Abstract
Purpose
To study the correlation between glaucomatous visual field (VF) defects assessed by standard automated perimetry (SAP) and peripapillary retinal nerve fiber layer (RNFL) thinning measured by spectral domain optical coherence tomography (OCT) using a modified OCT-based peripapillary RNFL structure–function map.
Patients and methods
Perimetric glaucoma patients and age-matched normal control subjects were recruited from a university hospital clinic. All eyes underwent testing with the Spectralis spectral domain OCT and SAP on the same day. An OCT-based correspondence map, which correlated VF areas with peripapillary RNFL sectors was created to evaluate the relationship between glaucomatous RNFL thinning and VF loss in six nerve fiber layer bundle areas. Correlations of RNFL thinning with corresponding VF defects were examined using Spearman rank-order correlations. To demonstrate the association between localized VF defects and RNFL thickness, the theoretical curves were made according to an established log-linear model. The measured RNFL thickness values and VF defects were presented in the same scatterplot for each sector.
Results
Fifty-six glaucoma patients and 85 normal subjects were included in the study. Significant association between localized VF loss and RNFL thinning was found in corresponding areas. Data from the current study fit well with established log-linear models, which compare RNFL thickness values with VF defects.
Conclusion
Analysis of RNFL thinning in eyes with localized glaucomatous VF defects showed good structure–function correlation in a new OCT-based structure–function correspondence map.
Introduction
Glaucoma is a group of diseases with characteristic optic neuropathy and associated visual field (VF) loss. Significant correlations have been described between glaucomatous structural changes of the optic disc and/or retinal nerve fiber layer (RNFL) and functional changes on VF testing.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Determining whether there is good correlation between localized VF defects and localized RNFL defects may be important in deciding whether glaucomatous progression has occurred and in understanding the pathogenesis of the disease.
The three imaging techniques that are currently used to examine the RNFL and optic nerve head (ONH) in glaucoma include confocal scanning laser ophthalmoscopy, optical coherence tomography (OCT), and scanning laser polarimetry.12 Because previous studies have suggested that structure–function associations are stronger with OCT measurements compared with the other two technologies,5, 12 the current study used the Spectralis spectral domain OCT (SD-OCT) (Heidelberg Engineering, Inc., Heidelberg, Germany) for RNFL thickness structural assessments.
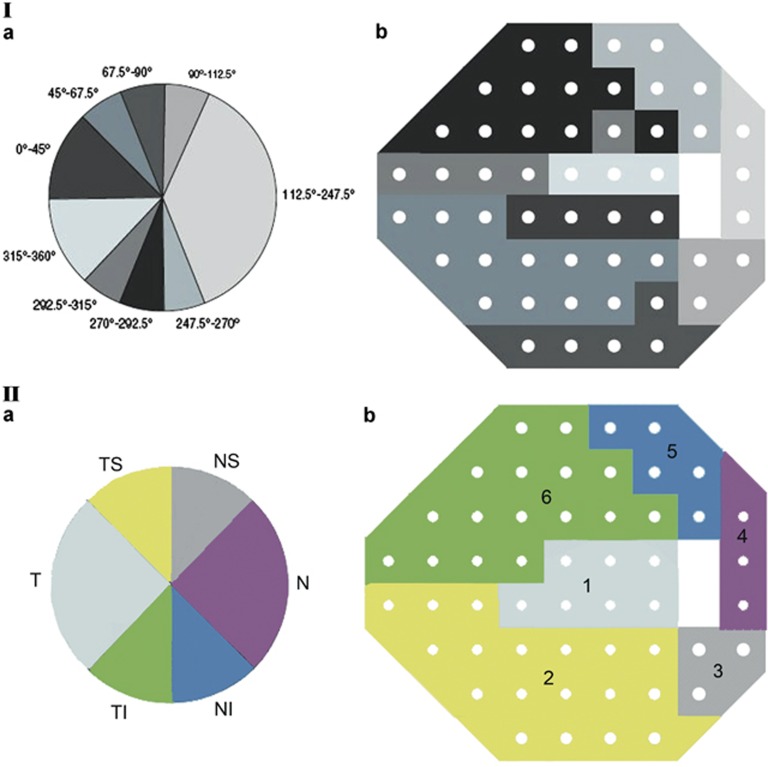
This paper, in particular, focuses on the topographic association between specific areas of glaucomatous VF loss and specific locations in the course of the RNFL bundles, and this topographic association can be delineated by structure–function correspondence maps. Many studies3, 4, 9, 10, 13, 15, 18 have used the Garway-Heath map,19 which maps VF test point locations to specific regions of the ONH. Weber et al20 described another structure–function map that correlated VF test points to optic disc margin locations, which were then extrapolated to OCT RNFL defects. Weber's method has since been used by others.6, 16 Because retinal nerve fibers do not project radially to the optic disc but rather arc into the optic disc, best RNFL-VF structure–function maps should not be assumed to be the same as ONH-VF structure–functions maps. Therefore, for this study, we modified and used a map established by Kanamori et al2 who evaluated the relationship between VF sensitivity and RNFL thickness as measured by Stratus OCT (Carl Zeiss Meditec, Dublin, CA, USA).
To our knowledge, the present study is the first that was designed to use an OCT-based structure–function correspondence map in order to assess the correlation between VF defects and SD-OCT RNFL thickness measurements using the Spectralis machine.
Material and methods
Participants and examinations
All subjects were recruited from the Glaucoma Service of a university hospital and were a part of the SD-OCT in Glaucoma Study population.
All subjects underwent a complete eye examination, which included history, visual acuity (VA) testing, refraction, Goldmann applanation tonometry, slit-lamp biomicroscopy, gonioscopy, central corneal thickness measurements by ultrasonic pachymetry, axial length, dilated ophthalmoscopy of the posterior segment, stereo disc photography (Visucam Pro NM (Carl Zeiss Meditec)), VF testing (Swedish Interactive Threshold Algorithm (SITA) 24-2 test of the Humphrey visual field analyzer 750i, Carl Zeiss Meditec), and peripapillary RNFL thickness measurements using the Spectralis OCT.
General inclusion criteria included patients with (1) a spherical equivalent between −5.0 diopters and +5.0 diopters, (2) best corrected VA of 20/40 or better, and (3) reliable VF testing (ie, with <33% fixation losses,21 <20% false positives, and 20% false negatives), and (4) intraocular pressures controlled within the 10–21 mm Hg range. The exclusion criteria included patients with (1) discernable congenital anomalies of the anterior chamber, (2) corneal scarring or opacities that would prevent adequate scanning, (3) diabetic proliferative or severe non-proliferative retinopathy, (4) VF loss attributed to a non-glaucoma condition, (5) a dilated pupil diameter of <2 mm, and (6) signal strength <15.
Normal volunteers were those without ocular disease except for mild cataract and those with normal disc appearances and VF test results. Normal VF test result criteria were modeled after the Ocular Hypertension Treatment Study (OHTS)21 and were defined as pattern standard deviation (PSD) >5% and glaucoma hemifield test (GHT) within normal limits. All glaucoma types, except for traumatic glaucoma, were included, as long as they fit the inclusion and exclusion criteria. Glaucoma patients were defined as having characteristic cupping of the optic nerve and corresponding glaucomatous VF defects. The VF was considered to be abnormal if it was classified as outside normal limits by the GHT or if three or more contiguous test locations in the pattern deviation plot were depressed significantly at the P<5% level with at least one at the P<1% level on the same side of the horizontal meridian.22 VF abnormalities were classified as mild (mean deviation (MD) >−6 decibels (dB)), moderate (−12 dB<MD≤−6 dB), or severe (MD≤−12 dB). If both eyes were normal or had glaucoma, one eye was selected randomly by the investigator, who was masked to the OCT results at the time the selection was made with a random number generator statistical table.
Spectralis OCT Peripapillary RNFL Measurement
All SD-OCT imaging was performed with the Spectralis OCT (version 4.0). The circular scan pattern was used for peri-papillary RNFL thickness measurements. The scan circle was 12 degrees in diameter, so the scan circle diameter in millimeters varied depending on the axial length. For a typical eye length, the circle was ∼3.5–3.6 mm in diameter.23 Sixteen frames were acquired with automatic real-time on. Scans were only included if there was a clear fundus image, which allowed for optic nerve and scan circle visibility before and during image acquisition, if the RNFL was visible with no interruptions, and if there was a continuous scan pattern without missing or blank areas.
The Spectralis OCT software calculates the average RNFL thickness of the overall globe (360 degrees), four quadrants (ie, temporal (T), superior (S), nasal (N), and inferior (I)), and four sectors (ie, superior-temporal (TS), superior-nasal (NS), inferior-nasal (NI), and inferior-temporal (TI)). The RNFL thickness values for T, TS, NS, N, NI, and TI were used in the present study. The local RNFL thickness deviation was calculated by subtracting the RNFL thickness values of glaucoma patients from the mean RNFL thickness values of age-matched normal subjects (control group) in all six sectors.
VF test
All patients had VF testing using the SITA standard 24-2 program of the Humphrey Field Analyzer 750 and Spectralis OCT RNFL thickness measurements on the same day. The VF defect values at the 52 test points in the total deviation plot were used and expressed in dB. Standard automated perimetry (SAP) total deviation points were grouped into six sectors: superonasal, nasal, inferonasal, inferotemporal, temporal, and superotemporal according to a structure–function correspondence map modified from the one described by Kanamori et al2 (Figure 1). Some RNFL sectors in their study were combined or increased to fit the six RNFL sectors in Spectralis OCT (Figure 1). The present study used mean values of six VF areas. To calculate the mean VF loss in these six areas, the anti-log values at all single test positions were calculated and then averaged.8, 9 For presentation and statistical analyses, these averaged values were converted back to the dB scale.9 Hood et al4 suggest that it is important to anti-log SAP values before averaging the values. On the basis of the knowledge that underlying ganglion cell number and differential light sensitivity expressed as 1/Lamberts has a linear relationship (r2=0.73),24 they provided the following rationale for anti-logging SAP values before averaging them. Suppose an arcuate region has two sections, one with a normal complement of the retinal ganglion cells (RGC) and the other with all the RGCs destroyed. Suppose further that the SAP total deviation was 0 dB in the region with the normal RGCs and −30 dB in the region without RGCs. The average of the total deviation values is −15 dB (∼1/30 on a linear scale), whereas the average after first taking the anti-log is about −3 dB (or 1/2 the normal value). In this example, it seems that the RNFL thickness values along the arcuate region would be closer to 1/2, rather than 1/30, of the normal value. Thus, averaging the anti-logged SAP values appears to be the best way to correlate functional VF defects with structural RNFL thickness values.4 Therefore, in the present study, the SAP total deviation values were used and anti-logged before averaging. Left eye data were converted into right eye format.
Figure 1.
Structure–function correspondence map generated by Kanamori et al2 (I) and modified structure–function correspondence map used in the present study (II).
Statistical analysis
Demographic characteristics of the normal and glaucoma groups were compared using χ2-tests and non-paired two-tailed Student's t-tests for categorical and continuous variables, respectively.
Correlations of RNFL thinning with VF defects values were examined using Spearman rank-order correlations. Statistical analyses were performed using SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to be statistically significant, and P<0.001 was considered to be highly statistically significant. A Bonferroni correction for multiple testing was used by multiplying the observed P-value with the number of comparisons within each analysis.25
To demonstrate the association between localized VF defects and RNFL thicknesses, the theoretical curves according to a log-linear model described by Hood3 were made. This log-linear model correlates glaucomatous VF defects with RNFL thinning.3 In this model, the measured RNFL thickness is TM (TM=TA0 × 10−D/10+TR).3, 4, 5 In this formula, TA0 is the RNFL thickness attributable to the RGC axons in the healthy normal state, D is the VF defect value (dB), and TR is the residual or base thickness, including glial cells and blood vessels. In normal eyes (assuming D=0), TM consists of two components: TA0 (normal RGC axon thickness) and TR (base thickness). In glaucoma patients, RGC axon thickness (TA) decreases with decreasing VF sensitivity, but TR does not change significantly. The linear relation between TA and the VF defect value D is TA=TA0 × 10−D/10. TR was determined for each sector as the median of the RNFL thickness when localized VF losses were >−10 dB; because when field losses are worse than −10 dB, the RNFL thickness reaches an asymptotic value according to the function formula (TM=TA0 × 10−D/10+TR).3, 5 TA0 is the RNFL thickness attributable to the RGC axons in the healthy normal state and was calculated by assuming D=0. TM in normal subjects was the average RNFL thickness of age-matched control subjects for each sector. In the present study, the theoretical curves using this log-linear model were calculated for each RNFL thickness sector. The 95% confidence interval was built by using±1.96 SD of TA0, taking into account the percentage of total residual RNFL thickness for the upper and lower boundaries.18 To evaluate the goodness of fit of the prediction curve to our data, the prediction curve and measured values of TM and D were presented in the same plot for each sector.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results
Fifty-six glaucoma patients and 85 normal subjects, for a total of 141 subjects, were recruited into the study. Table 1 showed the characteristics of the study populations. There were no significant differences between the glaucoma group and normal control group in terms of eye laterality, age, gender, race, and refractive error. Types of glaucoma included primary open-angle glaucoma (39 of 56 patients, 69.6%), normal tension glaucoma (4 of 56 patients, 7.1%), pseudoexfoliation glaucoma (5 of 56 patients, 8.9%), chronic angle closure glaucoma (4 of 56 patients, 7.1%), inflammatory glaucoma (1 of 56 patients, 1.8%), pigmentary glaucoma (1 of 56 patients, 1.8%), and juvenile open-angle glaucoma (2 of 56 patients, 3.6%). There were statistically significant differences in VF MD (P<0.001, t-test) and PSD (P <0.001, t-test) between normal subjects and glaucoma patients. Severity of VF defects was equally distributed among mild (21 of 56 patients, 37.5%), moderate (16 of 56 patients, 28.6%), and severe VF defects (19 of 56 patients, 33.9%).
Table 1. Demographics of the study population.
| Parameter | Normal control group | Glaucoma group | P-valuea |
|---|---|---|---|
| Number of participants | 85 | 56 | |
| Number of right eyes (percent total) | 60.0% | 62.5% | 0.766 |
| Age (mean±SD (range)) | 63.5±14.0 (18–90) | 68.1±12.7 (18–88) | 0.077 |
| Female (percent total) | 52.9% | 58.9% | 0.484 |
| Race (Caucasian, percent total) | 74.1% | 67.9% | 0.420 |
| Refractive error (spherical equivalent in diopters) (mean±SD) | −0.95±1.94 | −1.33±1.75 | 0.091 |
| VF mean deviation (mean±SD) dB | −1.25±1.74 | −9.62±8.68 | <0.001 |
| VF pattern SD (mean±SD) dB | 1.82±1.31 | 6.37±3.48 | <0.001 |
Abbreviations: dB, decibel; VF, visual field; SD, standard deviation.
When comparing normal and glaucoma groups, χ2-tests were used for categorical variables and non-paired two-tailed Student's t-tests were used for continuous variables.
The mean RNFL thickness values for the six sectors were shown in Table 2 and are separated into normal and glaucoma subjects. The RNFL thickness values in all six sectors were statistically different between normal and glaucoma groups (with Bonferroni correction) (Table 2).
Table 2. Spectralis optical coherence tomography retinal nerve fiber layer thickness measurements in normal eyes (control) and glaucomatous eyes.
| RNFL thickness (μm) | Normal (n=85) (mean±SD) | Glaucoma (n=56) (mean±SD) |
|---|---|---|
| Temporal RNFL | 70.3±10.6 | 54.1±13.4a |
| TS RNFL | 124.5±16.4 | 76.9±27.9a |
| NS RNFL | 98.7±19.9 | 68.4±22.9a |
| Nasal RNFL | 74.9±11.6 | 56.9±18.5a |
| NI RNFL | 103.9±20.8 | 72.7±29.0a |
| TI RNFL | 136.0±20.5 | 75.0±28.5a |
Abbreviations: NI, inferior-nasal; NS, superior-nasal; RNFL, retinal nerve fiber layer; SD, standard deviation; TI, inferior-temporal; TS, superior-temporal.
(P<0.0003) Significant differences noted when comparing the normal with the glaucoma group (analysis of variance after Bonferroni correction).
Mean values of localized VF defects and corresponding areas of RNFL thinning were summarized in Table 3. The deeper VF defects were in VF areas 2 and 6 that abutted the nasal meridian, and this corresponded to greater areas of RNFL thinning in the sectors (ie, TS and TI) associated with VF areas 2 and 6. With milder VF defects of 8.15 dB (area 3) and 8.30 dB (area 5) in NS and NI sectors, the associations were also strong with Spearman R-values of 0.696 and 0.771, respectively. There were significant correlations between localized VF defects and RNFL thinning in all six sectors (with Bonferroni correction) (Table 3). In comparisons of RNFL thickness with VF defect values, the highest Spearman correlation coefficient (−0.832) was achieved in the TI sector (VF area 6, Table 3).
Table 3. Correlations between peripapillary retinal nerve fiber layer (RNFL) thinning and visual field (VF) defect values in corresponding visual field areas in glaucoma patients.
| RNFL sector (VF area) | Value of localized VF defect (dB) (mean±SD) | Difference in RNFL thickness values between normal and glaucoma eyes (mean in microns±SD) | Spearman R (95% CI) | P-value |
|---|---|---|---|---|
| Temporal (area 1) | 6.47±6.05 | 16.2±13.4 | 0.464 (0.229, 0.648) | 0.013a |
| TS (area 2) | 9.47±8.92 | 47.6±27.9 | 0.803 (0.685, 0.880) | <0.0003 |
| NS (area 3) | 8.15±8.55 | 30.3±22.9 | 0.696 (0.530, 0.811) | <0.0003 |
| Nasal (area 4) | 6.78±7.07 | 18.0±18.5 | 0.493 (0.264, 0.669) | 0.003a |
| NI (area 5) | 8.30±8.90 | 31.2±29.0 | 0.771 (0.637, 0.860) | <0.0003 |
| TI (area 6) | 9.95±8.73 | 61.0±28.5 | 0.832 (0.728, 0.898) | <0.0003 |
Abbreviations: CI, confidence interval; dB, decibel; NI, inferior-nasal; NS, superior-nasal; SD, standard deviation; TI, inferior-temporal; TS, superior-temporal. Spearman's rank correlation coefficient was used (after Bonferroni correction).
The cutoff for significance with Bonferroni correction is 0.017.
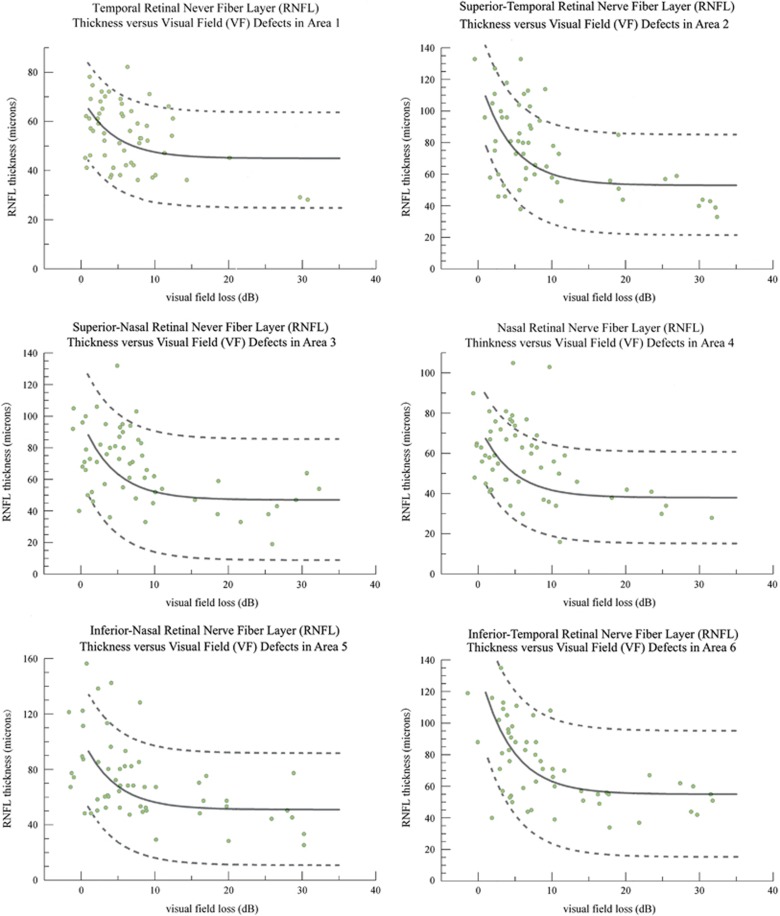
In order to calculate the theoretical curve (TM=TA0 × 10-D/10+TR ) that describes the association between RNFL thickness values and VF defects, the residual RNFL thickness (TR) for each sector was determined first. The residual RNFL thickness (TR) values were 45 μm (T), 53 μm (TS), 47 μm (NS), 38 μm (N), 51 μm (NI), and 55 μm (TI). Figure 2 shows the predicted curves (solid lines) and 95% confidence intervals (dashed lines) that depict the RNFL thickness (μm) and localized VF defect (dB scale) relationship for the six OCT RNFL sectors using our data and Hood's log-linear model. When the actual RNFL thickness values versus VF defects for all six sectors were plotted (Figure 2), most of the actual data were inside the 95% confidence interval of the prediction model, with 82–95% of values within the confidence interval. Forty-six eyes (82%) were inside the 95% confidence interval of the prediction model (TS RNFL sector versus VF defect in area 2, and nasal RNFL sector versus VF defect in area 4), and 53 eyes (95%) were inside the 95% confidence interval of the prediction model (NS RNFL sector versus VF defect in area 3).
Figure 2.
Structure–function correlation between local retinal nerve fiber layer (RNFL) thickness and corresponding visual field (VF) defects in all six sectors. The solid line in each graph represents the theoretical log-linear relationship between RNFL thickness (TM) and VF defect value (D). The dashed lines represent the 95% confidence intervals of the prediction curves. The measured values TM and D of each sector are presented in corresponding log-linear plot.
Discussion
Localized RNFL thinning as measured by OCT can be correlated to localized glaucomatous VF loss.2, 3, 4, 5, 6, 12, 14, 16, 17, 18 However, most of these studies used the Stratus time domain OCT to measure RNFL thickness.2, 3, 4, 6, 13, 14, 16, 17 Although in two of these studies, SD-OCT was used to evaluated RNFL thickness,5, 18 the structure–function correspondence maps made in those studies correlated VF test points to regions of the ONH. In the present study, we created a modified OCT-based structure–function map that shows significant correlation between localized areas of VF loss and SD-OCT measurements of RNFL thinning.
When designing a study that evaluates the relationship between structure and function in glaucoma, the best methods to interpret and present functional data need to be determined. SAP provides a few parameters (ie, raw threshold values (VF sensitivity), total deviation values, pattern deviation values, MD, and PSD), but which are best fit for functional assessments and statistical analyses? In previous structure–function relationship studies where localized VF defects were correlated to RNFL thickness changes, VF parameters used included mean raw threshold values,2, 9, 12, 14, 15, 17 total deviation values,1, 3, 4, 5, 13, 16, 18 and pattern deviation values.16 Total deviation values, which are age-corrected, are less influenced by age than raw threshold values, and they may be more sensitive to detect early diffuse loss compared with pattern deviation values.26 Therefore, total deviation values have theoretical advantages over mean raw threshold values and pattern deviation values. In the current study, total deviation values were used. We found high association between structure and function tests with highest Spearman R-values of 0.832 in the TI sector. In comparison, in the studies by Reus et al9 who used mean raw threshold, the highest Spearman R-value was 0.77 in the TS sector.
Another important consideration when designing a structure–function study is to determine which correspondence map would most accurately depict correlations between structural RNFL thinning and functional VF defects. The Garway-Heath structure–function map19 has been used in many studies3, 4, 9, 10, 13, 15, 18 and maps VF test points to regions of the ONH. In a study of 178 subjects (64 glaucomatous eyes, 26 ocular hypertension eyes and 88 normal eyes), Horn et al5 reported significant correlation between VF defects and RNFL thinning as measured by Spectralis OCT and GDx VCC, using the Garway-Heath map. The higher Spearman R for the Spectralis OCT group was achieved in the RNFL sector of TS sector (R: −0.81) and TI sector (R: −0.77), and the lowest Spearman R was for the RNFL nasal sector of (R: −0.47).5 In another study of 250 patients (78 normal eyes, 210 suspect eyes, 134 glaucomatous eyes) using the Garway-Heath map, Leite et al18 found that associations between RNFL thickness as measured by Cirrus SD-OCT (Carl Zeiss Meditec) and SAP VF sensitivity were statistically significant in all RNFL sectors except for the nasal sector, with the highest coefficient of determination (R2: 0.314) being the TS RNFL sector followed by the TI sector (R2: 0.259). Kanamori et al2 generated a new and different type of VF cluster map that correlated Stratus OCT RNFL sectors by grouping the VF test points with the highest relation to each RNFL sector. As OCT measures RNFL thickness away from the optic disc margin and as retinal nerve fibers do not project radially from the optic disc, the retinotopic orientation is different between a site on the optic disc margin and that on the concentric RNFL scan circle.2 Theoretically, Kanamori's map is more suitable in structure–function studies where RNFL thickness is measured by an OCT scan circle. For the present structure–function study, we modified the RNFL sector regions in Kanamori's study in order to fit the six RNFL sectors in the Spectralis OCT printout (Figure 1). For example, the sector regions of 90–112.5 degrees and 247.5–270 degrees in Kanamori's map were increased to 90–135 degrees and 225–270 degrees in our map (Figure 1). In our study, correlations between RNFL thinning and VF defects were statistically significant in all six RNFL sectors, with higher Spearman correlation coefficients associated with the TI sector (R: 0.832) and TS sector (R: 0.803) and lower Spearman correlation coefficients associated with the temporal sector (R: 0.464) and nasal sector (R: 0.493). Compared with Horn et al's (Spearman R: 0.47–0.81) and Leite et al's (R2: 0.259–0.314) SD-OCT RNFL studies, which were based on the Garway-Heath map, our study found slightly stronger correlation coefficients (Spearman R: 0.464–0.832) using Kanamori's OCT-based structure–function correspondence map. When evaluating glaucomatous structure–function relationships, Kanamori's map, which correlates VF defects with OCT RNFL circular scan measurements, may be a more accurate way to correlate RNFL structure and VF function.
In the past few years, many researchers focused on determining whether a linear or logarithmic relation best describes the relationship between RNFL changes and VF defects.3, 9, 12, 15, 17, 27 However, there is still no well-accepted mathematical model describing the structural–functional relationship in glaucoma. One of the reasons is that RNFL thickness is measured in a linear scale (μm), whereas VF is measured in a logarithmic scale (dB). Hood et al3, 4, 5 reported a log-linear model to describe the relationship between the glaucomatous OCT RNFL thinning and SAP sensitivity loss, and this log-linear model was described earlier in the Materials and methods section (TM=TA0 × 10-D/10+TR). The theoretical curve, based on the log-linear model, can be made by plotting the mean RNFL thickness against the mean field loss in the corresponding region.3 In this study, comparison of our data and Hood's prediction model demonstrated that most of the patients (82% in the TS and NI RNFL sectors to 95% in the NS RNFL sector) were inside the 95% prediction limits. Hood's log-linear model that compares RNFL thickness with VF defects is consistent with the data in our study. Our study supported that Hood's model may be a good model to describe the structure–function relationship in glaucoma. This log-linear model may be better than simple linear or logarithmic descriptions of the relationship between RNFL changes and VF defects.
In some structure–function studies, weak or no structure–function associations were found in groups with early glaucomatous damage.14, 18, 28, 29 Nouri-Mahdavi et al29 described that in early glaucoma, with their group average MD of -2.9 dB, no correlation was found between MD/PSD and RNFL average thickness using the OCT 2000 (Carl Zeiss Meditec). Ajtony et al14 reported that no structure–function correlation was detected in POAG eyes with PSD <1.9 dB and RNFL average thickness above 70 μm. Leite et al18 found weak-to-moderate structure–function associations with glaucoma patients with an average MD of -5 dB in their study. However, stronger associations were reported by Leung et al,17 as they found R2 values of 0.623 using the Stratus and 0.588 using the scanning laser polarimeter, and their study patients had more severe glaucoma with a mean MD of −11 dB. In our current study, we found strong structure–function associations, and the mean MD of glaucoma patients in our study was −9.62 dB. It has been shown that analysis of early glaucomatous, healthy individuals, and glaucoma suspect eyes have weaker correlation with VF sensitivity than analyses that include eyes with moderate to severe glaucoma patients, mainly because in healthy and suspect patients, the range of VF loss is narrower.14, 18, 28 On the other hand, in the current study, we found that with deterioration of VF, the RNFL thickness tends to be stable despite of changes in VF (Figure 2). Leite et al18 reported similar findings. In light of these studies, functional evaluation may be more critical in the follow-up of advanced glaucoma patients, but longitudinal studies are needed to address this issue.
One of the limitations of the current cross-sectional study was that we did not evaluate multiple measurements per subject over time, so causal relationships were not established, although we did found strong correlation between structural and functional tests. Longitudinal studies may be better for developing prediction models. Because our study only evaluated perimetric glaucoma patients, it is difficult to determine whether structural or functional glaucomatous damage occurs first.3, 4, 5 Future studies, which include pre-perimetric glaucoma patients, should be considered to better elucidate answers to these questions.
In conclusion, an OCT-based correspondence map can demonstrate statistically significant associations between localized VF defects and Spectralis SD-OCT RNFL thinning. Best correlations between VF defects and areas of RNFL thinning were found for the TI and TS sectors. In contrast to the classic Garway-Heath ONH and VF structure–function map, this study presents a novel structure–function map that is more suitable for OCT RNFL and VF structure–function correlations.
Acknowledgments
Gratitude is extended to Baojian Fan, PhD of the department of ophthalmology at the Harvard Medical School for statistical consultation. This study was supported in part by the National Institutes of Health (R01 EY14975-01).
The authors (HW, LC, TCC) declare no conflict of interest. Dr de Boer holds patents in SD-OCT technology, and NIDEK has supported his research.
References
- Horn FK, Mardin CY, Viestenz A, Junemann AG. Association between localized visual field losses and thickness deviation of the nerve fiber layer in glaucoma. J Glaucoma 2005; 14: 419–425. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Naka M, Nagai-Kusuhara A, Yamada Y, Nakamura M, Negi A. Regional relationship between retinal nerve fiber layer thickness and corresponding visual field sensitivity in glaucomatous eyes. Arch Ophthalmol 2008; 126: 1500–1506. [DOI] [PubMed] [Google Scholar]
- Hood DC, Anderson SC, Wall M, Kardon RH. Structure versus function in glaucoma: an application of a linear model. Invest Ophthalmol Vis Sci 2007; 48: 3662–3668. [DOI] [PubMed] [Google Scholar]
- Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res 2007; 26: 688–710 Epub 2007 Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn FK, Mardin CY, Laemmer R, Baleanu D, Juenemann AM, Kruse FE et al. Correlation between local glaucomatous visual field defects and loss of nerve fiber layer thickness measured with polarimetry and spectral domain OCT. Invest Ophthalmol Vis Sci 2009; 50: 1971–1977. [DOI] [PubMed] [Google Scholar]
- El Beltagi TA, Bowd C, Boden C, Amini P, Sample PA, Zangwill LM et al. Retinal nerve fiber layer thickness measured with optical coherence tomography is related to visual function in glaucomatous eyes. Ophthalmology 2003; 110: 2185–2191. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Shakiba S, Sample PA, Shahrokni S, van Horn S, Garden VS et al. Association between quantitative nerve fiber layer measurement and visual field loss in glaucoma. Am J Ophthalmol 1995; 120: 732–738. [DOI] [PubMed] [Google Scholar]
- Gulati V, Agarwal HC, Sihota R, Saxena R. Correlation analysis of visual field thresholds and scanning laser ophthalmoscopic optic nerve head measurements in glaucoma. Ophthalmic Physiol Opt 2003; 23: 233–242. [DOI] [PubMed] [Google Scholar]
- Reus NJ, Lemij HG. The Relationship between Standard automated perimetry and GDx Vcc measurements. Invest Ophthalmol Vis Sci 2004; 45: 840–845. [DOI] [PubMed] [Google Scholar]
- Bowd C, Zangwill LM, Weinreb RN. Association between scanning laser polarimetry measurements using variable corneal polarization compensation and visual field sensitivity in glaucomatous eyes. Arch Ophthalmol 2003; 121: 961–966. [DOI] [PubMed] [Google Scholar]
- Chen YY, Chen PP, Xu L, Ernst PK, Wang L, Mills RP. Correlation of peripapillary nerve fiber layer thickness by scanning laser polarimetry with visual field defects in patients with glaucoma. J Glaucoma 1998; 7: 312–316. [PubMed] [Google Scholar]
- Bowd C, Zangwill LM, Medeiros FA, Tavares IM, Hoffmann EM, Bourne RR et al. Structure-function relationships using confocal scanning laser ophthalmoscopy, optical coherence tomography, and scanning laser polarimetry. Invest Ophthalmol Vis Sci 2006; 47(7): 2889–2895. [DOI] [PubMed] [Google Scholar]
- Nagai-Kusuhara A, Nakamura M, Kanamori A, Negi A. Association of optic disc configuration and clustered visual field sensitivity in glaucomatous eyes with hemifield visual field defects. J Glaucoma 2009; 18: 62–68. [DOI] [PubMed] [Google Scholar]
- Ajtony C, Balla Z, Somoskeoy S, Kovacs B. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by optical coherence tomography. Invest Ophthalmol Vis Sci 2007; 48: 258–263. [DOI] [PubMed] [Google Scholar]
- Schlottmann PG, De Cilla S, Greenfield DS, Caprioli J, Garway-Heath DF. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by scanning laser polarimetry. Invest Ophthalmol Vis Sci 2004; 45: 1823–1829. [DOI] [PubMed] [Google Scholar]
- Yalvac IS, Altunsoy M, Cansever S, Satana B, Eksioglu U, Duman S. The correlation between visual field defects and focal nerve fiber layer thickness measured with optical coherence tomography in the evaluation of glaucoma. J Glaucoma 2009; 18: 53–61. [DOI] [PubMed] [Google Scholar]
- Leung CK, Chong KK, Chan WM, Yiu CK, Tso MY, Woo J et al. Comparative study of retinal nerve fiber layer measurement by StratusOCT and GDx VCC, II: structure/function regression analysis in glaucoma. Invest Ophthalmol Vis Sci 2005; 46(10): 3702–3711. [DOI] [PubMed] [Google Scholar]
- Leite MT, Zangwill LM, Weinreb RN, Rao HL, Alencar LM, Medeiros FA. Structure-function Relationships Using the Cirrus Spectral Domain Optical Coherence Tomograph and Standard Automated Perimetry. J Glaucoma 2012; 21(1): 49–54 doi:10.1097/IJG.0b013e31822af27a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology 2000; 107(10): 1809–1815. [DOI] [PubMed] [Google Scholar]
- Weber J, Ulrich H. A perimetric nerve fiber bundle map. Int Ophthalmol 1991; 15: 193–200. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol 1999; 117(5): 573–583. [DOI] [PubMed] [Google Scholar]
- Katz J, Sommer A, Gaasterland DE, Anderson DR. Comparison of analytic algortihms for detecting glaucomatous visual field loss. Arch Ophthalmol 1991; 109: 1684–1689. [DOI] [PubMed] [Google Scholar]
- Heidelberg Engineering. OCT Acquisition Window1 –Overview. Spectralis OCT QuickGuide (Software Version 4.0) 2008. p 10.
- Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between ganglion cell numbers and light sensitivity. Invest Ophthalmol Vis Sci 2000; 41: 1774–1782. [PubMed] [Google Scholar]
- Weisstein EW Bonferroni correction. MathWorld. Available at http://mathworld.wolfram.com/BonferroniCorrection.html.
- Artes PH, Chauhan BC, Keltner JL, Cello KE, Johnson CA, Anderson DR et al. Longitudinal and cross-sectional analyses of visual field progression in participants of the Ocular Hypertension Treatment Study. Arch Ophthalmol 2010; 128: 1528–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci 2002; 43: 2213–2220. [PubMed] [Google Scholar]
- Miglior S, Riva I, Guareschi M, Di Matteo F, Romanazzi F, Buffagni L et al. Retinal sensitivity and retinal nerve fiber layer thickness measured by optical coherence tomography in glaucoma. Am J Ophthalmol 2007; 144: 733–740. [DOI] [PubMed] [Google Scholar]
- Nouri-Mahdavi K, Hoffman D, Tannenbaum DP, Law SK, Caprioli J. Identifying early glaucoma with optical coherence tomography. Am J Ophthalmol 2004; 137: 228–235. [DOI] [PubMed] [Google Scholar]