Abstract
Purpose
To determine intraocular pharmacokinetic properties of intravitreally injected vascular endothelial growth factor (VEGF)-Trap in a rabbit model.
Methods
VEGF-Trap was intravitreally injected in 18 rabbit eyes. Eyes were enucleated 1 h and 1, 2, 5, 14, and 30 days after injections and immediately frozen at −80 °C. Concentration of VEGF-Trap in vitreous, aqueous humor, and retina/choroid was determined using an indirect enzyme-linked immunosorbent assay and analyzed to obtain pharmacokinetic properties.
Results
Maximum concentration of VEGF-Trap was achieved at 1 h in all three tissues. A one-compartment model of distribution was selected as the final model for all tissues studied. Estimated half-life of VEGF-Trap in vitreous, aqueous humor, and retinal/choroid was 87.1, 36.8, and 35.0 h, respectively, and estimated mean residence time was 125.7, 53.1, and 50.5 h, respectively. Area under the curve from time 0 to the end point was 10009.8, 3945.1, and 1189.3, respectively. Total exposure of the aqueous humor and retina/choroid to VEGF-Trap was 39.4% and 11.9% of vitreous exposure, respectively.
Conclusion
The vitreous half-life of VEGF-Trap is 3.63 days. This is shorter than that of bevacizumab (6.99 days) and longer than that of ranibizumab (2.51 days), as shown in studies using the same experimental settings. The concentration of VEGF-Trap peaked at 1 h after injections in all eye tissues studied.
Introduction
Anti-vascular endothelial growth factor (anti-VEGF) agents have been widely used in numerous diseases affecting sight, including exudative age-related macular degeneration (AMD), macular edema subsequent to retinal vein occlusion (RVO) or diabetic retinopathy, and neovascular glaucoma. Intravitreal ranibizumab injection (Lucentis; Genentech Inc., San Francisco, CA, USA), approved by the United States Food and Drug Administration for the treatment of exudative AMD1, 2 and macular edema subsequent to RVO,3 has been the first-line treatment for diseases affecting sight, and has shown notable efficacy in vision improvement.1, 2, 3, 4 In addition, intravitreal administration of bevacizumab (Avastin; Genentech Inc.) has been used to treat exudative AMD and other VEGF-associated conditions, although this drug is only approved for the systemic treatment of metastatic colon cancer.5
The novel anti-VEGF agent VEGF-Trap was developed for the treatment of VEGF-associated intraocular conditions, including exudative AMD and macular edema secondary to RVO and diabetic retinopathy.6, 7, 8, 9, 10 VEGF-Trap has greater binding affinity for VEGF than ranibizumab and bevacizumab,6 suggesting that it may have a substantially longer duration of action in the eye, as predicted by a mathematical model.11 Accordingly, it is hypothesized that VEGF-Trap will require less-frequent administration by intravitreal injection compared with ranibizumab and bevacizumab, although intraocular pharmacokinetic (PK) studies for VEGF-Trap are lacking. In one PK study, the intravitreal half-life of VEGF-Trap was found to be 4.58 days, as assessed by positron emission tomography/computed tomography imaging.12 Further, the manufacturer of VEGF-Trap, Regeneron Pharmaceuticals Inc. (Tarrytown, NY, USA), reports that the intravitreal half-life of VEGF-Trap-Eye (Aflibercept, Eylea) is 4.79 days.11 Nevertheless, comparable intraocular PK studies for VEGF-Trap are warranted, not only to assess the efficacy of VEGF-Trap, but also to understand the intraocular distribution and elimination of VEGF-Trap.
In the present study, we determined the intraocular PK properties of intravitreally injected VEGF-Trap in the vitreous, aqueous humor, and retina/choroid of the eye, using a conventional immunoassay in a rabbit model. This model was previously used for the study of intraocular PK properties of intravitreally injected bevacizumab and ranibizumab.13, 14 In addition, we assessed the differences in the PK properties and bioavailability of bevacizumab, ranibizumab, and VEGF-Trap by comparing our current results with the data from our previous studies.13, 14
Materials and methods
Generation of recombinant VEGF-Trap protein
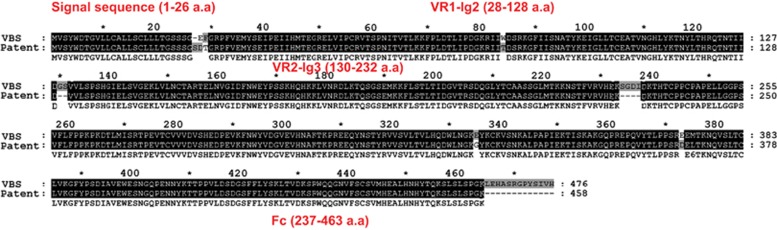
We generated VEGF-Trap based on a previous report from Regeneron Inc.6 An expression vector encoding a fusion protein of human VEGFR1-Ig2 (UniProt ID: P17948, 140S∼231D) and VEGFR2-Ig3 (UniProt ID: P35968, 226V∼327K) tagged with the human Fc domain was cloned into the pCMV-dihydrofolate reductase (dhfr) vector. The gene construct containing dhfr was transfected into dhfr-deficient CHO cells (CRL-9096, American Type Culture Collection, Manassas, VA, USA) to establish recombinant CHO stable cell lines expressing VEGF-Trap, as previously described.15 Briefly, transfected cells were selected using G418, and genes were amplified by gradually increasing methotrexate (0.001–0.5 μM). The cells were then grown in HyQSFM4CHO (Thermo Scientific, Waltham, MA, USA) media supplemented with 0.5 μM methotrexate. After 5 days, culture media containing VEGF-Trap was harvested, and VEGF-Trap was purified by protein A-sepharose affinity chromatography. Protein A-bound VEGF-Trap was eluted under acidic conditions with 0.2 M Glycine (pH 2.7), followed by immediate neutralization with 1 M Tris-HCl (pH 9.0). Purified proteins were dialyzed in 1 × phosphate-buffered saline (PBS), quantified using the Bradford assay, and visualized by Coomassie Blue staining of a SDS-polyacrylamide gel electrophoretic (SDS-PAGE) gel. The generated VEGF-Trap was composed of 476 amino acids and had a sequence similar to commercially available VEGF-Trap-Eye, aflibercept (Eylea; Regeneron Inc. and Bayer Healthcare Pharmaceuticals, Berlin, Germany), which was composed of 458 amino acids. The discordance between two protein sequences were observed in 5.3% of the sequences (Figure 1).
Figure 1.
Sequence of two vascular endothelial growth factor-Traps (VEGF-Traps). VBS indicates the VEGF-Trap sequence used in the present study and Patent indicates VEGF-Trap-Eye (Aflibercept, Eylea) sequence from the patent (Regeneron). The discordance in sequences of the two proteins occurred within 5.3% (25 out of 476 amino acids). VBS is a sequence constructed by the laboratory named ‘Vascular Biology and Stem Cell (VBS) laboratory' in the Korea Advanced Institute of Science and Technology (KAIST).
Animal experiments
Approval for the study was obtained from the Seoul National University Bundang Hospital Institutional Animal Care and Use Committee, and all procedures adhered to the guidelines of the Association for Research in Vision and Ophthalmology for research in animals.
A total of 18 eyes were obtained from 18 healthy New Zealand white rabbits weighing 1.5–2 kg. The intraocular PK of intravitreally injected VEGF-Trap was assessed by the same experimental design used in our previous studies.13, 14 Briefly, the animals were anesthetized with an intramuscular injection of 15 mg/kg of Zoletil (a mixture of tiletamine hydrochloride and zolazepam hydrochloride; Virbac Laboratories, Carros, France) and 5 mg/kg of xylazine hydrochloride. Furthermore, topical anesthesia (1% proparacaine hydrochloride ophthalmic eye drops; Alcaine; Alcon Laboratories Inc., Fort Worth, TX, USA) was administered after dilation of the eyes with phenylephrine hydrochloride and tropicamide eye drops (Mydrin-P; Santen Pharmaceutical Co., Osaka, Japan). After placing 5% povidone iodine on the periocular region and the conjunctiva of the right eye, VEGF-Trap (0.3 mg/0.03 ml) was intravitreally administered to the right eye 1 mm behind the surgical limbus in the superiotemporal quadrant by injection with a 30-gauge needle and a Hamilton syringe (Hamilton Company, Reno, NV, USA). Three rabbits were killed at each of the following time points: 1 h or 1, 2, 5, 14, or 30 days after injection. The right eyes were enucleated and immediately frozen at −80 °C until the immunoassay was performed. Prior to analysis, the frozen eyes were separated into three parts—the vitreous, aqueous humor, and retina/choroid. Aqueous humor samples were defrosted and the volume of the sample was measured. Vitreous samples were defrosted and solubilized in 1.0 ml PBS containing 1% bovine serum albumin (BSA) on a rotator overnight at 4 °C. The samples were centrifuged at 387 g for 10 min, as performed by Bakri et al16 The volume of the sample after centrifugation was measured. The frozen retina/choroid was weighed and the samples were defrosted and homogenized (CellLytic MT; C3228, Sigma-Aldrich, St. Louis, MO, USA), as described by Nomoto et al.17
VEGF-Trap immunoassay
Concentrations of VEGF-Trap in each compartment were measured using an indirect enzyme-linked immunosorbent assay, as described previously.13, 14, 16 Briefly, the 165 amino-acid variants of human recombinant VEGF (rVEGF) was immobilized on 96-well flat-bottom plates (Corning Inc., Corning, NY, USA). The human rVEGF was diluted to 1.0 μg/ml in 50 mM carbonate buffer (pH 9) and then aliquoted onto the 96-well plates (100 μl per well). Plates were incubated overnight at 4 °C, and then washed with 1 × PBS and blocked for 2–4 h at 4 °C with 1% BSA in 1 × PBS. After final washes, the plates were stored until dry at 4 °C.
Vitreous, aqueous humor, and retina/choroid samples were diluted with 0.1% BSA in 1 × PBS so as to be within the linear range of the assay, aliquoted onto the VEGF plates (100 μl per well), and incubated overnight at 4 °C. For each individual plate, a standard curve containing known VEGF-Trap concentrations (0.008–1000 μg/ml) was included. The bound VEGF-Trap was detected with 1 : 20 000 goat anti-human IgG/F(ab′)2 antibody labeled with horseradish peroxidase (Pierce Biotechnology Inc., Rockford, IL, USA). The diluted secondary antibody was incubated on the human rVEGF plate for 45 min at room temperature with agitation, followed by washing. The optical density was measured by detecting absorbance after treating the 3,3′,5,5′-tetramethyl benzidine substrate with hydrogen peroxide. The concentration of VEGF-Trap in our samples was calculated from the standard curve, which was constructed using the relative light signal from solutions of VEGF-Trap with known concentrations. The retina/choroid concentration of VEGF-Trap was calculated by dividing the weight of VEGF-Trap (μg) by that of the retina/choroid tissue (g).
PK data analysis
The concentrations of VEGF-Trap in the vitreous, aqueous humor, and retina/choroid were analyzed by one- and two-compartment models and a non-compartmental analysis using Phoenix WinNonlin software version 1.3 (Certara, St. Louis, MO, USA). The following equation was used for the one-compartment model: 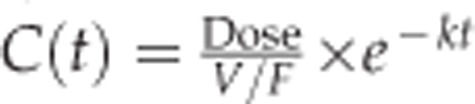 , where C(t) (μg/ml) denotes concentration at time t, V/F (ml) is the apparent volume of distribution, and k (1/h) indicates the elimination rate constant. For vitreous humor, F was assumed to 1. However, for the aqueous humor and retina/choroid, F was not assumed or estimated. The following equation was used for the two-compartment model: C(t)=A × e−αt+B × e−βt, where A (μg/ml) and B (μg/ml) are the back-extrapolated intercepts of the distribution and elimination phases, respectively. Both α (1/h) and β (1/h) represent the rate constants of the distribution and elimination phases, respectively. Half-life (t1/2, h), mean residence time (MRT, h), maximum concentration (Cmax, μg/ml), area under the concentration–time curve (AUC, μg × h/ml), and apparent clearance (CL/F, ml/h) were estimated by post hoc analysis. After analysis, model selection between the one- and two-compartment models was done based on the following criteria: (1) Akaike Information Criterion (AIC), (2) precision of parameter estimates, and (3) graphical analysis. The AIC was computed using the weighted residual sum of squares of model (WRSS) and the number of observations and parameters (N and P) used during the modeling as follows: AIC=N × log (WRSS)+2P. The AIC, precision of parameter estimate (SE, presented as the coefficient of variation (CV)), and goodness-of-fit plot including the predicted versus the observed concentrations were compared between the two models.
, where C(t) (μg/ml) denotes concentration at time t, V/F (ml) is the apparent volume of distribution, and k (1/h) indicates the elimination rate constant. For vitreous humor, F was assumed to 1. However, for the aqueous humor and retina/choroid, F was not assumed or estimated. The following equation was used for the two-compartment model: C(t)=A × e−αt+B × e−βt, where A (μg/ml) and B (μg/ml) are the back-extrapolated intercepts of the distribution and elimination phases, respectively. Both α (1/h) and β (1/h) represent the rate constants of the distribution and elimination phases, respectively. Half-life (t1/2, h), mean residence time (MRT, h), maximum concentration (Cmax, μg/ml), area under the concentration–time curve (AUC, μg × h/ml), and apparent clearance (CL/F, ml/h) were estimated by post hoc analysis. After analysis, model selection between the one- and two-compartment models was done based on the following criteria: (1) Akaike Information Criterion (AIC), (2) precision of parameter estimates, and (3) graphical analysis. The AIC was computed using the weighted residual sum of squares of model (WRSS) and the number of observations and parameters (N and P) used during the modeling as follows: AIC=N × log (WRSS)+2P. The AIC, precision of parameter estimate (SE, presented as the coefficient of variation (CV)), and goodness-of-fit plot including the predicted versus the observed concentrations were compared between the two models.
In addition, the Cmax, Tmax (time to Cmax), and AUClast in the vitreous, aqueous humor, and retina/choroid were also calculated using a non-compartmental method. In addition, the AUClast in the vitreous, aqueous humor, and retina/choroid for bevacizumab and ranibizumab was calculated using raw data from our previous studies,13, 14 using non-compartmental methods as stated above. Unfortunately, we had not measured the bevacizumab concentration in the retina/choroid during our previous study. Using the AUClast in each compartment, the total exposure of the aqueous humor and retina/choroid to VEGF-Trap, bevacizumab, and ranibizumab from the vitreous was estimated.
Results
Data were collected from 18 eyes taken from 18 rabbits. There was no evidence of ocular inflammation or other adverse events following treatment. Changes in the concentration and estimated amount of VEGF-Trap in the vitreous, aqueous humor, and retina/choroid over time are provided in Table 1. The Cmax of VEGF-Trap was observed at 1 h in both the aqueous humor and retina/choroid, as well in the vitreous. After 1 h, intravitreally injected VEGF-Trap was distributed as follows: 129.26±18.99 μg in the vitreous, 9.90±1.05 μg in the aqueous humor, and 0.61±0.24 μg in the retina/choroid. Approximately half of the total amount of VEGF-Trap observed in the vitreous at 1 h remained after 5 days. After 14 days, less than 3.9% of VEGF-Trap remained in the vitreous.
Table 1. Concentration and estimated amount of VEGF-Trap in the vitreous, aqueous humor, and retina of rabbits at 1 h and at 1, 2, 5, 14, and 30 days after intravitreal VEGF-Trap injection.
| Time |
Vitreous |
Aqueous humor |
Retina |
|||
|---|---|---|---|---|---|---|
| Conc (μg/ml) | Amount (μg) | Conc (μg/ml) | Amount (μg) | Conc (μg/g) | Amount (μg) | |
| 1 h | 86.2±12.7 | 129.3±19.0 | 49.5±5.3 | 9.9±1.1 | 25.6±10.2 | 0.6±0.2 |
| 1 days | 56.0±6.6 | 83.9±9.9 | 36.6±1.1 | 7.3±0.2 | 19.2±4.3 | 0.5±0.1 |
| 2 days | 46.6±1.5 | 69.9±2.2 | 14.4±1.4 | 2.9±0.3 | 8.4±6.2 | 0.2±0.1 |
| 5 days | 39.5±7.7 | 59.2±11.5 | 10.0±0.2 | 2.0±0.0 | 2.5±2.5 | 0.1±0.1 |
| 14 days | 3.4±0.2 | 5.0±0.3 | 2.1±0.3 | 0.4±0.1 | — | — |
| 30 days | 1.3±0.7 | 1.9±1.0 | 0.4±0.0 | 0.1±0.0 | — | — |
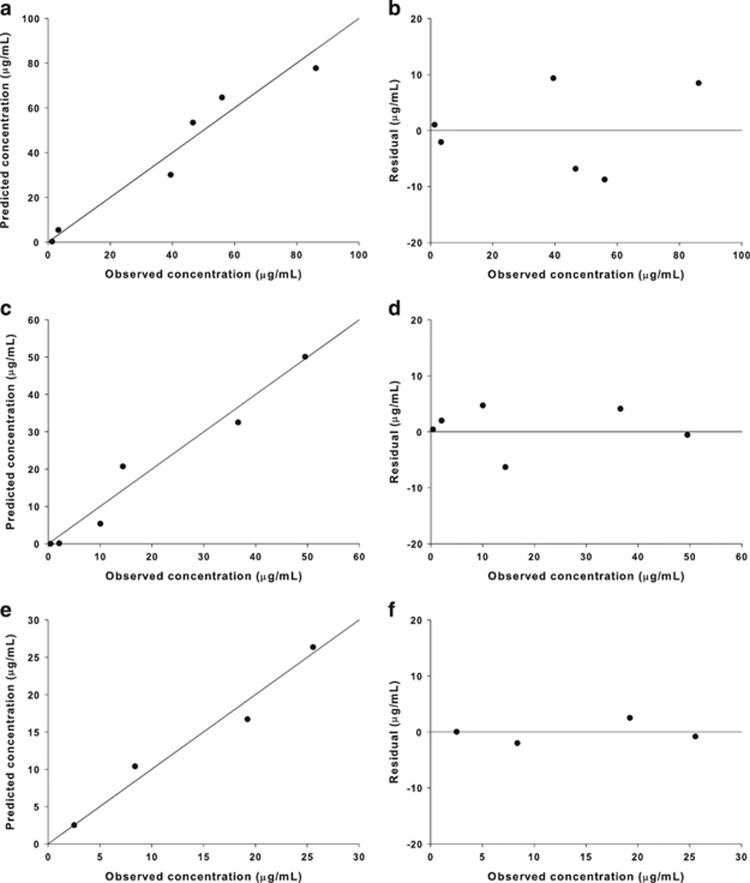
The one-compartment model was selected as the final model in all three eye tissues. This model had lower AIC values than the two-compartment model in the aqueous humor (30.5 vs 33.2) and retina/choroid (13.6 vs 17.6). Although the two-compartment model had lower AIC values than the one-compartment model in the vitreous (35.1 vs 38.0), the estimated parameters of this model had a large SE (CV% >100). A basic goodness-of-fit plot is presented in Figure 2 for the one-compartment model.
Figure 2.
Basic goodness-of-fit plot for the one-compartment model. (a and b) Vitreous, (c and d) aqueous humor, and (e and f) retina/choroid.
The estimated one-compartment models for the vitreous, aqueous humor, and retina/choroid were as follow: 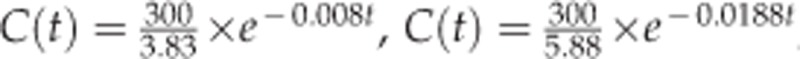 , and
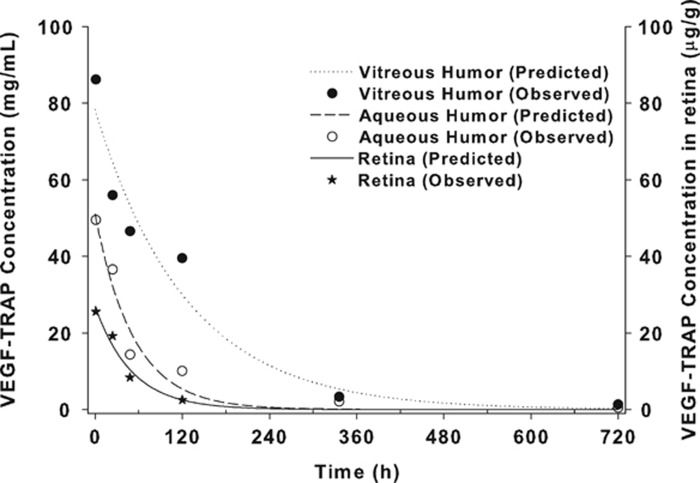
, and 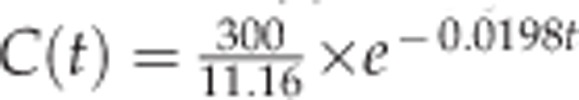 . The estimated half-life of VEGF-Trap in the vitreous, aqueous humor, and retina/choroid was 87.1, 36.8, and 35.0 h, respectively. MRT was estimated to be 125.7, 53.1 and 50.5 h for the vitreous, aqueous humor, and retina/choroid, respectively. The calculated AUCslast were 10009.8 for the vitreous, 3945.1 for the aqueous humor, and 1189.3 for the retina/choroid. Detailed PK parameters are provided in Table 2. The observed concentration–time data of VEGF-Trap at six time points and the fitted models in the vitreous, aqueous humor, and retina/choroid are provided in Figure 3.
. The estimated half-life of VEGF-Trap in the vitreous, aqueous humor, and retina/choroid was 87.1, 36.8, and 35.0 h, respectively. MRT was estimated to be 125.7, 53.1 and 50.5 h for the vitreous, aqueous humor, and retina/choroid, respectively. The calculated AUCslast were 10009.8 for the vitreous, 3945.1 for the aqueous humor, and 1189.3 for the retina/choroid. Detailed PK parameters are provided in Table 2. The observed concentration–time data of VEGF-Trap at six time points and the fitted models in the vitreous, aqueous humor, and retina/choroid are provided in Figure 3.
Table 2. Pharmacokinetic parameters of VEGF-Trap.
| PK parameters | Vitreous | Aqueous humor | Retina |
|---|---|---|---|
| T1/2 (h)a | 87.1 | 36.8 | 35.0 |
| MRT (h)a | 125.7 | 53.1 | 50.5 |
| Cmax (μg/ml)b | 86.2 | 49.5 | 25.6 |
| Tmax (h)b | 1 | 1 | 1 |
| AUClast (h × μg/ml)b | 10009.8 | 3945.1 | 1189.3 |
| V/F (ml)a | 3.83 | 5.88 | 11.16 |
| CL/F (ml/h)a | 0.03 | 0.11 | 0.22 |
Abbreviations: AUC, area under the curve; Cmax, observed maximum concentration; CL/F, apparent clearance; MRT, mean resident time; Tmax, time to Cmax; V/F, apparent volume of distribution.
One-compartmental analysis.
Non-compartmental method.
Figure 3.
VEGF-Trap concentration in the eye of rabbits. Points represent the observed concentrations and lines represent the estimated concentrations determined by the one-compartmental model.
The total exposure (AUClast) of the aqueous humor to VEGF-Trap, bevacizumab, and ranibizumab was 39.4, 31.7, and 28.7%, respectively, when compared with the vitreous. In contrast, the total exposure of the retina/choroid to VEGF-Trap and ranibizumab was 11.9 and 1.3%, respectively, when compared with the vitreous.
Discussion
The present study provides the intraocular distribution and PK profile of intravitreally administered VEGF-Trap using a rabbit model. The results show that the vitreous half-life of VEGF-Trap is 3.63 days and that intravitreally administered VEGF-Trap clears in parallel from the vitreous, aqueous humor, and retina/choroid. VEGF-Trap concentrations were highest in the aqueous humor and retina/choroid 1 h after intravitreal administration, indicating that intravitreally injected VEGF-Trap rapidly distributes to the aqueous humor and retina/choroid. In addition, the VEGF-Trap concentrations in the aqueous humor and retina/choroid 1 h post administration were about 60 and 30%, respectively, of the vitreous concentration. These results are important, as the retina/choroid is the major target tissue of VEGF-Trap treatment. Further, the anterior segment structures, which contain the aqueous humor, are also an important target of VEGF-Trap.
Evidence from our studies and others indicate that intravitreal VEGF-Trap has two mechanisms of excretion, either through drainage of the aqueous humor or through the retina/choroid.18 The estimated amount of VEGF-Trap at each time point suggests that most intravitreally administered VEGF-Trap is excreted through the aqueous humor. However, we could not estimate the exact amount of VEGF-Trap excreted in each way, because there is no data regarding the bioavailability between the vitreous, aqueous humor, retina/choroid, and systemic circulation, which is essential for generating a PK model covering all intraocular compartments concurrently.
Although previous reports indicate that the half-life of VEGF-Trap is 4.58–4.79 days,11, 12 there are no published reports using conventional immunoassays or rabbit models to assess the intraocular PK properties of VEGF-Trap. We estimated the half-life of VEGF-Trap to be 3.63 days and provided PK properties of VEGF-Trap for the first time using a conventional immunoassay in rabbits. Previous studies suggest the half-life of intravitreally administered drugs may be proportional to their molecular size.11, 18 The half-life of VEGF-Trap (145 kDa) was estimated to be 4 days, similar to the estimates in our study, based on the half-life of ranibizumab (48 kDa),14, 16, 19 bevacizumab (149 kDa),13, 17, 20, 21 and rituximab (145 kDa).22 In addition, we could directly compare the PK parameters for ranibizumab, bevacizumab, and VEGF-Trap, because we had conducted the PK studies for these three anti-VEGF agents using the same experimental methodologies. The vitreous half-life for ranibizumab, bevacizumab, and VEGF-Trap were 2.75, 7.06, and 3.63 days, respectively.13, 14 The elimination of vitreous bevacizumab consisted of two distinct phases, fitting well to the two-compartment model. Thus, the comparison of MRTs between these three anti-VEGF agents, rather than their half-lives, provides more accurate insights. The estimated MRT is 3.15 days for ranibizumab, 9.74 days for bevacizumab, and 5.24 days for VEGF-Trap (unpublished data for ranibizumab and bevacizumab). Thus, the PK values for VEGF-Trap are between those of ranibizumab and bevacizumab. Some evidence suggests that bevacizumab can form chains with VEGF, leading to large, multimeric complexes.23 This may partially explain the substantial differences in the half-lives and MRT between bevacizumab and VEGF-Trap, which have a similar molecular weight.
The ratio of the concentration of VEGF-Trap in the aqueous humor to the vitreous in this study ranged from 0.3 to 0.6. This was consistent with the ratio of ranibizumab as determined by Gaudreault et al,18, 19 but higher than in our previous studies.13, 14 In addition, using the concentration–time curves, the total exposure of the aqueous humor or retinal/choroid to each anti-VEGF agent after intravitreal injection could be determined and used to compare the PK properties of anti-VEGF agents. The total exposure of the aqueous humor after intravitreal injection was 28.7–39.4% of the vitreous exposure for all three experimental PK studies, including bevacizumab, ranibizumab, and VEGF-Trap. These results suggest the presence of a common excretion pathway through the aqueous humor for these drugs. However, the total exposure of the retina/choroid compared with the vitreous was quite different for ranibizumab and VEGF-Trap. The ratio for VEGF-Trap was 11.9%, whereas the ratio for ranibizumab was 1.3%, suggesting that VEGF-Trap has a higher bioavailability than ranibizumab. These results also indicate that distribution and uptake to the retina/choroid from the vitreous may depend on the characteristics of each anti-VEGF agent, such as molecular weight, charge, glycosylation, and binding sites, which also affect the bioavailability of agents. In addition, the presence and affinity of the Fc portion of anti-VEGF agents may affect the exposure ratio in the retina/choroid.24, 25, 26 We are currently assessing the role of Fc portion of anti-VEGF agents in intraocular distribution and elimination. We believe that this will improve our understanding of intraocular PK in anti-VEGF agents.
One limitation to our study was that the generated VEGF-Trap used for analysis is slightly different from commercially available VEGF-Trap-Eye. We generated VEGF-Trap based on a report from Regeneron Pharmaceuticals Inc.6, which is slightly different from commercially available VEGF-Trap-Eye (Eylea, Regeneron Pharmaceuticals Inc.). This may have resulted in some differences in the PK properties. However, the VEGF-binding domains (VR1-Ig2 and VR2-Ig3) are nearly identical, and there are only a few (5.3%) differences between the amino-acid sequences of VEGF-Trap used in this study and VEGF-Trap-Eye (Figure 1). Thus, the potential effects caused by this difference are likely not significant.
In this study, we provide for the first time the intraocular PK properties of VEGF-Trap, an anti-VEGF therapeutic, used for the first-line treatment of VEGF-associated intraocular pathologies.6, 7, 8, 9, 10 We expect that this study will provide relevant information for the administration of intravitreal VEGF-Trap for treatment planning and further studies.
Acknowledgments
This research was supported by the SK Telecom Grant (Grant 06-2013-092). The sponsor or funding organization had no role in the design or conduct of this research. We alone are responsible for the content and writing of the paper.
The authors declare no conflict of interest.
References
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1432–1444. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010; 117: 1102–1112 e1101. [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L et al. Ranibizumab for diabetic macular edema: results from 2 phase iii randomized trials: RISE and RIDE. Ophthalmology 2012; 119(4): 789–801. [DOI] [PubMed] [Google Scholar]
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012; 119: 1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 2002; 99: 11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R et al. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology 2012; 119: 1658–1665. [DOI] [PubMed] [Google Scholar]
- Heier JS, Clark WL, Boyer DS, Brown DM, Vitti R, Berliner AJ et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS Study. Ophthalmology 2014; 121(7): 1414–1420. [DOI] [PubMed] [Google Scholar]
- Korobelnik JF, Holz FG, Roider J, Ogura Y, Simader C, Schmidt-Erfurth U et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO Study. Ophthalmology 2014; 121: 202–208. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 2014; 121: 193–201. [DOI] [PubMed] [Google Scholar]
- Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol 2008; 92: 667–668. [DOI] [PubMed] [Google Scholar]
- Christoforidis JB, Williams MM, Kothandaraman S, Kumar K, Epitropoulos FJ, Knopp MV. Pharmacokinetic properties of intravitreal I-124-aflibercept in a rabbit model using PET/CT. Curr Eye Res 2012; 37: 1171–1174. [DOI] [PubMed] [Google Scholar]
- Ahn J, Kim H, Woo SJ, Park JH, Park S, Hwang DJ et al. Pharmacokinetics of intravitreally injected bevacizumab in vitrectomized eyes. J Ocul Pharmacol Ther 2013; 29(7): 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SJ, Ahn J, Park S, Kim H, Hwang DJ, Park JH et al. Intraocular pharmacokinetics of ranibizumab in vitrectomized versus nonvitrectomized eyes. Invest Ophthalmol Vis Sci 2014; 55: 567–573. [DOI] [PubMed] [Google Scholar]
- Jung K, Lee D, Lim HS, Lee SI, Kim YJ, Lee GM et al. Double anti-angiogenic and anti-inflammatory protein Valpha targeting VEGF-A and TNF-alpha in retinopathy and psoriasis. J Biol Chem 2011; 286: 14410–14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 2007; 114: 2179–2182. [DOI] [PubMed] [Google Scholar]
- Nomoto H, Shiraga F, Kuno N, Kimura E, Fujii S, Shinomiya K et al. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci 2009; 50: 4807–4813. [DOI] [PubMed] [Google Scholar]
- Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci 2005; 46: 726–733. [DOI] [PubMed] [Google Scholar]
- Gaudreault J, Fei D, Beyer JC, Ryan A, Rangell L, Shiu V et al. Pharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina 2007; 27: 1260–1266. [DOI] [PubMed] [Google Scholar]
- Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007; 114: 855–859. [DOI] [PubMed] [Google Scholar]
- Sinapis CI, Routsias JG, Sinapis AI, Sinapis DI, Agrogiannis GD, Pantopoulou A et al. Pharmacokinetics of intravitreal bevacizumab (Avastin(R)) in rabbits. Clin Ophthalmol 2011; 5: 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Csaky KG, Chan CC, Bungay PM, Lutz RJ, Dedrick RL et al. The pharmacokinetics of rituximab following an intravitreal injection. Exp Eye Res 2006; 82: 760–766. [DOI] [PubMed] [Google Scholar]
- Zhang A, Singh SK, Shirts MR, Kumar S, Fernandez EJ. Distinct aggregation mechanisms of monoclonal antibody under thermal and freeze-thaw stresses revealed by hydrogen exchange. Pharm Res 2012; 29: 236–250. [DOI] [PubMed] [Google Scholar]
- Kim H, Robinson SB, Csaky KG. FcRn receptor-mediated pharmacokinetics of therapeutic IgG in the eye. Mol Vis 2009; 15: 2803–2812. [PMC free article] [PubMed] [Google Scholar]
- Murinello S, Mullins RF, Lotery AJ, Perry VH, Teeling JL. Fcgamma receptor upregulation is associated with immune complex inflammation in the mouse retina and early age-related macular degeneration. Invest Ophthalmol Vis Sci 2014; 55: 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powner MB, McKenzie JA, Christianson GJ, Roopenian DC, Fruttiger M. Expression of neonatal Fc receptor in the eye. Invest Ophthalmol Vis Sci 2014; 55(3): 1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]