Abstract
Obstructive sleep apnea (OSA) is known to be independently associated with several cardiovascular diseases including hypertension, myocardial infarction, and stroke. To determine how OSA can increase cardiovascular risk, animal models have been developed to explore the underlying mechanisms and the cellular and end-organ targets of the predominant pathophysiological disturbance in OSA–intermittent hypoxia. Despite several limitations in translating data from animal models to the clinical arena, significant progress has been made in our understanding of how OSA confers increased cardiovascular risk. It is clear now that the hypoxic stress associated with OSA can elicit a broad spectrum of pathological systemic events including sympathetic activation, systemic inflammation, impaired glucose and lipid metabolism, and endothelial dysfunction, among others. This review provides an update of the basic, clinical, and translational advances in our understanding of the metabolic dysfunction and cardiovascular consequences of OSA and highlights the most recent findings and perspectives in the field.
Keywords: intermittent hypoxia, sleep apnea, translational medicine, cardiovascular disease
this article is part of a collection on 1st PanAmerican Congress of Physiological Sciences: Physiology Without Borders. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of complete or partial collapse of the upper airway during sleep, resulting in apneas or hypopneas, respectively. The futile efforts to breathe during obstructed events result in increased negative intrathoracic pressure, intermittent hypoxia (IH), and sleep fragmentation (19). OSA is a growing medical problem around the world, which is closely associated with the burden of obesity. Recent evidence from the well-established Wisconsin Cohort showed that among adults from 30 to 70 yr of age, ∼13% of men and 6% of women have moderate to severe forms of OSA [apnea-hypopnea index (AHI), ≥15 events/h of sleep] (103). The prevalence of OSA frequently surpasses 50% in patients with cardiovascular diseases, including hypertension (25, 96), metabolic syndrome (26), atrial fibrillation (42), and coronary artery disease (16, 45). More than a common comorbidity or just an epiphenomenon of overweight and obesity, increasing evidence suggests that OSA triggers several mechanisms that ultimately impact cardiovascular risk (29). Much of our knowledge about the pathophysiology of OSA is the result of translational studies conducted in humans and animals. In this review, we first briefly discuss the role of OSA as a cardiovascular risk factor followed by a critical analysis of the several animal models that have been developed to study the cardiovascular consequences of OSA. The translational evidence for mechanistic pathways contributing to the cardiovascular consequences of OSA is also examined. Finally, we assess the need for the development of new experimental models and address potential future directions for translational research on the impact of OSA on cardiovascular morbidity and mortality.
OSA as a Cardiovascular Risk Factor
The cardiovascular implications of OSA have gained growing prominence in the last three decades. Despite a limited awareness of the medical community (15), there is consistent evidence that severe OSA is independently associated with increased risk of poor cardiovascular outcome mainly because of myocardial infarction and stroke (77, 83, 141, 142). The vast majority of the studies showed a causal relationship between OSA severity (evaluated by AHI) and risk of cardiovascular events. However, most studies also showed that only severe OSA was significantly associated with increased mortality (44). Several randomized studies reported positive effects of OSA treatment with continuous positive airway pressure (CPAP) on intermediate outcomes such as blood pressure, endothelial dysfunction, arterial stiffness, or inflammation [reviewed in Drager et al (29)]. The impact of OSA treatment on hard outcomes such as fatal and nonfatal cardiovascular events in humans is limited so far to observational studies. However, observational studies consistently showed that the treatment of OSA with CPAP was associated with a significant decrease on the risk of cardiovascular events and mortality rates in severe OSA (9, 10, 43, 77, 79, 80). Current ongoing large multicenter randomized studies such as the Sleep Apnea Cardiovascular Endpoints (SAVE) trial (81) will be useful to elucidate whether the treatment of OSA with CPAP is really effective in reducing cardiovascular mortality.
Animal Models of OSA
Animal models have been used extensively to investigate both the pathogenesis and downstream organ- and tissue-specific outcomes of sleep apnea and IH. The current review focuses on animal models of natural or experimentally induced sleep apnea and IH, as well as briefly highlight the role of these models in shaping our understanding of the development of neurogenic hypertension, as one example of cardiovascular risk in patients with OSA.
Natural models of OSA.
One of the major limitations in the field of OSA is the lack of clinically relevant models of sleep apnea, particularly OSA. Early reports of spontaneous apnea in English bulldogs have not led to significant insights into the pathology of OSA, in part because apneas were largely limited to periods of rapid eye movement (REM) sleep (57). Similarly, the presence of central and obstructive apneas was not pursued in the initial studies of obese Yucatan (76) and Vietnamese pot-bellied (140) pigs that presented inspiratory airflow limitation. However, large animal models are complex, expensive, and genetically heterogeneous. In contrast, rodent models have advantages of the well-characterized genotypes and availability of inbred and transgenic animals.
Rodents exhibit periodic cessations in breathing that occur almost exclusively in the absence of diaphragmatic electromyography activity (122) and defined as spontaneous or more commonly post-sigh if preceded by a larger breath (14). The rates of these central apneas in rodents depend on the strain (41, 47) and expression of particular genes, including the monoamine serotonin (55) and orexin (87). Serotonin pathways have been implicated in central apnea generation using receptor subtype-specific pharmacological blockade (115). Interestingly, from a cardiovascular perspective, experimentally inducing hypotension reduces central apnea rates in rats (114), whereas spontaneously hypertensive rats exhibit an increase in spontaneous apneas during both non-REM and REM sleep (11).
Although obesity is a major risk factor for sleep apnea in humans, genetically obese mice do not exhibit higher central apnea rates than control lean mice (17). With respect to the upper airway, obese New Zealand mice have MRI-assessed increases in fat mass of tongue, soft palate, and lateral pharyngeal walls (5), and obese ob/ob mice have defects in upper airway neuromechanical control that can be reversed with leptin administration (105). Hernandez et al. (49) reported inspiratory flow limitation in the obese New Zealand mouse, but in contrast to mice, obese rats maintain a stable upper airway, even during REM sleep (133). In summary, rodents remain the animal model of choice to study the genesis of central sleep apnea and the cardiovascular consequences of the IH, but an ideal experimental model for the pathogenesis of OSA remains to be developed.
Experimentally induced models of sleep apnea.
In the absence of compelling natural models of upper airway obstruction, studies focusing on the pathophysiological consequences of OSA have either directly obstructed the upper airway or modeled the downstream physioconsequences of airway obstruction, including the most commonly employed intervention of IH. In the 1990s, two laboratories developed chronic dog models of experimentally induced airway obstruction to assess cardiovascular outcomes of OSA (63, 92). More recently, upper airway obstruction models have been extended to rats using either isolated head and body chambers in restrained rats (33) or a balloon inflation mechanism in the trachea of unrestrained rats (Fig. 1) (127). Depending of the OSA model, balloons last for a long time, cause few complications, allow induction of apnea during sleep, allow induction of apneas that start at a fixed point in the respiratory cycle, and elicit cardiorespiratory responses similar to those observed in humans (Fig. 1).
Fig. 1.

Cardiovascular and respiratory responses induced by obstructive apnea in rats in one of the available models. Shown are representative recordings of arterial pressure (AP), electrocardiogram (ECG), mean arterial pressure (MAP), heart rate (HR) and thoracic pressure (TP) over an episode of apnea induced by balloon inflation in the trachea of unrestrained rats. Modified with permission from Angheben et al. (1a) and Schoorlemmer et al. (127).
The classic model to study the consequences of OSA involves administration of IH to simulate the repetitive brief periods of hypoxia and reoxygenation that result from airway obstruction. The first experimental study of IH was performed by Fletcher et al. (35) in the early 1990s in an effort to understand the impact of the hypoxic component of OSA on the development of high blood pressure. In the ensuing years there have been over 1,000 studies of various forms of IH in rats and mice. The periods of IH, which are usually repetitively administered for 8–12 hr during the light or sleeping phase can also induce arousal responses in rodents (106) and induce a sleepy phenotype (143), mimicking other important characteristics of OSA; however, arousals are limited to periods where the IH occurs during sleep, and a significant proportion of IH occurs with the animal awake. Two laboratories have developed computer-controlled feedback systems to deliver hypoxia only during periods of sleep in mice (120, 135) and rats (46). Other limitations of the IH model include the absence of intermittent hypercapnia, although some studies have incorporated both stimuli (36, 91) and the absence of intrathoracic swings that occur with upper airway obstruction. Finally, there is a large variability among experimental models, which is also observed among clinical studies of patients with OSA, that make it difficult to standardize an ideal model of OSA. With this limitation in mind, it seems that the duration of exposure more likely to influence outcomes than the hypoxic nadir or the rate of hypoxic cycling (38).
Animal models of OSA and the development of hypertension.
The general acceptance of hypertension as an established outcome of OSA has, in part, been dependent on animal models demonstrating causality and uncovering mechanistic pathways impacting blood pressure. The landmark study from Toronto showed that 5 wk of experimentally induced airway obstruction during sleep in dogs elevated mean arterial blood pressure (MABP) by more than 10 mmHg within 5 wk and that MABP returned to a normotensive state within 3 wk of normal, unobstructed sleep (6). Multiple studies in mice and rats have also shown MABP increases of ∼10 mmHg within 7–10 days of IH exposure and that the hypertension can be sustained for several weeks (8, 68, 100, 101, 136). Although a diverse array of mechanisms have been implicated in the development of hypertension in rodents exposed to IH, hyperactivation of the carotid body-sympathetic-renal axis has both strong experimental support (2, 70, 101) and relevance to clinical OSA where patients are known to exhibit increased sympathetic nerve activity (SNA) (131). There is a consensus that experimental models of IH are characterized as a neurogenic model of hypertension. Animal models continue to make significant contributions to our understanding of tissue and organ pathology that result from OSA/IH. The ability to directly interrogate causality and either to exclude or to add comorbid features of OSA/IH in a controlled fashion will ensure that animal models continue to have long-term translational relevance to the field of sleep and breathing.
Autonomic Dysfunction and Elevated Blood Pressure in Sleep Apnea
Corroborating evidence from rodent models and clinical studies strongly suggest that IH and OSA, respectively, increase blood pressure. The Wisconsin Sleep Cohort showed an increased incidence of hypertension associated with OSA, even for mild/moderate levels of OSA (102). In a landmark paper from Somers et al. (131), patients with OSA were found to have higher levels of SNA during wakefulness, with further increases in blood pressure and sympathetic activity during sleep. The ability of CPAP therapy to reverse the elevations in SNA and blood pressure in patients with OSA, combined with data from animal models (147, 151), defines OSA/IH as a neurogenic model of hypertension.
In rodents, repetitive, rapid, and intermittent reduction of inspired O2 fraction in the IH model induces cyclical falls in the arterial Po2 with subsequent activation of the arterial chemoreceptors (18, 99, 100, 118). Because in IH models there is no upper airway obstruction and negative intrathoracic pressure swings, it is conceivable that the subsequent blood pressure elevations are due to the repeated episodes of activation of peripheral chemoreceptors and/or enhancement of the downstream chemoreflex responses.
Several studies have demonstrated that IH induces structural and functional alterations in the glomus chemosensitive cells located in the carotid body. Potentially, disturbances in glomus cell function could cause increased sensitivity and reactivity of the peripheral chemoreceptors, contributing to an enhancement of cardiorespiratory responses to hypoxia (66).
One mechanism by which IH may induce neurogenic hypertension is enhancement of central pathways activated by peripheral chemoreceptor stimulation. Particularly interesting are recent findings indicating changes in the sympathetic respiratory coupling in animals chronically exposed to IH (85, 151). The sympathetic and respiratory coupling is observed during inspiration, and this phenomenon is characterized as the sinus arrhythmia. In animals exposed to IH for 10 days, a large increase in the sympathetic activity during the late expiratory phase of the respiratory cycle is observed, indicating a significant change in sympathetic and respiratory coupling (84, 85, 148, 149, 150). Alterations in neurons of the respiratory network contribute to an increase in the frequency discharge of the rostral ventrolateral medulla presympathetic neurons and changes in the respiratory-sympathetic coupling (1, 84). Thus the primary changes in the IH model occur in the respiration pattern and then secondarily impact the frequency discharge of the presympathetic neurons, the sympathetic outflow, and ultimately arterial pressure. Similar changes in the respiratory pattern and the sympathetic-respiratory coupling were recently described in patients with OSA (34).
Metabolic Intermediates of the Cardiovascular Risk in Sleep Apnea
OSA, insulin resistance, and type 2 diabetes.
An independent association between OSA and insulin resistance was first described in observational studies by Brooks et al. (7) and Vgontzas et al. (144). In 2002, two groups of investigators published cross-sectional data in larger patient cohorts (52, 112). An independent relationship between insulin resistance and OSA was demonstrated in the Sleep Heart Health Study (113). The cross-sectional data from the Wisconsin Sleep cohort of patients with sleep apnea showed a significant increase in the prevalence of type 2 diabetes in patients with OSA. The odds ratio for having type 2 diabetes mellitus with an AHI of ≥15 versus <5 was 2.30 after adjustments for age, sex, and body habitus (116). A recent historical cohort study involving 8,678 patients showed that OSA and nocturnal desaturations are associated with incidence of type 2 diabetes (62), which was in line with several previous prospective studies (4, 78). OSA is highly prevalent in patients with type 2 diabetes (94). The prevalence of OSA (diagnosed by the AHI > 5/h) in patients who are obese with type 2 diabetes exceeds 80%, and the prevalence of moderate and severe OSA exceeds 50% (39). The severity of nocturnal intermittent hypoxemia has been associated with the impairment of insulin sensitivity and insulin secretion by pancreatic β-cells (110).
Numerous uncontrolled studies examined the effect of CPAP on glucose tolerance, insulin sensitivity in subjects with prediabetes, and glycemic control in patients with type 2 diabetes. The studies in prediabetics yielded contradictory results, whereas glycemic control in diabetics largely improved [reviewed in Pamidi and Tasali (94)]. However, a recent randomized clinical trial of 8 h of nightly CPAP showed significant improvement of insulin sensitivity in prediabetics after 2 wk of treatment, which emphasizes importance of CPAP adherence for metabolic outcomes (95).
Exposure mice to IH have been employed to determine the impact on glucose metabolism (117). In lean mice, short-term IH for 9–24 h increased fasting blood glucose levels, insulin resistance, glucose intolerance, and suppressed insulin secretion by the pancreatic β-cells (61, 69). After 4–6 wk of IH, suppression of insulin secretion persisted but glucose intolerance and insulin resistance subsided (69, 130, 145). In contrast, in mice with diet-induced and genetic obesity, IH induced severe glucose intolerance, and insulin resistance persisted even after chronic exposure (27, 107). Animal studies demonstrated that effects of IH on glucose metabolism are mediated by the sympathetic nervous system (SNS) (128, 129). As noted above, IH stimulates the carotid bodies, which in turn activates the central sympathetic outflow to the liver and increase the catecholamine efflux by the adrenal medulla, resulting in suppression of insulin secretion, stimulation of hepatic gluconeogenesis and glucose output (130). These changes are abolished by carotid body denervation and adrenal medullectomy (129, 130) (Fig. 2).Thus there is evidence in the literature suggesting that the hypoxic stress of OSA contributes to the development of insulin resistance and type 2 diabetes via SNS activation, but this causal link was not yet confirmed in humans.
Fig. 2.

Proposed pathways mediating prodiabetic effects of intermittent hypoxia (IH). IH upregulates carotid body chemoreflexes while downregulating baroreflexes. The augmented carotid sinus nerve activity excites brainstem sympathetic neurons, resulting in activation of the sympathetic nervous system efferent nerves. The enhanced sympathetic nervous system efferent output 1) increases the epinephrine efflux from the adrenal medulla, which inhibits pancreatic insulin secretion; 2) induces hypertension and endothelial dysfunction; 3) increase glycogenolysis and gluconeogenesis in the liver, augmenting the hepatic glucose output; and 4) induces adipose tissue lipolysis, increasing fatty acid flux to the liver and skeletal muscle, which causes insulin resistance. It is important to note that the impact of IH on glucose metabolism may also occur via non-carotid body pathways. FFA, free fatty acid. Modified with permission from Shin et al. (130).
OSA, dyslipidemia, and nonalcoholic fatty liver disease.
Relationships between OSA and dyslipidemia have not yet been fully characterized. The largest cross-sectional data set from the Sleep Heart Health Study showed that fasting levels of total serum cholesterol and triglycerides directly correlated and HDL cholesterol levels inversely correlated with the severity of OSA in men under 65 (88). More recently, Trzepizur et al. (139) reported independent associations between higher triglyceride and lower HDL cholesterol levels and the severity of nocturnal IH measured by the oxygen desaturation index in 2,081 patients. Sleep apnea has been associated with increased plasma free fatty acids (s) levels (3, 58), which were decreased by supplemental oxygen (58). The largest randomized clinical study of CPAP examining serum lipids was performed in 220 adults for 1 mo and showed a decrease in plasma total cholesterol by 10.8 mg/dl in the treated group, whereas triglyceride levels were unchanged (119). Studies by Chirinos et al. (13) showed that CPAP decreased serum triglycerides only when it was combined with weight loss. It is important to note that only one study examining the effect of OSA on postprandial lipids by Phillips et al. (104) reported a significant decrease in triglyceride and cholesterol with CPAP treatment. Postprandial lipids circulate in the form of triglyceride-rich chylomicrons. Postprandial hypertriglyceridemia confers risk of myocardial infarction, ischemic heart disease, stroke, and death (40, 90) and may contribute to OSA-related cardiovascular morbidity and mortality. The hypoxic stress of OSA increases levels of s and triglyceride-rich lipoproteins that are markers of insulin resistance (65). In fact, a recent study linked OSA-induced dyslipidemia to insulin resistance (75). Murine studies have also shown a predominant effect of IH on s, triglyceride-rich lipoproteins, fasting VLDL, and post-prandial chylomicrones (28, 123). Data from the rodent studies of IH suggested that 1) an increase in levels is related to SNA-induced excessive adipose tissue lipolysis (59) and 2) VLDL and chylomicrone hyperlipidemia is caused by inhibition of lipoprotein lipase, a key enzyme of lipoprotein clearance mediated by hypoxia inducible factor-1α transcriptionally activating an lipoprotein lipase (LPL)-inhibitor angiopoietin-like 4 in adipose tissues (30, 146). Thus the effect of OSA on serum lipids remains poorly characterized, but available data in the literature suggest that OSA may lead to proatherogenic dyslipidemia with selective increases of s and triglyceride rich lipoproteins. Therefore, SNS activation, insulin resistance, and adipose tissue hypoxia may be implicated in the pathogenesis of proatherogenic dyslipidemia in OSA.
Emergent evidence suggests that nonalcoholic fatty liver disease (NAFLD) is independently associated with OSA (60, 89). The severity of OSA predicts the presence of liver fibrosis on biopsy (60, 108). According to some studies we have available so far, CPAP had no effect on liver enzymes (64) or expression of hepatic steatosis by MRI or CT scan (50, 138). To our knowledge, no CPAP trial was conducted to evaluate the effect of OSA on biopsy-confirmed NAFLD.
In animal models, IH augments liver triglyceride content by increasing influx of s from adipose tissue and activating lipid biosynthetic pathways, sterol regulatory element binding protein 1 (SREBP-1), and a SREBP-1-regulated enzyme stearoyl CoA desaturase 1 (71, 72). Partial deficiency of hypoxia inducible factor-1α abolished SREBP-1 and stearoyl-CoA desaturase-1 upregulation and prevented triglyceride accumulation in the liver during IH (73). Mouse IH induces oxidative stress in the liver, activating NADPH oxidase (57), and causes inflammation activating a proinflammatory transcription factor NF-κB (124) and NF-kB regulated proinflammatory cytokines TNF-1α, IL-1β, IL-6 and macrophage inflammatory protein-2 (125). In mice on a high-fat diet, IH converted diet-induced hepatic steatosis to steatohepatitis (nonalcoholic steatohepatitis) and liver fibrosis (125). Thus there is experimental evidence of a strong independent association between OSA and NAFLD with nonalcoholic steatohepatitis and liver fibrosis backed by experimental evidence, but causal relationships between OSA and NAFLD have not been established.
Figure 2 shows the putative pathways mediating prodiabetic effects of IH.
Vascular Dysfunction and Atherosclerosis in Sleep Apnea
OSA is associated with endothelial dysfunction and altered repair mechanisms, (54, 61) increased arterial stiffness (22, 97), and premature development of atherosclerosis (20, 23, 24, 67, 82). Interventional studies, including several randomized trials, showed that OSA treatment with CPAP reversed or attenuated parameters of vascular dysfunction and atherosclerosis (21, 53, 54). Interestingly, markers of inflammation were associated with the vascular dysfunction in OSA (54, 82). Moreover, improvements in surrogate markers of atherosclerosis and arterial stiffness were improved by CPAP in parallel to significant decreases in inflammatory markers and sympathetic activation (21). Two main mechanisms underlying vascular dysfunction and atherosclerosis in patients with OSA–impaired lipid metabolism and inflammation–were elucidated mainly through application of rodent models of IH.
Lipid metabolism impairment and atherosclerosis.
The impact of IH on dyslipidemia and lipid metabolism was discussed in the previous section. Consistent evidence from animal models suggests the potential role of dyslipidemia on atherosclerosis in OSA. In a pivotal study, Savransky et al. (123) showed that IH promoted formation of fatty streaks and small mature plaques in the aortic arch and descending aorta of wild-type male C57BL/6J mice on a high-cholesterol diet. Combined exposure to IH and a high-cholesterol diet resulted in marked progression of dyslipidemia with further increases in serum total cholesterol and LDL cholesterol, increase in serum lipid peroxidation, and upregulation of an important hepatic enzyme of lipoprotein secretion, stearoyl-CoA desaturase-1(123). Consistently, dyslipidemia and atherosclerosis induced by IH were attenuated by deficiency of stearoyl CoA desaturase-1 (126). In a prone model of atherosclerosis [apolipoprotein E (ApoE) knockout (KO) mice under high-cholesterol diet], Jun et al. (56) showed that IH accelerated atherosclerotic plaque growth without affecting plaque composition. These observations in the animal model suggest that the harmful effects of IH on the vascular bed are potentiated when other risk factors for atherosclerosis are also present. Data from recent studies suggested that decreased lipoprotein clearance is a complementary mechanism of atherosclerosis induced by IH. As previously discussed, IH inhibits adipose tissue LPL because of the activation of angiopoietin-like 4 (Angptl4) (28). Using Angptl4-neutralizing antibody, Drager et al. (30) demonstrated the important role of lipoprotein clearance on atherogenesis in a model of sleep apnea. In vehicle-treated mice, IH increased adipose Angptl4 levels, inhibited adipose LPL, increased fasting levels of plasma triglycerides and very LDL cholesterol, and increased the size of atherosclerotic plaques. The effects of IH were abolished by the Angptl4-neutralizing antibody (Fig. 3).
Fig. 3.
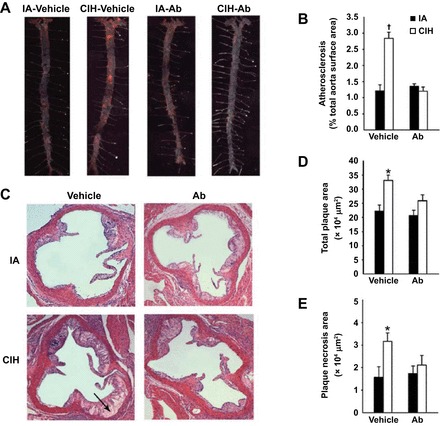
Effect of chronic IH (CIH) and angiopoietin-like-4 antibodies (Ab) on the atherosclerotic plaque size in en face preparations of the entire aorta (A and B) and in cross-sections of the aortic root of apolipoprotein E−/− mice (C–E). A: representative images of the entire aorta with atherosclerotic lesions stained in red. Sudan IV; original magnification, ×10. B: percentage of the total aortic surface covered by the atherosclerotic lesions. C: representative cross sections of the aortic root. Hematoxylin and eosin staining. Original magnification, ×100. Arrow points to plaque necrosis. D: total plaque cross-sectional area (in μm2). E: plaque necrosis area (in μm2). IA, intermittent air. *P < 0.05 for CIH vehicle vs. remaining groups. †P < 0.001 for CIH vehicle vs. remaining groups. Reprinted with permission from Drager et al. (38).
Thus there is consistent evidence from animal models that IH is an important trigger to induce atherosclerosis through dyslipidemia and lipid metabolism impairment, but we still need more data from clinical studies to confirm these findings in humans.
Inflammation and atherosclerosis.
Several studies have shown that patients with OSA have elevated markers of proinflammatory mediators and inflammatory markers with proatherogenic properties such as TNF-α, IL-6, IL-8, C-reactive protein, leukotriene B4 (LTB4), and adhesion molecules (ICAM-1, VCAM-1, L-selectin, SE-selectin, P-selectin) (26, 31, 93, 128, 134). Interestingly, Ryan et al. (121) showed the crucial role of NF-κB activation with the downstream consequences of production of inflammatory genes in response to IH in a translational study. Of note, patients with OSA have an increased NF-κB activity, a key transcription factor that elicits inflammatory pathways (51).
Recent experimental studies have pointed to the importance of inflammation in the vascular dysfunction and atherogenesis induced by OSA. Li et al. (74) exposed THP-1 cells (human monocytic cell line derived from acute monocytic leukemia) to IH (74). They found an increased production of LTB4 and the expression of 5-lipoxygenase and leukotriene A4 hydrolase, the key enzymes for producing LTB4. In addition, IH exposures promoted increased cellular cholesterol accumulation and foam cell formation. The LTB4 receptor 1 (BLT1) antagonist U-75302 markedly attenuated IH-induced changes. Furthermore, IH-induced lesion formation was markedly attenuated in BLT1−/−/ApoE−/− mice (74).
Exploring the potential role of NF-κB in the atherogenesis induced by OSA, Song and colleagues (132) studied wild type and mice deficient in the p50 subunit of NF-κB (p50-KO), fed normal chow diet or high-cholesterol diet, and exposed to sham or IH. P50 gene deletion diminished IH+ high-cholesterol diet-induced NF-κB activation and abolished IH+ high-cholesterol diet-induced atherosclerosis. P50 gene deletion inhibited vascular wall inflammation, reduced hepatic TNF-α level, attenuated the elevation in serum cholesterol level, and diminished macrophage foam cell formation induced by IH+ high-cholesterol diet exposure (132). Studying ApoE-KO mice or in both ApoE and p50 genes (ApoE-p50-double KO) exposed to sham or IH, the same group found that IH caused more pronounced atherosclerotic lesions in ApoE-p50-double KO mice than ApoE-KO mice in parallel to a greater elevation in serum cholesterol level, serum levels of TNF-α and IL-6, aortic TNF-α, and inducible nitric oxide synthase expression and aortic infiltration of Mac3-positive macrophages (32). Recently, Poulain and colleagues (109) examined the role of the visceral fat in modulating the inflammation and atherogenesis induced by IH. IH induced morphological (shrunken adipocytes), functional (increased uncoupling protein-1 expression), and inflammatory (increased macrophage recruitment and secretion of IL-6 and TNF-α) remodeling of epididymal adipose tissue. Hypoxic mice presented more severe dyslipidemia and atherosclerosis lesions. Epididymal lipectomy attenuated both IH-induced dyslipidemia and atherogenesis (109). In summary, animal models of OSA and cell culture provide evidence that OSA promotes vascular dysfunction and premature atherosclerosis via multiple pathways, including impaired metabolism and inflammation.
Perspectives
Nowadays OSA is a major public health concern. Clinical studies in patients and animal IH models indicate that OSA activates multiple intermediate pathways that lead to cardiovascular disease. Despite significant advances in our understanding of pathways promoting cardiovascular disease in patients with OSA, we need to be more innovative and develop new experimental models and strategies for a better understanding of the mechanisms underlying the cardiovascular disease induced by OSA. The potential effects of OSA on circadian variability sleep structure, sleep fragmentation, and deprivation have received little attention and deserve further investigation. We currently have limited understanding of the relative contributions of metabolic intermediates such as insulin resistance, hyperlipidemia, and inflammation on cardiovascular end points of atherosclerosis and hypertension. Although much progress has been made with respect to hypertension, there is a need to systematically explore the impact of OSA treatment on the progression and regression of atherosclerosis. Large prospective randomized studies are necessary to fully establish whether the treatment of OSA can decrease metabolic dysfunction and reduce cardiovascular events. Finally, translational approaches are essential for a better understanding of the causes, and they will be critical in helping the development of new strategies for the prevention and the treatment for OSA.
GRANTS
This work was supported by FundaçãoZerbini and Research grant from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2012/02953-2) to Dr. Luciano F. Drager and FAPESP (2013/06077-5) to Dr. Benedito H. Machado.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.F.D., V.Y.P., C.P.O., S.L.C., G.L.-F., and B.H.M. conception and design of research; L.F.D., V.Y.P., S.L.C., and B.H.M. prepared figures; L.F.D., V.Y.P., C.P.O., S.L.C., G.L.-F., and B.H.M. drafted manuscript; L.F.D., V.Y.P., C.P.O., S.L.C., G.L.-F., and B.H.M. edited and revised manuscript; L.F.D., V.Y.P., C.P.O., S.L.C., G.L.-F., and B.H.M. approved final version of manuscript.
REFERENCES
- 1.Almado CE, Leao RM, Machado BH. Intrinsic properties of rostral ventrolateral medulla presympathetic and bulbospinal respiratory neurons of juvenile rats are not affected by chronic intermittent hypoxia. Exp Physiol 99: 937–950, 2014. [DOI] [PubMed] [Google Scholar]
- 1a.Angheben JM, Schoorlemmer GH, Rossi MV, Silva TA, Cravo SL. Cardiovascular responses induced by obstructive apnea are enhanced in hypertensive rats due to enhanced chemoreceptor responsivity. PLoS One 9: e86868, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol 83: 95–101, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Barceló A, Piérola J, de la Peña M, Esquinas C, Fuster A, Sanchez-de-la-Torre M, Carrera M, Alonso-Fernandez A, Ladaria A, Bosch M, Barbé F. Free fatty acids and the metabolic syndrome in patients with obstructive sleep apnoea. Eur Respir J 37: 1418–1423, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med 122: 1122–1127, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennick MJ, Pack AI, Ko K, Kim E, Pickup S, Maislin G, Schwab RJ. Altered upper airway and soft tissue structures in the New Zealand Obese mouse. Am J Respir Crit Care Med 179: 158–169, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest 99: 106–109, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, Sullivan CE, Yue DK. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab 79: 1681–1685, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol 99: 2028–2035, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med 156: 115–122, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nuñez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med 189: 1544–1550, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Carley DW, Berecek K, Videnovic A, Radulovacki M. Sleep-disordered respiration in phenotypically normotensive, genetically hypertensive rats. Am J Respir Crit Care Med 162: 1474–1479, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Chin K, Nakamura T, Shimizu K, Mishima M, Nakamura T, Miyasaka M, Ohi M. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med 109: 562–567, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, Foster GD, Maislin G, Saif H, Broderick P, Chittams J, Hanlon AL, Pack AI. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med 370: 2265–2275, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christon J, Carley DW, Monti D, Radulovacki M. Effects of inspired gas on sleep-related apnea in the rat. J Appl Physiol 80: 2102–2107, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Costa LE, Uchôa CH, Harmon RR, Bortolotto LA, Lorenzi-Filho G, Drager LF. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart 101: 1288–1292, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Danzi-Soares NJ, Genta PR, Nerbass FB, Pedrosa RP, Soares FS, César LA, Drager LF, Skomro R, Lorenzi-Filho G. Obstructive sleep apnea is common among patients referred for coronary artery bypass grafting and can be diagnosed by portable monitoring. Coron Artery Dis 23: 31–38, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Davis EM, Locke LW, McDowell AL, Strollo PJ, O'Donnell CP. Obesity accentuates circadian variability in breathing during sleep in mice but does not predispose to apnea. J Appl Physiol 115: 474–482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Rio R, Moya EA, Iturriaga R. Carotid body and cardiorespiratory alterations in intermittent hypoxia: the oxidative link. Eur Respir J 36: 143–150, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophisiology of sleep apnea. Physiol Rev 90: 47–112, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 172: 613–618, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 176: 706–712, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest 131: 1379–1386, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Drager LF, Bortolotto LA, Maki-Nunes C, Trombetta IC, Alves MJ, Fraga RF, Negrao CE, Krieger EM, Lorenzi-Filho G. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis 208: 490–495, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Drager LF, Bortolotto LA, Krieger EM, Lorenzi-Filho G. Additive effects of obstructive sleep apnea and hypertension on early markers of carotid atherosclerosis. Hypertension 53: 64–69, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, Lorenzi-Filho G. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol 105: 1135–1139, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Drager LF, Lopes HF, Maki-Nunes C, Trombetta IC, Toschi-Dias E, Alves MJ, Fraga RF, Jun JC, Negrão CE, Krieger EM, Polotsky VY, Lorenzi-Filho G. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One 5: e12065, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 19: 2167–2174, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drager LF, Li J, Shin MK, Reinke C, Aggarwal NR, Jun JC, Bevans-Fonti S, Sztalryd C, O'Byrne SM, Kroupa O, Olivecrona G, Blaner WS, Polotsky VY. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J 33: 783–790, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol 62: 569–576, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans-Fonti S, Gay J, Sussan TE, Jun JC, Myers AC, Olivecrona G, Schwartz AR, Halberg N, Scherer PE, Semenza GL, Powell DR, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med 188: 240–248, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyugovskaya L, LavieP, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 165: 934–939, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Fang G, Song D, Ye X, Mao SZ, Liu G, Liu SF. Chronic intermittenthypoxia exposure induces atherosclerosis in ApoE knockout mice: role of NF-κB p50. Am J Pathol 181: 1530–1539, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Farré R, Nacher M, Serrano-Mollar A, Galdiz JB, Alvarez FJ, Navajas D, Montserrat JM. Rat model of chronic recurrent airway obstructions to study the sleep apnea syndrome. Sleep 30: 930–933, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fatouleh R, McKenzie DK, Macefield VG. Respiratory modulation of muscle sympathetic nerve activity in obstructive sleep apnoea. Exp Physiol 99: 1288–1298, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Fletcher EC, Lesske J, Qian W, Miller CC 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19: 555–561, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Fletcher EC, Bao G, Miller CC 3rd. Effect of recurrent episodic hypocapnic, eucapnic, and hypercapnic hypoxia on systemic blood pressure. J Appl Physiol 78: 1516–1521, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher EC. An animal model of the relationship between systemic hypertension and repetitive episodic hypoxia as seen in sleep apnoea. J Sleep Res 4: 71–77, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Foster GE, Poulin MJ, Hanly PJ. Intermittent hypoxia and vascular function: implications forobstructive sleep apnoea. Exp Physiol 92: 51–65, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Sunyer FX, Darcey V, Kuna ST; Sleep AHEAD. Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 32: 1017–1019, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA 300: 2142–2152, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Friedman L, Haines A, Klann K, Gallaugher L, Salibra L, Han F, Strohl KP. Ventilatory behavior during sleep among A/J and C57BL/6J mouse strains. J Appl Physiol 97: 1787–1795, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK. Association of atrial fibrillation and obstructive sleep apnea. Circulation 110: 364–367, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Rio F, Alonso-Fernández A, Armada E, Mediano O, Lores V, Rojo B, Fernández-Lahera J, Fernández-Navarro I, Carpio C, Ramírez T. CPAP effect on recurrent episodes in patients with sleep apnea and myocardial infarction. Int J Cardiol 168: 1328–1335, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Ge X, Han F, Huang Y, Zhang Y, Yang T, Bai C, Guo X. Is obstructive sleep apnea associated with cardiovascular and all-cause mortality? PLoS One 8: e69432, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geovanini GR, Gowdak LH, Pereira AC, Danzi-SoaresNde J, Dourado LO, Poppi NT, Cesar LA, Drager LF, Lorenzi-Filho G. OSA and depression are common and independently associated with refractory angina in patients with coronary artery disease. Chest 146: 73–80, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Hamrahi H, Chan B, Horner RL. On-line detection of sleep-wake states and application to produce intermittent hypoxia only in sleep in rats. J Appl Physiol 90: 2130–2140, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. J Appl Physiol 92: 1133–1140, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Hendricks JC, Kline LR, Kovalski RJ, O'Brien JA, Morrison AR, Pack AI. The English bulldog: a natural model of sleep-disordered breathing. J Appl Physiol 63: 1344–1350, 1987. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez AB, Kirkness JP, Smith PL, Schneider H, Polotsky M, Richardson RA, Hernandez WC, Schwartz AR. Novel whole body plethysmography system for the continuous characterization of sleep and breathing in a mouse. J Appl Physiol 112: 671–680, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoyos CM, Killick R, Yee BJ, Phillips CL, Grunstein RR, Liu PY. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax 67: 1081–1089, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Htoo AK, Greenberg H, Tongia S, Chen G, Henderson T, Wilson D, Liu SF: Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath 10: 43–50, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 165: 670–676, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleepapnea and response to treatment. Am J Respir Crit Care Med 169: 348–353, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 117: 2270–2278, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joseph V, Pequignot JM, Van Reeth O. Neurochemical perspectives on the control of breathing during sleep. Respir Physiol Neurobiol 130: 253–263, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 209: 381–386, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jun J, Savransky V, Nanayakkara A, Bevans Li S, Smith PL, Polotsky VY. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol 295: R1274–R1281, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jun JC, Drager LF, Najjar SS, Gottlieb SS, Brown CD, Smith PL, Schwartz AR, Polotsky VY. Effects of sleep apnea on nocturnal free fatty acids in subjects with heart failure. Sleep 34: 1207–1213, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jun JC, Shin MK, Devera R, Yao Q, Mesarwi O, Bevans-Fonti S, Polotsky VY. Intermittent hypoxia-induced glucose intolerance is abolished by alpha-adrenergic blockade or adrenal medullectomy. Am J Physiol Endocrinol Metab 307: E1073–E1083, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kallwitz ER, Herdegen J, Madura J, Jakate S, Cotler SJ. Liver enzymes and histology in obese patients with obstructive sleep apnea. J Clin Gastroenterol 41: 918–921, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 102: 2607–2610, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med 190: 218–225, 2014. [DOI] [PubMed] [Google Scholar]
- 63.Kimoff RJ, Makino H, Horner RL, Kozar LF, Lue F, Slutsky AS, Phillipson EA. Canine model of obstructive sleep apnea: model description and preliminary application. J Appl Physiol 76: 1810–1817, 1994. [DOI] [PubMed] [Google Scholar]
- 64.Kohler M, Pepperell JC, Davies RJ, Stradling JR. Continuous positive airway pressure and liver enzymes in obstructive sleep apnoea: data from a randomized controlled trial. Respiration 78: 141–146, 2009. [DOI] [PubMed] [Google Scholar]
- 65.Krauss RM, Siri PW. Dyslipidemia in type 2 diabetes. Med Clin North Am 88: 897–909, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. In Comprehensive Physiology. Hoboken, NJ: Wiley, 2011, p. 141–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kylintireas I, Craig S, Nethononda R, Kohler M, Francis J, Choudhury R, Stradling J, Neubauer S. Atherosclerosis and arterial stiffness in obstructive sleepapnea–a cardiovascular magnetic resonance study. Atherosclerosis 222: 483–489, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol 100: 1974–1982, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Lee EJ, Alonso LC, Stefanovski D, Strollo HC, Romano LC, Zou B, Singamsetty S, Yester KA, McGaffin KR, Garcia-Ocana A, O'Donnell CP. Time-dependent changes in glucose and insulin regulation during intermittent hypoxia and continuous hypoxia. Eur J Appl Physiol 113: 467–478, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia–influence of chemoreceptors and sympathetic nervous system. J Hypertens 15: 1593–1603, 1997. [DOI] [PubMed] [Google Scholar]
- 71.Li J, Grigoryev DN, Ye SQ, Thorne L, Schwartz AR, Smith PL, O'Donnell CP, Polotsky VY. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol 99: 1643–1648, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Li J, Thorne N L, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 97: 698–706, 2005. [DOI] [PubMed] [Google Scholar]
- 73.Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL, Polotsky VY. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1alpha. Physiol Genomics 25: 450–457, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Li RC, Haribabu B, Mathis SP, Kim J, Gozal D. Leukotriene B4 receptor-1 mediates intermittent hypoxia-induced atherogenesis. Am J Respir Crit Care Med 184: 124–131, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu A, Cardell J, Ariel D, Lamendola C, Abbasi F, Kim SH, Holmes TH, Tomasso V, Mojaddidi H, Grove K, Kushida CA, Reaven GM. Abnormalities of lipoprotein concentrations in obstructive sleep apnea are related to insulin resistance. Sleep 38: 793–799, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lonergan RP 3rd, Ware JC, Atkinson RL, Winter WC, Suratt PM. Sleep apnea in obese miniature pigs. J Appl Physiol 84: 531–536, 1998. [DOI] [PubMed] [Google Scholar]
- 77.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365: 1046–1053, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Marshall NS, Wong KK, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med 5: 15–20, 2009. [PMC free article] [PubMed] [Google Scholar]
- 79.Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, Soriano Y, Román-Sánchez P, Illa FB, Canal JM, Durán-Cantolla J. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med 180: 36–41, 2009. [DOI] [PubMed] [Google Scholar]
- 80.Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, Soler-Cataluña JJ, Almeida-Gonzalez C, De la Cruz Morón I, Durán-Cantolla J, Montserrat JM. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med 186: 909–916, 2012. [DOI] [PubMed] [Google Scholar]
- 81.McEvoy RD, Anderson CS, Antic NA, Chen B, He Q, Heeley E, Huang S, Huang Y, Wang J, Zhong N. The sleep apnea cardiovascular endpoints (SAVE) trial: rationale and start-up phase. J Thorac Dis 2: 138–143, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med 172: 625–630, 2005. [DOI] [PubMed] [Google Scholar]
- 83.Mooe T, Franklin KA, Holmström K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med 164: 1910–1913, 2001. [DOI] [PubMed] [Google Scholar]
- 84.Moraes DJ, Da Silva MP, Bonagamba LG, Mecawi AS, Zoccal DB, Antunes-Rodrigues J, Varanda WA, Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci 33: 19223–19237, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moraes DJ, Zoccal DB, Machado BH. Medullary respiratory network drives sympathetic overactivity and hypertension in rats submitted to chronic intermittent hypoxia. Hypertension 60: 1374–1380, 2012. [DOI] [PubMed] [Google Scholar]
- 86.Moraes DJ, Machado BH. Electrophysiological properties of laryngeal motoneurones in rats submitted to chronic intermittent hypoxia. J Physiol 593: 619–634, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol 102: 241–248, 2007. [DOI] [PubMed] [Google Scholar]
- 88.Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, Quan SF. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol 154: 50–59, 2001. [DOI] [PubMed] [Google Scholar]
- 89.Nobili V, Cutrera R, Liccardo D, Pavone M, Devito R, Giorgio V, Verrillo E, Baviera G, Musso G. Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am J Respir Crit Care Med 189: 66–76, 2014. [DOI] [PubMed] [Google Scholar]
- 90.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298: 299–308, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Norton CE, Jernigan NL, Kanagy NL, Walker BR, Resta TC. Intermittent hypoxia augments pulmonary vascular smooth muscle reactivity to NO: regulation by reactive oxygen species. J Appl Physiol 111: 980–988, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O'Donnell CP, King ED, Schwartz AR, Smith PL, Robotham JL. Effect of sleep deprivation on responses to airway obstruction in the sleeping dog. J Appl Physiol 77: 1811–1818, 1994. [DOI] [PubMed] [Google Scholar]
- 93.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol 94: 179–184, 2003. [DOI] [PubMed] [Google Scholar]
- 94.Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol 3: 126, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pamidi S, Wroblewski K, Stepien M, Sharif-Sidi K, Kilkus J, Whitmore H, Tasali E. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. A randomized controlled trial. Am J Respir Crit Care Med 192: 96–105, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 58: 811–817, 2011. [DOI] [PubMed] [Google Scholar]
- 97.Pedrosa RP, Barros IM, Drager LF, Bittencourt MS, Medeiros AK, Carvalho LL, Lustosa TC, Carvalho MM, Ferreira MN, Lorenzi-Filho G, Costa LO. OSA is common and independently associated with hypertension and increased arterial stiffness in consecutive perimenopausal women. Chest 146: 66–72, 2014. [DOI] [PubMed] [Google Scholar]
- 98.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol 96: 1236–1242, 2004. [DOI] [PubMed] [Google Scholar]
- 100.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peng YJ, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, VasavdaC, Kumar GK, Semenza GL, Prabhakar NR. Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J Physiol 592: 3841–3858, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342: 1378–1384, 2000. [DOI] [PubMed] [Google Scholar]
- 103.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177: 1006–1014, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. Am J Respir Crit Care Med 184: 355–361, 2011. [DOI] [PubMed] [Google Scholar]
- 105.Polotsky M, Elsayed-Ahmed AS, Pichard L, Harris CC, Smith PL, Schneider H, Kirkness JP, Polotsky V, Schwartz AR. Effects of leptin and obesity on the upper airway function. J Appl Physiol 112: 1637–1643, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med 7: 7–16, 2006. [DOI] [PubMed] [Google Scholar]
- 107.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Polotsky VY, Patil SP, Savransky V, Lan A, Fonti S, Frame LA, Steele KE, Schweizter MA, Clark JM, Torbenson MS, Schwartz AR. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med 179: 228–234, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poulain L, Thomas A, Rieusset J, Casteilla L, Levy P, Arnaud C, Dematteis M. Visceral white fat remodelling contributes to intermittent hypoxia-induced atherogenesis. Eur Respir J 43: 513–522, 2014. [DOI] [PubMed] [Google Scholar]
- 110.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med 179: 235–240, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 6: e1000132, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165: 677–682, 2002. [DOI] [PubMed] [Google Scholar]
- 113.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 160: 521–530, 2004. [DOI] [PubMed] [Google Scholar]
- 114.Radulovacki M, Trbovic S, Carley DW. Hypotension reduces sleep apneas in Zucker lean and Zucker obese rats. Sleep 19: 767–773, 1996. [DOI] [PubMed] [Google Scholar]
- 115.Radulovacki M, Trbovic SM, Carley DW. Serotonin 5-HT3-receptor antagonist GR 38032F suppresses sleep apneas in rats. Sleep 21: 131–136, 1998. [DOI] [PubMed] [Google Scholar]
- 116.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med 172: 1590–1595, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissueoxygen profiles and metabolic outcomes. J Appl Physiol 111: 881–890, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol 560: 577–586, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax 59: 777–782, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rubin AE, Polotsky VY, Balbir A, Krishnan JA, Schwartz AR, Smith PL, Fitzgerald RS, Tankersley CG, Shirahata M, O'Donnell CP. Differences in sleep-induced hypoxia between A/J and DBA/2J mouse strains. Am J Respir Crit Care Med 168: 1520–1527, 2003. [DOI] [PubMed] [Google Scholar]
- 121.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112: 2660–2667, 2005. [DOI] [PubMed] [Google Scholar]
- 122.Sato T, Saito H, Seto K, Takatsuji H. Sleep apneas and cardiac arrhythmias in freely moving rats. Am J Physiol Regul Integr Comp Physiol 259: R282–R287, 1990. [DOI] [PubMed] [Google Scholar]
- 123.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 175: 1290–1297, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Savransky V, Nanayakkara A, Vivero A, Li J, Bevans S, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia predisposes to liver injury. Hepatology 45: 1007–1013, 2007. [DOI] [PubMed] [Google Scholar]
- 125.Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol 293: G871–G877, 2007. [DOI] [PubMed] [Google Scholar]
- 126.Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser AB, Steele KE, Schweitzer MA, Patil SP, Bhanot S, Schwartz AR, Polotsky VY. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res 103: 1173–1180, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schoorlemmer GH, Rossi MV, Tufik S, Cravo SL. A new method to produce obstructive sleep apnoea in conscious unrestrained rats. Exp Physiol 96: 1010–1018, 2011. [DOI] [PubMed] [Google Scholar]
- 128.Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 105: 2462–2464, 2002. [DOI] [PubMed] [Google Scholar]
- 129.Shin MK, Han W, Bevans-Fonti S, Jun JC, Punjabi NM, Polotsky VY. The effect of adrenal medullectomy on metabolic responses to chronic intermittent hypoxia. Respir Physiol Neurobiol 203: 60–67, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shin MK, Yao Q, Jun JC, Bevans-Fonti S, Yoo DY, Han W, Mesarwi O, Richardson R, Fu YY, Pasricha PJ, Schwartz AR, Shirahata M, Polotsky VY. Carotid body denervation prevents fasting hyperglycemia during chronic intermittent hypoxia. J Appl Physiol 117: 765–776, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Song D, Fang G, Mao SZ, Ye X, Liu G, Gong Y, Liu SF. Chronic intermittenthypoxia induces atherosclerosis by NF-κB-dependent mechanisms. Biochim Biophys Acta 1822: 1650–1659, 2012. [DOI] [PubMed] [Google Scholar]
- 133.Sood S, Liu X, Liu H, Horner RL. Genioglossus muscle activity and serotonergic modulation of hypoglossal motor output in obese Zucker rats. J Appl Physiol 102: 2240–2250, 2007. [DOI] [PubMed] [Google Scholar]
- 134.Stanke-Labesque F, Pépin JL, de Jouvencel T, Arnaud C, Baguet JP, Petri MH, Tamisier R, Jourdil JF, Lévy P, Bäck M. Leukotriene B4 pathway activation and atherosclerosis in obstructive sleep apnea. J Lipid Res 53: 1944–1951, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O'Donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. J Appl Physiol 91: 2758–2766, 2001. [DOI] [PubMed] [Google Scholar]
- 136.Tahawi Z, Orolinova N, Joshua IG, Bader M, Fletcher EC. Altered vascular reactivity in arterioles of chronic intermittent hypoxic rats. J Appl Physiol 90: 2007–2013, 2001. [DOI] [PubMed] [Google Scholar]
- 137.Tanne F, Gagnadoux F, Chazouilleres O, Fleury B, Wendum D, Lasnier E, Lebeau B, Poupon R, Serfaty L. Chronic liver injury during obstructive sleep apnea. Hepatology 41: 1290–1296, 2005. [DOI] [PubMed] [Google Scholar]
- 138.Trenell MI, Ward JA, Yee BJ, Phillips CL, Kemp GJ, Grunstein RR, Thompson CH. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab 9: 679–687, 2007. [DOI] [PubMed] [Google Scholar]
- 139.Trzepizur W, Le Vaillant M, Meslier N, Pigeanne T, Masson P, Humeau MP, Bizieux-Thaminy A, Goupil F, Chollet S, Ducluzeau PH, Gagnadoux F; Institut de Recherche en Santé Respiratoire des Pays de la Loire (IRSR) Sleep Cohort Group. Independent association between nocturnal intermittent hypoxemia and metabolic dyslipidemia. Chest 143: 1584–1589, 2013. [DOI] [PubMed] [Google Scholar]
- 140.Tuck SA, Dort JC, Olson ME, Remmers JE. Monitoring respiratory function and sleep in the obese Vietnamese pot-bellied pig. J Appl Physiol 87: 444–451, 1999. [DOI] [PubMed] [Google Scholar]
- 141.Uchôa CH, Danzi-SoaresNde J, Nunes FS, de Souza AA, Nerbass FB, PedrosaRP, César LA, Lorenzi-Filho G, Drager LF. Impact of OSA on cardiovascular events after coronary artery bypass sugery. Chest 147: 1352–1360, 2015. [DOI] [PubMed] [Google Scholar]
- 142.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation 118: 955–960, 2008. [DOI] [PubMed] [Google Scholar]
- 143.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 27: 194–201, 2004. [DOI] [PubMed] [Google Scholar]
- 144.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 85: 1151–1158, 2000. [DOI] [PubMed] [Google Scholar]
- 145.Wang N, Khan SA, Prabhakar NR, Nanduri J. Impairment of pancreatic β-cell function by chronicintermittent hypoxia. Exp Physiol 98: 1376–1385, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yao Q, Shin MK, Jun JC, Hernandez KL, Aggarwal NR, Mock JR, Gay J, Drager LF, Polotsky VY. Effect of chronic intermittent hypoxia on triglyceride uptake in different tissues. J Lipid Res 54: 1058–1065, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zoccal DB, Bonagamba LG, Oliveira FR, Antunes-Rodrigues J, Machado BH. Increased sympathetic activity in rats submitted to chronic intermittent hypoxia. Exp Physiol 92: 79–85, 2007. [DOI] [PubMed] [Google Scholar]
- 148.Zoccal DB, Machado BH. Sympathetic overactivity coupled with active expiration in rats submitted to chronic intermittent hypoxia. Respir Physiol Neurobiol 174: 98–101, 2010. [DOI] [PubMed] [Google Scholar]
- 149.Zoccal DB, Machado BH. Coupling between respiratory and sympathetic activities as a novel mechanism underpinning neurogenic hypertension. Curr Hypertens Rep: 13: 229–236, 2011. [DOI] [PubMed] [Google Scholar]
- 150.Zoccal DB, Paton JF, Machado BH. Do changes in the coupling between respiratory and sympathetic activities contribute to neurogenic hypertension? Clin Exp Pharmacol Physiol 36: 1188–1196, 2009. [DOI] [PubMed] [Google Scholar]
- 151.Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol 586: 3253–3265, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


