Abstract
Objectives:
Elimination diets have been used for many years to treat irritable bowel syndrome (IBS). These approaches had fallen out of favor until a recent resurgence, which was based on new randomized controlled trial (RCT) data that suggested it might be effective. The evidence for the efficacy of dietary therapies has not been evaluated systematically. We have therefore conducted a systematic review to examine this issue.
Methods:
MEDLINE, EMBASE, and the Cochrane Controlled Trials Register were searched up to December 2013. Trials recruiting adults with IBS, which compared any form of dietary restriction or addition of an offending food group in patients already on a restricted diet vs. placebo, control therapy, or “usual management”, were eligible. Dichotomous symptom data were pooled to obtain a relative risk of remaining symptomatic after therapy as well as the number needed to treat with a 95% confidence interval.
Results:
We identified 17 RCTs involving 1,568 IBS patients that assessed elimination diets. Only three RCTs involving 230 patients met our eligibility criteria, all of which evaluated different approaches, and thus a meta-analysis could not be conducted.
Conclusions:
More evidence is needed before generally recommending elimination diets for IBS patients.
Introduction
Dietary restriction has long been recommended for lower gastrointestinal symptoms,1 and many irritable bowel syndrome (IBS) patients feel that their symptoms relate to food sensitivity.2 Initial research suggested that dietary restriction might be effective,3 but further studies reported that diet did not have a major role in IBS. The previous American College of Gastroenterology (ACG) monograph on IBS concluded that there was insufficient evidence to recommend exclusion diets in IBS and their routine use outside of a clinical trial was not recommended.5 Since the publication of this monograph there has been a resurgence of interest in how gluten sensitivity may have a role in IBS6 and new exclusion diets have emerged, such as restricting fermentable carbohydrates including oligosaccharides, disaccharides, monosaccharides, and polyols (termed the FODMAPs (Fermentable Oligo-Di-Monosaccharides and Polyols) diet).7 There have been randomized controlled trials (RCTs) evaluating these approaches, but there has been no systematic review summarizing the evidence.
Methods
Search strategy and study selection
A search of the medical literature was conducted using MEDLINE (1946 to December 2013), EMBASE and EMBASE Classic (1947 to December 2013), and the Cochrane central register of controlled trials. RCTs examining the effect of excluding factors from the diet or supplementing the diet with fiber in adult patients (over the age of 16 years) with IBS were eligible for inclusion (Box 1). We contacted the authors of studies that evaluated functional gastrointestinal disorders that could have included IBS, but did not report this group of patients separately, for further information. Similarly, we contacted original investigators of studies who did not report dichotomous data, but were otherwise eligible for inclusion in the systematic review, to explore whether these data were available.
Eligibility criteria.
Randomized controlled trials:
Adults (participants aged >16 years).
Diagnosis of IBS based on either a clinician's opinion, or meeting specific diagnostic criteria*.
Compared dietary manipulation by exclusion of specific foods or food groups with placebo diet or no therapy.
Minimum duration of therapy 7 days.
Minimum duration of follow-up 7 days.
Dichotomous assessment of response to therapy in terms of effect on global IBS symptoms or abdominal pain following therapy†.
*Manning, Kruis score, Rome I, II, or III.
†Preferably patient reported, but if this was not available, then as assessed by a physician or questionnaire data. If these data were not presented, then the trial was not eligible.
The literature search was performed as part of a broader exercise to inform an update of the ACG monograph on the management of IBS. Specifically, studies on IBS were identified with the terms irritable bowel syndrome and functional diseases, colon (both as medical subject heading (MeSH) and free-text terms), and IBS, spastic colon, irritable colon, or functional adj5 bowel (as free-text terms). These were combined using the set operator AND with diet, fat-restricted, diet, protein-restricted, diet, carbohydrate-restricted, diet, gluten-free diet, macrobiotic diet, vegetarian diet, macrobiotic diet, Mediterranean diet, fads, gluten, lactose intolerance, lactose, both as MeSH and free-text terms, or the free text terms FODMAP$, glutens, or food adj5 intolerance.
Articles in any language were eligible and were translated where appropriate. Abstracts were also eligible, and conference proceedings from United European Gastroenterology Week and Digestive Diseases Week between 2001 and 2013 were hand-searched to identify potentially eligible studies published only in abstract form. We also performed a recursive search of the literature from the bibliographies of all relevant studies retrieved from the electronic search. Two masked reviewers assessed potentially relevant articles using predesigned eligibility forms, according to the prospectively defined eligibility criteria (Box 1). We resolved any disagreement between investigators by consensus.
Outcome assessment
The primary outcome was defined as global improvement in IBS symptoms. If this was not available, then improvement in abdominal pain was taken as the primary outcome. If neither of these outcomes were reported, then the trial was not eligible. Where more than one definition was provided for improvement in the primary outcome, the most stringent definition with the lowest placebo response rate was taken. Secondary outcomes included quality of life and adverse events.
Data extraction
Two reviewers (PM and ACF) independently recorded data from eligible studies on to a Microsoft Excel spreadsheet (XP professional edition; Microsoft Corp, Redmond, WA). In addition to the primary outcome (Box 2), the following clinical data were extracted for each trial: setting (primary, secondary, or tertiary care-based), number of centers, country of origin, type of dietary restriction or fiber supplementation, duration of therapy, total number of adverse events reported, criteria used to define IBS, primary outcome measure used to define symptom improvement or cure following therapy, duration of follow-up, proportion of female patients, and proportion of patients according to predominant stool pattern. Data were extracted as intention-to-treat analyses, with all dropouts assumed to be treatment failures, wherever trial reporting allowed this.
Data extraction methodology.
Outcome of interest: Improvement in global IBS symptoms preferable, if not reported then improvement in abdominal pain.
Reporting of outcomes: Patient-reported preferable, if not available then investigator-reported.
Time of assessment: Upon completion of therapy.
Denominator used: True intention-to-treat analysis, if not available then all evaluable patients.
Cutoff used for dichotomization: Any improvement in global IBS symptoms or abdominal pain for Likert-type scales, investigator-defined improvement for continuous scales, if no investigator definition available then we used ≥1 s.d. decrease in symptom score from baseline to completion of therapy (we assessed if the use of any decrease in symptom score from baseline to completion of therapy altered our analysis).
Assessment of risk of bias
Two independent reviewers (PM and ACF) assessed risk of bias using the Cochrane handbook risk of bias tool.8 This evaluates the method of randomization, whether allocation was concealed, method of blinding, the completeness of follow-up, whether there was evidence of selective outcome reporting, and other biases.
Data synthesis and statistical analysis
If data permitted, we intended to summarize global IBS symptoms or abdominal pain persisting with intervention compared with control as a relative risk with 95% confidence intervals. If enough data were provided, these summary statistics would be pooled using a random-effects model,9 to allow for any heterogeneity between studies. Adverse events data was also summarized with relative risks if this information was provided.
Results
Efficacy of dietary intervention in the treatment of IBS
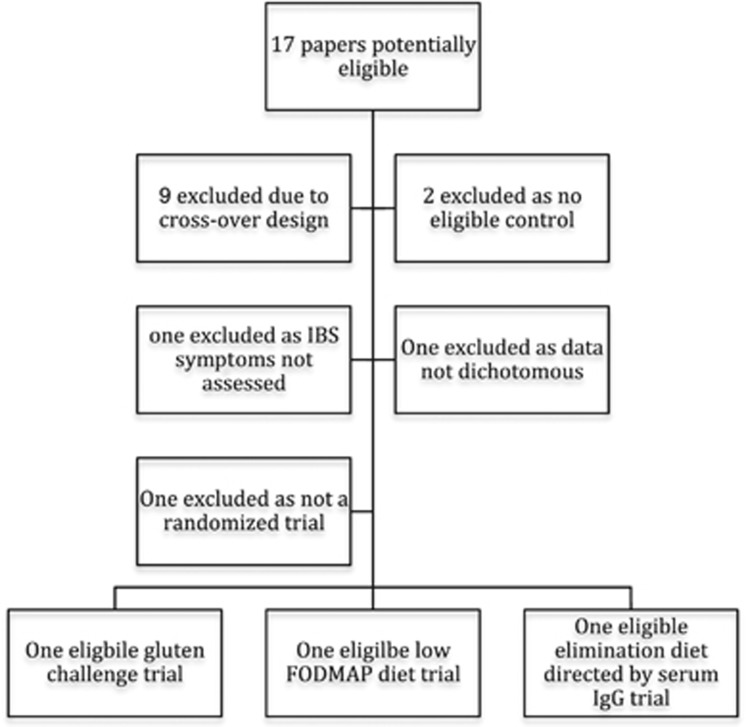
The search strategy provided 360 papers on dietary intervention to review. We identified 17 papers evaluating 1,568 IBS patients who were potential RCTs that evaluated dietary intervention in IBS.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Only three papers10, 11, 12 evaluating 230 patients were eligible, and agreement between reviewers regarding eligibility was perfect (κ-statistic=1.0) (Figure 1). The remaining 14 papers13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 were excluded for a variety of reasons outlined in Table 1. The eligible studies are summarized in Table 2, but each evaluated a different dietary intervention, thus precluding meta-analysis (Table 3).
Figure 1.
Flow chart of papers evaluated for the systematic review of food elimination diets in irritable bowel syndrome (IBS). IgG, immunoglobulin G.
Table 1. Reasons for exclusion of potentially eligible papers that were considered for the dietary intervention in IBS systematic review.
| Reference | Randomized | Design | Dietary intervention | Control | IBS symptoms assessed | Dichotomous data extractable | ≥7 Days therapy |
|---|---|---|---|---|---|---|---|
| King et al.13 | Yes | Crossover | Exclusion diet | Yes | Yes | No | Yes |
| Ong et al.14 | Yes | Crossover | Low FODMAPs diet | Yes | Yes | No | No |
| Bentley et al.15 | Yes | Crossover | Exclusion diet after sensitivity testing | Yes | Yes | No | Unclear |
| Carroccio et al.16 | Yes | Crossover | Empirical exclusion diet followed by sensitivity testing | Unclear | Yes | No | Yes |
| Shepherd et al.17 | Yes | Crossover | Low FODMAPs | Yes | Yes | No | Yes |
| Vazquez Roque et al.18 | Yes | Parallel | Gluten-free diet | Yes | No | No | Yes |
| Halmos et al.19 | Yes | Crossover | Low FODMAPs | Yes | Yes | No | Yes |
| Biesiekierski et al.20 | Yes | Crossover | Rechallenge of gluten after low FODMAPs diet | Yes | Yes | No | No |
| Berg et al.21 | Yes | Parallel | Fructose-reducing diet | “IBS diet”—unclear if this is another active therapy | Yes | No | Yes |
| Jones et al.22 | Yes | Crossover | Food rechallenge in food elimination responders | Yes | Yes | No | No |
| Carroccio et al.23 | No | No | Gluten challenge in wheat-sensitive patients | No | No | No | Yes |
| Lunardi et al.24 | Yes | Crossover | Oral chromoglycate in food-intolerant IBS patients—not an eligible intervention | Yes | Yes | No | Yes |
| Stefanini et al.25 | Yes | Parallel | Empirical food elimination diet | Sodium chromoglygate—not an eligible control | Yes | Yes | Yes |
| Piccinini et al.26 a | Yes | Parallel | Empirical food elimination diet | Sodium chromoglygate—not an eligible control | Yes | Yes | Yes |
FODMAPs, Fermentable Oligo-Di-Monosaccharides and Polyols; IBS, irritable bowel syndrome.
Possible duplicate paper as design similar, but intervention was for 3 weeks rather than 4 weeks.
Table 2. RCTs eligible for inclusion in the dietary intervention in IBS systematic review.
| Author | Design | Participants | Interventions | Methodology | Outcomes |
|---|---|---|---|---|---|
| Atkinson et al.10 | UK RCT, single center. | 150 Rome II IBS. Tested for food serum IgG assay. Recruited from secondary care; 83% female. | Diet avoiding foods that they were intolerant of, according to IgG assay or sham diet for 12 weeks. | Method of randomization and concealment of allocation not stated. Double-blind. Other IBS medications allowed. | Patients asked “Compared with your IBS before you started the food elimination diet, are you now: terrible, worse, slightly worse, no change, slightly better, better, or excellent?” Better or excellent was taken as significant improvement |
| Biesiekierski et al.11 | Australian RCT, single | 39 Rome III IBS patients intolerant of gluten, but celiac excluded. Recruited from newspaper advertisement; 89% female. | Diet spiked with 16 g gluten per day vs. placebo for 4 weeks. | Adequate method of randomization and concealment of allocation. Double-blind. No other IBS medications allowed. | Patients answering “no” to the question “Over the last week were your symptoms adequately controlled?” |
| Staudacher et al.12 | UK RCT, single center | 41 Rome III IBS. Recruited from secondary care. Bloating and/or diarrhea included, predominant constipation excluded. | FODMAPs diet vs. habitual diet for 4 weeks. | Method of randomization and concealment of allocation not stated. Open study—patients not blinded (unclear if researchers masked). | GI symptom rating scale. Patients asked “Were your symptoms adequately controlled over the previous week?” |
FODMAPs, Fermentable Oligo-Di-Monosaccharides and Polyols; GI, gastrointestinal; IBS, irritable bowel syndrome; IgG, immunoglobulin G; RCT, randomized controlled trial.
Table 3. RCTs of specific diets excluded from the dietary intervention in IBS systematic review.
| Reference | Design | Participants | Interventions | Methodology | Outcomes |
|---|---|---|---|---|---|
| King et al.13 | UK RCT, single center | 6 Female Rome I IBS and 6 female healthy controls. Tested for food serum IgG assay. Recruited from secondary care. Fecal excretion of fat, nitrogen, starch, and non-starch polysaccharide NSP was measured, and 24 h excretion of hydrogen and methane. | Restriction diet of exclusion of beef, all dairy products, yeasts, fruits, cereals, rice, caffeinated drinks, and tap water for 2 weeks vs. normal western diet in a crossover trial. | Method of randomization and concealment of allocation not stated. Patients not blinded—unclear if researchers masked). | For IB patients, the median symptom score was 4 (3–7) on the exclusion diet compared with 8 (IQR 5·25–10) for the standard diet (P=0.0001). Breath hydrogen production was greater in patients with IBS compared with controls, and in both groups gas production was reduced by an exclusion diet. |
| Ong et al.14 | Australian RCT, single center | 15 Rome III IBS patients and 15 healthy controls. Intolerant of gluten but celiac excluded. Healthy volunteers recruited from advertisement, IBS patients from secondary care clinic. 87% IBS patients female. Fourteen-hour excretion of hydrogen and methane measured. | High (50 g per day) vs. low (9 g per day) FODMAPs diet for 2 days in a crossover design. | Method of randomization and concealment of allocation not stated. Single-blind (patients not blinded). | Overall IBS symptom score was significantly higher for IBS patients during the high FOMAPs diet (median 6; range 2–9) compared with low FODMAPs diet (2; 0–7) (P=0.002). Breath hydrogen production was greater in patients with IBS than in controls. Breath hydrogen greater on high vs. low FODMAP diet in IBS and control patients. |
| Bentley et al.15 | UK RCT, single center | 27 Patients with clinically diagnosed of IBS. Recruited from secondary care; 81% female. All had skin prick tests for potential food allergy. | All patients asked to eat a diet of lamb, pears, and rice for an unspecified duration. Other foods then individually introduced at least 3 days apart, and if any caused symptoms, then the patient was exposed to a double-blind provocation trial with that food—three capsules with offending food and three with placebo in a random order. Patient needed to identify the challenge correctly on at least 5 occasions. | Method of randomization and concealment of allocation not stated. Double-blind. | 3/19 Patients who completed the study correctly identified offending foods in placebo controlled trial. All had a history of atopy and a positive skin prick test to inhalant allergens, although only 1/3 had positive skin prick test to relevant foods. |
| Carroccio et al.16 | Italian two center RCT | 160 Patients with Rome II IBS, 40 patients with other GI diseases, and 50 healthy controls. Recruited from secondary care; 79% female. Fecal tryptase, ECP and calprotectin measured. Serum IgE (total and food specific) also measured. | All patients had a 4-week elimination diet excluding cow's milk, eggs, wheat, tomatoes, and chocolate. All completed a daily diary. Those who responded to the diet were given a double-blind placebo controlled trial of cow's milk and then wheat proteins using capsules containing these agents or placebo. Capsules given for 2 weeks with 1 week wash out and then crossed over and patient deemed food sensitive if symptoms returned when given the active capsule. | Method of randomization and concealment of allocation not stated. Double-blind. | 70/160 (44%) IBS patients responded to open restriction diet. Forty of 70 (57%) were food sensitive according to the double-blind challenge (note with this design there is a 50% chance the patient will be “correct” if they guess with at least one of the food types so the importance of this finding is unclear. Interestingly, fecal ECP and tryptase (but not calprotectin) were higher in the food-sensitive IBS group compared with the nonsensitive IBS group. |
| Shepherd et al.17 | Australian single center RCT | 26 Patients with Rome II IBS and fructose malabsorption diagnosed with fructose hydrogen breath test. Symptoms responded to 3 months for a low FODMAPs diet. Recruited from secondary care dietetic practice. | Patients continued on a low FODMAPs diet. After run-in phase were blindly challenged with four drinks that were prepared to be high in fructose, fructans, fructans, and fructose mixed and glucose. The type of drink was administered randomly for 2 weeks. The dose of the agent was increased each 3 days from low to high and continued on the dose tolerated for 2 weeks. There was a washout period, and thus the patient was free of symptoms for 7 days before going on to the next phase in this crossover design. | Method of randomization and concealment of allocation not stated. Double-blind. | Overall IBS symptom score measured by visual analog scale was higher for fructose, fructans, and mixed groups compared with glucose group and this was reported to be statistically significant. Twenty to thirty percent felt their symptoms remained controlled on the fructose, fructans, and mixed groups compared with over 80% in the glucose group. |
| Vazquez Roque et al.18 | US single center RCT | Forty-five patients with Rome II IBS-D recruited from tertiary care clinic; 96% female. All took gluten before entering trial and no evidence of celiac disease on serology or duodenal biopsy. HLA genotyping was performed and large and small bowel transit was measured with scintigraphy. Lactulose/mannitol test for small and large bowel permeability was also measured. Tight junctions were quantified using real-time PCR from small bowel and rectal biopsies. | Twenty-three patients randomized to gluten-free diet, 22 randomized to gluten-containing diet for 4 weeks. Stool form was measured using Bristol Stool chart. Overall IBS symptoms were not measured (confirmed by the authors). | Method of randomization and concealment of allocation adequate. Single-blind (patients not blinded). | Patients on a gluten-free diet had marginally statistically significant lower frequency of bowel movements—~0.25 less bowel movements per day (P=0.04) with no difference in stool form or ease of passage. There was no significant effect on transit time, but there was a statistically significant increase in small (but not large) bowel permeability HLA-DQ2/8-positive patients. |
| Halmos et al.19 | Australian single center RCT | Thirty patients with Rome III IBS recruited by advertisement and eight healthy controls. Seventy percent IBS patients female. | Twenty-one days of a low FODMAPs diet or 21 days of typical Australian diet randomized in a crossover design with a 21-day washout period. Gastrointestinal symptoms were measured on a visual analog scale. | Method of randomization adequate and concealment of allocation not stated. Patients said to be blinded but over 80% IBS patients correctly identified the active diet. | Overall gastrointestinal symptoms less with the low FODMAPs diet (22.8 (95% CI=16.7–28.8) compared with the control diet (44.9 (95% CI=36.6–53.1), P<0.001. Effects were noted in IBS-C and IBS-D. The other IBS groups were too small to analyze. |
| Biesiekierski et al.20 | Australian single center RCT | Thirty-seven patients with Rome III IBS recruited by advertisement. Patients with normal duodenal biopsy and HLA-DQ2 and HLA-DQ8 negative. All thought their symptoms were gluten related and had adhered to a gluten-free diet for at least 6 weeks. | Initial 7-day run-in of low FODMAPS diet and gluten-free diet. Patients then given three diets—high gluten (16 g per day), low gluten (2 g per day), or placebo in random order for 1 week with a 2-week washout period in a crossover design. Symptoms were measured by visual analog scale. | Method of randomization and concealment of allocation adequate. Double-blind. | Significant reduction in symptoms scores during the open 7-day run-in of low FODMAPs diet (P<0.001, actual numbers are not given). No change in overall symptoms on either gluten-containing diets compared with no gluten—all continued on low FODMAPs diet. |
| Berg et al.21 | Norwegian three center RCT | 202 with Rome II IBS recruited from secondary care. All had a fructose hydrogen breath test but patients continued in the trial whatever the result; 75% female. | Two-week run-in with “IBS” diet—not clear what this was. Patients were then randomized to either carry on with their diet or also add a fructose-reduced diet with guidance from a study nurse. | Method of randomization adequate and concealment of allocation not stated. Not blinded. | One hundred and eighty-two patients completed the study. Significant reduction in overall symptoms in the fructose-reduced diet compared with no change in the control group. The effect was independent of the results of the fructose hydrogen breath test (63% positive). |
| Jones et al.22 | UK single center RCT | Twenty-five patients with clinical diagnosis IBS. Unclear how many were female. Plasma immune complexes, histamine, eosinophil count, and breath hydrogen excretion measured. Rectal PGE2 was measured. | All patients were asked to limit their diet to one meat, a single fruit, and distilled or spring water for 1 week. Those who responded were admitted to hospital for 4 days and asked to continue on the same foods, but were given breakfast via a nasogastric tube with or without offending foods in a randomized order for each day. No wash out. | Method of randomization not stated and concealment of allocation adequate. Double-blind. | Four refused to try the diet and 14/21 responded to diet. Six patients were admitted for the crossover trial for 4 days. Rectal PGE2 was significantly increased in patients with offending food challenges compared with controls. Symptoms not measured. |
CI, confidence interval; ECP, eosinophil cationic protein; FODMAPs, Fermentable Oligo-Di-Monosaccharides and Polyols; GI, gastrointestinal; HLA, human leukocyte antigen; IBS, irritable bowel syndrome; IgG, immunoglobulin G; IQR, interquartile range; NSP, non-starch polysaccharides; PGE2, prostaglandin E2; RCT, randomized controlled trial.
Gluten-free diet
There were four trials11, 18, 20, 23 in 400 IBS patients evaluating the impact of a gluten-free diet (GFD) on symptoms or a gluten challenge in patients who were sensitive to gluten (or in one case20 had a positive response to a low FODMAPs diet). Three trials11, 18, 23 reported that a GFD was effective in reducing IBS symptoms, or that a gluten challenge in those already on a GFD significantly increased symptoms. However, only one study11 met our eligibility criteria and the others were excluded because of a variety of reasons, including not assessing IBS symptoms,18 not treating for at least 7 days,20 or not being a randomized study.23 The eligible trial recruited 39 IBS patients who had normal duodenal biopsies and negative tissue transglutaminase and endomysial antibodies, but with symptom improvement on a GFD. Patients were instructed to continue on this diet and in addition each took a gluten-free muffin and two slices of gluten-free bread for 6 weeks. Patients were randomized to have this supplement spiked with gluten, so they would receive 16 g of gluten per day, or for the supplement to remain gluten free. The trial design was at low risk of bias. IBS symptoms were measured by visual analog scale. Thirty-four patients completed the study, and global symptoms, pain, bloating, and tiredness statistically significantly increased in the gluten group from every week after the first week in the gluten group. Furthermore, 13 (68%) of 19 patients in the gluten group reported that symptoms were not adequately controlled compared with 6 (40%) of 15 in the placebo group. The paper reported this difference as highly statistically significant using a generalizing estimating equation that controls for baseline variables (P=0.0001). This is a legitimate statistical approach, but if the authors had used another legitimate approach of using a Fisher's exact test, the results would not have been statistically significant (P=0.16), although this was not reported in the paper. The relative risk of symptoms occurring in the group challenged with gluten was 1.71 (95% confidence interval =0.91–3.62) according to these proportions.
FODMAPs diet
There were four RCTs12, 14, 17, 19 evaluating a low FODMAP diet in 112 IBS patients. Three RCTs14, 17, 19 were excluded, two as the data were not extractable for a meta-analysis17, 19 and one as they studied patients for <1 week.14 All these trials reported that a low FODMAP diet was effective in reducing IBS symptoms. The eligible trial12 evaluated 41 IBS patients and randomized them to 4 weeks of a low FODMAP diet or to continue on with their normal diet. There was a trend for all symptoms, except constipation or diarrhea, to improve, and this was statistically significant for bloating, borborygmi, and urgency. This study measured fecal microbiota using fluorescent in situ hybridization and found those on a low FODMAP diet had a significant reduction in bifidobacteria compared with controls. Overall, 13 (68%) of 19 patients randomized to the low FODMAP diet reported adequate control of their symptoms compared with 5 (23%) of 22 in the control group. This difference was statistically significant (P=0.005), but it is important to emphasize that this trial had a high risk of bias, as participants and researchers were not blinded and were aware who was on a low FODMAP diet.
Other exclusion diets
Nine RCTs10, 13, 15, 16, 21, 22, 24, 25, 26 evaluated other exclusion diets in 1,056 IBS patients. Most gave empirical exclusion diets that omitted a broad range of foods (e.g., one trial16 excluded cow's milk, eggs, wheat, tomato, or chocolate from the diet) but two10, 15 constructed the diet based on an individual's food sensitivity testing. Three of these trials were excluded because they evaluated sodium cromoglycate24 or compared an elimination diet with cromoglycate.25, 26 Five further13, 15, 16, 21, 22 trials were excluded as they did not have data that was extractable for a meta-analysis, usually because of a crossover design. Three of these trials16, 21, 22 were positive and two13, 15 were negative. One RCT10 was eligible and this evaluated 150 patients with Rome II IBS recruited from a single gastroenterology outpatient center in the United Kingdom. Celiac disease and lactose intolerance was excluded and patients underwent immunoglobulin G antibody testing to a panel of 29 food antigens. Food sensitivity was defined as an immunoglobulin G titer that was three times the level of normal. Patients were then randomized to a true diet in which they omitted all foods that they were intolerant of, according to their sensitivity testing, for 12 weeks, or a sham diet where patients were asked to avoid the same number of foods, but these were not related to their sensitivity testing. Subjects were most commonly intolerant of milk, yeast, egg, and wheat in sensitivity testing. The trial had an unclear risk of bias. Nineteen patients were lost to follow-up and 18/65 (28%) in the active intervention arm reported an improvement in symptoms compared with 11/66 (17%) in the control group, which was not statistically significant (P=0.14). Interestingly, 11 patients withdrew in the intervention arm because the diet was too restrictive compared with only three in the control arm. If only those who strictly followed their diet (24 (32%) of 75 of those allocated to the intervention group) were analyzed, then the mean IBS severity score was statistically significantly lower in the intervention group.
Discussion
Concerns regarding the quality of the underlying studies are particularly true for data relating to exclusion diets in IBS. There are a number of RCTs that have, in total, enrolled more than 1,500 IBS patients, but few provide data that can be synthesized using meta-analysis. The reasons for this are numerous, but one of the key factors relates to the use of the crossover design. This design is not, in itself, a problem for meta-analytic techniques, but data are rarely reported in a way that can be used to synthesize results.
Most published RCTs report positive results, but this should be interpreted with caution as the three trials that were eligible for this review all have their limitations. The only trial in this review that had a low risk of bias11 suggested that gluten exacerbates IBS symptoms in non-celiac IBS patients whose symptoms are already controlled with a GFD. This is the strongest evidence we have that a GFD may help some IBS patients, but the numbers recruited in this trial were modest and more data are needed so we can be confident of the estimate of effect. A low FODMAP diet has emerged as a new and interesting approach to the management of IBS. Four RCTs have evaluated this, but only one trial12 met the eligibility criteria and this was small and at high risk of bias as it was an open study. Although there is a great deal of interest in this approach,27 more data are needed before we can be confident this is effective in IBS patients. There are a large number of trials evaluating other elimination diets, but the only trial that was eligible for this review was negative.10
Some trials eliminate foods based on food allergy testing,10, 15 but this focuses the approach on the presumption that allergy is the underlying mechanism of action, which may or may not be true even if certain foods are the cause of IBS symptoms. There are a number of mechanisms whereby ingested food may cause gastrointestinal symptoms.28 The osmotic activity of the ingested food may encourage the influx of water, which may cause distention of the lumen.29 Distention may also be caused by gas produced from the fermentation of ingested food by the gut flora.30 Fermentation of food results in the release of many soluble molecules, such as short-chain fatty acids described above, which can have both pro- and anti-inflammatory properties. Ingested food also acts a substrate for the gut microbiome, and change in diet can lead to a change in the microbiome composition.31 This in turn could lead to an alteration in gut function, which, in turn, can lead to IBS symptoms.32 Support for this hypothesis comes from one study that did evaluate changes in the microbiome with a low FODMAP diet and reported a decrease in Bifidobacterium counts associated with this dietary intervention.12
The discussion of mechanisms through which diet can cause IBS symptoms is important as this directly affects the future design of food elimination trials. If the cause of symptoms relates to a direct effect of the food in the gut lumen, then the impact will be closely related to meals and should resolve within a relatively short time, if the offending food is removed from the diet. In this scenario, a crossover trial may be an appropriate design. However, if the mechanism relates to subtle manifestations of inflammation and/or changes in the gut microbiome, then this may take some time to return to baseline and a crossover design would not be appropriate. As the mechanism of dietary intervention in IBS is not known at this stage, it would be better if trials maintained a parallel group design to avoid problems with delayed or carry-over effects. Crossover designs should be relegated to treatment trials for rare disorders when subject accrual may be difficult. IBS is a common disorder affecting 10 to 20% of the population,33 and subject accrual should not be a problem
Despite promising data on the efficacy of dietary restriction in IBS, we suggest that this approach cannot be recommended strongly until more evidence is accumulated. These interventions are generally considered cheap and harmless, but a GFD is difficult to implement and is far from inexpensive; in fact, the food industry is projected to make US$16 billion annually in 2016 as it capitalizes on our concerns regarding gluten.34 Elimination diets can also be very restrictive for patients,35 highlighting the need for further data before such approaches are used widely in IBS.36
Acknowledgments
This study was performed to inform the American College of Gastroenterology Monograph on irritable bowel syndrome. We are grateful to Professor Camilleri and colleagues for providing additional data on their trial.
Guarantor of the article: Paul Moayyedi, MBChB, PhD, MPH, FACG.
Specific author contributions: ACF, EMMQ, BEL, AJL, YAS, LRS, EES, BMRS, and PM conceived the study. ACF and PM collected all data. ACF and PM analyzed and interpreted the data. PM drafted the manuscript. All authors commented on drafts of the paper. All authors have approved the final draft of the manuscript.
Financial support: American College of Gastroenterology.
Potential competing interests: Alexander C. Ford, Eamonn M.M. Quigley, Brian E. Lacy, Anthony J. Lembo, Yuri A. Saito, Lawrence R. Schiller, Edy E. Soffer, Brennan M.R. Spiegel, and Paul Moayyedi: none.
References
- Spiro HM. The irritable bowel 1958Conn Med 2009; 73: 41–45. [PubMed] [Google Scholar]
- Bohn L, Storsrud S, Tornblom H et al. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol 2013; 108: 634–641. [DOI] [PubMed] [Google Scholar]
- Petitpierre M, Gumowski P, Girard JP. Irritable bowel syndrome and hypersensitivity to food. Ann Allergy 1985; 54: 538–540. [PubMed] [Google Scholar]
- Zwetchkenbaum J, Burakoff R. The irritable bowel syndrome and food hypersensitivity. Ann Allergy 1988; 61: 47–49. [PubMed] [Google Scholar]
- Brandt LJ, Chey WD, Foxx-Orenstein AE et al. An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol 2009; 104 (Suppl I): S1–S35. [DOI] [PubMed] [Google Scholar]
- Carroccio A, Mansueto P, D'Almcamo A, Iacono G. Non-celiac wheat sensitivity as an allergic condition: personal experience and narrative review. Am J Gastroenterol 2013; 108: 1845–1852. [DOI] [PubMed] [Google Scholar]
- Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol 2013; 107: 657–666. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions: Version 5.0.2. Available at: www.cochrane-handbook.org2009; last accessed date May 2014.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- Atkinson W, Sheldon TA, Shaath N et al. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut 2004; 53: 1459–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesiekierski JR, Newnham ED, Irving PM et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol 2011; 106: 508–514. [DOI] [PubMed] [Google Scholar]
- Staudacher HM, Lomer MC, Anderson JL et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr 2012; 142: 1510–1518. [DOI] [PubMed] [Google Scholar]
- King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet 1998; 352: 1187–1189. [DOI] [PubMed] [Google Scholar]
- Ong DK, Mitchell SB, Barrett JS et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol 2010; 25: 1366–1373. [DOI] [PubMed] [Google Scholar]
- Bentley SJ, Pearson DJ, Rix KJ. Food hypersensitivity in irritable bowel syndrome. Lancet 1983; 2: 295–297. [DOI] [PubMed] [Google Scholar]
- Carroccio A, Brusca I, Mansueto P et al. Fecal assays detect hypersensitivity to cow's milk protein and gluten in adults with irritable bowel syndrome. Clin Gastroenterol Hepatol 2011; 9: 965–971. [DOI] [PubMed] [Google Scholar]
- Shepherd SJ, Parker FC, Muir JG et al. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol 2008; 6: 765–771. [DOI] [PubMed] [Google Scholar]
- Vazquez Roque MI, Camilleri M, Smyrk T et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 2013; 144: 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmos EP, Power VA, Shepherd SJ et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2013; 146: 67–75.e5. [DOI] [PubMed] [Google Scholar]
- Biesiekierski JR, Peters SL, Newnham ED et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013; 145: 320–328. [DOI] [PubMed] [Google Scholar]
- Berg LK, Fagerli E, Martinussen M et al. Effect of fructose-reduced diet in patients with irritable bowel syndrome, and its correlation to a standard fructose breath test. Scand J Gastroenterol 2013; 48: 936–943. [DOI] [PubMed] [Google Scholar]
- Jones VA, McLaughlan P, Shorthouse M et al. Food intolerance: a major factor in the pathogenesis of irritable bowel syndrome. Lancet 1982; 2: 1115–1117. [DOI] [PubMed] [Google Scholar]
- Carroccio A, Mansueto P, Iacono G, Soresi M, D'Alcamo A et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol 2012; 107: 1898–1906. [DOI] [PubMed] [Google Scholar]
- Lunardi C, Bambara LM, Biasi D, Cortina P, Peroli P et al. Double-blind cross-over trial of oral sodium cromoglycate in patients with irritable bowel syndrome due to food intolerance. Clin Exp Allergy 1991; 21: 569–572. [DOI] [PubMed] [Google Scholar]
- Stefanini GF, Saggioro A, Alvisi V et al. Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicentre study of 428 patients. Scand J Gastroenterol 1995; 30: 535–541. [DOI] [PubMed] [Google Scholar]
- Piccinini G, Feliciani M, Mazzetti M et al. A potential diagnostic role of a disodium chromoglycate course in irritable bowel syndrome. Int J Immunopathol Pharmacol 1990; 3: 107–112. [Google Scholar]
- Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol 2012; 107: 657–666. [DOI] [PubMed] [Google Scholar]
- Farre R, Tack J. Food and symptom generation in functional gastrointestinal disorders: physiological aspects. Am J Gastroenterol 2013; 108: 698–706. [DOI] [PubMed] [Google Scholar]
- Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol 2013; 108: 707–717. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zheng X, Cong Y et al. Bloating and distention in irritable bowel syndrome: the role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am J Gastroenterol 2013; 108: 1516–1525. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Morales EE, Overington J, Guerrero-Alba R et al. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: a role for PAR2. Am J Gastroenterol 2013; 108: 1634–1643. [DOI] [PubMed] [Google Scholar]
- Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol 2012; 107: 991–1000. [DOI] [PubMed] [Google Scholar]
- Strom S A big bet on gluten-free. Available at: http://www.nytimes.com/2014/02/18/business/food-industry-wagers-big-on-gluten-free.html?partner=rss&emc=rss&_r=1 (last accessed 21 February 2014).
- Sainsbury K, Mullan B, Sharpe L. A randomized controlled trial of an online intervention to improve gluten-free diet adherence in celiac disease. Am J Gastroenterol 2013; 108: 811–817. [DOI] [PubMed] [Google Scholar]
- Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BMR, Quigley EMM. American College of Gastroenterology Monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 2014; 109: S1–S26. [DOI] [PubMed] [Google Scholar]