Abstract
An effective regulation of metal ion homeostasis is essential for the growth of microorganisms in any environment and in pathogenic bacteria is strongly associated with their ability to invade and colonise their hosts. To gain a better insight into zinc acquisition in Group A Streptococcus (GAS) we characterized null deletion mutants of the adcA and lmb genes of Streptococcus pyogenes strain MGAS5005 encoding the orthologues of AdcA and AdcAII, the two surface lipoproteins with partly redundant roles in zinc homeostasis in Streptococcus pneumoniae. Null adcA and lmb mutants were analysed for their capability to grow in zinc-depleted conditions and were found to be more susceptible to zinc starvation, a phenotype that could be rescued by the addition of Zn2+ ions to the growth medium. Expression of AdcA, Lmb and HtpA, the polyhistidine triad protein encoded by the gene adjacent to lmb, during growth under conditions of limited zinc availability was examined by Western blot analysis in wild type and null mutant strains. In the wild type strain, AdcA was always present with little variation in expression levels between conditions of excess or limited zinc availability. In contrast, Lmb and HtpA were expressed at detectable levels only during growth in the presence of low zinc concentrations or in the null adcA mutant, when expression of lmb is required to compensate for the lack of adcA expression. In the latter case, Lmb and HtpA were overexpressed by several fold, thus indicating that also in GAS AdcA is a zinc-specific importer and, although it shares this function with Lmb, the two substrate-binding proteins do not show fully overlapping roles in zinc homeostasis.
Introduction
Zinc is the second most abundant transition metal in biological systems [1] [2]. In general, transition metals are key components of nearly 50% of all known enzymes with the dual role of being important structural elements as well as essential cofactors to an extensive range of enzymatic activities [3]. Specifically, zinc ions are present in many proteins involved in fundamental biological tasks, such as DNA polymerases, proteases, ribosomal proteins. However, despite its essential role in biology, Zn2+ can become toxic if accumulated to excess, as it competes with other metals for binding to active sites of enzymes thus disrupting normal metabolic processes. Hence, Zn2+ homeostasis in bacteria must rely on tightly regulated import and export mechanisms [4].
Two transport systems of importance in this context are the orthologous ATP binding cassette (ABC) transporters ZnuABC of Escherichia coli [5] and AdcABC of Streptococcus pneumoniae [6]. Acquisition of zinc from the environment is achieved by both transporters through their substrate-binding protein (SBP) component, a lipoprotein able to bind and transfer Zn2+ ions to the membrane-bound permease component of the transporter. In Treponema pallidum Zn2+-binding SBPs belong to two types, one showing promiscuous preference for metal binding (TroA) and the other specific for Zn2+ (ZnuA) [7]. Similarly, S. pneumoniae, as most streptococci, possesses two Zn2+-binding SBPs, AdcA and AdcAII, whose only partially redundant function in Zn2+ acquisition has been recently well characterized [8]. AdcA is more closely related to the high affinity zinc-specific ZnuA, typical of Gram-negative SBPs, although it contains significant structural differences. One such difference is the presence of an extended loop of ~200 amino acids at its C terminus containing a second Zn2+-binding domain with homology to the zinc-chaperone ZinT of Gram-negative bacteria [8]. AdcAII lacks this region and contains only the canonical amino-terminal Zn2+-binding domain [9]. Moreover, AdcAII requires the presence of polyhistidine triad proteins for the uptake of zinc in vivo [10], while AdcA can efficiently recruit zinc in the absence of Pht proteins [8].
Zinc homeostasis is crucial in any normal metabolic condition, however, it becomes particularly critical during infection, when both host and pathogen compete for the same essential metals. In addition, vertebrates have evolved defensive systems that deplete or flood infection sites with metal ions in order to eradicate the pathogen [11] [12]. Thus, during the most critical phases of their life cycle, namely interaction with the host, pathogens need the finest tuning of their metal homeostasis systems. A thorough characterization of all the components with a role in metal homeostasis in pathogenic bacteria would help considerably in providing an understanding of the mechanisms underlying bacterial infections. In this regard, recent work has characterized the role of two key components of the zinc efflux system in Group A Streptococcus (GAS), CzcD and GczA, demonstrating that zinc is crucial to the innate immunity response against GAS infection [13]. More recently, it has also been shown that zinc exerts its toxicity in GAS through inhibition of the central carbon metabolism and by causing disruption of capsule biosynthesis[14]. The essential role of zinc during infection had already be inferred in a previous report that describes how a GAS mutant that lacks Lsp, the only zinc receptor of GAS that has been structurally characterized[15], is attenuated in a murine subcutaneous ulcer model of infection[16].
Herein we report an attempt to gain a better insight into zinc acquisition in Group A Streptococcus by characterizing the orthologues of S. pneumoniae AdcA and AdcAII, named AdcA and Lmb, respectively, in Streptococcus pyogenes serotype M1 strain MGAS5005 [17]. It should be noted that Lmb has been previously named also Lsp, for lipoprotein of S. pyogenes [18], or Lbp, for laminin-binding protein [19], in S. pyogenes strains of different M types. Herein we chose to use the lmb denomination assigned to the M5005_Spy_1711 locus in strain MGAS5005 [17]. In summary, in streptococci the best-characterized components of the zinc uptake system are the S. pneumoniae AdcA and AdcAII lipoproteins whose orthologues in S. pyogenes are, respectively, AdcA, which shows 77% sequence conservation with the pneumococcal AdcA, and Lmb/Lsp/Lbp sharing 78% sequence homology with S. pneumoniae AdcAII. For both transporters the most conserved regions correspond to the domains identified as zinc-binding domains in the S. pneumoniae orthologues and, in particular, the metal-binding sites in Lbp and AdcAII are completely superimposable as determined by their structural characterization [15].
Null deletion mutants of the adcA and lmb genes were analysed for their capability to grow in zinc-depleted conditions. Expression of AdcA, Lmb and HtpA, the polyhistidine triad protein encoded by the gene adjacent to lmb, during growth under conditions of limited zinc availability was investigated by Western blot analysis in wild type and null mutant strains. Null mutants of adcA or lmb were more susceptible to zinc starvation, a phenotype that could be rescued by the addition of Zn2+ ions to the growth medium. In the wild type strain, AdcA was always present with little variation in expression levels between conditions of excess or limited zinc availability. In contrast, Lmb and HtpA were expressed at detectable levels only during growth in the presence of low zinc concentrations or in the null adcA mutant, where expression of lmb is required to compensate for the lack of adcA expression.
Hence, uptake of zinc in S. pyogenes strain MGAS5005 requires the action of both AdcA and Lmb only during growth in medium containing limiting amounts of Zn2+, whereas AdcA is always expressed and it possibly represents the main Zn2+ importer. Our results also suggest that these two SBPs must have partly redundant roles in zinc acquisition, as already described for AdcA and AdcAII in S. pneumoniae [8].
Materials and Methods
Ethics Statement
All animal studies were carried out in compliance with current Italian legislation on the care and use of animals in experimentation (Legislative Decree 116/92) and with the Novartis Animal Welfare Policy and Standards. Protocols were approved by the internal "Novartis Animal Ethical Committee" (approval number: AEC 200825) and authorized by the "Italian Ministry of Health" (authorization number: 21/2009-B).
Bacterial strains and growth conditions
The S. pyogenes M1T1 serotype strain MGAS5005 is a clinical isolate carrying a frameshift mutation resulting in production of a truncated CovS, the sensor kinase element of a two-component signal transduction system which negatively regulates virulence [20]. Strain MGAS5005 and the isogenic mutants MGAS5005∆adcA and MGAS5005∆lmb were grown in Todd Hewitt broth (THB, Difco) supplemented with 5% of yeast extract (THY) or in tryptic soy agar (TSA, Difco) medium supplemented with 5% of defibrinated ram blood (TSA-blood). The complemented strain MGAS5005-∆adcA/pMU1328-adcA was grown in THY medium in the presence of 1 μg ml-1 of erythromycin.
The Escherichia coli strain DH10B (Invitrogen) carrying the pJRS233-∆adcA construct and strain HB101 (Promega) carrying the pJRS233-∆lmb construct were grown in Luria Bertani (LB) broth containing 200 μg ml-1 or 100 μg ml-1 of erythromycin, respectively, at 30°C or 37°C.
DNA cloning
Deletion mutant strains were generated by allelic replacement using the temperature-sensitive shuttle vector pJRS233 (Perez-Casal et al., 1993). Suitable constructs were obtained by amplifying DNA fragments of 1 kb upstream and 1 kb downstream of the open reading frame of adcA or lmb using genomic DNA of strain SF370 as a template. The two 1 kb amplicons were joined in a single fragment by means of a SOEing PCR [21] and inserted between the BamHI and XhoI restriction sites of vector pJRS233.
Complementation of the MGAS5005 adcA null mutant was obtained as follows. The chromosomal locus encompassing 143 nucleotides upstream of the adcA open reading frame, the adcA coding region and the downstream 51 nucleotides was amplified from genomic DNA of strain MGAS5005, generating a blunt PCR product of 1732 bp. This was ligated into the SmaI-digested pMU1328 expression vector [22]. The sequence of primers designed for all cloning experiments is given in Table 1.
Table 1. Bacterial strains, plasmids and primers used in this study.
| Strains | Relevant characteristics | Reference or source |
| E. coli | ||
| HB101 | supE44 hsdS20 (rB-, mB-) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | Promega |
| DH10B | hsdS (rB- mB-) gal dcm | Invitrogen |
| S. pyogenes | ||
| MGAS5005 | M1T1 serotype clinical isolate, sequenced strain | [13] |
| MGAS5005∆adcA | adcA null mutant of MGAS5005 | This study |
| MGAS5005∆lmb | lmb null mutant of MGAS5005 | This study |
| Plasmids | Relevant characteristics | Reference |
| pJRS233 | temperature-sensitive shuttle vector used to create null mutants by allelic replacement, Ampr Eryr | [20] |
| pMU1328 | shuttle vector suitable for expression in streptococci, Eryr | [17] |
| Primers | Sequence (5’-3’)a | Application |
| P1forXhoI-adcA | GCGGCctcgagGAAGATTACCTTTGCTCAGCTGA | adcA null mutants |
| P2rev-adcA | GAAGATTTGCTTAGTGAGTTAAGAGATTCCTCCTTTGTTATTAACTG | |
| P3for-adcA | CAGTTAATAACAAAGGAGGAATCTCTTAACTCACTAAGCAAATCTTC | |
| P4forBamHI-adcA | GCGGCggatccTTCCTTGGTAGTGATAGCTGCAC | |
| P5for-adcA | GCCATTTAATACCATGGTGCC | |
| P6rev-adcA | CTGCAATCCTTAGGCGTTCTAA | |
| adcAko-ICfor | CCCAATCAATTTGGCATTGA | Amplification of ∆adcA locus |
| adcAko-ICrev | GTCATGGTTTCTTGCCATAA | |
| 5’adcA-Bamfor | GCTAAggatccGCAACTGCTTAGCC | Cloning of adcA into pMU1328 |
| 3’adcA-Salrev | GCGGCgtcgacAAAGAAAAAGCAAACCTCCTTAAAAG | |
| lmb-ko-Salfor | CATCAACTGATTTCAgtcgacGG | lmb null mutants |
| lmb-ko-P2rev | CTTCAACTGTTGATAGAGCACACCTTTTTTCATAGTACCTCC | |
| lmb-ko-P3for | GGAGGTACTATGAAAAAAGGTGTGCTCTATCAACAGTTGAAG | |
| 3'Spy1710-BglII | CGGTGTAGTagatctAAAAGTTC | |
| lmb-ko-ICfor | TGAATGAACCATTGTTTGCGAC | Amplification of ∆lmb locus |
| lmb-ko-ICrev | CCATGAGGCACCACATACCC |
a = restriction sites are indicated in lowercase and underlined.
The nucleotide sequences of PCR products, pJRS233 or pMU1328 constructs were determined using a BigDye Terminator V3.1 kit (Applied Biosystem) in an ABI PRISM 3700 Analyzer (Applied Biosystems).
Deletion mutants of adcA and lmb
MGAS5005 competent cells were prepared as described by Sitkiewicz & Musser [23] or by Kimoto & Taketo [24]. MGAS5005-∆adcA and MGAS5005-∆lmb mutants were obtained as described by Perez-Casal et al. [25]. Briefly, after electroporation, GAS cells were plated onto TSA-blood + 0.5 μg ml-1 erythromycin medium and incubated at 30°C for episomal replication of the temperature-sensitive vector pJRS233. Colonies were screened for the presence of the SOEing-generated insert and the positive ones were inoculated into 3 ml of THY + 1 μg ml-1 erythromycin and grown at 37°C to force integration of the vector into the specific chromosomal locus. Excision from the chromosome of pJRS233 carrying the wild type allele of adcA or lmb was obtained by growing sequential dilutions of cultures in THY medium without erythromycin and incubating them overnight at 37°C. Allelic replacement was confirmed by PCR amplification of the specific locus using primers external to the chromosomal region encompassing the allelic exchange site. The PCR products were subsequently verified by sequencing.
Growth in zinc depleted media
Bacterial cells were plated onto TSA-blood medium with the addition of 1 μg ml-1 erythromycin when required and incubated overnight at 37°C. A single colony was inoculated into 3 ml of THY and incubated overnight at 37°C. Each culture was then diluted 1:1000 into THY (with the addition of 1 μg ml-1 erythromycin when required) containing the appropriate concentration of the chelating agent Tetrakis-(2-Pyridylmethyl) ethylenediamine (TPEN, Sigma Aldrich) and also with the addition of ZnCl2 (Sigma-Aldrich). After incubation overnight at 37°C, growth was monitored spectrophotometrically at 600 nm. Experiments were performed in three independent replicates.
Preparation of protein extracts
Bacterial cells were inoculated into 10 ml of the appropriate medium and incubated at 37°C until they reached an optical density reading of 0.4. The culture was harvested by centrifugation at 6,000 rpm for 5 min and washed with PBS. The bacterial pellet was then suspended in 500 μl of 10 mM Tris-HCl pH 8.0 containing 200 U ml-1 of mutanolysin (Sigma Aldrich) and 2 mg ml-1 of lysozyme (Sigma Aldrich) and incubated for 1 h at 37°C with shaking. After centrifugation for 5 min at 13,000 rpm, the pellet was suspended in 150 μl of 10 mM Tris-HCl pH 8.0, 1 mM EDTA pH 8.0, 2% SDS and mixed by vortexing for 30 sec. Samples were diluted 1:4 with the Dissolving Buffer (Invitrogen) in the presence of a reducing agent, boiled for 5 min and stored at -20°C until needed. Ten micrograms of each total cell extract were loaded onto a 4–12% Novex Bis-Tris NuPAGE pre-casted gel (Invitrogen) in MES buffer (Invitrogen) and stained with SimplyBlue SafeStain (Invitrogen).
Western blot analysis
Five micrograms of total protein extracts were separated onto a 4–12% Novex Bis-Tris NuPAGE (Invitrogen) pre-casted gel and then transferred onto a nitrocellulose membrane with the iBlot apparatus (Invitrogen). Membranes were saturated with 10% skimmed milk in PBS-T buffer and probed with the appropriate antiserum, diluted in 1% skimmed milk in PBS-T buffer. Antisera against AdcA, Lmb and HtpA were raised in rabbits or mice immunised with purified recombinant proteins obtained as previously reported [26]. In our experimental conditions, the dilution used for each specific antiserum was 1:10,000 for rabbit anti-AdcA and 1:3,000 for mouse anti-Lmb or mouse anti-HtpA. After 1 h incubation at RT and 3 washes, an HRP-conjugated secondary antibody was added to the membrane at a 1:20,000 dilution in 1% skimmed milk PBS-T and incubated for 40 min under gentle agitation. The unbound antibody was washed away by 5 washes in the same buffer, the membrane was then overlaid with SuperSignal West Pico chemiluminescent substrate (Thermo Scientific Pierce) and exposed to a radiographic film. A marker for direct visualization of standard bands (MagicMark XP Western Protein Standard, Invitrogen) was used routinely for protein molecular mass estimation directly on Western blots. The antiserum specific for HtpA was found to give aspecific binding to a 37 kDa band present in all samples with an intensity that is directly correlated with the amount of total protein content of each sample, as evaluated by the Bradford method (Bio-Rad).
Results and Discussion
The ΔadcA mutant is more sensitive to zinc starvation
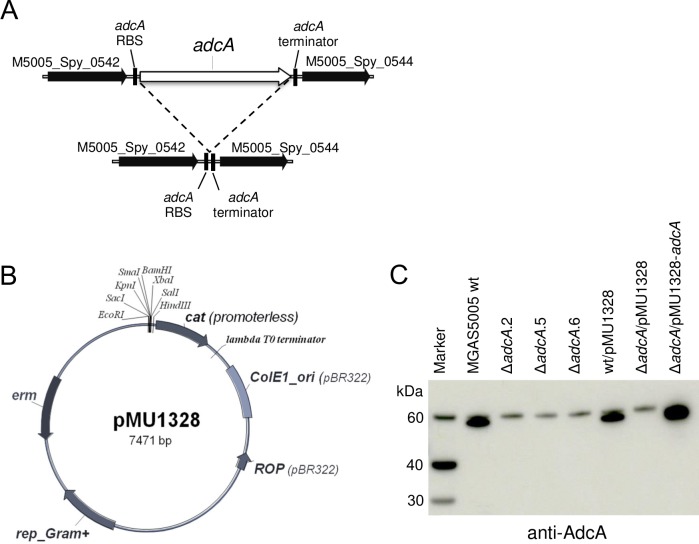
The gene product of adcA in the S. pyogenes strain MGAS5005 has been annotated as the orthologue of the high-affinity zinc uptake system protein ZnuA [17], known to be involved in zinc uptake both in Gram-negative [27] [28] and in Gram-positive bacteria [29]. Isogenic null mutants of adcA in strain MGAS5005 were generated as described in the Methods section by using the temperature-sensitive vector pJRS233 carrying the two regions flanking the adcA locus fused together to precisely delete the open reading frame assigned to AdcA (Fig 1A). One of the isogenic ΔadcA mutants was then used to obtain a strain complemented in trans by the wild type adcA gene carried on the expression vector pMU1328 [22]. The shuttle vector pMU1328, originally designed to identify promoter sequences in Streptococcus sanguis, S. lactis and S. cremoris [30], has been shown to be stably maintained in several Streptococcal species [31] [22]. Herein, we show that the pMU1328 replicon is suitable for the efficient expression of a homologous protein also in S. pyogenes. As is evident from Fig 1C, expression of AdcA was completely abolished in the ΔadcA mutant and approximately 2-fold higher than in the wild type strain when the same mutant was complemented with the pMU1328-adcA construct.
Fig 1. Construction of adcA null mutants.
(A) Schematic representation of the adcA locus in S. pyogenes MGAS5005 wild type and ΔadcA deletion mutants. (B) Map of plasmid pMU1328 used for complementation of the ΔadcA deletion mutants. (C) Western blot analysis of AdcA expression in total cell extracts of wild type, three independent ΔadcA null mutants and a ΔadcA complemented with the pMU1328-adcA construct using anti-AdcA specific antibodies.
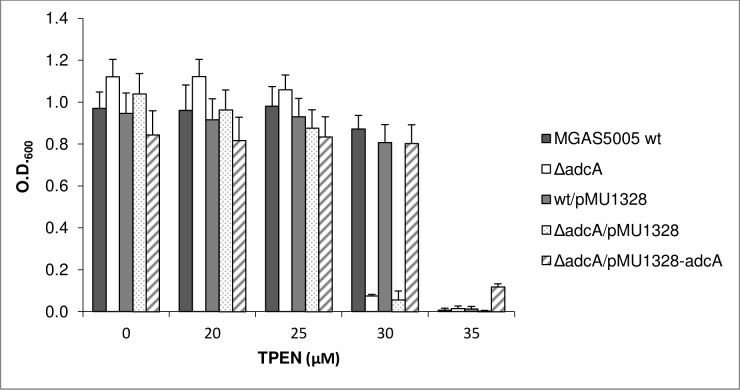
Since the gene product of adcA in other streptococci is known to be involved primarily in zinc uptake and homeostasis, we analysed the sensitivity to zinc starvation of the ∆adcA mutant and compared it with that of the wild type strain. The same mutant complemented with the pMU1328-adcA construct or carrying the empty pMU1328 vector were also included in our analysis as controls (Fig 2). The chelating agent N,N,N’,N’-Tetrakis-(2-pyridylmethyl)ethylenediamine (TPEN), a cell permeable high-affinity Zn2+ chelator used to reduce intra- and extra-cellular concentration of zinc in zinc homeostasis studies [32][16], was added at increasing concentrations to the growth medium of the ∆adcA mutant and MGAS5005 wild type strain. The ΔadcA mutant showed a higher susceptibility to zinc starvation. Growth was nearly abolished in the mutant by the addition of 30 µM TPEN, while it was only slightly decreased in the wild type or in the complemented strain (Fig 2). Complete inhibition of growth was observed at 35 μM TPEN for all strains, except for the ΔadcA mutant complemented with the pMU1328-adcA construct which showed some residual growth at 35 µM TPEN, possibly due to the higher level of AdcA expression in this strain (Fig 1C).
Fig 2. Susceptibility to Zn2+ starvation.
Susceptibility to Zn2+ starvation of wild type, ΔadcA null mutant and complemented strains grown in THY medium containing increasing concentrations of the chelating agent N,N,N’N’-Tetrakis (2-pyridylmethyl)-1,2-ethylenediamine (TPEN). Error bars represent the standard deviation.
Expression of Lmb and HtpA increases in ΔadcA mutants
The increased susceptibility to zinc starvation of the adcA null mutant strongly supports the notion that AdcA is indeed involved in zinc homeostasis in S. pyogenes, as already reported for other streptococci. In particular, in S. pneumoniae a recent characterization of AdcA has highlighted its complementarity in zinc homeostasis with AdcAII, the other Zn2+-binding SBP of streptococci, and demonstrated the cooperative functionality between the two importers [8]. Furthermore, immediately downstream of the gene coding for the AdcAII homologue Lmb in all the GAS strains characterized to date is located the gene coding for the histidine triad protein HtpA, a member of a family of proteins containing multiple copies of a histidine triad motif known to bind Zn2+ ions [33][34]. The gene coding for HtpA is highly conserved both in sequence and location also in GBS strains [33] and in S. pneumoniae, where adcAII is also located in the same operon as phtD, one of the four genes coding for a pneumococcal histidine triad protein [34][35]. For these reasons, we analysed the differences in expression levels of Lmb and HtpA in the ΔadcA mutant grown in zinc-depleted medium supplemented with increasing amounts of zinc ions (Fig 3). Firstly, it should be noted that in the wild type strain expression of AdcA was highest in medium supplemented with 10–15 μM of zinc ions, while at 20 μM ZnCl2 it was comparable with the level of expression observed in complete medium (Fig 3A). This suggests that limiting amounts of Zn2+ ions are able to induce only a small increase in expression of AdcA. On the contrary, Lmb and HtpA were expressed at significantly higher levels in both wild type and ΔadcA strains grown in the presence of 10–15 μM Zn2+ ions (Fig 3B and 3C). During growth in rich medium containing excess zinc ions (THB), expression of Lmb and HtpA was undetectable in wild type cells and low in the ΔadcA mutant. Moreover, Lmb and HtpA were expressed at higher levels in the ΔadcA mutant grown in the presence of 20 μM Zn2+ ions, a growth condition that presumably requires a functional AdcA for zinc homeostasis, which in a ΔadcA mutant could only be provided by an increased expression of Lmb and, consequently, of HtpA.
Fig 3. Expression of Lmb and HtpA in ΔadcA mutants.
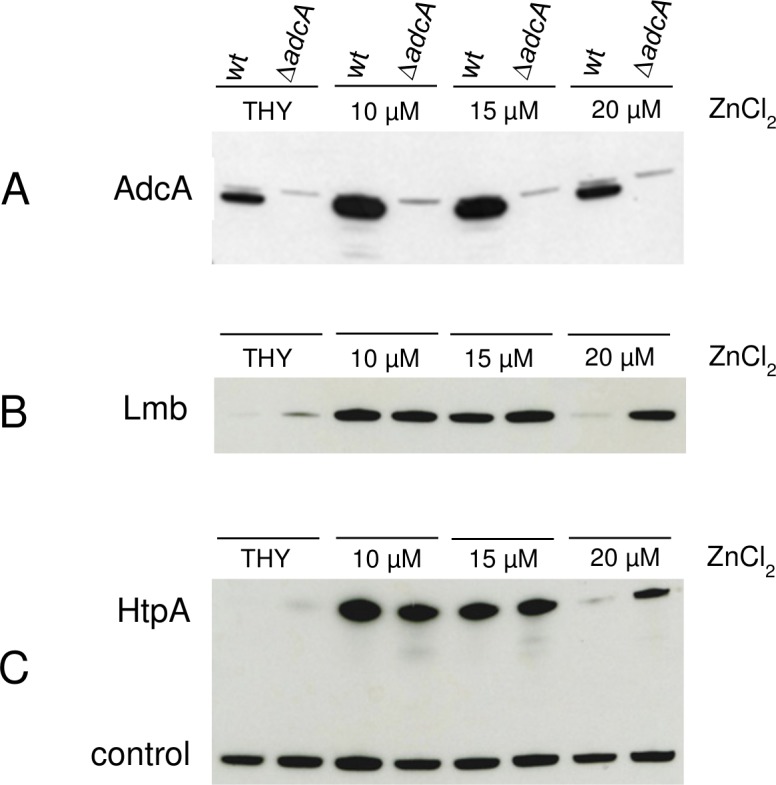
Western blot analysis of total cell extracts from S. pyogenes MGAS5005 wild type and ΔadcA null mutant grown in complete medium (THY) or in zinc-depleted medium (THY + 35 μM TPEN) containing increasing amounts of ZnCl2. (A) Western blot using anti-AdcA specific antibodies. (B) Western blot using anti-Lmb specific antibodies. (C) Western blot using anti-HtpA specific antibodies.
The Δlmb mutant is as sensitive to zinc starvation as the ΔadcA strain
An in-frame deletion mutant of lmb/lsp has already been described in the S. pyogenes strain HSC5 [36] and shown to have a role in zinc homeostasis and pathogenesis [16]. However, due to the variability in genetic composition between different S. pyogenes strains [37] and the complexity of zinc homeostasis control in any biological system, we generated a Δlmb mutant in strain MGAS5005 in order to investigate the contribution of Lmb to zinc uptake in the same genetic background used for characterizing AdcA. The map of the locus in the lmb null mutant is outlined in Fig 4A and the Western blot analysis carried out to confirm the absence of Lmb expression in the mutant strain is given in Fig 4B.
Fig 4. Construction of Δlmb null mutants.
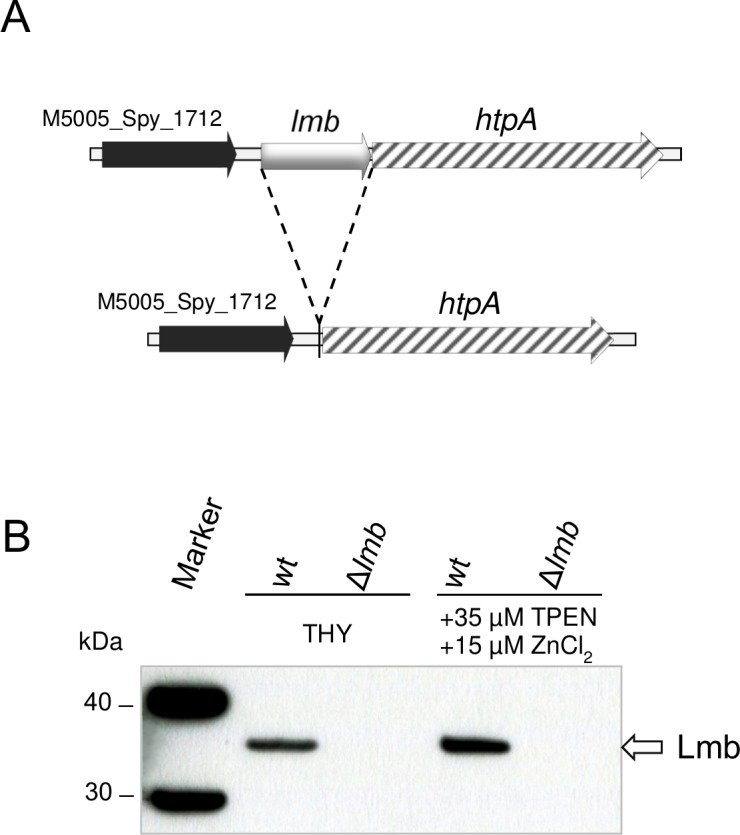
(A) Schematic representation of the lmb locus in S. pyogenes MGAS5005 wild type and Δlmb deletion mutants. (B) Western blot analysis of Lmb expression using anti-Lmb specific antibodies in total cell extracts of wild type and of a Δlmb null mutant grown in THY medium or in THY with the addition of 35 μM TPEN and 15 μM ZnCl2. The latter growth medium was tested since it was observed to give higher expression of Lmb in our experimental conditions.
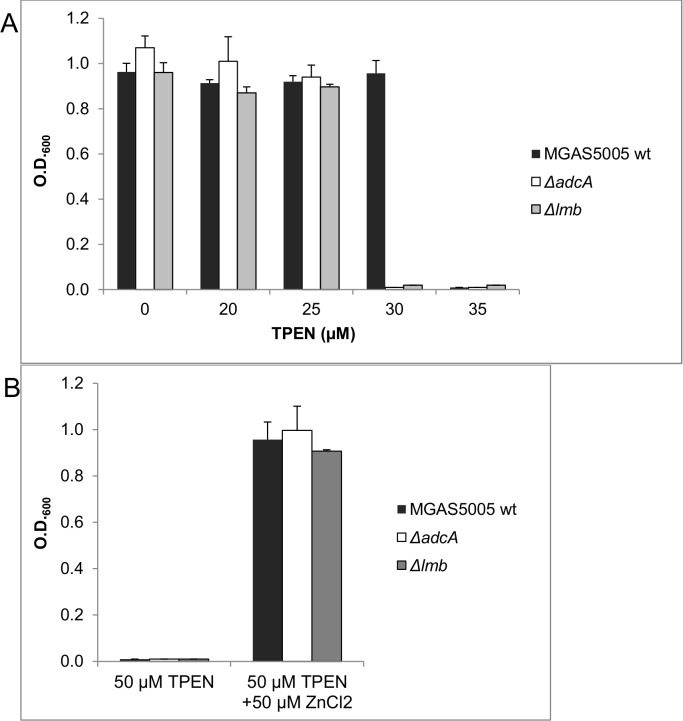
Loss of adcA or lmb expression in the two null mutants did not significantly impair their growth rates in THY medium with respect to that of the wild type strain (S1 Fig). Furthermore, growth in the presence of increasing concentrations of TPEN, for both ΔadcA and Δlmb mutants, was also comparable with that of the wild type strain at concentrations up to 25 μM TPEN. Conversely, at 30 μM TPEN only the wild type strain was able to grow and at 35μM TPEN growth was completely inhibited for all strains (Fig 5A). Addition of equimolar amounts of Zn2+ ions to the zinc-depleted medium could rescue growth of both ΔadcA and Δlmb mutant strains (Fig 5B), thus confirming that in S. pyogenes these two SBPs have overlapping roles in zinc acquisition, as already described in S. pneumoniae [8].
Fig 5. Inhibition of growth by TPEN is rescued by the addition of Zn2+ ions.
(A) Susceptibility to Zn2+ starvation of wild type, ΔadcA and Δlmb null mutants grown in THY medium containing increasing concentrations of the chelating agent TPEN. (B) Inhibition of growth by 50 μM TPEN is rescued by the addition of either 50 μM Zn2+ in wild type as well as in ΔadcA and Δlmb null mutants. Error bars represent the standard deviation.
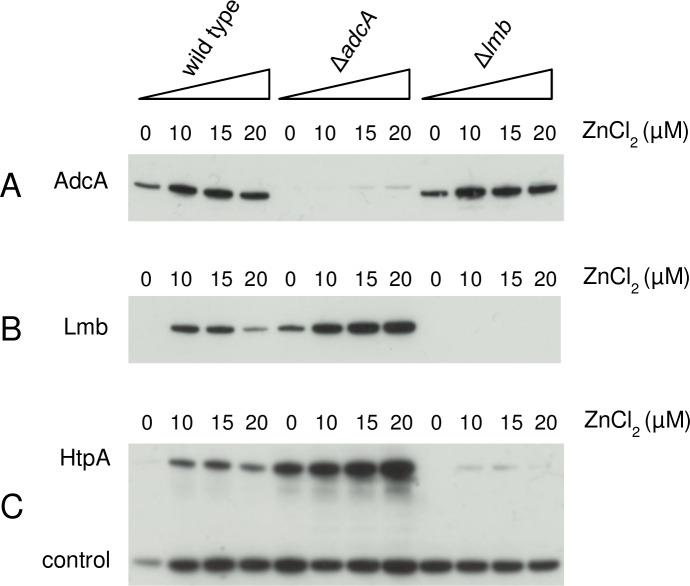
Wild type S. pyogenes MGAS5005 and isogenic mutant strains lacking adcA or lmb were analysed for expression of AdcA, Lmb and HtpA during growth in complete medium (THY) or in zinc-depleted medium (THY + 35 μM TPEN) containing increasing amounts of ZnCl2. The immunoblot analyses presented in Fig 6 confirm the results obtained for the ΔadcA mutant (Fig 3). Lmb and HtpA were both expressed at higher levels in the ΔadcA mutant, strongly supporting the assumption that also in S. pyogenes AdcA and Lmb have overlapping roles in zinc acquisition [8] (Fig 6B and 6C).
Fig 6. Expression of AdcA, Lmb and HtpA during growth in zinc-depleted medium.
Western blot analysis of total cell extracts from S. pyogenes MGAS5005 wild type, ΔadcA and Δlmb null mutants grown in complete medium (THY) or in zinc-depleted medium (THY + 35 μM TPEN) containing increasing amounts of ZnCl2. (A) Western blot using anti-AdcA specific antibodies. (B) Western blot using anti-Lmb specific antibodies. (C) Western blot using anti-HtpA specific antibodies.
An additional outcome of this analysis is the observation that expression of Lmb and HtpA was not detectable in the wild type strain grown in THY (lanes 0 in Fig 6B and 6C). As this condition probably represents growth in the presence of excess zinc ions, we can conclude that the main importer of zinc ions in S. pyogenes is AdcA. In fact, AdcA is always expressed in wild type cells, with little variation in the level of expression during growth in media with a different content of Zn2+ ions or in the absence of a functional Lmb, as in the case of the Δlmb mutant (Fig 6A). In contrast, Lmb may represent the zinc importer expressed only during growth in zinc-depleted media while, in the absence of AdcA as in the ΔadcA mutant, it needs to be overexpressed only during growth in an excess of Zn2+ ions. This implies that in normal conditions, when the concentration of Zn2+ ions is 20 μM or higher, AdcA is sufficient to maintain a physiological homeostasis of zinc ions, while in zinc-depleted growth conditions both importers are required for the maintenance of a correct influx of zinc ions.
Conversely, an excess of intracellular free zinc ions is known to be highly toxic, thus the need to overexpress both Lmb and HtpA in the ΔadcA mutant may be due to the different zinc-binding capabilities of AdcA and Lmb. Indeed, a recent characterization of AdcA and AdcAII (Lmb) of S. pneumoniae has shown that, as previously reported for the orthologous SBPs in other bacteria [7] [38] [39], AdcA has two zinc-binding domains [8] while AdcAII contains only one zinc-binding site [9]. Although the affinity and stoichiometry of zinc binding to AdcA and Lmb in S. pyogenes have not been determined, it is reasonable to assume that they are similar to those reported for other streptococci during growth in vitro. Thus, if AdcA and Lmb contribute to the maintenance of a low intracellular content of free Zn2+ ions through their capability of binding zinc, expression of Lmb should increase at least two-fold in the absence of AdcA in order to provide the correct number of zinc binding sites. In fact, a greater than three-fold increase of Lmb expression was observed in the ΔadcA mutant, particularly during growth in the presence of zinc ions in excess (Fig 6B). Interestingly, the increase of HtpA expression was even higher in the same conditions (Fig 6C). These variations in level of expression are confirmed by the quantitative analysis presented in S2 Fig.
As the function of S. pyogenes HtpA has yet to be clarified, we propose that, by analogy with the role described for the pneumococcal histidine triad proteins [40][35][10], the main task of HtpA is to facilitate Zn2+ acquisition exclusively through Lmb. Indeed, since the null lmb mutant obtained in this work also lacked expression of HtpA (Fig 6C), but could grow at a normal rate and with levels of AdcA expression very similar to those observed in the wild type strain grown in the same conditions (Fig 6A), it may be inferred that acquisition of Zn2+ ions through AdcA does not require HtpA. Whether AdcA is capable of binding zinc unaided or needs the cooperation of another histidine triad protein remains to be investigated. Future work will be focused on studying the interplay between the different SBPs involved in zinc ion uptake and the histidine triad proteins that presumably contribute to the maintenance of zinc homeostasis in S. pyogenes.
Supporting Information
Strains were grown in THY medium and the increase in cell density over time was measured spectrophotometrically. Overnight cultures were diluted at a ratio of 1:2 in fresh THY and grown in a static environment for 2 hours. The growing cells were then diluted to an OD600 of 0.03 in fresh THY. The cells were statically grown in a microaerobic environment and the OD600 of the culture was measured at regular intervals.
(TIF)
Relative intensities of the bands detected in Western blot analysis of total cell extracts from S. pyogenes MGAS5005 wild type, ΔadcA and Δlmb null mutants grown in complete medium (THY) or in zinc-depleted medium (THY + 35 μM TPEN) containing increasing amounts of zinc ions. The relative intensities were determined on the basis of two independent experiments using the Phoretix 1D software (Cleaver Scientific Ltd., Rugby, United Kingdom). The data were normalised on the control bands of each experiment (see Fig 6). Error bars represent the standard deviation.
(TIF)
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by GSK Vaccines S.r.l. The authors confirm that the GSK Vaccines S.r.l funder provided support in the form of salaries for authors VT RR CLG, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of all authors are articulated in the ‘author contributions’ section.
References
- 1.Coleman JE. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61: 897–946. 10.1146/annurev.bi.61.070192.004341 [DOI] [PubMed] [Google Scholar]
- 2.Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. J Proteome Res. 2006;5: 3173–3178. [DOI] [PubMed] [Google Scholar]
- 3.Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem. 2008;13: 1205–18. 10.1007/s00775-008-0404-5 [DOI] [PubMed] [Google Scholar]
- 4.Braymer JJ, Giedroc DP. Recent developments in copper and zinc homeostasis in bacterial pathogens. Curr Opin Chem Biol. 2014;19: 59–66. 10.1016/j.cbpa.2013.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patzer SI, Hantke K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol. 1998;28: 1199–210. [DOI] [PubMed] [Google Scholar]
- 6.Dintilhac A, Alloing G. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal. Mol Microbiol. 1997;25: 727–739. Available: http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1997.5111879.x/abstract [DOI] [PubMed] [Google Scholar]
- 7.Desrosiers DC, Sun YC, Zaidi AA, Eggers CH, Cox DL, Radolf JD. The general transition metal (Tro) and Zn2+ (Znu) transporters in Treponema pallidum: analysis of metal specificities and expression profiles. Mol Microbiol. 2007/06/22 ed. 2007;65: 137–152. [pii] 10.1111/j.1365-2958.2007.05771.x [DOI] [PubMed] [Google Scholar]
- 8.Plumptre CD, Eijkelkamp B a, Morey JR, Behr F, Couñago RM, Ogunniyi AD, et al. AdcA and AdcAII employ distinct zinc acquisition mechanisms and contribute additively to zinc homeostasis in Streptococcus pneumoniae. Mol Microbiol. 2014;91: 834–51. 10.1111/mmi.12504 [DOI] [PubMed] [Google Scholar]
- 9.Loisel E, Jacquamet L, Serre L, Bauvois C, Ferrer JL, Vernet T, et al. AdcAII, a new pneumococcal Zn-binding protein homologous with ABC transporters: biochemical and structural analysis. J Mol Biol. 2008;381: 594–606. 10.1016/j.jmb.2008.05.068 [DOI] [PubMed] [Google Scholar]
- 10.Plumptre CD, Hughes CE, Harvey RM, Eijkelkamp B a, McDevitt C a, Paton JC. Overlapping functionality of the Pht proteins in zinc homeostasis of Streptococcus pneumoniae. Infect Immun. 2014;82: 4315–24. 10.1128/IAI.02155-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. Elsevier Ltd; 2010;14: 218–24. 10.1016/j.cbpa.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker KW, Skaar EP. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev. 2014;38: 1235–49. 10.1111/1574-6976.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ong CY, Gillen CM, Barnett TC, Walker MJ, McEwan AG. An antimicrobial role for zinc in innate immune defense against group A streptococcus. J Infect Dis. 2014;209: 1500–8. 10.1093/infdis/jiu053 [DOI] [PubMed] [Google Scholar]
- 14.Ong CY, Walker MJ, McEwan AG. Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes. Sci Rep. 2015;5: 10799 10.1038/srep10799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linke C, Caradoc-Davies TT, Young PG, Proft T, Baker EN. The laminin-binding protein Lbp from Streptococcus pyogenes is a zinc receptor. J Bacteriol. 2009;191: 5814–23. 10.1128/JB.00485-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weston BF, Brenot A, Caparon MG. The metal homeostasis protein, Lsp, of Streptococcus pyogenes is necessary for acquisition of zinc and virulence. Infect Immun. 2009;77: 2840–8. 10.1128/IAI.01299-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, Ricklefs SM, et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events. J Infect Dis. 2005;192: 771–82. 10.1086/432514 [DOI] [PubMed] [Google Scholar]
- 18.Elsner A, Kreikemeyer B, Braun-Kiewnick A, Spellerberg B, Buttaro BA, Podbielski A. Involvement of Lsp, a member of the LraI-lipoprotein family in Streptococcus pyogenes, in eukaryotic cell adhesion and internalization. Infect Immun. 2002;70: 4859–69. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=128222&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terao Y, Kawabata S, Kunitomo E, Nakagawa I, Hamada S. Novel laminin-binding protein of Streptococcus pyogenes, Lbp, is involved in adhesion to epithelial cells. Infect Immun. 2002;70: 993–7. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=127702&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumby P, Whitney AR, Graviss E a., DeLeo FR, Musser JM. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2: 0041–0049. 10.1371/journal.ppat.0020005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horton RM. PCR-mediated recombination and mutagenesis. SOEing together tailor-made genes. Mol Biotechnol. 1995;3: 93–9. 10.1007/BF02789105 [DOI] [PubMed] [Google Scholar]
- 22.Lo Sapio M, Hilleringmann M, Barocchi M a, Moschioni M. A novel strategy to over-express and purify homologous proteins from Streptococcus pneumoniae. J Biotechnol. Elsevier B.V.; 2012;157: 279–86. 10.1016/j.jbiotec.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 23.Sitkiewicz I, Musser J. Expression microarray and mouse virulence analysis of four conserved two-component gene regulatory systems in group A Streptococcus. Infect Immun. 2006;74: 1339–51. 10.1128/IAI.74.2.1339-1351.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimoto H, Taketo A. Efficient electrotransformation system and gene targeting in pyogenic streptococci. Biosci Biotechnol Biochem. 2003;67: 2203–9. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Casal J., Price J. A., Maguin E., Scott JR. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol Microbiol. 1993;8: 809–819. [DOI] [PubMed] [Google Scholar]
- 26.Galeotti CL, Bove E, Pezzicoli A, Nogarotto R, Norais N, Pileri S, et al. Surface interactome in Streptococcus pyogenes. Mol Cell Proteomics. 2012;11: 10.1074/mcp.M111.015206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desrosiers DC, Bearden SW, Mier I, Abney J, Paulley JT, Fetherston JD, et al. Znu is the predominant zinc importer in Yersinia pestis during in vitro growth but is not essential for virulence. Infect Immun. 2010;78: 5163–77. 10.1128/IAI.00732-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabbianelli R, Scotti R, Ammendola S, Petrarca P, Nicolini L, Battistoni A. Role of ZnuABC and ZinT in Escherichia coli O157:H7 zinc acquisition and interaction with epithelial cells. BMC Microbiol. 2011;11: 36 10.1186/1471-2180-11-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayle L, Chimalapati S, Schoehn G, Brown J, Vernet T, Durmort C. Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Mol Microbiol. 2011;82: 904–16. 10.1111/j.1365-2958.2011.07862.x [DOI] [PubMed] [Google Scholar]
- 30.Achen MG, Davidson BE, Hillier a J. Construction of plasmid vectors for the detection of streptococcal promoters. Gene. 1986;45: 45–49. 10.1016/0378-1119(86)90130-7 [DOI] [PubMed] [Google Scholar]
- 31.Lakshmidevi G, Davidson BE, Hillier AJ. Molecular characterization of promoters of the Lactococcus lactis subsp. cremoris temperate bacteriophage BK5-T and identification of a phage gene implicated in the regulation of promoter activity. Appl Environ Microbiol. 1990;56: 934–42. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=184325&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shumaker DK, Vann LR, Goldberg MW, Allen TD, Wilson KL. TPEN, a Zn2+/Fe2+ chelator with low affinity for Ca2+, inhibits lamin assembly, destabilizes nuclear architecture and may independently protect nuclei from apoptosis in vitro. Cell Calcium. 1998;23: 151–164. Available: http://www.ncbi.nlm.nih.gov/pubmed/9601611 [DOI] [PubMed] [Google Scholar]
- 33.Kunitomo E, Terao Y, Okamoto S, Rikimaru T, Hamada S, Kawabata S. Molecular and biological characterization of histidine triad protein in group A streptococci. Microbes Infect. 2008;10: 414–23. 10.1016/j.micinf.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 34.Loisel E, Chimalapati S, Bougault C, Imberty A, Gallet B, Di Guilmi AM, et al. Biochemical characterization of the histidine triad protein PhtD as a cell surface zinc-binding protein of pneumococcus. Biochemistry. 2011;50: 3551–8. 10.1021/bi200012f [DOI] [PubMed] [Google Scholar]
- 35.Plumptre CD, Ogunniyi AD, Paton JC. Polyhistidine triad proteins of pathogenic streptococci. Trends Microbiol. Elsevier Ltd; 2012;20: 485–93. 10.1016/j.tim.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 36.Brenot A, Weston BF, Caparon MG. A PerR-regulated metal transporter (PmtA) is an interface between oxidative stress and metal homeostasis in Streptococcus pyogenes. Mol Microbiol. 2007;63: 1185–1196. 10.1111/j.1365-2958.2006.05577.x [DOI] [PubMed] [Google Scholar]
- 37.Carroll RK, Beres SB, Sitkiewicz I, Peterson L, Matsunami RK, Engler DA, et al. Evolution of diversity in epidemics revealed by analysis of the human bacterial pathogen group A Streptococcus. Epidemics. 2011;3: 159–70. 10.1016/j.epidem.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 38.Wei B, Randich AM, Bhattacharyya-Pakrasi M, Pakrasi HB, Smith TJ. Possible regulatory role for the histidine-rich loop in the zinc transport protein, ZnuA. Biochemistry. 2007;46: 8734–43. 10.1021/bi700763w [DOI] [PubMed] [Google Scholar]
- 39.Yatsunyk LA, Easton JA, Kim LR, Sugarbaker SA, Bennett B, Breece RM, et al. Structure and metal binding properties of ZnuA, a periplasmic zinc transporter from Escherichia coli. J Biol Inorg Chem. 2008;13: 271–88. 10.1007/s00775-007-0320-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao Z-Q, Zhang Y-M, Pan X-Z, Wang B, Chen J-Q. Insight into the evolution of the histidine triad protein (HTP) family in Streptococcus. PLoS One. 2013;8: e60116 10.1371/journal.pone.0060116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Strains were grown in THY medium and the increase in cell density over time was measured spectrophotometrically. Overnight cultures were diluted at a ratio of 1:2 in fresh THY and grown in a static environment for 2 hours. The growing cells were then diluted to an OD600 of 0.03 in fresh THY. The cells were statically grown in a microaerobic environment and the OD600 of the culture was measured at regular intervals.
(TIF)
Relative intensities of the bands detected in Western blot analysis of total cell extracts from S. pyogenes MGAS5005 wild type, ΔadcA and Δlmb null mutants grown in complete medium (THY) or in zinc-depleted medium (THY + 35 μM TPEN) containing increasing amounts of zinc ions. The relative intensities were determined on the basis of two independent experiments using the Phoretix 1D software (Cleaver Scientific Ltd., Rugby, United Kingdom). The data were normalised on the control bands of each experiment (see Fig 6). Error bars represent the standard deviation.
(TIF)
Data Availability Statement
All relevant data are within the paper.