Abstract
Gastroesophageal reflux disease (GERD) is a highly prevalent chronic condition where in stomach contents reflux into the esophagus causing symptoms, esophageal injury, and subsequent complications. Proton pump inhibitors (PPI) remain the mainstay of therapy for acid suppression. Despite their efficacy, significant proportions of GERD patients are either partial or non-responders to PPI therapy. Patients should be assessed for mechanisms that can lead to PPI failure and may require further evaluation to investigate for alternative causes. This monograph will outline a diagnostic approach to the PPI non-responder, review mechanisms associated with PPI failure, and discuss therapeutic options for those who fail to respond to PPI therapy.
CASE STUDY
MJ is a 37-year-old woman sent for a fourth opinion in gastroenterology. She describes having “horrible” heartburn symptoms for >10 years. She is been treated with multiple medications and although each initially seemed to improve her symptoms she quickly became “resistant” and her symptoms all returned. More specifically, she has tried over-the-counter agents such as antacids and histamine-type-2 receptor antagonists (H2RAs), in addition to all six proton pump inhibitors (PPIs). Each PPI trial lasted 4–8 weeks and consisted of both once-daily therapy and b.i.d. therapy. Sucralfate did not provide any significant benefit, nor did an empiric trial of metoclopramide. Three separate upper endoscopies have been normal, including biopsies from the distal and mid-esophagus (all were performed on PPI therapy). A 48-h wireless pH capsule study performed on a twice-daily PPI was normal, as was a 24-h impedance-pH probe (also performed on b.i.d. PPI therapy). Her other medical problems include migraine headaches, temporomandibular joint syndrome, interstitial cystitis and irritable bowel syndrome with constipation predominance. She is not allergic to any medication although she notes that she is frequently sensitive to medications. She does not smoke cigarettes and has 2–3 glasses of wine each week. She underwent appendectomy as a child and underwent laparoscopic cholecystectomy 3 years ago for chronic upper abdominal pain (the pathology was normal and no gallstones were identified). Her weight has remained stable during this time period (body mass index=24 kg/m2). Her physical examination is unrevealing. She is frustrated by her symptoms and wonders why she has these symptoms and whether other tests are necessary or other treatments available. She states that she has done a lot of research on the topic and believes that she is an excellent candidate for anti-reflux surgery. As a clinician, how do you explain the persistent symptoms to this patient? What are potential etiologies for a PPI non-responder? What treatment options are available?
INTRODUCTION: SCOPE OF THE PROBLEM
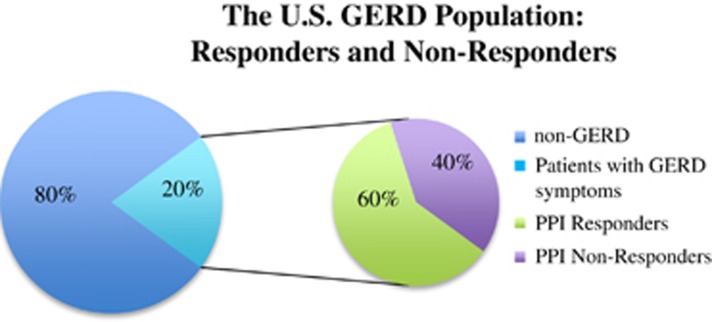
Gastroesophageal reflux disease (GERD) is a common chronic condition, affecting approximately 20% of the American adult population.1, 2 GERD is characterized by a number of symptoms, the two most common being frequent heartburn and acid regurgitation. Untreated or undertreated gastroesophageal reflux (GER) can lead to complications including esophageal erosions, strictures, esophageal adenocarcinoma, and impaired quality of life.3 GER was the most frequent outpatient diagnosis with almost nine million visits in 2009.4 The direct cost of treating GERD makes it the most costly gastrointestinal disease in the United States; figures from 2002 estimate that GERD management was associated with costs of up to $9.3 billion, whereas indirect costs are thought to be considerably more.5
The treatment of GERD advanced greatly in the late 1980s with the introduction of proton pump inhibitors (PPIs), which have now become the mainstay of therapy for acid suppression.6 Despite their efficacy, several studies have shown that a significant proportion of GERD patients are either partial or non-responders to PPI therapy, whereby their heartburn and/or regurgitation symptoms are not relieved by either a standard (single) or double-dose PPI during a minimum trial of 8 weeks.7 El-Serag et al.3 reviewed 19 studies looking at GERD patients treated with PPIs, and found an overall prevalence of partial and non-responders of up to 45% in observational studies. Non-randomized studies revealed a prevalence of 17% when defined as “persisting troublesome heartburn” and 28% when defined as troublesome regurgitation; randomized studies revealed prevalence rates of 32% and 28%, respectively.3
Partial responders and non-responders are distinguished by the extent in which they relieve symptom alleviation. When evaluating the extent or response, it is important to evaluate relevant symptoms of GER, such as heartburn and regurgitation, independently of other dyspeptic-like symptoms such as nausea, bloating, belching, and early satiety. A small number of clinical trials have demonstrated that PPIs can be effective in relieving dyspeptic-like symptoms, although such symptoms are not directly related to acid reflux. The mechanisms by which PPIs improve these symptoms remains unclear, but PPIs may have other therapeutic effects aside from reducing intraluminal acidity. Nevertheless, unrealistic expectations commonly exist for complete resolution of these dyspeptic-like symptoms, which are unfortunately still less likely to respond to anti-reflux therapy relative to typical symptoms, and contribute greatly toward disease burden and reduce quality of life.
In addition, failure to respond to a PPI is also associated with increased health-care costs. These increased costs occur because of a number of factors including repetitive use of resources including outpatient visits, diagnostic procedures, and prescription drugs.8 In addition, persistence of reflux symptoms may cause significant distress, with even mild symptoms being reported to cause a reduction in quality of life.9 This negative impact on daily functioning has been reported by Bytzer et al.9 using the Quality of Life in Reflux and Dyspepsia questionnaire.
Patients with GERD are typically classified as either erosive esophagitis (EE) or non-erosive reflux disease (NERD).10 These patients display varying responses to PPI therapy, with NERD patients generally having lower response rates while representing the largest proportion of patients with refractory heartburn.10 On the other hand, individuals with EE, who make up 30–40% of GERD patients, are much more likely to have a symptomatic response to PPI, with a response rate of 56% at 4 weeks when treated with a PPI administered once daily.11
This review will outline a diagnostic approach for the PPI non-responder described in the case study, review pathophysiologic mechanisms associated with PPI failure, and discuss therapeutic options for GERD patients who fail to respond to PPI therapy.
MECHANISM AND FACTORS INVOLVED IN PPI FAILURE
Compliance
The initial, key diagnostic step when first evaluating a patient with GERD symptoms unresponsive to PPI therapy is to evaluate the compliance of PPI therapy by assessing both the drug dosage and timing of medication administration.6 Studies have found PPI compliance to be quite low in patients with GERD, with adherence of only 55% 1 month after receiving the initial prescription, with a further decrease to just 30% at 6 months.7 In a study by Dickman et al.,12 which studied the clinical characteristics shared by non-responders, it was found that lack of response was associated with reduced compliance, and that non-responders were significantly less likely to have implemented lifestyle modifications, such as weight control and avoiding late night meals. Non-compliance should be suspected in patients who had full symptom resolution for a period and then develop a recurrence of symptoms.13 Of the two main subgroups of GERD, NERD patients have the lowest compliance rate for PPI therapy, with the following reasons most commonly reported for non-compliance: insufficient knowledge regarding the condition, side effects, and wanting personal control.14
In regards to the proper timing of administration, PPIs should ideally be administered 30 min before breakfast.15 Unfortunately, many health-care providers commonly fail to provide this critical piece of information, leaving patients unaware.13 As suboptimal timing can reduce PPI efficacy, Gunaratnam et al.16 evaluated the prevalence of inappropriate dosing. Suboptimal dosing was described as being >60 min before meals, following meals, at bedtime, or as needed, whereas optimal dosing was described as being up to 60 min before or with meals. In this study, it was found that only 46% of patients were dosed optimally.16 Of the remaining 54%, 39% took their PPI dose >60 min before meals, whereas 30% took their PPI following meals, 28% took their PPI at bedtime, and 4% as needed.16 The dose was timed appropriately in order to maximize the suppression of gastric acid in only 12% of patients.16 These results are supported by a study by Barrison et al.17 who found that almost 70% of primary care providers in the United States, and 20% of gastroenterologists, did not consider drug administration in relation to meals as significant or advised administration at bedtime.
Reduced PPI bioavailability and increased PPI metabolism
The bioavailability of the various PPIs differs greatly; however, differences in pharmacokinetic properties have not been shown to be clinically relevant, as clinically meaningful differences in efficacy have not been proven.13 However, genetic differences in drug metabolism may have a significant role in PPI efficacy. Hepatic cytochrome P450 2C enzymes are key factors in the metabolism of PPIs.6 Genetic variations in these enzymes and their ability to metabolize PPIs may alter total plasma drug concentration, thereby leading to differences in acid suppression among GERD patients.6 Those individuals who rapidly metabolize PPIs will have reduced gastric acid suppression and lower rates of healing when compared with normal metabolizers.6 Rapid metabolizers are more common among Asian populations with 12–20% being affected, compared with only 2–6% in Caucasian populations.6
There is considerable genetic variation in the ability to metabolize PPIs based on patients' cytochrome P450 2C enzymes. Although both lansoprazole and omeprazole are thought to be metabolized by the cytochrome expressed in patients with genotype CYP2C19, another study suggests esomeprazole is preferentially metabolized through the CYP3A4 pathway.6, 18 This difference was suggested in a study by Schwab et al.,18 which provides a theoretical framework for rapid metabolizers to overcome an inadequate response to treatment by changing PPIs.
Functional bowel disorder
Functional heartburn compromises 50% of NERD patients and is more common in young, non-obese, female patients.3, 19 The Rome committee for functional esophageal disorders defines functional heartburn as sporadic retrosternal burning pain in the absence of structural abnormalities, pathologic acid reflux, or motility disorders.19 There are two main subgroups of functional heartburn.19 The first is referred to as esophageal hypersensitivity and makes up 40% of patients with functional heartburn. These patients, despite having esophageal acid exposure within the physiological range, demonstrate a temporal relationship between symptoms and acid reflux events.19 The increasing use of impedance-pH studies may have contributed to a greater recognition of this group of patients. Patients within this subgroup may respond to therapy if given high doses of PPIs.19
The second subgroup, termed non-esophageal reflux disease, consists of patients where there is little correlation between acid reflux events and symptoms, which raises the issue of whether symptoms are the result of non-acid stimuli or influences besides luminal stimuli. Therefore, in this group, instead of increasing the dose of PPI, alternate treatment options, such as selective serotonin reuptake inhibitors and tricyclic anti-depressants are recommended (see Treatment section below).
There are numerous possible explanations for the development of esophageal hypersensitivity, including dilated intercellular spaces, central sensitization, and esophageal afferent sensitization. Intercellular space dilatation has been observed in both animal and human models after esophageal acid exposure.19 Dilated intercellular spaces can help explain the presence of symptoms in the absence of esophageal changes, as this finding is independent of esophageal inflammation, which is characterized by higher paracellular permeability, potentially allowing chemosensitive nociceptors to be activated.6, 19 Yet another explanation is central nervous system sensitization, which may lead to both visceral and somatic hypersensitivity. In some patients, injury to the distal esophageal mucosa in the presence of acid may cause central sensitization and elevated visceral pain sensation that continues long after the initial injury has healed. This process occurs because of hyperexcitability of spinal cord neurons, the result of acid-induced tissue injury causing activation of nociceptive C fibers.19
It has also been demonstrated that even transient acidification of the esophagus can cause persistent esophageal sensitivity to a variety of sensory modalities.20 Hobston et al.20 evaluated 15 NERD patients and 15 matched controls, with all participants undergoing esophageal manometry, 24- h pH monitoring, evoked central nervous system potential, and sensory testing. From these investigations, it was found that pain thresholds were lower, and esophageal evoked potentials were significantly shorter, in reflux-negative NERD patients compared with both controls and reflux-positive patients.20 These findings provide a rationale to support the role of esophageal visceral hyperalgesia in patients with non-esophageal reflux disease and may help in the development of targeted therapy.20
Non-acid reflux
Non-acid GER involves the reflux of gastric contents with a pH >4.13 Esophageal hypersensitivity involves the perception of “not-abnormal reflux,” which may include episodes of non-acid reflux; these may be influenced by the degree of esophageal distension and/or higher reflux volumes.7 Duodenogastroesophageal reflux (DGER) is the reflux of duodenal contents into the esophagus, which can potentially be diagnosed by measuring the bilirubin level in the refluxate,13 although accurate measurement is problematic. Bile reflux is uncommon, accounting for only 10–15% of non-acid reflux.6
The reason why refluxate with higher pH values cause reflux symptoms is not well understood, but it has been proposed that esophageal hypersensitivity and mechanical distension may be involved.11 Moreover, the proximal extension of reflux contents in the esophagus is an important factor in symptomatic reflux unresponsive to PPI, possibly due to either elevated sensitivity in the proximal esophagus or a “summation effect” secondary to greater recruitment of nociceptors along the esophagus.11 It has also been established that the esophageal transition zone of striated and smooth muscle has a higher sensitivity to mechanical stimulation compared with the distal esophagus smooth muscle.11 In a multicenter study, results indicated that 37% of patients on at least a twice-daily PPI, with study day symptoms, had a positive symptom index (SI) for non-acid reflux.21
Gasiorowska et al.22 sought to determine whether the frequency of DGER and/or weakly acidic reflux differed between PPI responders and non-responders. This study consisted of 24 PPI non-responders and 23 successful responders, and it was found that 82% of successful PPI responders had abnormal DGER compared with 67% of PPI failure patients.22 It was therefore concluded that no significant difference exists in the degree of DGER/acid exposure at the time of treatment between PPI responders and non-responders.22 Reflux symptoms in non-responders are more typically associated with acid reflux compared with DGER, and the presence of DGER in non-responders is more likely a PPI phenomenon rather than a unique failure phenomenon.22
Nocturnal reflux
Gastric acid secretion follows a circadian rhythm, with a decrease in intragastric pH during the nighttime fasting period, and a rise toward dawn and postprandially.23 Nocturnal acid breakthrough is a phenomenon defined as a drop in gastric pH <4 for at least 1 h at night in a patient on a twice-daily PPI.24 Nocturnal gastric acid secretion in the presence of twice-daily PPI therapy occurs in 60–80% of patients.6 It is thought that this nocturnal activity serves as a protective mechanism against gastric nitrosamines and bacteria; however, it may lead to acid breakthrough at a time when the esophagus is not protected and vulnerable.6 As a result, augmenting gastric acid suppression by adding a nighttime histamine-type-2 receptor antagonist (H2RA), in addition to using a PPI twice daily, has been suggested, and has been shown to improve control of daytime and nocturnal breakthrough.24 Peghini et al.25 looked at the use of a third dose of omeprazole at bedtime compared with bedtime ranitidine in a small study of 12 patients receiving twice-daily omeprazole. Following 1 week of omeprazole 20 mg twice-daily treatment, participants underwent intragastric pH monitoring with varying bedtime treatments with omeprazole, ranitidine, or placebo.25 It was found that nocturnal secretion was best controlled by bedtime ranitidine, suggesting that nocturnal breakthrough is likely mediated by histamine.25
Similarly, in a study by Rackoff et al.,24 56 GERD patients taking both PPI twice daily and nighttime H2RA were reviewed. Unlike the study by Peghini et al., where intragastric pH monitoring was performed, Rackoff et al.24 focused instead on symptom reporting. These patients were given a questionnaire to assess their overall symptoms, nocturnal symptoms, sleep disturbance, and the duration of therapy. They found that an H2RA improved overall symptoms in 72%, whereas nocturnal reflux symptoms improved in 74% and sleep disturbance was reduced in 67% whereas 13% stopped the H2RA because of reduced improvement after 1 month of treatment.24
Although these two studies demonstrated gastric acid suppression and symptom improvement with the use of a nighttime H2RA, controversy exists as tolerance to H2RAs has been demonstrated. Fackler et al.26 determined that when an H2RA was added to twice-daily omeprazole, nocturnal gastric acid exposure returned to pH levels seen when PPIs were used alone after 1 week of H2RA therapy. Therefore, it was concluded that H2RAs are beneficial in defending against nocturnal reflux, but this temporary effect lasts only 1 week before tolerance develops.26 As H2RA use may be ineffective at controlling nocturnal breakthrough after >1 week, an alternative approach proposed is to treat at-risk patients intermittently upon exposure to reflux stimuli, such as large fatty meals.6
Functional dyspepsia
Functional dyspepsia is diagnosed using the Rome criteria in conjunction with a normal upper endoscopy. It is often difficult to separate patients with GERD symptoms vs. those with functional dyspepsia symptoms. Substantial overlap exists epidemiologically, symptomatically, and even diagnostically. Despite the frequency of overlap among these two populations, few studies have been reported. The goal is to identify those who will respond to acid-suppressive therapy, this often renders a further diagnostic dilemma in patients who are PPI non-responders.27
Delayed gastric emptying
As the number of patients with diabetes mellitus and/or narcotic use increases, the amount of evidence regarding the role of delayed gastric emptying in refractory GERD also grows.8 Dickman et al.12 evaluated GERD patients who had received a PPI once or twice a day for a minimum of 3 months, and assessed symptoms of gastroparesis using the Gastroparesis Cardinal Symptom Index (GCSI) questionnaire. The 245 patients included in the study were divided into three groups representing patients with a full response to PPI once daily (group A), patients who did not respond to PPI once daily (group B), and patients who failed to respond to a PPI twice daily (group C), respectively.12 The GCSI was significantly different across these three groups, with lower values in group A compared with either group B or C (a lower score indicates less severe symptoms). The prevalence of a very high GCSI (score ≥27) was 6.3%, 7.7%, and 19% in groups A, B, and C, respectively.12 Unfortunately, despite worsening GCSI scores in patients with refractory symptoms on twice-daily PPI, gastric emptying scans were not measured.12 Moreover, Kudara et al.28 assessed 15 individuals with EE on lansoprazole 30 mg q.d., evaluating each patients' gastric emptying after 8 weeks of treatment, and found that 4 of the patients who continued to be symptomatic had significant delays in gastric emptying in comparison with the remaining 11 who showed response. Although interesting, this study is limited by the small sample size, the inclusion of only patients with EE, and the absence of a gastric emptying scan at baseline.28
Psychological comorbidity
Stress, emotional, and psychological states are concomitant factors that can lead to treatment failure, both through lack of adherence to a PPI regimen and by altering the response to treatment.13 In a study by Van der Velden et al.,29 it was found that 73% of patients with PPI treatment failure had mental health problems with somatization, in comparison with 15% of patients who responded to treatment. Somatization and psychological problems have also been linked with worsening of symptom intensity and higher discomfort with esophageal acid and balloon distension studies.3 Psychological comorbidities can alter esophageal perception, allowing low intensity stimuli within the esophagus to be perceived as painful.19
Although there are no studies demonstrating rising acid secretion in response to stress, 64% of patients with GERD labeled stress as an important cause of reflux exacerbation, whereby stress relieving interventions have been linked to symptom improvement ratings.19 Also, high anxiety levels are predictive of poor symptom response, especially in those without reflux esophagitis.3
Becher and El-Serag30 performed a systematic review, which included nine studies with a total of 14,774 non-responders, to evaluate the relationship between persistent symptoms and health-related quality of life. This review demonstrated that patients with persistent reflux have decreased health both physically and mentally. PPI non-responders had clinically significant lower mental health at baseline when compared with responders, suggesting a bi-directional relationship whereby lower mental health could be a cause or an effect of persistent symptoms. Furthermore, in patients with persistent symptoms, high anxiety levels were important features of reduced mental health compared with patients who respond to PPI.30 Consequently, psychological comorbidities may have an important role in treatment failure, and treatment approaches that focus on underlying psychosocial problems should help to improve the response of these patients to PPIs.11
Helicobacter pylori
Studies have observed a significant reduction in gastric acid production in patients infected by Helicobacter pylori while on PPI therapy.31 The greater acid suppression observed in H. pylori-positive patients is thought to result from proximal migration of the bacteria that occurs with PPI therapy.13 To test the theory that healing and symptom resolution would be better in H. pylori-infected individuals secondary to increased intragastric pH with PPI treatment, Holtmann et al.31 studied 971 patients in a double-blind randomized trial with endoscopic confirmed esophagitis; H. pylori status was determined using a 13C-urea breath test. All patients received pantoprazole 40 mg q.d. for 4 weeks and underwent endoscopy at the 4- and 8-week mark. In the H. pylori-positive patients, healing rates were significantly higher after 4 (86.6% vs. 76.3% P=0.0005) and 8 weeks (96.4% vs. 91.8% P<0.004). Relief of symptoms after 4 weeks was also significantly (P<0.05) better in H. pylori-infected patients than in uninfected patients.31
Eosinophilic Esophagitis
Eosinophilic esophagitis (EoE) should be considered in the differential diagnosis of those with GERD refractory to treatment,6 as these patients are usually unresponsive to once and even twice-daily PPI therapy, and usually require further evaluation with upper endoscopy.32 The patients more commonly affected by EoE are young males who often have associated food allergies and/or asthma who present with a history of occasional dysphagia with solids and/or food impaction. In order to diagnose EoE, endoscopy must be performed, which may demonstrate esophageal rings, white exudates, and longitudinal furrows.6 A meta-analysis of endoscopic findings in EoE from 100 publications involving 4,678 patients with EoE and 2,742 control patients found that the sensitivity, specificity, and predictive values of endoscopic findings alone are insufficient as a diagnostic criteria.33 Therefore, proximal and distal esophageal biopsies should be performed, with evidence of >15–20 eosinophils per high-power field.6
The cost effectiveness of performing biopsy during endoscopy has been debated. The American Gastroenterology Association do not recommend biopsy in patients with exclusively refractory GERD symptoms, only in patients with dysphagia, because of the high prevalence of EoE in this group.32 However, in a study performed by Miller et al.,32 biopsy at the time of upper endoscopy in those with refractory GERD without dysphagia was found to be cost effective, with roughly 7% of patients within this group found to have EoE, of which 45% would have been missed in the absence of biopsies. These undiagnosed patients would continue to be symptomatic with increased health-care costs incurred through unsuccessful PPI use, clinic visits, and diagnostics tests, as well as diminished quality of life.32
Although the pathogenesis is unclear, it is thought that EoE may either be an atypical form of GERD or a T-helper cell cytokine response following exposure to allergens or food.6 Patients suspected of EoE should be given a 2-month course of PPI to exclude PPI esophageal eosinophilia (this entity is considered distinct from EoE but not necessarily GERD). To determine whether reflux is contributing to eosinophilia additional evaluation, such as pH testing, may be indicated. Topical steroids are recommended for those who fail to respond to PPI therapy.33
Extraesophageal symptoms
GERD symptoms can be divided into esophageal/typical symptoms, such as regurgitation and heartburn, or extraesophageal/atypical symptoms, such as globus, chronic cough, and/or hoarseness. The Montreal Consensus has acknowledged links between GERD and these extraesophageal symptoms; however, despite multiple epidemiological studies identifying a link, causality cannot be concluded. These disorders are often multifactorial, with GER likely a co-factor rather than the etiology. Consequently, it is very important that patients with these atypical symptoms have proper evaluation for non-GERD causes, with work up for allergy, pulmonary, and/or ENT disorders.
Aerophagia
Aerophagia is a functional gastrointestinal syndrome characterized by repetitive troublesome belching secondary to excessive air swallowing.34 Aerophagia can result in abdominal discomfort and may be associated with gastric visceral hypersensitivity.34 Consequently, the symptoms of aerophagia often overlap with other functional upper GI disorders.34 Aerophagia does not respond to PPI treatment, unless it is accompanied by true acid reflux.
Globus
The association between globus, the sensation of a lump or foreign body within the throat, and GERD has been debated in Western countries for many years.35 In patients with globus sensation and laryngeal symptoms, the prevalence of GERD is high, with symptom improvement with PPI administration. Although this finding is suggestive of an association, it may actually be that the laryngeal symptoms are associated with GERD.35
Zollinger–Ellison syndrome
Zollinger–Ellison is a gastrin-producing tumor that develop because of the proliferation of non-beta islet cells in the pancreas, which ultimately leads to excessive secretion of acid from the stomach parietal cells. Although esophageal reflux has not been commonly described in Zollinger–Ellison syndrome, a prospective study by Richter et al.36 demonstrated a high prevalence of GERD in patients with this condition. Out of a group of 15 patients with Zollinger–Ellison syndrome, 9 patients had objective findings of GERD.36 Esophageal disease was the initial manifestation in five of these patients.36 Consequently, in patients with intractable heartburn despite anti-reflux measures, weight loss, dietary changes, and antacids, Zollinger–Ellison should be considered.36
Rumination syndrome
Rumination syndrome is a behavioral condition characterized by postprandial regurgitation. Symptoms of postprandial regurgitation are often mistaken for GERD. It is been recommended to obtain a combined manometry-impedence test to help distinguish this entity from GERD.37 Treatment consists of behavioral therapy and reassurance.
Diagnostic evaluation
Diagnostic testing in PPI refractory GERD patients is primarily used to identify persistent acid reflux, recognize alarming anatomical and histological abnormalities of the upper GI tract, and detect functional heartburn.
Upper endoscopy
Upper endoscopy is the common initial test used clinically to evaluate patients with GERD who have failed PPI treatment. The American Society of Gastrointestinal Endoscopy (ASGE) has further endorsed this clinical strategy. The goal is to identify histological or anatomical abnormalities that can explain patients' refractoriness to treatment. However, in general, the value of endoscopy in discovering GERD-related findings in patients with refractory GERD symptoms is low. A recent study evaluated endoscopic and histologic findings and the association between those failing to respond to once-daily PPI compared with those on no treatment. A total of 105 subjects (mean age 54.7±15.7 years, 34 women) were enrolled in the PPI treatment failure group and 91 (mean age 53.4±15.8 years, 23 women) were enrolled in the no treatment group.38 Anatomical findings during upper endoscopy were significantly more common in the no treatment group compared with the refractory GERD group (55.2% vs. 40.7%, P=0.04). GERD-related findings were significantly more common in the no treatment group compared with the refractory GERD group (EE: 30.8% vs. 6.7%, respectively, P<0.05). Thus, upper endoscopy seems to have a low diagnostic yield in this patient population. Some researchers disagree with this assessment, arguing that endoscopic evaluation is helpful in ruling out pill and infectious esophagitis, EoE (4–6% in PPI refractory patients) and other rare cases of refractory GERD such as Zollinger–Ellison syndrome.7
Esophageal pH monitoring
A widely available and accepted test for measuring esophageal acid exposure remains ambulatory pH monitoring.39, 40 Prolonged (48–96 h) wireless pH monitoring as compared with the catheter-based systems increases the likelihood of detecting reflux disease in patients undergoing symptom evaluation.41 Prakash et al.41 studied 157 subjects who had undergone pH monitoring with the wireless system for symptom evaluation. Acid exposure time, SI, and a measure of reflux-associated symptom probability were calculated after days 1 and 2 of recording. Data revealed extending recording time increased symptom recording in subjects by 6.8% and doubled the number of symptoms available for association with acid reflux events.
The question of whether to perform testing “on” or “off” therapy is often confusing and multiple studies have been conducted to better assess this predicament. In a study by Zerbib et al.,42 71 patients were evaluated while on PPI and 79 patients off of PPI. This study concluded that ambulatory reflux monitoring is of higher yield when it is performed after stopping PPIs, as 52% of patients off PPI had a positive symptom association probability (SAP), compared with only 31% while on PPI.42 Moreover, roughly half of patients found to have negative SAP on PPI demonstrated positive SAP while off PPI.42 In a similar study by Hemminck et al.,43 30 patients with symptoms of regurgitation, heartburn, and/or chest pain despite treatment with twice-daily PPI had ambulatory 24-h pH impedence monitoring both on PPI, as well as after 7 days without PPI. Reflux episodes were recorded, as well as SAP.43 This study found that PPI therapy did not impact the total number of reflux episodes, but did result in weaker acidic reflux episodes (48±31 on PPI, 24±17 off PPI) with fewer acid reflux episodes (20±25 on PPI, 49±34 off PPI).43 Symptom association analysis demonstrated 15 patients with positive SAP while off PPI, whereas only 11 patients had positive SAP while on PPI; however, this difference was not statistically significant.43
Based on the findings of these recent studies, in order to demonstrate or exclude GERD in patients with PPI-resistant symptoms; ambulatory 24-h impedence-pH monitoring should be performed “off” PPI therapy44 offering the best chance to assess correlation between symptoms and reflux episodes. Once reflux has been identified as the source of symptoms, measurement of esophageal acid exposure while on PPI therapy can be useful,43 as it provides useful information regarding the efficacy of acid-suppressive treatment and may reveal a positive association between symptoms and weakly acidic reflux episodes, which is a possible indication for anti-reflux surgery.
On the other hand, performing the test “on” PPI therapy provides useful information regarding the efficacy of acid-suppressive treatment and may reveal a positive association between symptoms and weakly acidic reflux episodes, which is a possible indication for anti-reflux surgery.45
An alternative approach is to tailor based on the clinical presentation.45 For example, in patients with low probability of GERD (extraesophageal symptoms without typical symptoms) monitoring without PPI is preferred as this enables GERD to be ruled out. In contrast, those with higher probability of GERD (typical symptoms with partial response), testing on PPI makes it possible to evaluate ongoing reflux despite therapy.45
Additional data may be obtained by inclusion of a symptom–reflux correlation measure such as the SI, and/or SAP, both of which may help determine the relationship between heartburn episodes and acid reflux events, regardless of whether the pH test is normal or abnormal. It is important to note that such symptom association analysis can only be implemented when symptoms are of sudden onset, but cannot be used for more longstanding symptoms of globus or hoarseness. Various methods have been developed to help quantify this temporal relationship and patients with a positive relationship are more likely to respond to therapy. SI is one such measure, whereby the percentage of reflux-related symptom events is calculated, with a threshold of ≥50%. The problem with this method is it does not take into account the total number of reflux episodes. An additional method is SAP, which depicts the probability that the observed temporal relation did not occur by chance by calculating the statistical relationship between symptoms and events using Fisher's exact test (threshold ≥95%).
Nevertheless, Slaughter et al.46 showed that SI or SAP can be over-interpreted in a cross-sectional study of 254 patients refractory to PPI therapy. Although 70% of the studied population had reflux rates <10%, SI and SAP values were determined by chance occurrences rather than reflux events and symptoms. The values also varied significantly from day to day. Overall, pH-monitoring studies have shown that PPI refractory patients have an abnormal number of reflux events and higher sensitivity to all types of reflux including acidic, weakly acidic, mixed and propagated.47, 48
Impedence-pH monitoring
Typical and atypical symptoms not responding to PPIs remain the main indication for performing impedence-pH testing. The advantage of combined impedence-pH test is to distinguish NERD from those patients with functional heartburn and esophageal hypersensitivity.7 It also allows for the detection of most GER episodes and identify acidic, weakly acidic, and weakly alkaline reflux.44 Interestingly, a postprandial impedence-pH study in patients who failed PPI twice daily, at baseline and during therapy, documented a shift from primarily acidic reflux to mostly weakly acidic reflux. Unlike untreated patients, regurgitation became the predominant symptom in patients who failed PPI twice daily.42 A recent multichannel intraluminal impedence-pH study in refractory GERD patients while on PPI therapy showed that up to 68% of heartburn episodes were associated with weakly acidic reflux.42 High esophageal proximal extent was the only important factor associated with reflux perception. Zerbib et al.42 reported results of multichannel intraluminal impedence-pH monitoring in a group of 150 patients, 79 patients off vs. 71 on twice a day PPI treatment. In patients off PPI, multichannel intraluminal impedence-pH monitoring added little value to patients' diagnosis when compared with pH monitoring alone (5–10%). Adding impedence to pH monitoring in patients on PPI therapy improved the diagnostic yield by 15–20%, resulting in better symptom correlation analysis than during the pH test alone.
Esophageal manometry
In a study of 76 patients with PPI refractory non-esophageal reflux disease, manometry was performed during PPI treatment, identifying 25% with an underlying esophageal motility disorder (Izawa et al.).49 No significant differences were noted between the groups with esophageal motility disorder and non-esophageal motility disorder in their 24-h intraesophageal pH holding time, mean number of GER episodes, or mean number of proximal reflux episodes, suggesting that some PPI refractory NERD patients have underlying esophageal motility disorders. Conventional or high-resolution manometry should be performed in order to rule out severe motor disorders including EoE as mentioned above, achalasia, scleroderma, and other connective tissue diseases.7 The clinical utility of esophageal motility is highest in patients with symptoms of dysphagia and chest pain.50
Gastric emptying scan
Delayed gastric emptying is an important contributor to GERD with a prevalence of 40% among GERD patients.13 Although data are limited, delayed gastric emptying has been shown to increase daily and postprandial liquid/mixed reflux events.51 In addition, many patients with symptoms of reflux often overlap with non-GERD causes such as gastroparesis; therefore, if there is clinical suspicion of delayed gastric emptying contributing to reflux symptoms then a 4-h gastric emptying scan should be obtained.
Treatment
After ensuring compliance and appropriate dosing, transitioning to an alternative PPI agent should be considered. In a multicenter, randomized double-blind study, evaluating patients with ongoing symptoms despite therapy with lansoprazole 30 mg daily.52 Patients were subsequently randomized to receive 8 weeks of single-dose esomeprazole (40 mg daily) or have their lansoprazole dose increased to 30 mg b.i.d.52 The percentage of days free of heartburn symptoms from day 8 until end of treatment were measured and it was found that switching to an alternative PPI was as effective as doubling the dose of PPI (54.4% compared with 57.5%, respectively).52
Therapeutic options in patients who are considered to be PPI non-responders should be based on the results of a diagnostic workup that includes at least an upper endoscopy and impedance-pH test while a patient is on double-dose PPI, or a wireless pH capsule study while patient is off PPI therapy.52 These diagnostic tests can stratify the PPI non-responder patient population to those with weakly acidic reflux, residual acidic reflux, or functional heartburn as the underlying mechanism for their symptoms.53 Subsequently, targeted therapeutic modalities could be tailored to each patient.
Lifestyle modifications
Several therapeutic options should be considered in all patients who have failed PPI treatment regardless of their diagnostic stratification. Lifestyle modification should be considered in all patients as an adjunct to any anti-reflux treatment. Various lifestyle changes have been proposed in the literature but most of them lack evidence to support their use in clinical practice.54 However, because of the lack of evidence is primarily due to absence or paucity of interventional trials, it is common practice to recommend a variety of lifestyle modifications. A recent study demonstrated that lifestyle modification counseling was associated with a significant improvement in health-related quality of life scores at the start of the medical therapy for GERD.55 Interestingly, there has been inconsistent data about the value of alcohol and tobacco cessation,56 avoidance of citrus juices, spicy food, carbonated beverages, or caffeine.57 The only lifestyle modifications that have been shown to improve symptoms and/or esophageal inflammation include, weight loss, elevating the head of the bed, and avoidance of late night meals (at least 3 h prior bedtime).54, 58, 59, 60 Unfortunately, studies assessing the value of lifestyle modifications in the context of PPI non-responders are still unavailable.
PPI non-responders, who demonstrate weakly acidic reflux as the underlying cause of their symptom, should be considered for the following therapeutic modalities, transient lower esophageal sphincter relaxation reducers, pro-motility compounds, endoscopic treatment for GERD, and anti-reflux surgery15 (Figures 1 and 2 and Table 1). As esophageal hypersensitivity has an important role in symptom generation of PPI non-responders with weakly acidic reflux, pain modulators may also provide a therapeutic option22, 61, 62 (Table 2). Non-responders, who demonstrate residual acidic reflux as the underlying cause of their symptoms, should be re-evaluated for lifestyle modifications and proper compliance/adherence as well as considered for other anti-reflux medications such as sucralfate, gaviscon, and H2RAs. In addition, endoscopic treatment for GERD and anti-reflux surgery should be also entertained. The role of pain modulators in this group of patients remains to be elucidated. The last group of PPI non-responders includes patients with functional heartburn. In this group of patients, pain modulators are likely to be the most efficacious therapeutic strategy.
Figure 1.
The US GERD population: responders and non-responders. GERD, gastroesophageal reflux disease; PPI, proton pump inhibitor.
Figure 2.
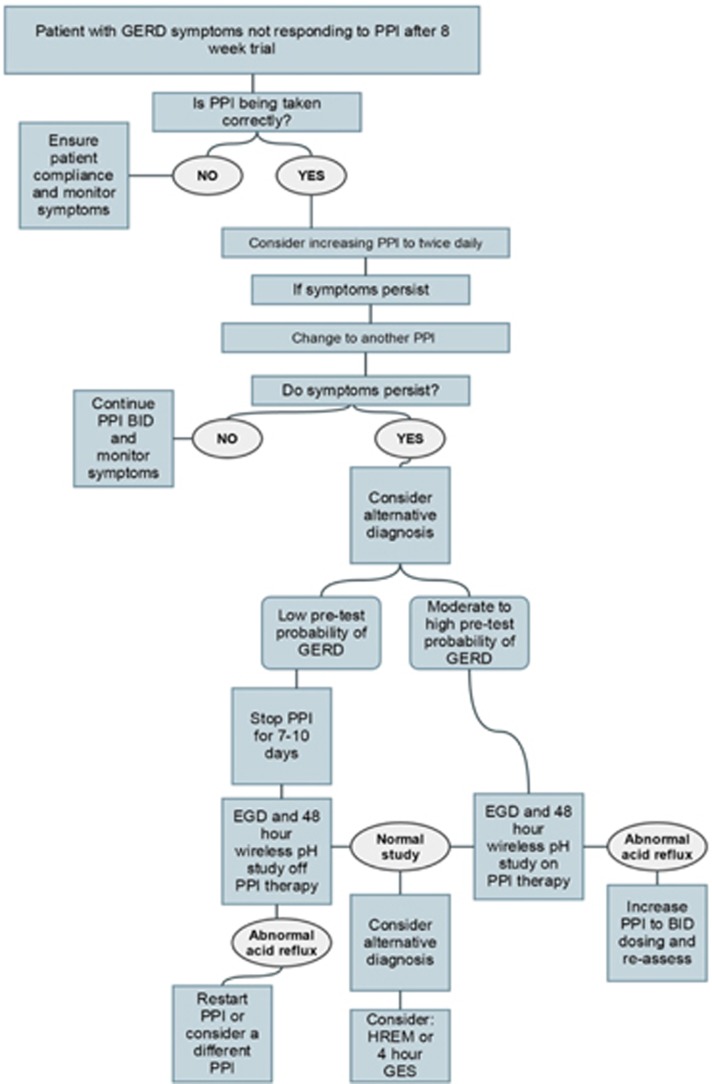
Diagnostic algorithm for PPI non-responders. EGD, esophagogastroduodenoscopy; GERD, gastroesophageal reflux disease; GES, gastric emptying scan; HREM, high-resolution esophageal manometry; PPI, proton pump inhibitor.
Table 1. Causes of PPI failure.
| Non-compliance |
| Improper dosing/timing |
| Rapid PPI metabolism |
| Reduced PPI bioavailability |
| Weakly acid reflux |
| Nocturnal reflux |
| Duodenogastroesophageal/bile reflux |
| Functional heartburn |
| Esophageal hypersensitivity |
| Esophageal motility disorders |
| Eosinophilic esophagitis |
| Rumination syndrome |
| Aerophagia |
| Globus |
| Psychological comorbidity |
| Functional dyspepsia |
| Delayed gastric emptying |
| Helicobacter pylori infection status |
| Zollinger–Ellison syndrome |
PPI, proton pump inhibitor.
Reference Fass.8
Table 2. Therapeutic options for PPI non-responders.
| Weakly acidic reflux | Residual acidic reflux | Functional heartburn | |
|---|---|---|---|
| Lifestyle modifications | ✓ | ✓ | |
| Compliance/adherence | ✓ | ✓ | |
| Baclofen | ✓ | ✓ | |
| Endoscopic treatment | ✓ | ✓ | |
| Anti-reflux surgery | ✓ | ✓ | |
| Sucralfate | ✓ | ✓ | |
| Gaviscon | ✓ | ✓ | |
| H2RA | ✓ | ||
| Pain modulators | ✓ | ✓ | |
| Psychological intervention | ✓ |
H2RA, histamine-type-2 receptor antagonist; PPI, proton pump inhibitor.
Sucralfate
Sucralfate is an aluminum salt of a sulfate disaccharide that acts by producing a protective mucosal effect through the inhibition of pepsin and ulcer formation.63 Evidence about the value of sucralfate in healing EE has been inconsistent64, 65, 66, 67, 68 and presently we are still devoid of any data about its role in non-responders. However, it is possible that PPI non-responder patients with residual acidic reflux may benefit from sucralfate.
Gaviscon
Gaviscon is an alginate-based formulation, which in the presence of gastric acid forms a gel. The formulation also contains sodium bicarbonate, which in the presence of acid transforms to carbon dioxide. This latter product is subsequently taken into the gel precipitate forming a floating raft on top of the gastric content.69 It has been suggested that the “acid pocket” is an important contributing factor for refractory postprandial reflux symptoms despite adequate acid suppression as documented by pH studies. In several case-controlled trials of small cohorts of patients, gaviscon has been shown to form a gel-like barrier over gastric contents, thereby displacing or eliminating the “acid pocket”.70, 71 The value of gaviscon in patients with refractory GERD has been scarcely studied. However, owing to its potential effect on the “acid pocket”, which appears to have a role in non-responders, it is possible that the drug may be efficacious.
HISTAMINE 2 RECEPTOR ANTAGONISTS
It has been proposed that H2RAs could be added at bedtime to patients who have failed PPI twice daily.25 Originally, it has been suggested that the effect of H2RA in PPI non-responders is through the reduction of intragastric nocturnal acid breakthrough duration. However, nocturnal acid breakthrough is unlikely to have an important effect on symptomatic GER events during sleep. In addition, concerns have been raised about the rapid development of tolerance after only 1 week of H2RA treatment. Furthermore, combining PPI once daily with an H2RA at bedtime provides significantly less acid suppression as compared with a PPI twice daily.26
TRANSIENT LOWER ESOPHAGEAL SPHINCTER RELAXATION REDUCERS: GABA-B AGONISTS
Baclofen, a GABA-B agonist, is the only transient lower esophageal sphincter relaxation reducer that is currently available as an add-on therapy for non-responders.72, 73 Doses of up to 20 mg three times daily can be used in patients with GERD who are refractory to PPI treatment.74 However, fatigue, drowsiness, headache, and nausea can be limiting side effects.
Prokinetics
Prokinetic agents have been proposed to improve GERD-related symptoms by different potential mechanisms that include improvement in esophageal peristalsis, acceleration of esophageal acid clearance, increase in lower esophageal sphincter basal pressure and improve gastric emptying. The clinical benefit of prokinetics as the sole treatment for GERD has been modest at best. Moreover, their use has been hampered by many adverse effects.10 Prokinetics have been suggested to improve GERD-related symptoms as an add-on therapy in patients who failed PPI treatment.52
Endoscopic treatment
The majority of endoscopic procedures for GERD treatment have been discontinued over the last decade because of unacceptable side effects, modest or lack of long-term efficacy, cost, time invested, and lack of reversibility.75, 76 Currently, only two endoluminal procedures are readily available. EsophyX (EndoGastric Solutions, Redmond, WA), which is used to perform trans-oral incisionless fundoplication and the Stretta procedure (Mederi Therapeutics, Greenwich, CT), which delivers radiofrequency energy into the lower part of the esophagus.77 Overall, these endoscopic procedures have been shown to be an effective therapeutic modality in carefully selected GERD patient. In several studies, both endoscopic techniques were efficacious in reducing the dose or completely eliminate the need for PPI treatment in non-responders.
Anti-reflux surgery
Refractory GERD has been reported as the most common indication for laparoscopic anti-reflux surgery in nearly 90% of the patients.78 Despite the high efficacy of anti-reflux surgery for preventing all reflux events, the utilization of this procedure has been declining over the last decade because of concerns related to short- and long-term complications need for reoperation, and recurrence of GERD-related symptoms.79, 80, 81 The magnetic sphincter augmentation device that comprises a ring of interlinked titanium beads with magnetic core (LINX Reflux Management System Thorax Medical, Shoreview, MN) is placed laparoscopically around the gastroesophageal junction in order to augment the lower esophageal sphincter competence.39 The LINX procedure provides an alternative option to the traditional anti-reflux surgery in a carefully selected patient population. A significant reduction in the PPI dose has been reported in most patients undergoing the LINX procedure.82
Alternative therapies/visceral pain modulators/psychological treatment
Pain modulators or visceral analgesics have been shown to significantly improve symptoms in patients with functional heartburn and those with refractory GERD, by acting at the central nervous system and/or peripherally at the sensory afferent level.83, 84 Pain modulators are given in a non-mood-altering dose and they include, tricyclic anti-depressants selective serotonin reuptake inhibitors, triazolopyridine (trazodone), serotonin norepinephrine reuptake inhibitors and others.
Several psychological treatment modalities such a cognitive behavioral therapy, hypnotherapy, biofeedback, and muscle relaxation techniques have all been shown to improve GERD solely or in combination with medical or surgical anti-reflux treatment.85, 86, 87, 88 Finally, acupuncture has been shown to be effective in controlling symptoms of patients with GERD who failed symptomatically to respond to PPI once daily.89
CONCLUSION
In the United States PPIs, as a class, are the third highest selling medication, with annual expenditures of over 13 billion dollars. The majority of PPI costs can be attributed to treating patients with GERD, although PPIs are commonly used for a variety of other conditions (i.e., functional dyspepsia, globus, and nausea). Unfortunately, not all patients respond to PPI therapy (the PPI non-responder), which represents both a challenge and a conundrum to health-care providers. Challenges arise because patients remain symptomatic despite taking a medication prescribed with the intent of improving symptoms, they are exposed to increased risks with the use of an unnecessary medication, they are subjected to increased costs to the use of ineffective medications and they suffer from reduced quality of life.90 The conundrum for the provider is how to efficiently evaluate and treat these patients.91, 92, 93, 94 As outlined in this review, a critical question for health-care providers when faced with a PPI non-responder is to determine why the patient is taking the PPI. PPIs are wonderfully effective agents at treating EE, and are quite effective at treating GERD (with symptoms of heartburn typically responding better than regurgitation). However, PPIs are significantly less effective (i.e., functional dyspepsia) or ineffective (i.e., irritable bowel syndrome) at treating a host of other disorders. This first, critical question is even more complicated than it appears, as reflux symptoms are nonspecific, and are frequently confused with other disorders that respond poorly to PPIs (i.e., functional dyspepsia, EoE). Answering this question not infrequently requires testing (e.g., upper endoscopy, high-resolution esophageal manometry, and pH testing) to ensure an accurate diagnosis and also to assess whether the dose of PPI used is correct. If the indication is appropriate and the dose is correct, then compliance needs to be addressed. As outlined, several studies have consistently shown that patients do not take PPIs correctly, and prescribers frequently fail to provide the correct information to ensure PPI success. Finally, for those patients who fail to respond to a PPI, treating visceral pain, visceral hypersensitivity, and co-existing psychological distress eventually leads to symptom improvement, and should be considered as treatment options our patient presented earlier.
Guarantor of the article: Zilla H. Hussain, MD.
Specific author contributions: Emily E. Henderson, Carla Maradey-Romerao, and Nina George: collected data and helped write manuscript; Ronnie Fass and Brian E. Lacy: helped write and revise manuscript.
Financial support: None.
Potential competing interests: None.
References
- Locke GR, Fett SL, Zinsmeister AR et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 1997; 112: 1448–1456. [DOI] [PubMed] [Google Scholar]
- Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis 1976; 21: 953–956. [DOI] [PubMed] [Google Scholar]
- El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther 2010; 32: 720–737. [DOI] [PubMed] [Google Scholar]
- Peery AF, Dellon ES, Lund J et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012; 143: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler RS, Everhart JE, Donowitz M et al. The burden of selected digestive diseases in the United States. Gastroenterology 2002; 122: 1500–1511. [DOI] [PubMed] [Google Scholar]
- Richter JE. How to manage refractory GERD. Nat Clin Pract Gastroenterol Hepatol 2007; 4: 658–664. [DOI] [PubMed] [Google Scholar]
- Cicala M, Emerenziani S, Guarino MP et al. Proton pump inhibitor resistance, the real challenge in gastro-esophageal reflux disease. World J Gastroenterol 2013; 19: 6529–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. Proton pump inhibitor failure-what are the therapeutic options? Am J Gastroenterol 2009; 104: S33–S38. [DOI] [PubMed] [Google Scholar]
- Bytzer P, Veldhuyzen van Zanten S, Mattsson H et al. Partial symptom-response to proton pump inhibitors in patients with non-erosive reflux disease or reflux oesophagitis – a post hoc analysis of 5796 patients. Aliment Pharmacol Ther 2012; 36: 635–643. [DOI] [PubMed] [Google Scholar]
- Maradey-Romero CFR. New and future drug development for gastroesophageal reflux disease. J Neurogastroenterol Motil 2014; 20: 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershcovici T, Fass R. An algorithm for diagnosis and treatment of refractory GERD. Best Pract Res Clin Gastroenterol 2010; 6: 923–936. [DOI] [PubMed] [Google Scholar]
- Dickman R, Boaz M, Aizic S et al. Comparison of clinical characteristics of patients with gastroesophageal reflux disease who failed proton pump inhibitor therapy versus those who fully responded. J Neurogastroenterol Motil 2011; 17: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R, Shapiro M, Dekel R et al. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease – where next? Aliment Pharmacol Ther 2005; 22: 79–94. [DOI] [PubMed] [Google Scholar]
- Hungin AP, Rubin G, O'Flanagan H. Factors influencing compliance in long-term proton pump inhibitor therapy in general practice. Br J Gen Pract 1999; 49: 463–464. [PMC free article] [PubMed] [Google Scholar]
- Fass R. Therapeutic options for refractory gastroesophageal reflux disease. J Gastroenterol Hepatol 2012; 27: 3–7. [DOI] [PubMed] [Google Scholar]
- Gunaratnam NT, Jessup TP, Inadomi J et al. Sub-optimal proton pump inhibitor dosing is prevalent in patients with poorly controlled gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2006; 23: 1473–1477. [DOI] [PubMed] [Google Scholar]
- Barrison AF, Jarboe LA, Weinberg BM et al. Patterns of proton pump inhibitor use in clinical practice. Am J Med 2001; 111: 469–473. [DOI] [PubMed] [Google Scholar]
- Schwab M, Hofmann U, Schaeffeler E et al. Esomeprazole-induced healing of gastroesophageal reflux disease is unrelated to the genotype of CYP2C19: evidence from clinical and pharmacokinetic data. Clin Pharmacol Ther 2005; 78: 627–634. [DOI] [PubMed] [Google Scholar]
- Fass R. Functional heartburn: the stimulus, the pain, and the brain. Gut 2002; 51: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson AR, Furlong PL, Azi Q. Esophageal afferent pathway sensitivity in non-erosive reflux disease. Neurogastroenterol Motil 2008; 20: 877–883. [DOI] [PubMed] [Google Scholar]
- Mainie I, Tutuian R, Shay S et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006; 55: 1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiorowska A, Navarro-Rodriguez T, Wendel C et al. Comparison of the degree of duodenogastroesophageal reflux and acid reflux between patients who failed to respond and those who were successfully treated with a proton pump inhibitor once daily. Am J Gastroenterol 2009; 104: 2005–2013. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith C, Rohss K, Bokelund Singh S et al. The effects of dose and timing of esomeprazole administration on 24-h, daytime and night-time acid inhibition in healthy volunteers. Aliment Pharmacol Ther 2010; 32: 1249–1256. [DOI] [PubMed] [Google Scholar]
- Rackoff A, Agrawal A, Hila A et al. Histamine-2 receptor antagonists at night improve gastroesophageal reflux disease symptoms for patients on proton pump inhibitor therapy. Dis Esophagus 2005; 18: 370–373. [DOI] [PubMed] [Google Scholar]
- Peghini PL, Katz PO, Castell DO. Ranitidine controls nocturnal gastric acid breakthrough on omeprazole: a controlled study in normal subjects. Gastroenterology 1998; 115: 1335–1339. [DOI] [PubMed] [Google Scholar]
- Fackler WK, Ours TM, Vaezi MF et al. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology 2002; 122: 625–632. [DOI] [PubMed] [Google Scholar]
- Quigley EMM, Lacy BE. Overlap of functional dyspepsia and GERD — diagnostic and treatment implications. Nat Rev Gastroenterol Hepatol 2013; 10: 175–186. [DOI] [PubMed] [Google Scholar]
- Kudara N, Chiba T, Orii S et al. Gastric emptying of patients with persistent reflux symptoms and erosive esophagitis under PPI therapy. Neurogastroenterol Motil 2004; 16: 654. [Google Scholar]
- van der Velden AW, Quarte-ro AO, Grobbee DE et al. Maintenance treatment for GERD: residual symptoms are associated with psychological distress. Digestion 2008; 77: 207–213. [DOI] [PubMed] [Google Scholar]
- Becher A, El-Serag H. Systematic review: the association between symptomatic response to proton pump inhibitors and health-related quality of life in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2011; 34: 618–627. [DOI] [PubMed] [Google Scholar]
- Holtmann G, Cain C, Malfertheiner P. Gastric Helicobacter pylori infection accelerates healing of reflux esophagitis during treatment with the proton pump inhibitor pantoprazole. Gastroenterology 1999; 117: 11–16. [DOI] [PubMed] [Google Scholar]
- Miller SM, Goldstein JL, Gerson LB. Cost-effectiveness model of endoscopic biopsy for eosinophilic esophagitis in patients with refractory GERD. Am J Gastroenterol 2011; 106: 1439–1445. [DOI] [PubMed] [Google Scholar]
- Kim HP, Vance RB, Shaheen NJ et al. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitkara DK, Bredenoord AJ, Rucker MJ et al. Aerophagia in adults: a comparison with functional dyspepsia. Aliment Pharmacol Ther 2005; 22: 855–858. [DOI] [PubMed] [Google Scholar]
- Tokashiki R, Yamaguchi H, Nakamura K et al. Globus sensation caused by gastroesophageal reflux disease. Auris Nasus Larynx 2002; 29: 347–351. [DOI] [PubMed] [Google Scholar]
- Richter JE, Pandol SJ, Castell DO et al. Gastroesophageal reflux disease in the Zollinger-Ellison syndrome. Ann Intern Med 1981; 95: 37–43. [DOI] [PubMed] [Google Scholar]
- Kessing BF, Smout AJ, Bredenoord AJ. Current diagnosis and management of the rumination syndrome. J Clin Gastroenterol 2014; 48: 478–483. [DOI] [PubMed] [Google Scholar]
- Poh CH, Gasiorowska A, Navarro-Rodriguez T et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc 2010; 71: 28–34. [DOI] [PubMed] [Google Scholar]
- Bautista JM, Wong WM, Pulliam G et al. The value of ambulatory 24 hr esophageal pH monitoring in clinical practice in patients who were referred with persistent gastroesophageal reflux disease (GERD)-related symptoms while on standard dose anti-reflux medications. Dig Dis Sci 2005; 50: 1909–1915. [DOI] [PubMed] [Google Scholar]
- Hirano I, Richter JE. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol 2007; 102: 668–685. [DOI] [PubMed] [Google Scholar]
- Prakash C, Clouse RE. Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2005; 3: 329–334. [DOI] [PubMed] [Google Scholar]
- Zerbib F, Roman S, Ropert A et al. Esophageal pH-impedance monitoring and symptom analysis in GERD: a study in patients off and on therapy. Am J Gastroenterol 2006; 101: 1956–1963. [DOI] [PubMed] [Google Scholar]
- Hemmink GJ, Bredenoord AJ, Weusten BL et al. Esophageal pH-impedance monitoring in patients with therapy-resistant reflux symptoms: “on” or “off” proton pump inhibitor? Am J Gastroenterol 2008; 103: 2446–2453. [DOI] [PubMed] [Google Scholar]
- Zerbib F, Duriez A, Roman S et al. Determinants of gastro-oesophageal reflux perception in patients with persistent symptoms despite proton pump inhibitors. Gut 2008; 57: 156–160. [DOI] [PubMed] [Google Scholar]
- Mainie I, Tutuian R, Agrawal A et al. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg 2006; 93: 1483–1487. [DOI] [PubMed] [Google Scholar]
- Slaughter JC, Goutte M, Rymer JA et al. Caution about overinterpretation of symptom indexes in reflux monitoring for refractory gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2011; 9: 868–874. [DOI] [PubMed] [Google Scholar]
- Pritchett JM, Aslam M, Slaughter JC et al. Efficacy of esophageal impedance/pH monitoring in patients with refractory gastroesophageal reflux disease, on and off therapy. Clin Gastroenterol Hepatol 2009; 7: 743–748. [DOI] [PubMed] [Google Scholar]
- Ribolsi M, Emerenziani S, Petitti T et al. Increased frequency and enhanced perception of reflux in non-erosive reflux disease patients non-responders to proton pump inhibitors. Dig Liver Dis 2012; 44: 549–554. [DOI] [PubMed] [Google Scholar]
- Izawa S, Funaki Y, Iida A et al. The role of gastroesophageal reflux in relation to symptom onset in patients with proton pump inhibitor-refractory nonerosive reflux disease accompanied by an underlying esophageal motor disorder. Digestion 2014; 89: 61–67. [DOI] [PubMed] [Google Scholar]
- Lacy BE, Paquette L, Robertson DJ et al. The clinical utility of esophageal manometry. J Clin Gastroenterol 2009; 43: 809–815. [DOI] [PubMed] [Google Scholar]
- Gourcerol G, Benanni Y, Boueyre E et al. Influence of gastric emptying on gastro-esophageal reflux: a combined pH-impedance study. Neurogastroenterol Motil 2013; 25: 800–e634. [DOI] [PubMed] [Google Scholar]
- Fass RS, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut 2009; 58: 295–309. [DOI] [PubMed] [Google Scholar]
- Hershcovici T, Fass R. Step-by-step management of refractory gastresophageal reflux disease. Dis Esophagus 2013; 26: 27–36. [DOI] [PubMed] [Google Scholar]
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166: 965–971. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, Ashida K, Miwa H et al. The impact of lifestyle modification on the health-related quality of life of patients with reflux esophagitis receiving treatment with a proton pump inhibitor. Am J Gastroenterol 2009; 104: 1106–1111. [DOI] [PubMed] [Google Scholar]
- Thomas E, Wade A, Crawford G et al. Randomised clinical trial: relief of upper gastrointestinal symptoms by an acid pocket-targeting alginate–antacid (Gaviscon double action) – a double-blind, placebo-controlled, pilot study in gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2014; 39: 595–602. [DOI] [PubMed] [Google Scholar]
- Johnson T, Gerson L, Hershcovici T et al. Systematic review: the effects of carbonated beverages on gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2010; 31: 607–614. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Machida A, Watanabe Y et al. Association between dinner-to-bed time and gastro-esophageal reflux disease. Am J Gastroenterol 2005; 100: 2633–2636. [DOI] [PubMed] [Google Scholar]
- Khan BA, Sodhi JS, Zargar SA et al. Effect of bed head elevation during sleep in symptomatic patients of nocturnal gastroesophageal reflux. J Gastroenterol Hepatol 2012; 27: 1078–1082. [DOI] [PubMed] [Google Scholar]
- Singh M, Lee J, Gupta N et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: a prospective intervention trial. Obesity 2013; 21: 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman R, Maradey-Romero C, Fass R. The role of pain modulators in esophageal disorders – no pain no gain. Neurogastroenterol Motil 2014; 26: 603–610. [DOI] [PubMed] [Google Scholar]
- Maradey–Romero C, Fass R. Antidepressants for functional esophageal disorders: evidence- or eminence-based medicine? Clin Gastroenterol Hepatol 2014; 13: 260–262. [DOI] [PubMed] [Google Scholar]
- Richter JE. Review article: the management of heartburn in pregnancy. Aliment Pharmacol Ther 2005; 22: 749–757. [DOI] [PubMed] [Google Scholar]
- Carling L, Cronstedt J, Fau - Engqvist A et al. Sucralfate versus placebo in reflux esophagitis. A double-blind multicenter study. Scand J Gastroenterol 1988; 23: 1117–1124. [DOI] [PubMed] [Google Scholar]
- Laitinen S, Stahlberg M, Kairaluoma MI et al. Sucralfate and alginate/antacid in reflux esophagitis. Scand J Gastroenterol 1985; 20: 229–232. [DOI] [PubMed] [Google Scholar]
- Simon B, Mueller P. Comparison of the effect of sucralfate and ranitidine in reflux esophagitis. Am J Med 1987; 83: 43–47. [DOI] [PubMed] [Google Scholar]
- Simon B, Ravelli GP, Goffin H. Sucralfate gel versus placebo in patients with non-erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther 1996; 10: 441–446. [DOI] [PubMed] [Google Scholar]
- Williams RM, Orlando R, Bozymski EM et al. Multicenter trial of sucralfate suspension for the treatment of reflux esophagitis. Am J Med 1987; 83: 61–66. [DOI] [PubMed] [Google Scholar]
- Mandel KG, Daggy BP, Brodie DA et al. Review article: alginate-raft formulations in the treatment of heartburn and acid reflux. Aliment Pharmacol Ther 2000; 14: 669–690. [DOI] [PubMed] [Google Scholar]
- Kwiatek MA, Roman S, Fareeduddin A et al. An alginate-antacid formulation (Gaviscon double action liquid) can eliminate or displace the postprandial “acid pocket” in symptomatic GERD patients. Aliment Pharmacol Ther 2011; 34: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohof WO, Bennink RJ, Smout AJ et al. An alginate-antacid formulation localizes to the acid pocket to reduce acid reflux in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 11: 1585–1591. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA. GABAB receptors in the brain-gastroesophageal axis. Am J Physiol Gastrointestinal Liver Physiol 2001; 281: G311–G315. [DOI] [PubMed] [Google Scholar]
- Frisby CL, Mattsson JP, Jensen JM et al. Inhibition of transient lower esophageal sphincter relaxation and gastroesophageal reflux by metabotropic glutamate receptor ligands. Gastroenterology 2005; 129: 995–1004. [DOI] [PubMed] [Google Scholar]
- Koek GH, Sifrim D, Lerut T et al. Effect of the GABAB agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut 2003; 52: 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. Alternative therapeutic approaches to chronic proton pump inhibitor treatment. Clin Gastroenterol Hepatol 2012; 10: 338–345. [DOI] [PubMed] [Google Scholar]
- Reavis K, Nguyen A. Endolumenal approaches to gastroesophageal reflux disease. In: Nguyen NT, Scott-Conner CEH editors. The SAGES Manual. Springer: : New York, 2012, p 247–260. [Google Scholar]
- Maradey-Romero C, Kale H, Fass R. Nonmedical therapeutic strategies for nonerosive reflux disease. J Clin Gastroenterol 2014; 48: 584–589. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Peterli R, Guenin MO et al. Laparoscopic antireflux surgery: long-term outcomes and quality of life. J Laparoendosc Adv Surg Tech 2006; 16: 557–561. [DOI] [PubMed] [Google Scholar]
- Armstrong D, Bennett JR, Blum AL et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 1996; 111: 85–92. [DOI] [PubMed] [Google Scholar]
- Schwartz MP, Smout AJ. Review article: the endoscopic treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2007; 26: 1–6. [DOI] [PubMed] [Google Scholar]
- Spechler S, Lee E, Ahnen D et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA 2001; 285: 2331–2338. [DOI] [PubMed] [Google Scholar]
- Ganz R, Peters JH, Horgan S. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med 2013; 368: 2038–2040. [DOI] [PubMed] [Google Scholar]
- Fass R. Functional heartburn: what it is and how to treat it. Gastrointest Endosc Clin N Am 2009; 19: 23–33. [DOI] [PubMed] [Google Scholar]
- Maradey-Romero C, Fass R. New therapies for non-cardiac chest pain. Curr Gastroenterol Rep 2014; 16: 1–8. [DOI] [PubMed] [Google Scholar]
- Kamolz T, Granderath F, Bammer T et al. Psychological intervention influences the outcome of laparoscopic antireflux surgery in patients with stress-related symptoms of gastroesophageal reflux disease. Scand J Gastroenterol 2001; 36: 800–805. [DOI] [PubMed] [Google Scholar]
- Klein KB, Spiegel D. Modulation of gastric acid secretion by hypnosis. Gastroenterology 1989; 96: 1383–1387. [DOI] [PubMed] [Google Scholar]
- McDonald-Haile J, Bailey MA, Schan CA et al. Relaxation training reduces symptom reports and acid exposure in patients with gastroesophageal reflux disease. Gastroenterology 1994; 107: 61–69. [DOI] [PubMed] [Google Scholar]
- Shay S, Johnson LF, Wong RK et al. Rumination, heartburn, and daytime gastroesophageal reflux. A case study with mechanisms defined and successfully treated with biofeedback therapy. J Clin Gastroenterol 1986; 8: 115–126. [PubMed] [Google Scholar]
- Dickman R, Schiff E, Holland A et al. Clinical trial: acupuncture vs. doubling the proton pump inhibitor dose in refractory heartburn. Aliment Pharmacol Ther 2007; 26: 1333–1344. [DOI] [PubMed] [Google Scholar]
- Hamzat H, Sun H, Ford JC et al. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging 2012; 29: 681–690. [DOI] [PubMed] [Google Scholar]
- Sifrim D, Castell D, Dent J et al. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut 2004; 53: 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavina L, Saino G, Bona D et al. Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg 2008; 12: 2133–2140. [DOI] [PubMed] [Google Scholar]
- Colgan SM, Faragher EB, Whorwell PJ. Controlled trial of hypnotherapy in relapse prevention of duodenal ulceration. Lancet 1988; 331: 1299–1300. [DOI] [PubMed] [Google Scholar]
- Gordon A, Berelowitz M, Bremner CG. Biofeedback improvement of lower esophageal sphincter pressures and reflux symptoms. J Clin Gastroenterol 1983; 5: 235–237. [DOI] [PubMed] [Google Scholar]