Abstract
Purpose
To compare the efficacy of ranibizumab 0.5-mg and 2.0-mg intravitreal injections for persistent diabetic macular edema (DME) previously treated with bevacizumab.
Methods
In all, 43 patients with residual center-involved DME following intravitreal bevacizumab were included in this 12-month prospective, nonrandomized, multicenter study. Enrolled patients received three monthly ranibizumab 0.5-mg injections. At month 3, patients with residual macular edema switched to three monthly injections of ranibizumab 2.0-mg. Assessments included monthly visual acuity and spectral-domain optical coherence tomography.
Results
Mean visual acuity improved by +6.4 letters at month 3 and +8.8 letters at month 6. Mean central subfield thickness (CST) decreased by –113 μm at month 3 and –165 μm at month 6. Before enrollment, 29/43 (67.4%) patients showed <10% CST reduction following monthly bevacizumab treatment. After three monthly ranibizumab 0.5-mg injections, 22/29 (75.9%) patients showed >10% reduction in CST, whereas 6 showed <10% reduction. Of these six, three (50%) showed >10% reduction in CST after switching to three monthly ranibizumab 2.0-mg doses. No serious adverse events were observed to month 6.
Conclusion
Ranibizumab 0.5-mg or 2.0-mg may improve visual and anatomic outcomes in patients with DME who demonstrated minimal or no response to bevacizumab therapy. Moreover, increased dosage of ranibizumab (2.0-mg) may provide additional benefit over ranibizumab 0.5-mg in some patients. However, 2.0-mg ranibizumab is not currently commercially licensed or available.
Introduction
Vision loss associated with diabetic macular edema (DME) is a significant public health problem that affects individuals psychologically, socially, and economically. In developed countries, DME is among the most common sight-threatening complications of diabetic retinopathy. The incidence of DME increases with severity and duration of diabetes, ranging from 3 to 20%.1
The pathogenesis of DME is not wholly understood. Histological findings include loss of pericytes and endothelial cells, together with capillary basement membrane thickening. Eventual microaneurysm formation paired with breakdown of the blood–retinal barrier lead to vascular leakage and macular edema.2, 3 Vascular endothelial growth factor (VEGF) is believed to play an important role in the pathogenesis of DME.4, 5, 6
The Early Treatment Diabetic Retinopathy Study (ETDRS) provided strong evidence for the treatment of clinically significant DME with macular laser therapy.7 This treatment was shown to reduce the risk of moderate vision loss by 50% however, significant visual gains were not common. VEGF inhibition for the treatment of DME has shown promising results with significant visual gains, resulting in a paradigm shift in the treatment of DME.8, 9
Bevacizumab (Avastin; Genentech, Inc., South San Francisco, CA, USA) is a fully humanized recombinant antibody that binds all isoforms of VEGF-A.10 Bevacizumab is currently used off-label to treat DME. Several studies support the efficacy of bevacizumab compared with focal macular laser.11, 12, 13, 14, 15, 16 In the BOLT trial, patients with persistent DME following macular laser therapy were randomized to bevacizumab vs continuing laser therapy. Patients treated with bevacizumab gained 8.6 letters vs 2.5 letters in those receiving macular laser therapy (P=0.005).15 Despite favorable results, there remain patients who either do not respond or only partially respond to intravitreal bevacizumab treatment.
Ranibizumab (Lucentis; Genentech, Inc., South San Francisco, CA, USA) is a monoclonal antibody fragment derived from the same murine antibody as bevacizumab, but with a different development path than bevacizumab.17 The goal in the development of ranibizumab was, in part, to produce a molecule that could diffuse more efficiently through the retina after intraocular injection for the treatment of age-related macular degeneration (AMD).18 Decreased systemic half-life and resulting systemic toxicity of an antibody fragment relative to a full-length antibody was another driving force in the development of this molecule.18 Relative to bevacizumab, ranibizumab has been reported to have a somewhat shorter vitreous half-life, but has 5- to 20-fold higher biologic activity.19, 20 This increased activity, coupled with the smaller molecular size of ranibizumab, may further benefit eyes with DME. The US Food and Drug Administration approval for ranibizumab 0.3-mg for the treatment of DME was granted in August 2012.21
Ranibizumab has been shown to be efficacious in the treatment of DME in numerous clinical trials.8, 9, 22, 23, 24, 25, 26 The REEF study was designed to evaluate visual and anatomic outcomes with intravitreal ranibizumab 0.5-mg in patients with DME who partially responded to or failed to respond to treatment with intravitreal bevacizumab 1.25-mg. Patients who failed to respond to ranibizumab 0.5-mg after 3 months were further treated with ranibizumab 2.0-mg to determine whether a higher dose of ranibizumab provided additional treatment benefit. Higher-dose ranibizumab has previously been shown to be effective in recalcitrant cases of other VEGF-mediated diseases including exudative AMD and radiation retinopathy.27, 28
Materials and methods
Study design
The REEF study is an open-label, prospective, multicenter, pilot clinical trial of intravitreal ranibizumab 0.5-mg and 2.0-mg in patients with DME who were previously treated with intravitreal bevacizumab 1.25-mg. The REEF study was conducted in compliance with the Helsinki Declaration of 1975 (as revised in 1983) and patients provided written consent before enrollment. REEF is registered on ClinicalTrials.gov with the identifier NCT01292798.
Patients
Inclusion criteria included center-involved DME, defined as central subfield thickness (CST) more than 300 μm on spectral-domain optical coherence tomography (SD-OCT; Spectralis; Heidelberg Engineering, Heidelberg, Germany). Before the study, all patients received at least two consecutive injections of intravitreal bevacizumab administered less than 7 weeks apart and within 1 year of the baseline study visit. Patients were divided into two groups based on their response (partial vs nonresponse) to pre-enrollment bevacizumab injections. A partial response was defined as a reduction of more than 10% in CST, but CST more than 300 μm or evidence of cystic edema on SD-OCT. Nonresponse was defined as a reduction less than 10% in CST (at 4–6 weeks following the second bevacizumab injection) compared with initial values observed before consecutive bevacizumab injections. Best-corrected visual acuity was measured on retro-illuminated standardized distance visual acuity charts (ETDRS visual acuity charts at 4 m). Included patients had baseline best-corrected visual acuity from 20/32 to 20/400 (Snellen equivalent; 19–73 ETDRS letters inclusive). Exclusion criteria included elevated blood pressure (>180/110, systolic >180 mm Hg, or diastolic >110 mm Hg); evidence of vitreoretinal interface abnormality on ocular examination or SD-OCT that may contribute to macular edema; and/or intravitreal corticosteroids or laser photocoagulation within 6 months before the baseline study visit.
Treatment
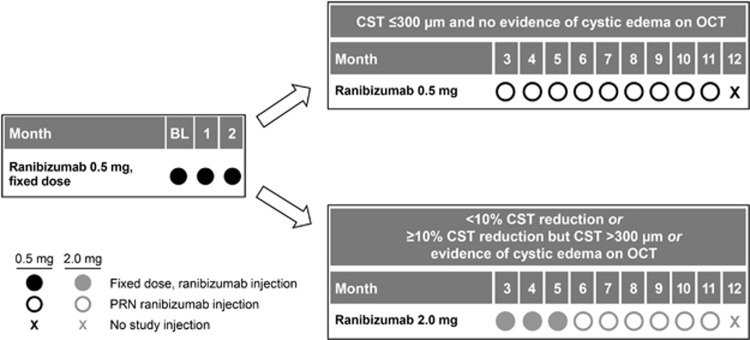
All patients received three consecutive monthly intravitreal injections of ranibizumab 0.5-mg (baseline, month 1, and month 2). Beginning at month 3, patients with persistent DME received three consecutive monthly intravitreal injections of ranibizumab 2.0-mg (months 3, 4, and 5). Patients with a fluid-free macula at month 3 continued on ranibizumab 0.5-mg through month 12, wherein treatment was administered on an as-needed basis for recurrent DME documented on SD-OCT (Figure 1).
Figure 1.
REEF study design. BL, baseline; PRN, pro re nata (as-needed).
At each monthly study visit, standardized visual acuity, SD-OCT, and a complete ophthalmic examination were completed. Fundus photography and fluorescein angiography were performed quarterly. Additionally, the Work Productivity and Activity Impairment: Specific Health Problem (WPAI:SHP) questionnaire29, 30 was administered at baseline, month 3, and month 12 to measure impairment in work and activities among patients with DME.
Assessments and outcome measures
The primary outcome measures were mean change in visual acuity at 6 and 12 months. Secondary outcome measures included mean change in 1-mm CST and rate of partial and complete response to the ranibizumab 0.5-mg and 2.0-mg doses. Herein, we present the primary and secondary outcomes at 6 months, including WPAI:SHP data through 12 months. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Statistical methods
Basic descriptive statistical analyses were applied to the data. Basic student's t tests were used to compare means for visual acuity and retinal thickness. SPSS statistics software (version 21.0.0.0; IBM, Armonk, NY, USA) was used for all statistical analyses.
Results
In all, 43 patients (43 eyes) were enrolled into this clinical trial (Table 1). Included patients had a mean (standard deviation (SD)) age of 64.0 (±10.1) years and 23 (53.5%) patients were male. Twenty patients had proliferative diabetic retinopathy and 23 patients had nonproliferative diabetic retinopathy. In addition to previous bevacizumab treatment, patients had previously received a mean (SD) of 0.3 (±0.9) intravitreal triamcinolone injections, and 20 of 43 eyes had prior focal photocoagulation with a mean (SD) of 2.5 (±2.1) sessions of laser treatment. Patients received a mean (SD) of 4.7 (±2.4) intravitreal bevacizumab injections before the study, and the mean (SD) 1-mm CST before and after two consecutive bevacizumab injections was 475.8 (±128.7) μm and 469.5 (±133.1) μm, respectively (Supplementary Figure 1).
Table 1. Patient baseline characteristics and pre-trial demographics.
| Study population | |
|---|---|
| Patients, n | 43 |
| Mean (SD) age, years | 64.0 (10.1) |
| Sex (male:female), n | 23 : 20 |
| Mean (SD) ETDRS VA, letters | 58.8 (9.7) |
| Mean (SD) CST, μm | 500.6 (129.1) |
| Mean (SD) retinal volume, mm3 | 9.9 (1.9) |
| Mean (SD) previous bevacizumab injections | 4.7 (2.4) |
| Mean (SD) previous lasers of macula | 2.5 (2.1) |
| Mean (SD) previous intravitreal triamcinolone injections | 0.3 (0.9) |
| Diabetic retinopathy severity | PDR: n=20; NPDR: n=23 |
Abbreviations: CST, central subfield thickness; ETDRS, Early Treatment Diabetic Retinopathy Study; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; SD, standard deviation; VA, visual acuity.
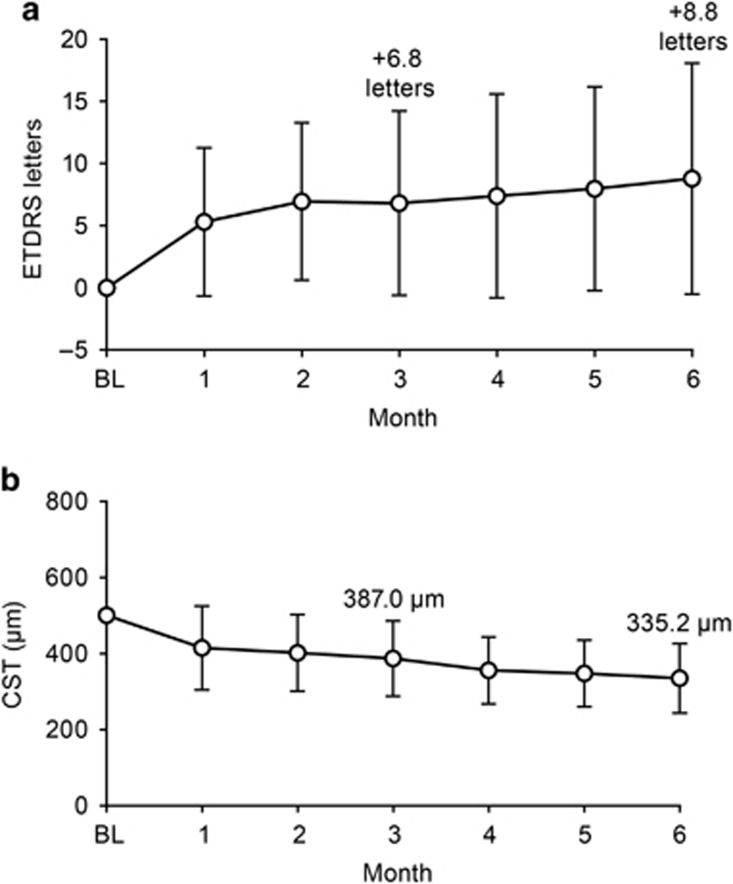
The mean (SD) visual acuity at baseline was 58.8 (±9.7) letters and improved by a mean of +6.8 (±7.4) letters at month 3 and by +8.8 (±9.3) letters at month 6. The improvement in visual acuity from baseline was statistically significant at both time points (P<0.0001) (Figure 2a). At month 3, 25/43 (58.1%) patients improved by 5 or more letters, 15/43 (34.9%) patients improved by 10 or more letters, and 5/43 (11.6%) patients improved by 15 or more letters from baseline. At month 6, 31/43 (72.1%) patients improved by 5 or more letters, 23/43 (53.5%) patients improved by 10 or more letters, and 9/43 (20.9%) patients improved by 15 or more letters from baseline.
Figure 2.
(a) Mean change in visual acuity through 6 months compared with baseline. (b) Mean CST from baseline to month 6. Baseline through month 6: n=42; 4 patients did not receive ranibizumab 2.0-mg during months 3 to 5. Vertical bars±standard deviation of the mean. BL, baseline; ETDRS, Early Treatment Diabetic Retinopathy Study.
The mean (SD) 1-mm CST at baseline was 500.6 (±129.1) μm. At month 3, it decreased to 387.0 (±99.2) μm (P<0.001), and further decreased to 335.2 (±91.5) μm at month 6 (P<0.001) (Figure 2b).
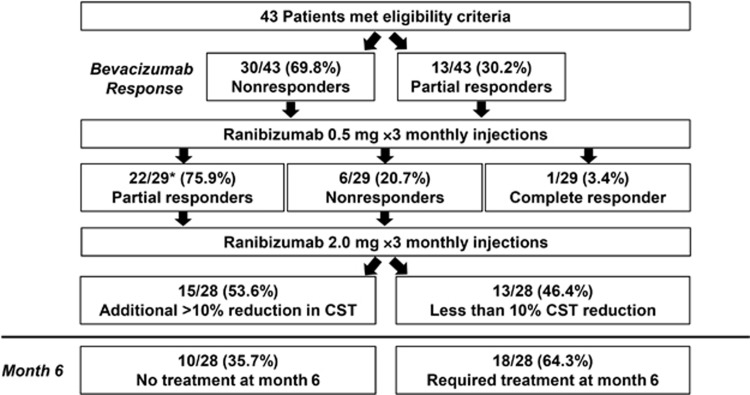
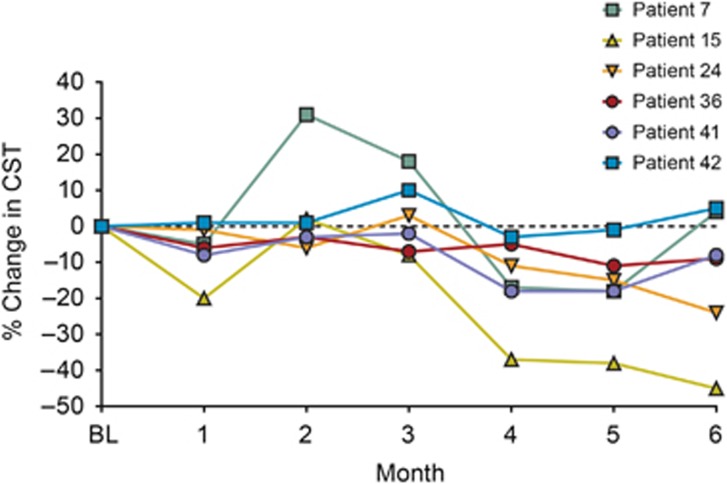
There were 13 partial responders and 30 nonresponders to treatment with intravitreal bevacizumab administered before enrollment in REEF. After nonresponders received three intravitreal ranibizumab 0.5-mg injections (at baseline and months 1 and 2), 1 patient showed complete resolution of DME, 22 patients showed partial resolution of DME, and 6 patients demonstrated no resolution or had worsening of DME compared with baseline. One patient died because of acute hypoxemic respiratory failure after 1 month in the study and was not included in the analysis. At months 3, 4, and 5, 28/29 bevacizumab nonresponders received three consecutive monthly intravitreal ranibizumab 2.0-mg injections. Of these 28 patients, 15 (53.6%) demonstrated an additional reduction of DME (>10% reduction compared with CST at month 3), and 13 (46.4%) showed no resolution or had worsening of DME. At month 6, 10 (35.7%) patients did not require additional treatment for DME (Figure 3). In the six patients who showed a reduction of less than 10% in CST following monthly bevacizumab and three doses of ranibizumab 0.5-mg, the administration of three doses of ranibizumab 2.0-mg resulted in three (50.0%) patients achieving a reduction of more than 10% in CST (Figure 4).
Figure 3.
Complete, partial, and minimal or nonresponse following three ranibizumab 0.5-mg injections and three ranibizumab 2.0-mg injections. *One death, patient not included in analysis.
Figure 4.
Percent change in CST through 6 months in six nonresponders following three ranibizumab 0.5-mg injections. The six patients showed minimal reduction in CST following three consecutive ranibizumab 0.5-mg injections; three of six patients showed reduction in CST following ranibizumab 2.0-mg treatment. BL, baseline.
All patients were stratified according to the number of pre-trial bevacizumab injections received (≤4 vs ≥5 bevacizumab injections in total). The mean (SD) change in visual acuity was +9.1 (±9.0) letters in the four or fewer injections group compared with +8.3 (±9.8) letters in the five or more group; this difference was not statistically significant (P=0.538). Mean (SD) CST reduction in the four or fewer injections group was –189.4 (±148.1) μm vs –122.0 (±90.2) μm in the five or more group; this difference also was not statistically different (P=0.404).
In the partial responder group, 11 (84.6%) patients treated with intravitreal injections of ranibizumab 0.5-mg for 3 months showed partial or no reduction and 2 (15.4%) showed complete resolution of DME. The 11 patients were treated with intravitreal ranibizumab 2.0-mg for 3 months; of these, 7 (63.6%) showed further reduction (>10% compared with CST value at month 3) and 5 (45.5%) did not require treatment at month 6.
Outcomes from the WPAI:SHP questionnaire showed that at baseline, 27.5% of all patients were employed; this percentage was slightly increased at month 3 and month 12 (30.8% and 29.7%, respectively). In all patients, both overall work impairment and activity impairment were reduced at month 12 compared with baseline. The percentage of patients who reported overall work impairment decreased from 23.3 to 15.0%, and reported activity impairment decreased from 24.3 to 16.8% (Supplementary Figure 2).
Discussion
In this prospective trial of patients with DME with partial or no response to intravitreal bevacizumab, ranibizumab 0.5-mg was effective at further improving vision and anatomic outcomes in some patients. Moreover, follow-up treatment with ranibizumab 2.0-mg provided additional efficacy in some patients who did not respond or partially responded to ranibizumab 0.5-mg.
In the study group, mean visual acuity increased with a corresponding decrease in CST at month 3 following three doses of ranibizumab 0.5-mg. After receiving the 0.5-mg doses, mean visual acuity further increased in patients with persistent DME with a corresponding decline in mean CST at month 6 following treatment with ranibizumab 2.0-mg.
The exact mechanism for the visual and SD-OCT gains seen following the switch from bevacizumab to ranibizumab is unclear. The smaller molecular size, increased binding affinity, and higher potency of ranibizumab are plausible explanations. Similar benefits also have been reported after switching from bevacizumab to ranibizumab in AMD with refractory choroidal neovascular membranes.31, 32 However, the increased VEGF levels in patients with DME relative to AMD33, 34 provides further support to the reasoning that increased VEGF inhibition may be of benefit in DME cases refractory to bevacizumab treatment.
The added benefit of high-dose ranibizumab (2.0-mg) in this trial provides an alternative treatment strategy for patients with refractory DME. Higher dose delivery may be achieved either by administering a higher concentration of drug or by more frequent dosing. It is important to note that 2.0-mg ranibizumab is not commercially licensed or available at this time. Stewart et al35 explored this concept in patients with AMD. Their results showed that ranibizumab dosed monthly at 2.0-mg produced a higher initial peak of VEGF binding activity, but a lower trough of activity at 28 days compared with ranibizumab 0.5-mg dosed every 2 weeks. The use of high-dose ranibizumab for the treatment of recalcitrant AMD has been shown to be beneficial by other investigators as well.27 It appears that, based on our study, this higher peak binding activity does impart some benefit on refractory DME, though our study did not explore the potential benefits of increased trough binding activity at 28 weeks gained by every 2-week dosing.
The safety of both ranibizumab 0.5-mg and 2.0-mg in this trial was consistent with previous trials. No increased incidence of ocular or systemic adverse events was seen. Given the higher rate of comorbidity associated with diabetes, diabetic patients are inherently at higher risk for arterial thromboembolic events than their AMD counterparts.36, 37, 38 Moreover, patients with DME often require bilateral injections, resulting in a theoretically higher risk of anti-VEGF class effects. The results of Phase III ranibizumab studies have shown increased number of adverse events potentially related to systemic VEGF inhibition in patients treated with the higher ranibizumab dose of 0.5-mg relative to the 0.3-mg dose when used in a monthly regimen.8, 39 This difference was not statistically significant, but given equal efficacy in the population studied, the manufacturer chose to successfully seek US Food and Drug Administration approval for the lower dose of ranibizumab (0.3-mg monthly) for the treatment of DME. It is noteworthy that the cerebrovascular event curves of the 0.5- and 0.3-mg doses studied in the RIDE and RISE Phase III studies do not start to separate until late in the second year of continuous monthly treatment. In the present study, after month 3, ranibizumab was used in an as-needed manner, and not monthly, to 12 months in patients with a fluid-free macula. It is plausible that using as-needed ranibizumab at higher concentrations does not result in safety differences seen in previous monthly trials, though more research is needed to truly evaluate this issue.
Limitations of the present study include the lack of a control group and small sample size. It is unclear how patients would have responded to continued monthly dosing with either bevacizumab 1.25-mg or ranibizumab 0.5-mg; it is possible that continued monthly treatment with either agent could have resulted in improvement. Certainly, the addition of a control group to evaluate continued monthly treatments would have been ideal, though with the small enrollment scope of this study, this was not feasible.
The enrollment criteria of nonresponse or partial response to anti-VEGF therapy is controversial and an admitted limitation in this anti-VEGF switch study. It is important to note that the study protocol was developed in late 2009 to early 2010; at this time, limited prospective studies and results on the treatment of DME with anti-VEGF agents were available. The definition of nonresponse has evolved since then. In the present study, the mean number of injections of bevacizumab received before enrollment was 4.7, which is considerably higher than the two injections required for study eligibility. Moreover, no difference was seen in visual acuity or SD-OCT outcomes when patients were stratified based on the number of pre-trial bevacizumab injections (≤4 vs ≥5 bevacizumab injections).
It also is unclear how patients would have responded to an increased dose of bevacizumab. Further investigation is needed to truly determine whether the differences seen in visual acuity and CST are reproduced. The authors look forward to the results of the pending Diabetic Retinopathy Clinical Research Network Protocol T, a comparative effectiveness study of intravitreal aflibercept, bevacizumab, and ranibizumab for DME (ClinicalTrials.gov identifier: NCT01627249).
Persistent DME following intravitreal anti-VEGF agents is a challenging and, unfortunately, not uncommon scenario. Bevacizumab provides mean gains in visual acuity and CST; however, a subset of patients have DME that is refractory to bevacizumab treatment. This subset of patients may derive further visual gain from switching treatment to ranibizumab at various dosages.
Acknowledgments
Support for third-party medical writing assistance for this manuscript, provided by Suzanne Douthwaite, BSc (Hons), Envision Scientific Solutions, was provided by Genentech, Inc. This investigator-sponsored trial (IST) was funded in part through a grant from Genentech, Inc. (FVF4978s) and by the California Retina Research Foundation.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
Meeting presentations: Portions of these data were presented at the 2013 Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, Seattle, WA, USA (May 6, 2013); the 16th Annual Club Vit Meeting, Southampton, Bermuda (July 2, 2013); the 2013 American Society of Retina Specialists (ASRS) Annual Meeting, Toronto, Ontario, Canada (August 28, 2013); and the 2013 American Academy of Ophthalmology (AAO) Annual Meeting, New Orleans, LA, USA (November 18, 2013).
Dr Dhoot is a consultant for Regeneron. Dr Pieramici is a consultant for Genentech, Inc., Alimera, Allergan, Santen, and ThromboGenics. Dr Castellarin is a consultant for Genentech, Inc. and has a personal financial interest with Alcon. Dr Couvillion has received research funding from Genentech, Inc. Dr Steinle is a speaker for Regeneron. Dr Bennett has received research funding from Genentech, Inc. M. Rabena has received honoraria from Genentech, Inc. Dr Avery is a consultant for Alcon, Allergan, Genentech, Inc., Notal Vision, Novartis, Ophthotech, QLT, Regeneron, and Replenish; has received financial support from Allergan and Genentech, Inc.; has received research funding from Allergan, Genentech, Inc., Novartis, and Regeneron; has a personal financial interest with Alexion, I-Tech JV Development, Novartis, Regeneron, and Replenish; and holds a patent with Replenish. Drs Nasir and See declare no potential conflicts of interest.
Supplementary Material
References
- Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep 2012; 12: 346–354. [DOI] [PubMed] [Google Scholar]
- Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol 1999; 14: 223–232. [DOI] [PubMed] [Google Scholar]
- Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care 2003; 26: 2653–2664. [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Tatlipinar S, Shah SM, Haller JA, Quinlan E, Sung J et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol 2006; 142: 961–969. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994; 331: 1480–1487. [DOI] [PubMed] [Google Scholar]
- Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina 2006; 26: 352–354. [DOI] [PubMed] [Google Scholar]
- Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 1985; 103: 1796–1806. [PubMed] [Google Scholar]
- Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L et alRISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012; 119: 789–801. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO et alRESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011; 118: 615–625. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004; 25: 581–611. [DOI] [PubMed] [Google Scholar]
- Soheilian M, Ramezani A, Bijanzadeh B, Yaseri M, Ahmadieh H, Dehghan MH et al. Intravitreal bevacizumab (Avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina 2007; 27: 1187–1195. [DOI] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research NetworkScott IU, Edwards AR, Beck RW, Bressler NM, Chan CK et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007; 114: 1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology 2009; 116: 1142–1150. [DOI] [PubMed] [Google Scholar]
- Solaiman KA, Diab MM, Abo-Elenin M. Intravitreal bevacizumab and/or macular photocoagulation as a primary treatment for diffuse diabetic macular edema. Retina 2010; 30: 1638–1645. [DOI] [PubMed] [Google Scholar]
- Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema. 24-month data: report 3. Arch Ophthalmol 2012; 130: 972–979. [DOI] [PubMed] [Google Scholar]
- Arevalo JF, Sanchez JG, Wu L, Maia M, Alezzandrini AA, Brito M et al. Pan-American Collaborative Retina Study Group. Primary intravitreal bevacizumab for diffuse diabetic macular edema: the Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology 2009; 116: 1488–1497, 1497.e1. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wiesmann C, Fuh G, Li B, Christinger HW, McKay P et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol 1999; 293: 865–881. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti–vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006; 26: 859–870. [DOI] [PubMed] [Google Scholar]
- Chong V. Biological, preclinical and clinical characteristics of inhibitors of vascular endothelial growth factors. Ophthalmologica 2012; 227(suppl 1): 2–10. [DOI] [PubMed] [Google Scholar]
- Krohne TU, Liu Z, Holz FG, Meyer CH. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am J Ophthalmol 2012; 154(682-686): e2. [DOI] [PubMed] [Google Scholar]
- Lucentis [package insert]. South San Francisco, CA: Genentech, Inc. 2014.
- Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV et alREAD-2 Study Group. Two-year outcomes of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study. Ophthalmology 2010; 117: 2146–2151. [DOI] [PubMed] [Google Scholar]
- Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 2010; 33: 2399–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL 3rd, Friedman SM et alDiabetic Retinopathy Clinical Research Network. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2011; 118: 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do DV, Nguyen QD, Khwaja AA, Channa R, Sepah YJ, Sophie R et alREAD-2 Study Group. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol 2013; 131: 139–145. [DOI] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research NetworkElman MJ, Qin H, Aiello LP, Beck RW, Bressler NM et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology 2012; 119: 2312–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff CC, Brown DM, Chen E, Major JC, Croft DE, Mariani A et alSAVE Study Group. SAVE (Super-dose Anti-VEGF) trial: 2.0 mg ranibizumab for recalcitrant neovascular age-related macular degeneration: 1-year results. Ophthalmic Surg Lasers Imaging Retina 2013; 44: 121–126. [DOI] [PubMed] [Google Scholar]
- Finger PT, Chin KJ. High-dose (2.0 mg) intravitreal ranibizumab for recalcitrant radiation retinopathy. Eur J Ophthalmol 2013; 23: 850–856. [DOI] [PubMed] [Google Scholar]
- Reilly Associates. Work Productivity and Activity Impairment Questionnaire: Specific Health Problem V2.0 (WPAI:SHP). Updated August 18, 2010. Available at http://www.reillyassociates.net/WPAI_SHP.html. Accessed July 29, 2014.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4: 353–365. [DOI] [PubMed] [Google Scholar]
- Ehlers JP, Spirn MJ, Shah CP, Fenton GL, Baker PS, Regillo CD et al. Ranibizumab for exudative age-related macular degeneration in eyes previously treated with alternative vascular endothelial growth factor inhibitors. Ophthalmic Surg Lasers Imaging 2010; 41: 182–189. [DOI] [PubMed] [Google Scholar]
- Kent JS, Iordanous Y, Mao A, Powell AM, Kent SS, Sheidow TG. Comparison of outcomes after switching treatment from intravitreal bevacizumab to ranibizumab in neovascular age-related macular degeneration. Can J Ophthalmol 2012; 47: 159–164. [DOI] [PubMed] [Google Scholar]
- Pfister M, Koch FH, Cinatl J, Rothweiler F, Schubert R, Singh P et al. Cytokine determination from vitreous samples in retinal vascular diseases [article in German]. Ophthalmologe 2013; 110: 746–754. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol 2002; 133: 70–77. [DOI] [PubMed] [Google Scholar]
- Stewart MW, Rosenfeld PJ, Penha FM, Wang F, Yehoshua Z, Bueno-Lopez E et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina 2012; 32: 434–457. [DOI] [PubMed] [Google Scholar]
- Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med 2004; 164: 1422–1426. [DOI] [PubMed] [Google Scholar]
- Abbott RD, Donahue RP, MacMahon SW, Reed DM, Yano K. Diabetes and the risk of stroke. The Honolulu Heart Program. JAMA 1987; 257: 949–952. [PubMed] [Google Scholar]
- Haffner SM, Lehto S, Rönnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–234. [DOI] [PubMed] [Google Scholar]
- Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L et alRIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013; 120: 2013–2022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.