Esophageal varices | 452 (68) | 240 (65) | 212 (71) | 0.08 |
| Variceal bleeding | 175 (26) | 76 (21) | 99 (33) | <0.001 |
| Creatinine (nl, 50–115 μmol/l) | 94±3 | 84±3 | 107±6 | 0.001 |
| INR (nl, 0.8–1.2) | 1.4±0.2 | 1.4±0.2 | 1.5±0.3 | 0.03 |
| Albumin (nl, 35–50 g/l) | 33±0.3 | 33±0.4 | 32±0.3 | 0.3 |
| Bilirubin (nl, <20 μmol/l) | 80±6 | 66±6 | 97±10 | 0.008 |
| Sodium (nl 133–146 μmol/l) | 136±0.2 | 137±0.3 | 136±0.3 | 0.06 |
| MELD | 14±0.3 | 13±0.5 | 15±0.5 | 0.001 |
| Child–Pugh (A/B/C) | 105/371/193 | 65/222/84 | 40/149/109 | <0.001 |
| Child–Pugh (points) | 8.9±0.1 | 8.6±0.2 | 9.2±0.2 | 0.008 |
| Etiology of cirrhosis | ||||
| Alcohol | 153 (23) | 70 (19) | 83 (28) | 0.1 |
| HCV | 265 (40) | 154 (42) | 111 (37) | |
| AILDa | 51 (8) | 29 (8) | 22 (7) | |
| HBV | 43 (6) | 23 (6) | 20 (6.5) | |
| NASH-Cryptogenic | 152 (23) | 91 (25) | 61 (21) | |
| Othersb | 5 (1) | 4 (1) | 1 (0.5) | |
| Concomitant HCC | 289 (43) | 171 (46) | 118 (40) | 0.1 |
AILD, autoimmune liver disease; BMI, body mass index; F, female; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; INR, international normalized ratio; L3 SMI, lumbar third skeletal muscle index; M, male; MELD, Model for End-Stage Liver Disease; NASH, non-alcoholic steatohepatitis. Numbers in parentheses are percentages.
Includes autoimmune hepatitis, primary biliary cirrhosis and primary sclerosing cholangitis.
Includes alpha-1-antitrypsin deficiency, hemochromatosis, and Wilson disease.
Survival in cirrhotic patients with and without sarcopenia
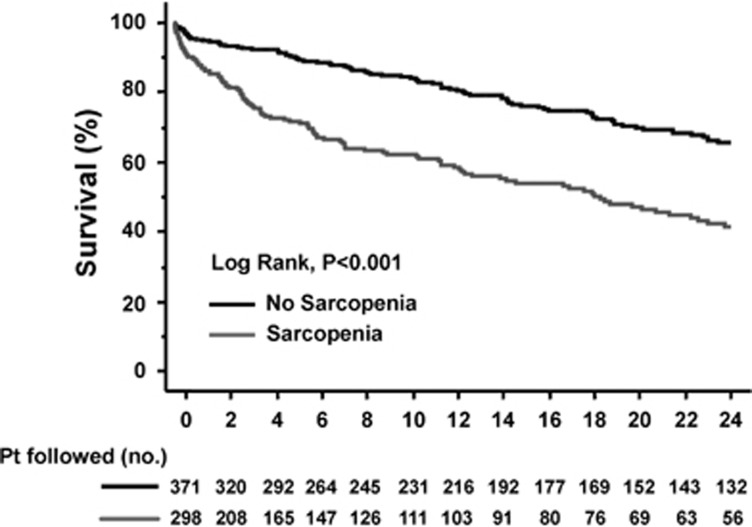
During a mean follow-up of 22±1 months (median, 11 months; range, 0.5–164 months), 229 patients received a liver transplant (34%) and 259 died (39%). Mean survival was shorter in patients with sarcopenia compared with non-sarcopenic patients (20±3 vs. 95±24 months, P<0.001). The estimated 3-month probability of survival was 81% in patients with sarcopenia compared with 93% in patients without sarcopenia. The 6-month and 1-year probabilities of survival were 72% and 61%, compared with 89% and 83% in sarcopenic and non-sarcopenic patients, respectively (Figure 3).
Figure 3.
Kaplan–Meier curves indicating the survival of patients with (—) and without (—) sarcopenia. The 3-month estimated probabilities of survival in patients with and without cirrhosis were 80% and 93%, respectively. Corresponding figures at 6 and 12 months were 71% and 90% and 53% and 83%, respectively (all P<0.00005 by log-rank tests).
Features associated with mortality in cirrhotic patients
By univariate Cox regression analysis of patients' clinical and biochemical characteristics, the presence of ascites (P<0.001), refractory ascites (P<0.001), and sarcopenia (P<0.001) were significantly associated with mortality in patients with cirrhosis. Also, higher levels of creatinine (P=0.02), bilirubin (P<0.001), INR (P<0.001), MELD (P<0.001) and Child–Pugh scores (P=0.001) and lower levels of albumin (P<0.001), sodium (P=0.007), and L3 SMI (P<0.001) were significantly associated with mortality in patients with cirrhosis (Table 2).
Table 2. Features associated with mortality by univariate cox analysis in patients with cirrhosis.
| Features associated with mortality by univariate analysis | Death (n=259) | Alive or censored (n=410) | HR | 95% CI | P-value |
|---|---|---|---|---|---|
| Age (years) | 56±1 | 57±0.5 | 0.99 | 0.98–1.01 | 0.2 |
| Gender (M:F) | 172:87 | 282:128 | 1.01 | 0.78–1.31 | 0.9 |
| BMI (kg/m2) | 28±0.5 | 27±0.5 | 0.99 | 0.98–1.02 | 0.8 |
| Ascites | 206 (80) | 275 (67) | 2.39 | 1.77–3.24 | <0.001 |
| Refractory ascites | 99 (38) | 120 (29) | 2.47 | 1.91–3.19 | <0.001 |
| Hepatic encephalopathy | 84 (32) | 167 (41) | 1.11 | 0.85–1.44 | 0.4 |
| Severe hepatic encephalopathy | 20 (8) | 39 (10) | 1.11 | 0.70–1.77 | 0.6 |
| Esophageal varices | 168 (65) | 284 (69) | 1.02 | 0.79–1.32 | 0.9 |
| Variceal bleeding | 51 (20) | 124 (30) | 0.84 | 0.62–1.14 | 0.3 |
| Creatinine (nl, 50–115 μmol/l) | 103±5 | 88±4 | 1.01 | 1.00–1.02 | 0.02 |
| INR (nl, 0.8–1.2) | 1.5±0.3 | 1.4±0.2 | 2.50 | 2.03–3.07 | <0.001 |
| Albumin (nl, 35–50 g/l) | 31±0.4 | 33±0.3 | 0.95 | 0.93–0.97 | <0.001 |
| Bilirubin (nl, <20 μmol/l) | 95±9 | 69±7 | 1.04 | 1.03–1.04 | <0.001 |
| Sodium (nl, 133–146 μmol/l) | 135±0.4 | 136±0.2 | 0.95 | 0.93–0.98 | <0.001 |
| L3 SMI (cm2/m2) | 49±1 | 51±1 | 0.97 | 0.96–0.99 | <0.001 |
| Sarcopenia | 141 (54) | 157 (38) | 2.31 | 1.81–2.96 | <0.001 |
| MELD score | 16±1 | 13±1 | 1.08 | 1.06–1.10 | <0.001 |
| Child–Pugh (points) | 8.9±0.1 | 8.6±0.2 | 1.11 | 1.04–1.18 | 0.001 |
CI, confidence interval; BMI, body mass index; F, female; HR, hazard ratio; INR, international normalized ratio; L3 SMI, lumbar third skeletal muscle index; M, male; MELD, Model for End-Stage Liver Disease.
Numbers in parentheses are percentages.
In the multivariate Cox analysis including MELD, sodium, albumin, the presence of ascites and sarcopenia, only MELD (hazard ratio (HR) 1.05, P<0.001), ascites (HR 1.61, P=0.008), albumin (HR 0.98, P=0.04), and sarcopenia (HR 2.26, P<0.001) were independently associated with mortality (Table 3). In a second model that included these variables plus L3 SMI as a continuous variable (instead of the dichotomous variable sarcopenia), L3 SMI (HR 0.98, P=0.001) was independently associated with mortality. The results for the remaining variables were largely unchanged compared with the primary model (data not shown).
Table 3. Features associated with mortality by multivariate cox analysis in patients with cirrhosis.
| Features associated with mortality by multivariate analysis | Death (n=259) | Alive or censored (n=410) | HR | 95% CI | P-value |
|---|---|---|---|---|---|
| Ascites | 206 (80) | 275 (67) | 1.61 | 1.13–2.30 | 0.008 |
| Albumin (nl, 35–50 g/l) | 31±0.4 | 33±0.3 | 0.98 | 0.96–0.99 | 0.04 |
| Sodium (nl, 133–146 μmol/l) | 135±1 | 136±1 | 0.96 | 0.95–1.02 | 0.3 |
| Sarcopenia | 139 (54) | 159 (39) | 2.26 | 1.73–2.94 | <0.001 |
| MELD score | 16±1 | 13±1 | 1.05 | 1.03–1.07 | <0.001 |
CI, confidence interval; HR, hazard ratio; MELD, Model for End-Stage Liver Disease. Numbers in parentheses are percentages.
Variables included in the multivariate analysis were ascites, albumin, sodium, sarcopenia, and MELD score. Child–Pugh, international normalized ratio, bilirubin, creatinine and lumbar third skeletal muscle index were not included to avoid colinearity.
Prediction of mortality using MELD and MELD-sarcopenia
Table 4 includes the c-statistics of MELD and MELD-sarcopenia for the prediction of overall and 3-, 6-, and 12-month mortality. In the entire patient cohort (n=669), MELD-sarcopenia outperformed MELD at each time point. For 3-month mortality, the c-statistics were 0.82 (95% confidence interval (CI) 0.78–0.87) for MELD and 0.85 (95% CI 0.81–0.88) for MELD-sarcopenia (P=0.13). Corresponding figures for 1-year mortality were 0.73 (95% CI 0.69–0.77) and 0.77 (95% CI 0.73–0.80), respectively (P=0.03). Likewise, in subgroup analyses restricted to patients with refractory ascites and a history of variceal hemorrhage, MELD-sarcopenia tended to outperform MELD, although not all analyses were statistically significant. In analyses stratified according to the MELD score, differences in discrimination between MELD-sarcopenia and MELD were greatest in patients with low MELD scores (<15) (Table 4).
Table 4. C-statistics of MELD, MELD-sarcopenia, and MELD-L3 SMI for the prediction of mortality in patients with cirrhosis.
| Patient cohort | 3-Month mortality | 6-Month mortality | 1-Year mortality | Overall mortality |
|---|---|---|---|---|
| All patients (n=669) | ||||
| MELD | 0.82 (0.78–0.87) | 0.77 (0.72–0.81) | 0.73 (0.69–0.77) | 0.70 (0.66–0.74) |
| MELD-sarcopenia | 0.85 (0.81–0.88) | 0.80 (0.76–0.84) | 0.77 (0.73–0.80) | 0.73 (0.70–0.77) |
| P-value vs. MELD | 0.13 | 0.10 | 0.03 | <0.01 |
| MELD-L3 SMI | 0.84 (0.80–0.89) | 0.79 (0.75–0.84) | 0.75 (0.71–0.79) | 0.72 (0.68–0.75) |
| P-value vs. MELD | 0.17 | 0.02 | 0.07 | 0.07 |
| Patients with refractory ascites (n=219) | ||||
| MELD | 0.71 (0.64–0.77) | 0.70 (0.63–0.76) | 0.69 (0.63–0.75) | 0.69 (0.63–0.75) |
| MELD-sarcopenia | 0.74 (0.68–0.80) | 0.74 (0.68–0.80) | 0.73 (0.68–0.79) | 0.73 (0.67–0.78) |
| P-value vs. MELD | 0.01 | 0.06 | <0.01 | 0.01 |
| MELD-L3 SMI | 0.74 (0.67–0.80) | 0.73 (0.67–0.80) | 0.72 (0.66–0.78) | 0.72 (0.66–0.78) |
| P-value vs. MELD | 0.03 | 0.10 | 0.12 | 0.19 |
| Patients with variceal hemorrhage (n=175) | ||||
| MELD | 0.68 (0.56–0.81) | 0.66 (0.54–0.78) | 0.65 (0.54–0.76) | 0.62 (0.52–0.71) |
| MELD-sarcopenia | 0.73 (0.63–0.83) | 0.73 (0.64–0.82) | 0.72 (0.64–0.81) | 0.70 (0.62–0.77) |
| P-value vs. MELD | 0.21 | 0.10 | <0.01 | 0.02 |
| MELD-L3 SMI | 0.72 (0.59–0.85) | 0.69 (0.57–0.81) | 0.68 (0.57–0.79) | 0.64 (0.54–0.74) |
| P-value vs. MELD | 0.22 | 0.36 | 0.26 | 0.33 |
| Patients with severe hepatic encephalopathy (n=59) | ||||
| MELD | 0.76 (0.67–0.86) | 0.71 (0.60–0.82) | 0.70 (0.60–0.81) | 0.70 (0.59–0.80) |
| MELD-sarcopenia | 0.73 (0.61–0.85) | 0.71 (0.59–0.83) | 0.71 (0.60–0.83) | 0.71 (0.60–0.83) |
| P-value vs. MELD | 0.47 | 0.99 | 0.85 | 0.81 |
| MELD-L3 SMI | 0.79 (0.70–0.89) | 0.75 (0.65–0.85) | 0.74 (0.64–0.84) | 0.74 (0.64–0.84) |
| P-value vs. MELD | 0.35 | 0.17 | 0.17 | 0.02 |
| Patients with HCC (n=289) | ||||
| MELD | 0.86 (0.77–0.95) | 0.75 (0.65–0.84) | 0.69 (0.61–0.76) | 0.67 (0.61–0.73) |
| MELD-sarcopenia | 0.86 (0.78–0.93) | 0.78 (0.70–0.86) | 0.74 (0.68–0.80) | 0.72 (0.66–0.77) |
| P-value vs. MELD | 0.95 | 0.53 | 0.04 | 0.08 |
| MELD-L3 SMI | 0.87 (0.78–0.95) | 0.76 (0.67–0.85) | 0.70 (0.62–0.77) | 0.68 (0.62–0.74) |
| P-value vs. MELD | 0.81 | 0.56 | 0.69 | 0.50 |
| Patients without HCC (n=380) | ||||
| MELD | 0.79 (0.73–0.84) | 0.76 (0.71–0.81) | 0.74 (0.69–0.79) | 0.71 (0.67–0.76) |
| MELD-sarcopenia | 0.82 (0.77–0.86) | 0.79 (0.75–0.83) | 0.77 (0.73–0.81) | 0.74 (0.70–0.78) |
| P-value vs. MELD | 0.06 | 0.04 | 0.11 | 0.27 |
| MELD-L3 SMI | 0.81 (0.76–0.87) | 0.79 (0.74–0.84) | 0.78 (0.73–0.82) | 0.74 (0.69–0.78) |
| P-value vs. MELD | 0.16 | 0.09 | 0.03 | 0.06 |
| Patients listed for transplant (n=494) | ||||
| MELD | 0.85 (0.81–0.90) | 0.79 (0.73–0.84) | 0.74 (0.69–0.79) | 0.72 (0.67–0.76) |
| MELD-sarcopenia | 0.86 (0.82–0.91) | 0.82 (0.77–0.86) | 0.79 (0.75–0.83) | 0.76 (0.72–0.80) |
| P-value vs. MELD | 0.41 | 0.33 | <0.01 | 0.01 |
| MELD-L3 SMI | 0.87 (0.82–0.91) | 0.81 (0.76–0.87) | 0.77 (0.71–0.82) | 0.74 (0.69–0.78) |
| P-value vs. MELD | 0.41 | 0.20 | 0.13 | 0.12 |
| Patients not listed for transplant (n=175) | ||||
| MELD | 0.81 (0.73–0.89) | 0.78 (0.71–0.85) | 0.74 (0.67–0.80) | 0.70 (0.64–0.76) |
| MELD-sarcopenia | 0.84 (0.78–0.90) | 0.80 (0.74–0.86) | 0.75 (0.69–0.81) | 0.71 (0.66–0.77) |
| P-value vs. MELD | 0.19 | 0.62 | 0.49 | 0.56 |
| MELD-L3 SMI | 0.83 (0.76–0.91) | 0.80 (0.73–0.86) | 0.75 (0.68–0.81) | 0.71 (0.65–0.76) |
| P-value vs. MELD | 0.27 | 0.35 | 0.38 | 0.58 |
| Patients with MELD <15 (n=438) | ||||
| MELD | 0.69 (0.56–0.82) | 0.62 (0.52–0.71) | 0.61 (0.54–0.68) | 0.59 (0.53–0.64) |
| MELD-sarcopenia | 0.85 (0.77–0.92) | 0.73 (0.65–0.81) | 0.70 (0.64–0.76) | 0.67 (0.62–0.71) |
| P-value vs. MELD | 0.02 | 0.02 | 0.04 | 0.02 |
| MELD-L3 SMI | 0.82 (0.68–0.95) | 0.71 (0.61–0.81) | 0.67 (0.60–0.75) | 0.63 (0.57–0.68) |
| P-value vs. MELD | 0.02 | 0.04 | 0.01 | 0.14 |
| Patients with MELD ≥15 (n=231) | ||||
| MELD | 0.71 (0.64–0.77) | 0.69 (0.63–0.75) | 0.68 (0.62–0.74) | 0.67 (0.61–0.72) |
| MELD-sarcopenia | 0.71 (0.64–0.77) | 0.70 (0.64–0.76) | 0.69 (0.64–0.75) | 0.68 (0.63–0.73) |
| P-value vs. MELD | 0.98 | 0.68 | 0.54 | 0.63 |
| MELD-L3 SMI | 0.72 (0.66–0.78) | 0.71 (0.65–0.77) | 0.70 (0.65–0.76) | 0.69 (0.64–0.74) |
| P-value vs. MELD | 0.24 | 0.16 | 0.13 | 0.06 |
HCC, hepatocellular carcinoma; L3-SMI, lumbar third skeletal muscle index; MELD, Model for End-Stage Liver Disease. Numbers in parentheses are 95% confidence intervals.
Finally, the inclusion of L3 SMI as a continuous variable (in MELD-L3 SMI) yielded similar findings in patients with hepatic encephalopathy and MELD<15; however, its general performance was inferior than using sarcopenia as dichotomous variable (MELD-sarcopenia) (Table 4).
DISCUSSION
Our study indicates that sarcopenia is present in almost one-half of patients with cirrhosis evaluated for liver transplantation. As sarcopenia is independently associated with a twofold risk of mortality, the modification of MELD to include sarcopenia (MELD-sarcopenia) is associated with improvement in the prediction of mortality in patients with cirrhosis. The observed benefit of modifying MELD to include sarcopenia was greatest in patients with low MELD scores, who are traditionally deemed to have a low risk of death. The importance of sarcopenia is reflected by the fact that if present it is equivalent to adding 10 points to the MELD score.
Our results are similar to a recent study that showed that evaluation of the transversal psoas muscle thickness at the level of the umbilicus is predictive of mortality in cirrhotic patients independent of MELD and MELDNa. In this study, the authors developed a similar score combining MELD and the transversal psoas muscle thickness divided by height and they found that discrimination of this novel score (referred to as MELD-psoas) was superior to that of MELD. Similar to our study, the score had improved performance in the subgroup of patients with refractory ascites.20 However, to the best of our knowledge, there is actually no evidence confirming that the cross-sectional area of psoas muscles has a good correlation with the whole lumbar or the whole body muscle areas. Moreover, the location of the umbilicus may change owing to ascites, so that measures may be recorded at different levels in these patients. In light of this potential limitation, we used the L3 SMI, which has been shown to be the best single imaging correlate of whole body muscle mass.21 Also, related to our study, previous experience has shown that moderate ascites informed mortality risk prediction in cirrhotic patients awaiting liver transplantation, particularly in patients with low MELD scores (<21). Therefore, the presence of moderate ascites should prompt clinicians to consider strategies to expand access to transplantation.22
As current scores to evaluate prognosis in patients with cirrhosis need appropriate modifications, previous studies have evaluated the importance of other biochemical parameters. For example, serum sodium has been shown to be an independent risk factor for mortality in patients with cirrhosis,4, 23 and several studies have reported that the addition of the serum sodium concentration to generate the MELDNa score is more accurate than MELD for predicting short-term mortality on the waiting list.4, 24, 25 Kim and colleagues reported that the use of MELDNa has the potential to prevent a significant proportion of deaths that occur within 90 days of waiting list registration. However, serum sodium is highly variable in cirrhotic patients prescribed diuretics, and in our study, sodium was not significantly associated with mortality in the multivariate analysis after adjustment for sarcopenia and other potential confounders (Table 3).
Additional studies have reported a negative prognostic impact of hypoalbuminemia among liver transplant candidates after adjusting for the MELD score and serum sodium concentration. In these studies, the incorporation of serum albumin in the MELDNa score to generate the five-variable MELD (5vMELD) improved the prediction of short-term mortality compared with MELD and MELDNa among patients awaiting liver transplantation.5, 26 This finding may reflect the fact that albumin is an indirect measure of the nutritional status of patients with cirrhosis. In our study, albumin was independently associated with mortality in the multivariate analysis; however, the statistical weight of sarcopenia was greater (Table 3).
At present, several methods are available to evaluate body composition and estimate muscle mass in patients with cirrhosis; however, most of these techniques have limitations primarily owing to subjectivity and limited reproducibility. In this regard, muscularity assessment based on cross-sectional CT imaging has emerged as an attractive index of nutritional status in patients with cirrhosis particularly owing to its objective nature.8, 9 These CT analyses are not influenced by fluid overload or overweight/obesity that are frequently present in patients with decompensated cirrhosis. Moreover, sarcopenia reflects a chronic detriment in the general physical condition, rather than acute severity of liver disease.27
This study emphasizes that, despite the fact that sarcopenia is not included in conventional scores for prognosis in patients with cirrhosis, its presence should alert clinicians to the same extent as other complications, such as ascites, hepatic encephalopathy, and variceal hemorrhage.28, 29
Importantly, there is growing evidence that extreme sarcopenia using different operational definitions, such as the lowest quartile of the total psoas area,12 lowest tertile of the total psoas area,11 lowest sixtile of the L3 SMI,30 or low skeletal muscle mass defined as <90% of the standard using bioelectrical impedance analysis,10 is associated with posttransplant mortality. Therefore, giving some priority to those patients with sarcopenia before they develop extreme muscle depletion may help to decrease mortality in a subgroup of patients with cirrhosis, without having a negative impact on survival postliver transplantation.28
In this study, we find a better performance MELD-sarcopenia at 1 year, rather than at 3 and 6 months. We suspect this is due to the relatively small size of our cohort and small number of events during the early follow-up period. Moreover, we did not find a significant improvement in performance of the MELD-sarcopenia score in patients with severe hepatic encephalopathy despite the fact that sarcopenia is associated with an increased risk of hepatic encephalopathy,31 and this complication increases the risk of mortality, even more than the development of ascites or variceal hemorrhage.32 This finding may be due to the fact that only 9% of our patients had severe hepatic encephalopathy or that these patients are too ill for sarcopenia to have an additive benefit on mortality prediction.
The inclusion of L3 SMI as a continuous variable (in MELD-L3 SMI) yielded inferior performance compared with MELD-sarcopenia. We hypothesize that this is related to our use of sarcopenia as a dichotomous variable according to gender and height, which gives more statistical strength to this variable.
One limitation of our study is that we used a definition of sarcopenia based on cutoff values that have been validated in populations with different malignancies rather than cirrhotic patients. Nevertheless, the values we used were derived from optimum stratification of the L3 SMI, finding the most significant P-values to define gender-specific cut points associated with mortality.19 Moreover, in previous studies in patients with cirrhosis, we have demonstrated that these values are useful to distinguish cirrhotic patients in whom sarcopenia is associated with a higher risk of mortality,8, 9 and that these cut points are very similar to a preliminary experience.33 Nevertheless, validation of sarcopenia values in cirrhosis is warranted.
In summary, sarcopenia is frequently present in patients with cirrhosis undergoing evaluation for liver transplantation and is independently associated with a higher risk of mortality. Modification of MELD to include sarcopenia (MELD-sarcopenia) is associated with an improvement in the prediction of mortality in patients with cirrhosis, mostly in those with low MELD scores. Additional validation in larger cohorts of patients with cirrhosis is necessary to corroborate our findings prior to widespread adoption of this novel score for liver allograft allocation. Moreover, it would be important to determine to what extent sarcopenia adversely affects survival following liver transplantation.
Study Highlights
APPENDIX
FORMULAS FOR PROGNOSTIC INDICES
MELD-Sarcopenia=MELD+10.35 × Sarcopenia
MELD-L3 SMI=MELD−0.3065 × L3 SMI
Guarantor of the article: Aldo J. Montano-Loza, MD, MSc, PhD.
Specific author contributions: Aldo J. Montano-Loza: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; study supervision. Andres Duarte-Rojo: analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Judith Meza-Junco: analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Vickie E. Baracos: analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Michael B. Sawyer: analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Jack X.Q. Pang: analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Crystal Beaumont: computed tomography analysis, critical revision of the manuscript for important intellectual content. Nina Esfandiari: computed tomography analysis, critical revision of the manuscript for important intellectual content. Robert P. Myers: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; study supervision.
Financial support: This study has been funded with a Clinical Research Award from the American College of Gastroenterology Institute, 2011.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Presented in part at the Annual Liver Meeting of the American Association for the Study of Liver Diseases, 4 November 2013, Washington, DC, USA.
Supplementary Material
References
- Freeman RB Jr, Wiesner RH, Harper A et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl 2002; 8: 851–858. [DOI] [PubMed] [Google Scholar]
- Wiesner R, Edwards E, Freeman R et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003; 124: 91–96. [DOI] [PubMed] [Google Scholar]
- Brown RS Jr, Lake JR. The survival impact of liver transplantation in the MELD era, and the future for organ allocation and distribution. Am J Transplant 2005; 5: 203–204. [DOI] [PubMed] [Google Scholar]
- Kim WR, Biggins SW, Kremers WK et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008; 359: 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RP, Shaheen AA, Faris P et al. Revision of MELD to include serum albumin improves prediction of mortality on the liver transplant waiting list. PLoS One 2013; 8: e51926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755–763. [DOI] [PubMed] [Google Scholar]
- Lang T, Streeper T, Cawthon P et al. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 2010; 21: 543–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano-Loza AJ, Meza-Junco J, Prado CM et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012; 10: 166–173 173 e1. [DOI] [PubMed] [Google Scholar]
- Meza-Junco J, Montano-Loza AJ, Baracos VE et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013; 47: 861–870. [DOI] [PubMed] [Google Scholar]
- Kaido T, Ogawa K, Fujimoto Y et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant 2013; 13: 1549–1556. [DOI] [PubMed] [Google Scholar]
- Krell RW, Kaul DR, Martin AR et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl 2013; 19: 1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesbe MJ, Patel SP, He K et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010; 211: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TM, Overgard EB, Cohen AE et al. Nutrition assessment and management in advanced liver disease. Nutr Clin Pract 2013; 28: 15–29. [DOI] [PubMed] [Google Scholar]
- O'Brien A, Williams R. Nutrition in end-stage liver disease: principles and practice. Gastroenterology 2008; 134: 1729–1740. [DOI] [PubMed] [Google Scholar]
- Mitsiopoulos N, Baumgartner RN, Heymsfield SB et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1998; 85: 115–122. [DOI] [PubMed] [Google Scholar]
- Kvist H, Sjostrom L, Tylen U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes 1986; 10: 53–67. [PubMed] [Google Scholar]
- Vehmas T, Kairemo KJ, Taavitsainen MJ. Measuring visceral adipose tissue content from contrast enhanced computed tomography. Int J Obes Relat Metab Disord 1996; 20: 570–573. [PubMed] [Google Scholar]
- Mourtzakis M, Prado CM, Lieffers JR et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008; 33: 997–1006. [DOI] [PubMed] [Google Scholar]
- Martin L, Birdsell L, Macdonald N et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013; 31: 1539–1547. [DOI] [PubMed] [Google Scholar]
- Durand F, Buyse S, Francoz C et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol 2014; 60: 1151–1157. [DOI] [PubMed] [Google Scholar]
- Shen W, Punyanitya M, Wang Z et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004; 97: 2333–2338. [DOI] [PubMed] [Google Scholar]
- Somsouk M, Kornfield R, Vittinghoff E et al. Moderate ascites identifies patients with low model for end-stage liver disease scores awaiting liver transplantation who have a high mortality risk. Liver Transpl 2011; 17: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinchoc M, Kamath PS, Gordon FD et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000; 31: 864–871. [DOI] [PubMed] [Google Scholar]
- Biggins SW, Kim WR, Terrault NA et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006; 130: 1652–1660. [DOI] [PubMed] [Google Scholar]
- Ruf AE, Kremers WK, Chavez LL et al. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl 2005; 11: 336–343. [DOI] [PubMed] [Google Scholar]
- Myers RP, Tandon P, Ney M et al. Validation of the five-variable Model for End-stage Liver Disease (5vMELD) for prediction of mortality on the liver transplant waiting list. Liver Int 2014; 34: 1176–1183. [DOI] [PubMed] [Google Scholar]
- Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle 2012; 3: 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano-Loza AJ. Muscle wasting: a nutritional criterion to prioritize patients for liver transplantation. Curr Opin Clin Nutr Metab Care 2014; 17: 219–225. [DOI] [PubMed] [Google Scholar]
- Montano-Loza AJ. New concepts in liver cirrhosis: clinical significance of sarcopenia in cirrhotic patients. Minerva Gastroenterol Dietol 2013; 59: 173–186. [PubMed] [Google Scholar]
- Montano-Loza AJ, Meza-Junco J, Baracos VE et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl 2014; 20: 640–648. [DOI] [PubMed] [Google Scholar]
- Merli M, Giusto M, Lucidi C et al. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis 2012; 28: 281–284. [DOI] [PubMed] [Google Scholar]
- Jepsen P, Ott P, Andersen PK et al. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010; 51: 1675–1682. [DOI] [PubMed] [Google Scholar]
- Montano-Loza AJ, Meza-Junco J, Prado CMM et al. New cutoff values for sarcopenia for predicting 6-months mortality in cirrhotic patients. J Hepatol 2013; 58: S95. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.