Abstract
Scarlet fever is one of a variety of diseases caused by group A Streptococcus (GAS). During 2011, a scarlet fever epidemic characterized by peak monthly incidence rates 2.9–6.7 times higher than those in 2006–2010 occurred in Beijing, China. During the epidemic, hospital-based enhanced surveillance for scarlet fever and pharyngitis was conducted to determine characteristics of circulating GAS strains. The surveillance identified 3,359 clinical cases of scarlet fever or pharyngitis. GAS was isolated from 647 of the patients; 76.4% of the strains were type emm12, and 17.1% were emm1. Almost all isolates harbored superantigens speC and ssa. All isolates were susceptible to penicillin, and resistance rates were 96.1% to erythromycin, 93.7% to tetracycline, and 79.4% to clindamycin. Because emm12 type GAS is not the predominant type in other countries, wider surveillance for the possible spread of emm12 type GAS from China to other countries is warranted.
Keywords: scarlet fever, pharyngitis, group A Streptococcus, GAS, emm type, superantigen, antimicrobial drug susceptibility, bacteria, epidemic, surveillance, Beijing, People’s Republic of China, China
Streptococcus pyogenes, also known as group A Streptococcus (GAS), is a common human pathogen that can induce a wide spectrum of diseases, ranging from noninvasive diseases, such as pharyngitis, scarlet fever, and impetigo, to invasive diseases, such as erysipelas, cellulitis, pneumonia, bacteremia, necrotizing fasciitis, and toxic shock syndrome. Moreover, GAS can cause rheumatic fever and acute poststreptococcal glomerulonephritis (1,2). In the late1980s, a change in the epidemiology of invasive GAS diseases and the emergence of streptococcal toxic shock syndrome were documented (3,4), and the current number of invasive GAS disease cases worldwide is high (2).
Many virulence factors contribute to the pathogenesis of GAS diseases (1,5). However, the matrix (M) protein, encoded by the emm gene, has the most critical role, mainly by antiphagocytic mechanisms (6,7), and the amino-terminal region of M protein is the most promising target for designing a vaccine (8,9). emm gene sequencing is a standard method for typing the M protein (10), but the distribution of emm types varies greatly by geographic location, time, and collection site of clinical specimens (9,11–14).
Streptococcal pyrogenic exotoxins also play a major role in the pathogenesis of GAS infections by acting as superantigens. When these exotoxins cross-link major histocompatibility complex class II molecules and T cell receptors, they trigger intense activation of a subset of T cells within a specific β-chain variable region. This process induces a tremendous release of a series of cytokines and may lead to cell, tissue, and organ damage (15,16).
Several antimicrobial drugs effectively treat GAS infections (1). In recent years, however, considerable attention has been given worldwide to the issue of antimicrobial drug–resistant GAS. Macrolide-resistant GAS strains have been isolated from various regions of the world (17–19). Macrolides are used as an alternative treatment for GAS in patients allergic to penicillin, and clindamycin, in combination with β-lactam antimicrobial drugs, is a recommended treatment for invasive GAS disease (20). Thus, it is critical that surveillance for macrolide- and clindamycin-resistant GAS be continued.
In China, scarlet fever is the only GAS disease reported by the National Notifiable Infectious Disease Surveillance System (NNIDSS) (21). According to NNIDSS, the incidence of scarlet fever in Beijing, China, before 2011 had persistently remained within normal threshold limits. However, in late spring 2011, an epidemic of scarlet fever occurred in Beijing and many other regions of China. In response to the epidemic, enhanced surveillance for GAS diseases was conducted in Beijing during May–July 2011 to determine characteristics of the circulating GAS strains.
Methods
National Notifiable Infectious Disease Surveillance System
China established NNIDSS in 2004, after the 2003 outbreak of severe acute respiratory syndrome. At that time, NNIDSS covered 37 infectious diseases, which were classified into 3 categories (A–C), in a descending order according to disease severity; scarlet fever belonged to category B.
A clinical case of scarlet fever is defined as acute illness onset with fever, pharyngitis, and sandpaper-like red rash with or without strawberry tongue, Pastia lines, or circumoral pallor. In China, clinicians and hospitals are to report clinical cases of scarlet fever to NNIDSS within 6 hours of diagnosis. To respond to the 2011 epidemic of scarlet fever and to monitor its severity, each clinical case of scarlet fever reported in Beijing was followed for 3 weeks after the onset of disease.
Enhanced Surveillance for GAS Diseases
Enhanced surveillance for GAS diseases was conducted in the pediatric clinics of 36 hospitals within Beijing’s 18 districts during May–July 2011. The surveillance system was designed and managed by the Beijing Center for Disease Prevention and Control (Beijing CDC). The Beijing CDC laboratory and 18 collaborating district laboratories were involved. The study was approved by the Institutional Review Board and the Human Research Ethics Committee of Beijing CDC.
The surveillance included children with scarlet fever or pharyngitis diagnosed by clinicians in the surveillance hospitals. All children with scarlet fever were invited to participate in the study after their parent(s)/guardian(s) gave informed consent. Each week, 10 children with pharyngitis were randomly selected from each hospital to participate in the study. Trained clinic staff used a standardized questionnaire to collect information (e.g., sex, age, date of illness onset, date medical care was sought, clinical symptoms and signs, and antimicrobial drug treatment) for each study participant. In addition, at each clinic, trained personnel obtained pharyngeal swab samples from study participants, and, the same day, a designated hospital staff member collected and sent all specimens to the corresponding district laboratory. The collaborating district laboratories isolated and identified GAS strains and then sent the isolates to Beijing CDC for emm typing, superantigen determination, and antimicrobial drug susceptibility testing. Patients with scarlet fever or pharyngitis from whom GAS was isolated were identified as confirmed GAS patients.
Isolation and Identification of GAS Strains
After pharyngeal swab samples arrived at a collaborating district laboratory, they were immediately spread onto 5% sheep blood agar plates and incubated overnight at 37°C in 5% CO2. We tested β-hemolytic isolates for susceptibility to bacitracin and used the Streptococcal Grouping Kit (Oxoid Ltd., Basingstoke, UK) to determine the Lancefield group for each isolate.
emm Typing
All GAS isolates were subjected to emm typing, as described (22). We extracted DNA and amplified and sequenced the 5′ region of the emm gene by using recommended primers and cycling conditions (22). We aligned the sequence of the 5′ region of the emm gene with sequences in the Blast-emm database (www.cdc.gov/ncidod/biotech/strep/strepblast.htm; Centers for Disease Control and Prevention, Atlanta, GA, USA). The emm type and subtype of GAS isolates were identified on the basis of the 90 bases encoding the N terminal 30 residues of the processed M protein and the exact 150 base sequences encoding the N terminal 50 residues of the mature M protein, respectively. The fairly conserved 30 bases encoding the last 10 residues of the M protein signal sequence were referred to for identifying the start of the sequence encoding the mature M protein.
Superantigen Detection
Thirteen superantigens (speA–speC, speF–speM, smeZ, and ssa) were detected by subjecting each GAS isolate to real-time PCR. Specific PCR primers were used to amplify the gene of each superantigen in a 40-μL real-time PCR reaction system under the following cycling condition: 2 min at 94°C, followed by 40 cycles of 15 s at 93°C and 60 s at 55°C. Superantigen profiles were investigated for various emm types of GAS isolates.
Antimicrobial Drug Susceptibility Testing
We used the VITEK2 Compact (bioMérieux, Marcy l'Etoile, France) to test all GAS isolates for susceptibility to penicillin, ampicillin, quinupristin-dalfopristin, linezolid, vancomycin, tigecycline, levofloxacin, erythromycin, clindamycin, and tetracycline. We estimated the minimal inhibitory concentration of each antimicrobial drug for individual GAS isolates and determined the corresponding susceptibility according to the 2011 criteria of the Clinical and Laboratory Standards Institute (23).
Statistical Analysis
We entered data by using Microsoft Excel 2003 software (Microsoft Corp., Redmond, WA, USA) and analyzed data by using the SPSS 16.0 statistical package (SPSS Inc., Chicago, IL, USA). The mean and SD were calculated for continuous variables, and percentages were calculated for categorical variables. Differences in distributions of emm types and superantigens of GAS isolates were compared between subgroups of participants by using the χ2 test or Fisher exact test. In addition, we compared antimicrobial drug susceptibilities by strain emm type by using the χ2 test. We used multivariate unconditional logistic regression analyses to determine factors associated with various clinical signs of scarlet fever in patients with confirmed GAS infection. All statistical tests were 2-sided, and statistical significance was defined as p<0.05.
Results
Comparison of the 2011 Epidemic and 2006–2010 Cluster Outbreaks
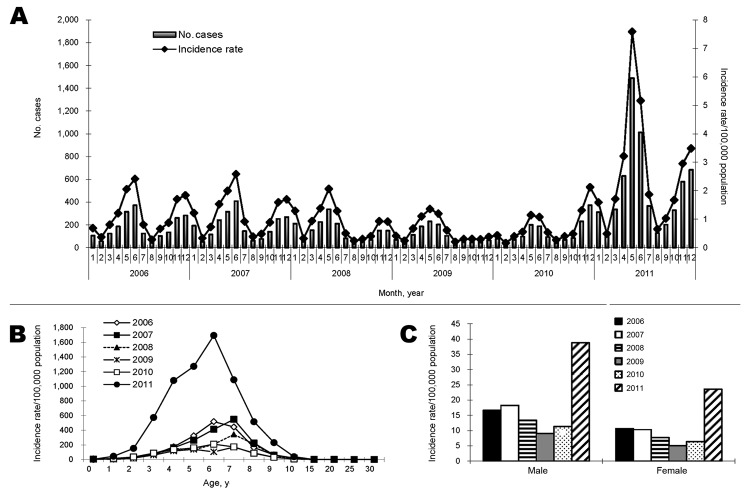
According to NNIDSS, the annual number of scarlet fever cases in Beijing during 2006–2010 ranged from 1,193 to 2,264, and annual incidence rates ranged from 7.0 cases to 14.3 cases/100,000 population. In 2011, however, the number of scarlet fever cases in Beijing rose to 6,152, and the incidence rate rose to 31.4 cases/100,000 population. Peak monthly incidence rates in 2011 were 2.9–6.7 times those in 2006–2010. During the epidemic and during 2006–2010, scarlet fever cases peaked twice yearly: 1 peak occurred in early summer, and a second, less pronounced peak occurred in winter, except in 2010, when the winter peak was more pronounced (Figure, panel A).
Figure.
Scarlet fever incidence, Beijing, China, 2006–2011, as reported in the National Notifiable Infectious Disease Surveillance System. A) Number of cases and incidence rate by month. B) Incidence rate by age. C) Incidence rate by sex.
Scarlet fever primarily affected children 3–8 years of age during the epidemic and nonepidemic years (Figure, panel B). During these years, the ratio of males to females in Beijing was 1.5:1.8, respectively, and the annual incidence of scarlet fever was persistently higher among males than females (Figure, panel C).
In Beijing, a cluster of scarlet fever was defined as onset of >2 clinical cases within a 7-day period in a school or kindergarten. According to this definition, 37–131 clusters (85–316 cases) occurred during 2006–2010 in Beijing, and 401 clusters (1,116 cases) occurred during 2011. In addition, case-patient follow-up showed that all cases reported during May–December, 2011, were fully resolved 3 weeks after illness onset without complications.
Characteristics of Children Enrolled in Enhanced Surveillance
A total of 3,359 children, representing 972 clinical cases of scarlet fever and 2,387 cases of pharyngitis, were enrolled in enhanced surveillance for GAS disease. Of the 3,359 enrollees, ≈41.0% were female. The mean age was 5.8 years (SD ± 3.3 years). The mean interval from illness onset to first medical visit was 1.5 days (SD ± 1.5 days). Approximately 41.2% of the children received antimicrobial drugs at home before seeking medical care.
Of the 3,359 enrollees, 647 (19.2%) were confirmed to have GAS infection. GAS was isolated from clinical samples for 44.2% (430/972) of the children with scarlet fever and from 9.1% (217/2,387) of the children with pharyngitis.
emm Subtypes and Superantigens
emm12 type accounted for 76.4% (494/647) of all GAS isolates. The leading emm subtypes were emm12.0 (65.7%), emm1.0 (16.8%), emm12.19 (5.7%), and emm12.1 (2.5%). There was a statistically significant difference in the proportion of emm12 type GAS in children <5 years of age and those >5 years of age (p = 0.004) and in the proportion of emm1 scarlet fever cases and pharyngitis cases (p = 0.042) (Table 1).
Table 1. SAgs and emm types of group A Streptococcus circulating during a scarlet fever epidemic, Beijing, China, 2011*.
| emm type or SAg | No. (%) patients, by age |
p value | No. (%) patients, by sex |
p value | No. (%) patients, by clinical diagnosis |
p value | Total, n = 647 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| <5 y, n = 246 | >5 y, n = 401 | Male, n = 402 | Female, n = 245 | Scarlet fever, n = 430 | Pharyngitis, n = 217 | |||||
| emm type | ||||||||||
| 12 | 203 (82.5) | 291 (72.6) | 0.004 | 303 (75.4) | 191 (78.0) | 0.453 | 331 (77.0) | 163 (75.1) | 0.599 | 494 (76.4) |
| 1 | 34 (13.8) | 77 (19.2) | 0.078 | 73 (18.2) | 38 (15.5) | 0.386 | 83 (19.3) | 28 (12.9) | 0.042 | 111 (17.1) |
| Other† |
9 (3.7) |
33 (8.2) |
0.022
|
26 (6.5) |
16 (6.5) |
0.975 |
16 (3.7) |
26 (12.0) |
<0.001
|
42 (6.5) |
| SAg | ||||||||||
| SpeA | 47 (19.1) | 96 (23.9) | 0.150 | 91 (22.6) | 52 (21.2) | 0.675 | 99 (23.0) | 44 (20.3) | 0.427 | 143 (22.1) |
| SpeB | 246 (100.0) | 401 (100.0) | NA | 402 (100.0) | 245 (100.0) | NA | 430 (100.0) | 217 (100.0) | NA | 647 (100.0) |
| SpeC‡ | 243 (98.8) | 400 (99.8) | 0.156 | 398 (99.0) | 245 (100.0) | 0.303 | 427 (99.3) | 216 (99.5) | 1.000 | 643 (99.4) |
| SpeF‡ | 245 (99.6) | 400 (99.8) | 1.000 | 401 (99.8) | 244 (99.6) | 1.000 | 429 (99.8) | 216 (99.5) | 1.000 | 645 (99.7) |
| SpeG‡ | 246 (100.0) | 399 (99.5) | 0.528 | 400 (99.5) | 245 (100.0) | 0.529 | 428 (99.5) | 217 (100.0) | 0.554 | 645 (99.7) |
| SpeH | 192 (78.0) | 301 (75.1) | 0.387 | 299 (74.4) | 194 (79.2) | 0.164 | 326 (75.8) | 167 (77.0) | 0.747 | 493 (76.2) |
| SpeI | 190 (77.2) | 302 (75.3) | 0.578 | 301 (74.9) | 191 (78.0) | 0.373 | 327 (76.0) | 165 (76.0) | 1.000 | 492 (76.0) |
| SpeJ | 47 (19.1) | 92 (22.9) | 0.249 | 87 (21.6) | 52 (21.2) | 0.900 | 99 (23.0) | 40 (18.4) | 0.180 | 139 (21.5) |
| SpeK ‡ | 1 (0.4) | 3 (0.7) | 1.000 | 1 (0.2) | 3 (1.2) | 0.155 | 0 | 4 (1.8) | 0.012 | 4 (0.6) |
| SpeL ‡ | 2 (0.8) | 5 (1.2) | 0.715 | 4 (1.0) | 3 (1.2) | 1.000 | 2 (0.5) | 5 (2.3) | 0.046 | 7 (1.1) |
| SpeM | 5 (2.0) | 9 (2.2) | 0.857 | 8 (2.0) | 6 (2.4) | 0.697 | 9 (2.1) | 5 (2.3) | 0.862 | 14 (2.2) |
| SmeZ‡ | 245 (99.6) | 400 (99.8) | 1.000 | 401 (99.8) | 244 (99.6) | 1.000 | 428 (99.5) | 217 (100.0) | 0.554 | 645 (99.7) |
| Ssa | 241 (98.0) | 391 (97.5) | 0.705 | 394 (98.0) | 238 (97.1) | 0.477 | 427 (99.3) | 205 (94.5) | <0.001 | 632 (97.7) |
*Boldface indicates statistical significance. SAg, superantigen. †Other emm types included types 4, 11, 22, 75, and 89. ‡Fisher exact test was used.
All of the GAS isolates harbored speB, and almost all possessed speC, speF, speG, smeZ, and ssa. Approximately 20% of the isolates harbored speA or speJ, and ≈75% possessed speH or speI; however, the percentage of isolates that harbored speK, speL, or speM was extremely low (Table 1). A total of 25 profiles of superantigens were found in the GAS isolates. Of the 494 emm12 isolates, 411 (83.2%) had the following superantigen profile: speA (−), speB (+), speC (+), speF (+), speG (+), speH (+), speI (+), speJ (−), speK (−), speL (−), speM (−), smeZ (+), ssa (+). Of the 111 emm1 isolates, 93 (83.8%) had the following superantigen profile: speA (+), speB (+), speC (+), speF (+), speG (+), speH (−), speI (−), speJ (+), speK (−), speL (−), speM (−), smeZ (+), ssa (+).
Antimicrobial Drug Susceptibility
All GAS strains isolated during the scarlet fever epidemic were susceptible to penicillin, ampicillin, quinupristin-dalfopristin, linezolid, vancomycin, and tigecycline, and 96.6% of them were susceptible to levofloxacin. Resistance to erythromycin, tetracycline, and clindamycin was found in 96.1%, 93.7%, and 79.4% of the isolates, respectively. A statistically significant difference was found between the percentage of emm1 and emm12 strains resistant to clindamycin (87.4% vs. 77.9%, respectively; p = 0.025) but not between the percentage of those resistant to erythromycin (99.1% vs. 96.4%, respectively; p = 0.134) or tetracycline (97.3% vs. 93.1%, respectively; p = 0.097).
Factors Associated with Clinical Signs of Scarlet Fever
The odds of having strawberry tongue was higher for GAS-infected study participants <5 years of age (odds ratio [OR] 2.04, 95% CI 1.46–2.83; p < 0.001). The odds of having a red rash was higher for participants infected with an emm1 versus emm12 type strain (OR 1.63, 95% CI 1.01–2.62; p = 0.046) and for participants <5 years of age (OR 2.52, 95% CI 1.74–3.65; p<0.001). Compared with patients with emm12 type strains, those with emm1 type strains had a higher probability of having Pastia lines (OR 1.80, 95% CI 1.02–3.16; p = 0.043) or circumoral pallor (OR 2.15, 95% CI 1.17–3.93; p = 0.013) (see Table 2, Appendix, wwwnc.cdc.gov/EID/article/19/6/12-1020-T1.htm, for details).
Table 2. Results of multivariate analyses for factors associated with 4 clinical signs of scarlet fever in patients with confirmed GAS infection, Beijing, China, 2011*.
| Factor | Clinical sign of scarlet fever† |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strawberry tongue |
Red rash |
Pastia lines |
Circumoral pallor |
||||||||||||
| Yes, n = 293 | No, n = 354 | OR (95% CI), p value | Yes, n = 430 | No, n = 217 | OR (95% CI), p value | Yes, n = 79 | No, n = 568 | OR (95% CI), p value | Yes, n = 63 | No, n = 584 | OR (95% CI), p value | ||||
| emm type | |||||||||||||||
| 12 | 232 (79.2) | 262 (74.0) | Ref | 331 (77.0) | 163 (75.1) | Ref | 57 (72.2) | 437 (76.9) | Ref | 43 (68.3) | 451 (77.2) | Ref | |||
| 1 | 51 (17.4) | 60 (16.9) | 1.03 (0.68–1.58), 0.884 | 83 (19.3) | 28 (12.9) | 1.63 (1.01–2.62), 0.046 | 20 (25.3) | 91 (16.0) | 1.80 (1.02–3.16), 0.043 | 18 (28.6) | 93 (15.9) | 2.15 (1.17–3.93), 0.013 | |||
| Other‡ |
10 (3.4) |
32 (9.0) |
0.40 (0.19–0.84), 0.016
|
|
16 (3.7) |
26 (12.0) |
0.35 (0.18–0.68), 0.002
|
|
2 (2.5) |
40 (7.0) |
0.40 (0.09–1.72), 0.218 |
|
2 (3.2) |
40 (6.8) |
0.54 (0.12–2.31), 0.402 |
| Age <5 y |
138 (47.1) |
108 (30.5) |
2.04 (1.46–2.83), <0.001
|
|
193 (44.9) |
53 (24.4) |
2.52 (1.74–3.65), <0.001
|
|
35 (44.3) |
211 (37.1) |
1.40 (0.86–2.27), 0.182 |
|
27 (42.9) |
219 (37.5) |
1.29 (0.75–2.22), 0.356 |
| Female sex |
105 (35.8) |
140 (39.5) |
1.09 (0.78–1.51), 0.630 |
|
157 (36.5) |
88 (40.6) |
1.09 (0.77–1.55), 0.615 |
|
36 (45.6) |
209 (36.8) |
0.65 (0.40–1.05), 0.079 |
|
28 (44.4) |
217 (37.2) |
0.69 (0.40–1.18), 0.171 |
| Interval >2 d§ | 51 (17.4) | 39 (11.0) | 1.87 (1.18–2.97), 0.008 | 61 (14.2) | 29 (13.4) | 1.19 (0.73–1.95), 0.489 | 7 (8.9) | 83 (14.6) | 0.58 (0.26–1.31), 0.190 | 3 (4.8) | 87 (14.9) | 0.29 (0.09–0.94), 0.040 | |||
*Four multivariate unconditional logistic regression analyses were separately conducted to examine factors for each clinical sign of scarlet fever. Scarlet fever and pharyngitis patients from whom GAS was isolated were identified as confirmed GAS patients. Boldface font indicates statistical significance. GAS, group A Streptococcus; OR, odds ratio; Ref, reference. †Data are number (%) patients unless otherwise indicated. ‡Other emm types included types 4, 11, 22, 75, and 89. §Interval between illness onset and hospital visit.
Discussion
We found that an epidemic of scarlet fever occurred in Beijing in 2011. emm12 was the predominant type among the circulating GAS strains, and resistance to erythromycin, tetracycline, and clindamycin was high among the isolates.
Although the incidence of scarlet fever in Beijing in 2011 was much higher than that in preceding years, the basic characteristics, including the seasonality of epidemic peaks, the most vulnerable age group, and the difference in susceptibility to infection by sex, did not change. In Beijing, the incidence of scarlet fever usually peaks twice a year in 2 distinct seasons—summer and winter—with the highest peak in early summer; however, it is well-recognized that scarlet fever most commonly occurs in winter/spring in other locations (24,25). This finding indicates that in China, GAS is transmitted more easily in early summer than in winter, possibly because in early summer, compared with winter, each year an emm type that is relatively new to young children sweeps through a population of children that does not have an effective level of population immunity. In addition, the winter peak in 2010 was higher than the summer peak, which implies that this epidemic of scarlet fever started at the end of 2010.
Among the GAS strains we identified circulating in Beijing during 2011, types emm12 and emm1 were most predominant (≈76% and ≈17%, respectively); these percentages were higher (≈40%) and lower (>40%), respectively, than those reported in China during previous years (13,26,27). Scarlet fever outbreaks also occurred in Hong Kong and Shanghai, China, during 2011, and emm12 was the predominant circulating type (28–30). These findings suggest that some intrinsic factors might have facilitated the spread of emm12 strains and led to the epidemic. Two previously unreported genomic insertions (64.9 kb and 46.4 kb) were identified in emm12 GAS strains isolated during the 2011 scarlet fever outbreak in Hong Kong. However, analysis of emm12 strains isolated during 2005–2010 showed that the insertions were also present in those strains (28), and the study concluded that mobile genetic elements, environmental factors, and host immune status might have contributed to the 2011 scarlet fever outbreak in Hong Kong.
emm12 is not known to be the exclusively predominant GAS type in other countries (9,31,32), but it is possible that the emm12 type strains circulating in China in 2011 could spread to other regions. Therefore, surveillance for the increased presence of emm12 strains outside of China is warranted. A 30-valent GAS vaccine that contains the most prevalent emm types found in our study (emm12 and emm1) is under development (8); such a vaccine would help prevent and control the spread of GAS diseases in China.
Superantigens in the GAS isolates in our study were similar to those found in isolates from other studies, except for speC and ssa. In contrast to findings in other studies, we found that almost all emm1 GAS strains from the 2011 Beijing epidemic harbored speC, and ssa became the primary superantigen of emm1 and emm12 isolates (11,32,33). Consistent with findings in earlier studies in other locations, we found that an extremely low number of GAS isolates harbored speK, speL, or speM (11,33,34).
All GAS isolates from the 2011 Beijing epidemic were susceptible to penicillin, a standard antimicrobial drug for the treatment of scarlet fever. However, >96% and ≈94% of the isolates were resistant to erythromycin and tetracycline, respectively, and ≈80% were resistant to clindamycin. These findings compare with earlier findings of 99.5%, 97.1%, and 99.5% resistance to erythromycin, tetracycline, and clindamycin, respectively (27). The extent of the resistance of GAS strains to erythromycin and tetracycline in many other countries has been very low (11,17,18). The high resistance rate in China might be attributed to the overuse of antimicrobial drugs in humans or animals.
In our study, the odds of having strawberry tongue or red rash with GAS infection was higher for younger patients. This could indicate that older patients had acquired partial immunity to GAS from a previous exposure or infection, resulting in milder clinical manifestation of the disease during subsequent infection.
This study had limitations. First, to track case outcomes, we followed up on the scarlet fever case-patients for 3 weeks after illness onset, which may not have allowed sufficient time to capture later-occurring complications; thus, outcome profiles may have been incomplete. Second, the enhanced surveillance in Beijing did not include invasive GAS diseases, so we could not report on all GAS strains circulating during the 2011 scarlet fever epidemic.
The 2011 scarlet fever epidemic in Beijing was characteristic of other scarlet fever epidemics and occurred after an abnormal peak winter incidence of the disease in 2010. emm12 type GAS became predominant. The level of GAS resistance to clindamycin was lower than that to erythromycin and tetracycline.
Acknowledgments
This study was supported by grants from the Beijing Nova Program of the Beijing Science and Technology Commission (2011047) and the National Key Program for Infectious Disease of China (2012ZX10004215-003-001).
Biography
Dr Yang is an infectious disease epidemiologist at the Beijing Center for Disease Prevention and Control and at the School of Public Health and Family Medicine, Capital Medical University. His research interests are the surveillance of infectious diseases and early warning and intervention strategies for infectious diseases outbreaks.
Footnotes
Suggested citation for this article: Yang P, Peng X, Zhang D, Wu S, Liu Y, Cui S, et al. Characteristics of group A Streptococcus strains circulating during scarlet fever epidemic, Beijing, China, 2011. Emerg Infect Dis [Internet]. 2013 Jun [date cited]. http://dx.doi.org/10.3201/eid1906.121020
These authors contributed equally to this article.
References
- 1.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. 10.1128/CMR.13.3.470-511.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. 10.1016/S1473-3099(05)70267-X [DOI] [PubMed] [Google Scholar]
- 3.Hoge CW, Schwartz B, Talkington DF, Breiman RF, MacNeill EM, Englender SJ. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock–like syndrome. A retrospective population-based study. JAMA. 1993;269:384–9. 10.1001/jama.1993.03500030082037 [DOI] [PubMed] [Google Scholar]
- 4.Cone LA, Woodard DR, Schlievert PM, Tomory GS. Clinical and bacteriologic observations of a toxic shock–like syndrome due to Streptococcus pyogenes. N Engl J Med. 1987;317:146–9 and. 10.1056/NEJM198707163170305 [DOI] [PubMed] [Google Scholar]
- 5.Bisno AL, Brito MO, Collins CM. Molecular basis of group A streptococcal virulence. Lancet Infect Dis. 2003;3:191–200. 10.1016/S1473-3099(03)00576-0 [DOI] [PubMed] [Google Scholar]
- 6.Whitnack E, Beachey EH. Inhibition of complement-mediated opsonization and phagocytosis of Streptococcus pyogenes by D fragments of fibrinogen and fibrin bound to cell surface M protein. J Exp Med. 1985;162:1983–97. 10.1084/jem.162.6.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horstmann RD, Sievertsen HJ, Knobloch J, Fischetti VA. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc Natl Acad Sci U S A. 1988;85:1657–61. 10.1073/pnas.85.5.1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein–based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine. 2011;29:8175–8. 10.1016/j.vaccine.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9:611–6. 10.1016/S1473-3099(09)70178-1 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Introduction to emm typing: M protein gene (emm) typing Streptococcus pyogenes. 2008. [cited 2012 Apr 25]. http://www.cdc.gov/ncidod/biotech/strep/M-ProteinGene_typing.htm
- 11.Meisal R, Andreasson IK, Hoiby EA, Aaberge IS, Michaelsen TE, Caugant DA. Streptococcus pyogenes isolates causing severe infections in Norway in 2006 to 2007: emm types, multilocus sequence types, and superantigen profiles. J Clin Microbiol. 2010;48:842–51. 10.1128/JCM.01312-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007;45:853–62. 10.1086/521264 [DOI] [PubMed] [Google Scholar]
- 13.Ma Y, Yang Y, Huang M, Wang Y, Chen Y, Deng L, et al. Characterization of emm types and superantigens of Streptococcus pyogenes isolates from children during two sampling periods. Epidemiol Infect. 2009;137:1414–9. 10.1017/S0950268809002118 [DOI] [PubMed] [Google Scholar]
- 14.Tewodros W, Kronvall G. M protein gene (emm type) analysis of group A beta-hemolytic streptococci from Ethiopia reveals unique patterns. J Clin Microbiol. 2005;43:4369–76. 10.1128/JCM.43.9.4369-4376.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev. 2008;225:226–43. 10.1111/j.1600-065X.2008.00681.x [DOI] [PubMed] [Google Scholar]
- 16.Sriskandan S, Faulkner L, Hopkins P. Streptococcus pyogenes: insight into the function of the streptococcal superantigens. Int J Biochem Cell Biol. 2007;39:12–9. 10.1016/j.biocel.2006.08.009 [DOI] [PubMed] [Google Scholar]
- 17.Montes M, Ardanuy C, Tamayo E, Domenech A, Linares J, Perez-Trallero E. Epidemiological and molecular analysis of Streptococcus pyogenes isolates causing invasive disease in Spain (1998–2009): comparison with non-invasive isolates. Eur J Clin Microbiol Infect Dis. 2011;30:1295–302. 10.1007/s10096-011-1226-x [DOI] [PubMed] [Google Scholar]
- 18.Tanz RR, Shulman ST, Shortridge VD, Kabat W, Kabat K, Cederlund E, et al. Community-based surveillance in the United States of macrolide-resistant pediatric pharyngeal group A streptococci during 3 respiratory disease seasons. Clin Infect Dis. 2004;39:1794–801 . 10.1086/426025 [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Shen X, Chang H, Huang G, Fu Z, Zheng Y, et al. High macrolide resistance in Streptococcus pyogenes strains isolated from children with pharyngitis in China. Pediatr Pulmonol. 2009;44:436–41. 10.1002/ppul.20976 [DOI] [PubMed] [Google Scholar]
- 20.Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18:1096–100. 10.1097/00006454-199912000-00014 [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Health of China. Incidence of notifiable infectious diseases in China in May, 2011. [cited 2012 Apr 25]. http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohjbyfkzj/s3578/201106/52004.htm
- 22.Centers for Disease Control and Prevention. Streptococcus pyogenes emm sequence database. 2008. [cited 2012 Apr 25]. http://www.cdc.gov/ncidod/biotech/strep/protocol_emm-type.htm
- 23.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twenty-first international supplement M100-S21. Wayne (PA): The Institute; 2011. [Google Scholar]
- 24.Theresa Lamagni JD, George R, Efstratiou A. Analysis of epidemiological patterns during a century of scarlet fever. 2008. [cited 2012 Apr 25]. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1229594253740
- 25.World Health Organization. Western Pacific Region. Scarlet fever: July 2011. [cited 2012 Apr 25]. http://www.wpro.who.int/mediacentre/factsheets/fs_20120301_ScarletFever/en/index.html
- 26.Liang Y, Shen X, Huang G, Wang C, Shen Y, Yang Y. Characteristics of Streptococcus pyogenes strains isolated from Chinese children with scarlet fever. Acta Paediatr. 2008;97:1681–5. 10.1111/j.1651-2227.2008.00983.x [DOI] [PubMed] [Google Scholar]
- 27.Liang Y, Liu X, Chang H, Ji L, Huang G, Zhou F, et al. Epidemiological and molecular characteristics of clinical isolates of Streptococcus pyogenes from Chinese children between 2005 and 2008. J Med Microbiol. 2012;61:975–83. 10.1099/jmm.0.042309-0 [DOI] [PubMed] [Google Scholar]
- 28.Tse H, Bao JY, Davies MR, Maamary P, Tsoi HW, Tong AH, et al. Molecular characterization of the 2011 Hong Kong scarlet fever outbreak. J Infect Dis. 2012;206:341–51. 10.1093/infdis/jis362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luk EY, Lo JY, Li AZ, Lau MC, Cheung TK, Wong AY, et al. Scarlet fever epidemic, Hong Kong, 2011. Emerg Infect Dis. 2012;18:1658–61. 10.3201/eid1810.111900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M, Yao W, Wang X, Li Y, Chen M, Wang G, et al. Outbreak of scarlet fever associated with emm12 type group A Streptococcus in 2011 in Shanghai, China. Pediatr Infect Dis J. 2012;31:e158–62. 10.1097/INF.0b013e31825874f3 [DOI] [PubMed] [Google Scholar]
- 31.Shea PR, Ewbank AL, Gonzalez-Lugo JH, Martagon-Rosado AJ, Martinez-Gutierrez JC, Rehman HA, et al. Group A Streptococcus emm gene types in pharyngeal isolates, Ontario, Canada, 2002–2010. Emerg Infect Dis. 2011;17:2010–7. 10.3201/eid1711.110159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luca-Harari B, Darenberg J, Neal S, Siljander T, Strakova L, Tanna A, et al. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2009;47:1155–65. 10.1128/JCM.02155-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Commons R, Rogers S, Gooding T, Danchin M, Carapetis J, Robins-Browne R, et al. Superantigen genes in group A streptococcal isolates and their relationship with emm types. J Med Microbiol. 2008;57:1238–46. 10.1099/jmm.0.2008/001156-0 [DOI] [PubMed] [Google Scholar]
- 34.Rivera A, Rebollo M, Miro E, Mateo M, Navarro F, Gurgui M, et al. Superantigen gene profile, emm type and antibiotic resistance genes among group A streptococcal isolates from Barcelona, Spain. J Med Microbiol. 2006;55:1115–23. 10.1099/jmm.0.46481-0 [DOI] [PubMed] [Google Scholar]