Abstract
The incidence of Clostridium difficile infection (CDI) has been rising in hospitals, long-term care facilities, and within the community. Cases have been more severe with more complications, deaths, and higher healthcare-associated costs. With the emergence of a hypervirulent strain of C. difficile and the increasing prevalence of community-acquired CDI among healthy patients without traditional risk factors, the epidemiology of C. difficile has been evolving. This changing epidemiology requires a change in management. Taking into account new risk factors for CDI and growing subpopulations of affected individuals, diagnostic, treatment, and prevention approaches need to be adjusted.
Introduction
The incidence of Clostridium difficile has been rising steadily over the past two decades with subsequent increases in mortality, prolonged hospital stays with higher level care, and a substantial rise in healthcare costs.1, 2 In fact, not only is C. difficile infection (CDI) the most common cause of hospital-acquired diarrhea, it has replaced methicillin-resistant Staphylococcus aureus as the most common hospital-acquired infection overall.2, 3, 4
C. difficile is a gram-positive, rod-shaped, spore-forming anaerobe with the ability to produce enterotoxin A and cytotoxin B (linked to the tcdA and tcdB genes, respectively), which are responsible for its virulence. Toxin B activates monocyte cytotoxin release and is significantly more potent than enterotoxin A, which causes local tissue injury.5 C. difficile is known to cause disease through release of these two toxins. Clinically, there is a wide spectrum of C. difficile presentations ranging from asymptomatic carriage to severe, life threatening, fulminant colitis, and toxic megacolon.6, 7, 8
Traditionally, C. difficile strains unable to produce one or both of these toxins were considered as non-pathogenic; however, there is now evidence that some strains may also produce a binary toxin transcribed from the ctdA and ctdB genes.9 The significance of this binary toxin on the pathogenicity of C. difficile is unknown. The BI/NAP1/027 strain produces this toxin as well as markedly increased levels of Toxin A and Toxin B, which may contribute to its hypervirulence.10 This new development may explain the rising incidence and severity of CDI.
Changing Epidemiology
While C. difficile has been linked to disease since at least the 1970s, it was not until 2000 that the incidence and severity of CDI has shown a steady rise.4 This has continued to the current time.
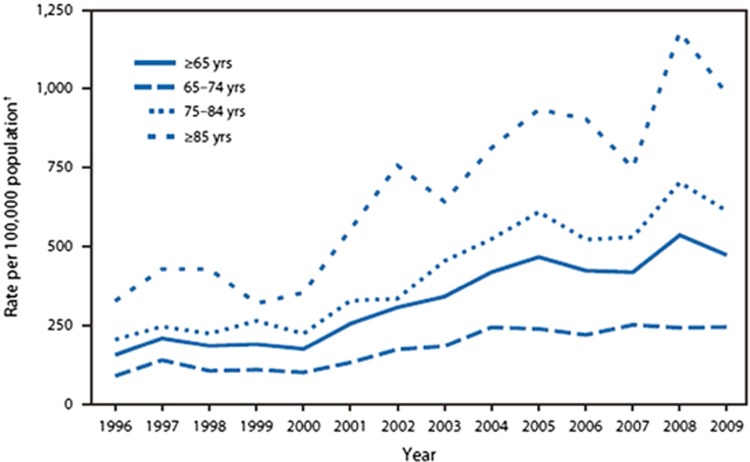
The greatest increase in cases has been seen in hospital settings. From the 1990s to 2000, CDI rates remained relatively stable across all age groups as shown in Figure 1. Since then, the incidence of CDI has increased from approximately 30–40 cases per 100,000 population in the mid 1990s to 84 cases per 100,000 population in 2005.4 McDonald et al. describe an increase in CDI from 264,000 cases in 1996 to 978,000 cases in 2003.4 Similarly, the US Agency for Healthcare Research and Quality's Healthcare Cost and Utilization Project identifies 24,200 hospital stays for patients with a principal CDI diagnosis (85,700 for CDI among all-listed diagnoses) in 1993 compared with 110,600 hospital stays in 2009 for a primary CDI diagnosis (336,600 for CDI among all-listed diagnoses).11 Although this database is a limited, nationwide sample of inpatient hospital stays, it illustrates a four-fold increase in the number of hospitalizations over a 16-year time period. CDI was associated with at least 1% of all hospital stays in 2009.11
Figure 1.
Rates of Clostridium difficile infection among hospitalized patients aged ≥65 years, by age group—National Hospital Discharge Survey, United States, 1996–2009 (from Centers for Disease Control and Prevention. Morb Mortal Wkly Rep (MMWR) 2011;60(34):1171.
Using patient discharge data, similar trends are seen. Nationally, among adult, non-maternal hospital discharges, there were 7.4 per 1,000 hospitalizations for CDI in 2003 compared with 13.5 per 1,000 in 2012.12 Surveillance efforts have also demonstrated increased morbidity and mortality among CDI patients. Death certificates with enterocolitis due to C. difficile as the primary cause of death increased from 793 in 1999 to 7,483 in 2008; the age-adjusted death rate rose 15%.13 Similar trends in CDI incidence have been seen in Canada and throughout Europe.14, 15, 16
Changing Populations at Risk
Several subpopulations are disproportionately affected by C. difficile and have higher associated morbidity and mortality. These include the elderly, immunocompromised individuals (including patients receiving chemotherapy or following solid organ transplantation), patients with inflammatory bowel disease (IBD), and patients with significant co-morbidities beyond CDI. Table 1 lists the various risk factors for CDI.
Table 1. Risk factors for Clostridium difficile infection.
| Antibiotic exposure |
| Older age (65 or older) |
| Prior, lengthy hospitalization or long-term care facility exposure |
| Comorbidities: malignancy, cystic fibrosis, inflammatory bowel disease, diabetes mellitus, cirrhosis, chronic kidney disease, immunodeficiency, solid organ or hematopoietic stem cell transplantation |
| Other medication exposure: chemotherapy, immunosuppressants, proton pump inhibitors |
| Prior gastrointestinal surgery |
| Consumption of processed meats |
| Presence of gastrostomy or jejunostomy tube |
While the elderly have always been at increased risk of developing CDI, much of the rise in hospital-acquired cases has been seen in patients over the age of 65. In fact, the rate of CDI discharge diagnoses was seven-fold higher in patients ≥65 years compared with patients aged 45–64 years (P<0.001).4 Patients with CDI were nearly 20 years older (67.9 years vs. 48.1 years) and patients ≥85 years had the highest rate (1,089 per 100,000 population), compared with only 11 per 100,000 for patients under 18 years old.11 In 2008, C. difficile ranked as the 18th leading cause of death among persons aged ≥65; 93% of C. difficile-associated deaths occurred in persons aged ≥65.13 As the US population continues to age, it is not unexpected that CDI rates among persons ≥65 years old will continue to rise.4, 17 Moreover, comparing 2000 with 2003, a greater number of hospitalized patients with a CDI discharge diagnosis were transferred to a long-term care facility.4 Various potential explanations for this rise among the elderly have been proposed, including residence at long-term care facilities, increased use of antibiotics and proton pump inhibitors, and decreased host defenses.4, 18
Patients with IBD are also at increased risk of developing CDI. In this population, CDI prevalence was 1.7% compared with 0.4% of the general population. Among IBD patients, those with ulcerative colitis had a higher CDI incidence (2.8%) compared with Crohn's disease patients (1.0%).19 Therefore, any IBD patient presenting with flare symptoms should be tested for C. difficile toxin to rule out disease. It remains unclear whether this higher CDI prevalence is related to increased antibiotic exposure, more frequent contact with healthcare settings, or an immunocompromised state.2
Multiple theories have been proposed to explain the overall rise in CDI—hospitalized patients are often older and sicker, there is increased use of broader spectrum antibiotics (e.g., fluoroquinolones resulting in resistance patterns), and inadequate prevention measures in healthcare settings.2, 4, 17, 18 It is not known if the widespread use of waterless, alcohol-based hand gels contributes to the nosocomial spread of C. difficile, although several studies have shown no increase in CDI with increased use of these hand gels in lieu of handwashing.20, 21 There is also increased testing among patients who present with diarrhea.
Emergence of Community Strains
Despite the rise in CDI-related hospitalizations worldwide, the population affected appears to have evolved since 2000. Historically, CDI has been associated with antibiotic use, although there is a growing number of patients who present with diarrheal symptoms and are diagnosed with CDI without any prior antimicrobial exposure.22, 23 These are frequently healthy adults without significant medical comorbidity. Therefore, it has become reality that prior antibiotic exposure can no longer be assumed. Furthermore, it is hypothesized that there is an emergence of community strains of C. difficile. To better characterize CDI by exposure setting and timing, disease classifications have been established as described in Table 2.5, 24
Table 2. CDI classification and symptom onset.
| Disease classification | Symptom onset |
|---|---|
| HCF onset, HCF-associated CDI | >48 h after admission but before discharge from an HCF |
| Community-associated CDI | Community onset or within 48 h of HCF admission, if symptom onset was >12 weeks after last HCF discharge |
| Community onset, HCF-associated CDI | Community onset or within 48 h of HCF admission, if symptom onset was ≤4 weeks after last HCF discharge |
| Indeterminate CDI | Does not meet criteria for above disease classifications |
| Unknown CDI | Lack of available data; unable to classify |
Although CDI-related discharges started to stabilize in 2009, overall CDI rates have continued to rise.11 This finding is mostly attributable to community acquisition diagnosed in outpatient care settings. It is estimated that community-acquired CDI may now account for up to 40% of CDI cases.3 These patients tend to be younger and healthier without recent hospitalization, antibiotic exposure or co-morbidities.3, 25
The mode of transmission within the community remains unclear. Exposure to C. difficile is through the fecal–oral route followed by an incubation period that may lead to infection, colonization, or complete clearance. Otten et al. suggest that exposure does not necessarily dictate disease—some persons become colonized, others develop clinical disease, some may develop disease with clearance, and some persons may have relapse.26 The determinants of which category a person falls into are unclear. For those persons who develop disease, infection may be cleared as vegetative cells and spores are eradicated, but infection may recur.
There are four proposed sources of C. difficile exposure within the community—person–person contact, animal–person contact, food contamination, and environment–person contact.26 Since infected persons shed C. difficile, person–person transmission is the most reasonable hypothesis for community-level acquisition; this mode of transmission has been clearly demonstrated in hospital settings.27 Situations have also been identified by which employees carry spores home exposing family members to C. difficile (e.g., healthcare workers, daycare centers, and veterinary clinics). Animals also shed C. difficile, including dogs, cats, horses, and calves, which should be considered as a potential mode of transmission in agricultural settings.26 Various studies have identified C. difficile in food products, including ground meat and water, although there is no clear evidence of disease transmission.28, 29, 30 Finally, environmental surfaces may become contaminated which is a common concern in healthcare settings, but extends to the community.26, 27 This lack of a clear understanding of how community-acquired CDI is transmitted illustrates the need for a multidisciplinary approach including improved home cleaning practices and hand hygiene at the hospital and community level; the impact on food safety practices and animal exposure is less defined.
A major factor in the development of community-acquired CDI was the identification of the hypervirulent, toxigenic strain, BI/NAP1/O27.5 Spiking between 2002 and 2006 as an international epidemic, this new strain has resulted in more severe clinical presentations requiring longer hospitalizations and resultant rises in healthcare costs. In particular, this strain has been linked to severe cases of CDI, especially among the elderly. This is likely related to 16 times greater toxin A production and 23 times greater toxin B production with high rates of fluoroquinolone resistance.31 The strain was initially identified following outbreaks in Canada, the United States, and Europe.32, 33 Since then, there have been other strains, such as PCR ribotypes 001, 018, 078, and 106 which have had associated outbreaks.34
There has also been a rise of proton pump inhibitors therapy among outpatients. There is increasing epidemiologic evidence that the long-term use of proton pump inhibitors may increase the risk of CDI, but perhaps not recurrent CDI (RCDI).35, 36 The mechanisms for this are not known, but may be related to a higher gastric pH decreasing colonization resistance.34
Changing Management
While the C. difficile organism and the populations it targets have both been evolving over the past 20 years, science has not stalled. There has been the development of new diagnostic testing strategies for C. difficile. In the 1990s, immunoassays were the primary diagnostic modality; however, current testing approaches have been modified to include more sensitive and specific molecular tests, such as polymerase chain reaction (PCR) that allows for earlier detection and more rapid treatment. This nucleic acid amplification test is more sensitive than toxin enzyme immunoassay tests and C. difficile culture and more specific than glutamate dehydrogenase testing; it is also widely available and has become the test of choice for rapid diagnosis of CDI.1 Despite the increased cost of PCR compared with immunoassays, it is a more cost-effective testing choice as it offers earlier detection, increased accuracy, and allows for more rapid treatment; it also allows for isolation of CDI patients and prevents further spread of infection.6 Despite this, one challenge with PCR is the risk of overdiagnosis, particularly in asymptomatic carriers who may be less likely to spread spores and contribute to the infection burden.37, 38, 39, 40 Additionally, this improved, highly sensitive, and specific test may explain some of the increased identification of cases and explain the rise in CDI prevalence. Since widespread use of C. difficile PCR may also pick up asymptomatic carriers, testing is only indicated in symptomatic patients.
As CDI has emerged as a common hospital-acquired infection and has become increasingly more prevalent in the community, the approach to reduction of risk factors and management has become more challenging requiring more creative solutions.
Traditionally, metronidazole and vancomycin have been the mainstays of CDI treatment with the newer antibiotic, fidaxomicin, as an alternative. Patients treated with fidaxomicin had lower recurrence rates of CDI compared with vancomycin in clinical trials; however, its use has been limited somewhat by its high cost. Treatment regimens are often dictated by disease severity as detailed in Table 3.1
Table 3. Treatment for C. difficile infection.
| CDI | Clinical presentation | Regimen | Considerations |
|---|---|---|---|
| Mild–moderate | Diarrhea No signs of severe or complicated disease | Metronidazole 500 mg p.o. t.i.d. × 10 daysa Alternative: Fidaxomicin 200 mg p.o. b.i.d. × 10 days | Avoid in pregnancy or with breast-feeding. Change to vancomycin if no response within 72 h |
| Severe | Albumin <3 g/dl Abdominal distention WBC >15,000 | Vancomycin 125 mg p.o. q.i.d. × 10 daysa | Increase to 250–500 mg p.o. q.i.d. if poor response |
| Complicated | Fever Significant leukocytosis (>35,000) or leukopenia <2,000) Hypoalbuminemia Abdominal distention +/− ileus +/− shock with hypotension or evidence of end-organ failure +/− elevation in serum lactate (>2.2 mmol/l) or C-reactive protein | Metronidazole 500 mg IV t.i.d. and vancomycin 500 mg p.o. q.i.d. +/− vancomycin enemas 500 mg p.r. q.i.d. | Includes patients with ileus, recent abdominal surgery, or inability to take p.o. These patients should be admitted to the intensive care unit. Consider surgery consult to consider surgical approaches to treatment |
| Recurrent | CDI episode within 8 weeks of previous episode | 1st recurrence: repeat metronidazole, vancomycin, or fidaxomicin 2nd recurrence: pulsed vancomycin regimen: 125 mg q.i.d. × 10 days, then 125 mg daily every 3 days × 10 doses | Consider FMT after 3 recurrences |
CDI, Clostridium difficile infection; FMT, fecal microbiota transplantation; IV, intravenous; RCT, randomized control trial; WBC, white blood cell. Adapted from refs. 1 and 6.
Recent guidelines recommend a 10-day course of therapy for treatment of mild-to-moderate CDI; this is also the length of therapy in the RCT of these drugs.1 If the patient is not clinically improved, then therapy can be extended or changed.
Despite effective treatment options for primary CDI, recurrence rates are 10–20%, with rates up to 40–65% after a first recurrence.41 With recurrence, treatment is often more challenging and clinical presentations may be more severe. A first recurrence can be treated with the same initial antibiotic (i.e., vancomycin, metronidazole, or fidaxomicin), unless the recurrence is severe in which case vancomycin is indicated. There is interest in fidaxomicin as a treatment for RCDI since recurrence rates for primary CDI were lower than for vancomycin, but the drug has not been studied for RCDI specifically. Other regimens include the use of the adjunct probiotic, Saccharomyces boulardii and intravenous immune globulin.42, 43 There is ongoing research into a monoclonal antibody to toxins A and B to treat recurrences and several vaccines are in development as well.44, 45, 46
Furthermore, as research into the intestinal microbiome continues to progress, we are learning more about the complex, interdependent ecosystem responsible for food digestion, immune system activation, and protection from invasive bacteria, also known as colonization resistance.47 Research on the microbiome continues to grow, particularly over the past several years. C. difficile is a prime example where altered microbiota contribute to disease; therefore, strategies to restore the intestinal microbiome may be effective as a treatment modality. Given the high rates of CDI recurrence and lack of uniformly successful therapy, there has been growing interest in fecal microbiota transplantation (FMT). FMT is a treatment that directly alters—and restores—the gut microbiome. FMT is the delivery of stool from a healthy donor into the colon of a patient with RCDI, either by the upper gastrointestinal route, such as nasoduodenal infusion or into the colon by enema or colonoscopy. Meta-analyses of the hundreds of published cases and series show an efficacy of 90% and a landmark randomized control trial showed efficacy in RCDI patients given stool by the nasoduodenal route compared with vancomycin therapy; a more recent randomized control trial also shows colonoscopic infusion to be more effective than vancomycin.48, 49, 50, 51 FMT with colonoscopic delivery has been demonstrated to be a cost-effective intervention for RCDI compared with oral vancomycin.52
While treatment of CDI is an important area for ongoing research, prevention efforts also need to be enhanced to counteract the growing epidemic, both for the individual patient and in interrupting transmission.
There is interest in the role of probiotics for prevention. While there is good evidence for two probiotics that prevent antibiotic-associated diarrhea (Lactobacillus rhamnosus GG and S. boulardii), there is less evidence that they prevent CDI.53 A meta-analysis concluded that there was moderate efficacy for probiotics in prevention of CDI, but this combined studies of many different probiotics or mixtures of probiotics in varied clinical settings.54 Moreover, a large randomized control trial of Lactobacillus spp. and Bifidobacter spp. in elderly inpatients showed no efficacy in the prevention of CDI.55 Probiotics have a good safety profile (although they should not be given to immune compromised patients for risk of bloodstream infections), but the data at this time are insufficient to recommend a specific product and which patient populations might benefit the most.
In healthcare settings, prevention measures include private hospital rooms for CDI patients, dedicated hospital equipment (e.g., stethoscopes), personal protective equipment (e.g., gowns and gloves), and availability of sinks with soap for handwashing or alcohol hand gels. There also needs to be thoughtful use of antibiotics to prevent development of resistance patterns.
Although preventive efforts have traditionally been a focus in hospital settings with comprehensive environmental cleaning with sodium hypochlorite (bleach), this thinking should be expanded to community settings.56 Affected patients should disinfect their home with a water/bleach (9:1 ratio of water to bleach) combination to ensure eradication of C. difficile spores and to help limit community transmission.
C. difficile infection prevalence is increasing for various reasons as discussed above. As a result, we need to refocus our prevention and management strategies. The future is promising, however, with ongoing research and development of better testing modalities, more effective medications, novel therapies including monoclonal antibodies and vaccines, and success with old therapies, such as FMT. We have implemented effective prevention strategies in the hospital setting to limit the spread of infection. As community-acquired CDI has emerged, we need to extend these strategies to the household.
Study Highlights

References
- Surawicz CM, Brandt LJ, Binion DG et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108: 478–498. [DOI] [PubMed] [Google Scholar]
- Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol 2010; 4: 409–416. [DOI] [PubMed] [Google Scholar]
- Khanna S, Pardi DS, Aronson SL et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol 2012; 107: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis 2006; 12: 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KB, Napolitano LM. Clostridium difficile infection: update on diagnosis, epidemiology, and treatment strategies. Surg Infect (Larchmt) 2014; 15: 490–502. [DOI] [PubMed] [Google Scholar]
- Surawicz CM. Clostridium difficile infection: risk factors, diagnosis and management. Curr Treat Options Gastroenterol 2015; 13: 121–129. [DOI] [PubMed] [Google Scholar]
- Bartlett JG, Chang TW, Gurwith M et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med 1978; 298: 531–534. [DOI] [PubMed] [Google Scholar]
- Bartlett JG. Historical perspectives on studies of Clostridium difficile and C. difficile infection. Clin Infect Dis 2008; 46 Suppl 1: S4–S11. [DOI] [PubMed] [Google Scholar]
- Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis 2012; 55 (Suppl 2): S65–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136: 1913–1924. [DOI] [PubMed] [Google Scholar]
- Lucado J, Gould C, Elixhauser A. Clostridium difficile Infections (CDI) in Hospital Stays, 2009: Agency for Healthcare Research and Quality; 2012, cited 19 January 2015. Available from http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf. [PubMed]
- Steiner C, Barrett M, Sun Y, Weiss A. HCUP Projections: Clostridium difficile Hospitalizations 2003-2014: US Agency for Healthcare Research and Quality; 2014. HCUP Projections Report #2014-03. Available from http://www.hcup-us.ahrq.gov/reports/projections/2014-03.pdf.
- Minino A, Xu J, Kochanek K. Deaths: Preliminary Data for 2008 Hyattsville. National Center for Health Statistics: MD, USA, 2010; 59 Available from http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_02.pdf. [PubMed] [Google Scholar]
- Pépin J, Valiquette L, Alary ME et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ 2004; 171: 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio A, Vaque-Rafart J, Calbo-Torrecillas F et al. Increasing rates in Clostridium difficile infection (CDI) among hospitalised patients, Spain 1999-2007. Euro Surveill 2008;13:pii:18943. [PubMed] [Google Scholar]
- Burckhardt F, Friedrich A, Beier D et al. Clostridium difficile surveillance trends, Saxony, Germany. Emerg Infect Dis 2008; 14: 691–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Pardi DS. Clostridium difficile infection: new insights into management. Mayo Clin Proc 2012; 87: 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007; 102: 2047–2056. [DOI] [PubMed] [Google Scholar]
- Ricciardi R, Ogilvie JW, Roberts PL et al. Epidemiology of Clostridium difficile colitis in hospitalized patients with inflammatory bowel diseases. Dis Colon Rectum 2009; 52: 40–45. [DOI] [PubMed] [Google Scholar]
- Boyce JM, Ligi C, Kohan C et al. Lack of association between the increased incidence of Clostridium difficile-associated disease and the increasing use of alcohol-based hand rubs. Infect Control Hosp Epidemiol 2006; 27: 479–483. [DOI] [PubMed] [Google Scholar]
- Gordin FM, Schultz ME, Huber RA et al. Reduction in nosocomial transmission of drug-resistant bacteria after introduction of an alcohol-based handrub. Infect Control Hosp Epidemiol 2005; 26: 650–653. [DOI] [PubMed] [Google Scholar]
- Taori SK, Wroe A, Hardie A et al. A prospective study of community-associated Clostridium difficile infections: the role of antibiotics and co-infections. J Infect 2014; 69: 134–144. [DOI] [PubMed] [Google Scholar]
- Naggie S, Frederick J, Pien BC et al. Community-associated Clostridium difficile infection: experience of a Veteran Affairs Medical Center in southeastern USA. Infection 2010; 38: 297–300. [DOI] [PubMed] [Google Scholar]
- McDonald LC, Coignard B, Dubberke E et al. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol 2007; 28: 140–145. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Severe Clostridium difficile-associated disease in populations previously at low risk—four states, 2005. Morb Mortal Wkly Rep 2005; 54: 1201–1205. [PubMed] [Google Scholar]
- Otten AM, Reid-Smith RJ, Fazil A, Weese JS. Disease transmission model for community-associated Clostridium difficile infection. Epidemiol Infect 2010; 138: 907–914. [DOI] [PubMed] [Google Scholar]
- Kim KH, Fekety R, Batts DH et al. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. J Infect Dis 1981; 143: 42–50. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Palacios A, Staempfli HR, Duffield T et al. Clostridium difficile in retail ground meat, Canada. Emerg Infect Dis 2007; 13: 485–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DG, Rodriguez-Palacios A. Transmission of Clostridium difficile in foods. Infect Dis Clin North Am 2013; 27: 675–685. [DOI] [PubMed] [Google Scholar]
- Songer JG, Trinh HT, Killgore GE et al. Clostridium difficile in retail meat products, USA, 2007. Emerg Infect Dis 2009; 15: 819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambol SP, Merrigan MM, Lyerly D et al. Toxin gene analysis of a variant strain of Clostridium difficile that causes human clinical disease. Infect Immun 2000; 68: 5480–5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessa FC. Community-associated Clostridium difficile infection: how real is it? Anaerobe 2013; 24: 121–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo VG, Poirier L, Miller MA et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005; 353: 2442–2449. [DOI] [PubMed] [Google Scholar]
- Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol 2011; 37: 178–187. [DOI] [PubMed] [Google Scholar]
- Dial S, Delaney JA, Barkun AN et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005; 294: 2989–2995. [DOI] [PubMed] [Google Scholar]
- Khanna S, Aronson SL, Kammer PP et al. Gastric acid suppression and outcomes in Clostridium difficile infection: a population-based study. Mayo Clin Proc 2012; 87: 636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donskey CJ, Sunkesula VC, Jencson AL et al. Utility of a commercial PCR assay and a clinical prediction rule for detection of toxigenic Clostridium difficile in asymptomatic carriers. J Clin Microbiol 2014; 52: 315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 2015; 313: 398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox MH. Overcoming barriers to effective recognition and diagnosis of Clostridium difficile infection. Clin Microbiol Infect 2012; 18 Suppl 6: 13–20. [DOI] [PubMed] [Google Scholar]
- Su WY, Mercer J, Van Hal SJ et al. Clostridium difficile testing: have we got it right? J Clin Microbiol 2013; 51: 377–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland LV, Surawicz CM, Rubin M et al. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol 1999; 20: 43–50. [DOI] [PubMed] [Google Scholar]
- Surawicz CM, McFarland LV, Greenberg RN et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis 2000; 31: 1012–1017. [DOI] [PubMed] [Google Scholar]
- O'Horo J, Safdar N. The role of immunoglobulin for the treatment of Clostridium difficile infection: a systematic review. Int J Infect Dis 2009; 13: 663–667. [DOI] [PubMed] [Google Scholar]
- Lowy I, Molrine DC, Leav BA et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010; 362: 197–205. [DOI] [PubMed] [Google Scholar]
- Karczewski J, Zorman J, Wang S et al. Development of a recombinant toxin fragment vaccine for Clostridium difficile infection. Vaccine 2014; 32: 2812–2818. [DOI] [PubMed] [Google Scholar]
- Foglia G, Shah S, Luxemburger C et al. Clostridium difficile: development of a novel candidate vaccine. Vaccine 2012; 30: 4307–4309. [DOI] [PubMed] [Google Scholar]
- Vindigni SM, Broussard EK, Surawicz CM. Alteration of the intestinal microbiome: fecal microbiota transplant and probiotics for Clostridium difficile and beyond. Expert Rev Gastroenterol Hepatol 2013; 7: 615–628. [DOI] [PubMed] [Google Scholar]
- van Nood E, Vrieze A, Nieuwdorp M et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368: 407–415. [DOI] [PubMed] [Google Scholar]
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011;53:994–1002. [DOI] [PubMed] [Google Scholar]
- Kassam Z, Hundal R, Marshall JK et al. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med 2012; 172: 191–193. [DOI] [PubMed] [Google Scholar]
- Cammarota G, Masucci L, Ianiro G et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther 2015; 41: 835–843. [DOI] [PubMed] [Google Scholar]
- Konijeti GG, Sauk J, Shrime MG et al. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis. Clin Infect Dis 2014; 58: 1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surawicz CM. Probiotics, antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in humans. Best Pract Res Clin Gastroenterol 2003; 17: 775–783. [DOI] [PubMed] [Google Scholar]
- Johnston BC, Ma SS, Goldenberg JZ et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med 2012; 157: 878–888. [DOI] [PubMed] [Google Scholar]
- Allen SJ, Wareham K, Wang D et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2013; 382: 1249–1257. [DOI] [PubMed] [Google Scholar]
- Hacek DM, Ogle AM, Fisher A et al. Significant impact of terminal room cleaning with bleach on reducing nosocomial Clostridium difficile. Am J Infect Control 2010; 38: 350–353. [DOI] [PubMed] [Google Scholar]