Abstract
Mechanical ventilation is a life-saving intervention for patients in respiratory failure. Unfortunately, prolonged ventilator support results in diaphragmatic atrophy and contractile dysfunction leading to diaphragm weakness, which is predicted to contribute to problems in weaning patients from the ventilator. While it is established that ventilator-induced oxidative stress is required for the development of ventilator-induced diaphragm weakness, the signaling pathway(s) that trigger oxidant production remain unknown. However, recent evidence reveals that increased plasma levels of angiotensin II (ANG II) result in oxidative stress and atrophy in limb skeletal muscles. Using a well-established animal model of mechanical ventilation, we tested the hypothesis that increased circulating levels of ANG II are required for both ventilator-induced diaphragmatic oxidative stress and diaphragm weakness. Cause and effect was determined by administering an angiotensin-converting enzyme inhibitor (enalapril) to prevent ventilator-induced increases in plasma ANG II levels, and the ANG II type 1 receptor antagonist (losartan) was provided to prevent the activation of ANG II type 1 receptors. Enalapril prevented the increase in plasma ANG II levels but did not protect against ventilator-induced diaphragmatic oxidative stress or diaphragm weakness. In contrast, losartan attenuated both ventilator-induced oxidative stress and diaphragm weakness. These findings indicate that circulating ANG II is not essential for the development of ventilator-induced diaphragm weakness but that activation of ANG II type 1 receptors appears to be a requirement for ventilator-induced diaphragm weakness. Importantly, these experiments provide the first evidence that the Food and Drug Administration-approved drug losartan may have clinical benefits to protect against ventilator-induced diaphragm weakness in humans.
Keywords: muscle atrophy, respiratory muscles, weaning, reactive oxygen species
mechanical ventilation (MV) is used clinically to maintain blood gas homeostasis in patients when the ventilatory capacity of their respiratory system fails. Common indications for MV include respiratory failure, heart failure, coma, and surgery. While MV can be a life-saving intervention, prolonged MV leads to diaphragm weakness due to both diaphragmatic atrophy and impaired contractile function, which is commonly termed ventilator-induced diaphragm dysfunction (VIDD) (25, 28, 34, 44). VIDD is clinically important because MV-induced diaphragmatic weakness can contribute to problems in weaning patients from the ventilator (9). The consequences of difficult weaning are extended stays in the intensive care unit along with increased patient morbidity and mortality (28). At present, an accepted standard of clinical care to prevent VIDD does not exist, and determining the mechanism(s) responsible for VIDD is a required first step for the development of effective countermeasures to preclude VIDD.
Animal experiments reveal that VIDD occurs in response to increased production of reactive oxygen species (ROS) in diaphragm fibers; this MV-induced surge in diaphragmatic ROS production is a required upstream signal to activate muscle proteases and increase proteolysis (1, 17, 27, 37, 46, 48). Although MV-induced ROS production can occur at several locations within diaphragm fibers, evidence indicates that both NADPH oxidase and mitochondria are sites of ROS production in the diaphragm during prolonged MV (15, 18, 23).
While it is established that increased ROS production plays a required role in the development of VIDD, the mechanism(s) responsible for MV-induced increases in diaphragmatic ROS production remain unknown. A potential cause of the MV-induced increase in diaphragmatic ROS production is elevated plasma levels of angiotensin II (ANG II). ANG II is a peptide hormone involved in the renin-angiotensin system that facilitates the regulation of blood pressure and fluid balance. Moreover, increased circulating ANG II can promote cellular ROS production by binding to ANG II type 1 (AT1) receptors with the resultant activation of NADPH oxidase and associated increase in ROS production leading to oxidative stress (36, 49). Evidence also indicates that ANG II-mediated activation of NADPH oxidase promotes increased mitochondrial ROS production via a cross talk mechanism (8). Importantly, infusion of ANG II in rodents results in higher ROS production in limb skeletal muscles along with increased protease activation and muscle fiber atrophy (4, 5, 7, 36). In this regard, our preliminary experiments confirm that prolonged MV results in increased plasma ANG II levels in rats (unpublished). Nonetheless, it is unknown if this MV-induced increase in plasma ANG II levels plays a required role in the development of diaphragmatic oxidative stress and VIDD. Therefore, with the use of an animal model of MV, these experiments tested the hypothesis that increases in circulating ANG II are responsible for MV-induced increases in diaphragmatic oxidative stress and the subsequent development of VIDD. Cause and effect was determined using two independent pharmacological approaches with different actions on ANG II signaling. In one experiment, MV-induced increases in circulating ANG II were prevented by administration of an angiotensin-converting enzyme (ACE) inhibitor (enalapril). In the second experiment, ANG II signaling was blocked by treatment of animals with an AT1 receptor antagonist (losartan).
METHODS
Experimental Animals
These experiments used adult female (4-6 mo old) Sprague-Dawley rats as the experimental model. The rat was chosen as the experimental model due to the similarities between the rat and human diaphragm in both anatomical and physiological parameters. We arbitrarily elected to use female animals in these experiments because VIDD develops rapidly in both male and female rats (38). Animals were maintained on a 12:12-h light-dark cycle and provided food and water ad libitum throughout the experimental period. All animals were housed at the University of Florida Animal Care Services Center, and the Animal Care and Use Committee of the University of Florida approved these experiments.
Experimental Design
To test the hypothesis that prevention of MV-induced increases in circulating ANG II or blocking the AT1 receptor will diminish MV-induced increases in mitochondrial ROS emission in the diaphragm and prevent VIDD, rats were randomly assigned to one of six experimental groups (n = 10/group): 1) 12 h spontaneous breathing (SB), animals injected with saline; 2) 12 h SB, animals treated with ACE inhibitor (enalapril); 3) 12 h SB, animals treated with AT1 receptor antagonist (losartan); 4) 12 h of MV, animals injected with saline; 5) 12 h of MV, animals treated with ACE inhibitor (enalapril); and 6) 12 h of MV, animals treated with AT1 receptor antagonist (losartan).
Experimental Protocol: Mechanical Ventilation and SB
Animals in the SB and MV groups were acutely anesthetized with pentobarbital sodium (60 mg/kg ip). After a surgical plane of anesthesia was reached, the animals were tracheostomized using aseptic techniques. An arterial catheter was placed in the carotid artery for monitoring of blood pressure and withdrawal of blood samples for measurement of blood gases and pH. Furthermore, a venous catheter with a “y” connector was placed in the jugular vein for the both continuous infusion of anesthesia and saline. Note that to maintain relatively constant arterial blood pressure in animals anesthetized at a surgical plane of anesthesia requires infusion of small amounts of saline during the 12-h experimental period. Animals in the MV group and SB group (without drugs) received ∼1.2–1.6 ml/h saline, whereas the MV animals treated with losartan and enalapril required higher rates of saline infusion (i.e., 2.2–2.5 ml/h) to maintain blood pressure during the experimental period.
The SB animals were allowed to spontaneously breath for 12 h while the MV animals were mechanically ventilated using a full-support MV mode and a positive pressure-driven venLETTERS TO THE EDITORtilator (Siemens) for 12 h (see online supplement for details) (2, 53). The 12-h duration of MV was selected because this duration is associated with diaphragmatic contractile dysfunction, myofiber atrophy, increased rates of proteolysis, and oxidative stress (28).
Drug Administration
Animals treated with the AT1 receptor antagonist losartan received an intraperitoneal priming dose (30 mg/kg) followed by intravenous infusion (100 ug/kg/min, infusion rate 0.30 ml/h) during the 12-h experimental period in both the mechanically ventilated and the SB animals. Animals treated with the ACE inhibitor enalapril received an intraperitoneal priming injection (40 mg/kg) followed by an intravenous infusion (100 ug/kg/min, infusion rate 0.30 ml/h) during the 12-h experimental period.
Biochemical Measurements
Plasma levels of ANG II, IL-6, and corticosterone.
To measure the plasma levels of ANG II, interleukin-6 (IL-6), and corticosterone during the experiment, blood samples were collected at several time points: 1) at the start of SB/MV, 2) 6 h after initiating SB/MV, 3) 9 h after initiating SB/MV, and 4) 12 h after initiating SB/MV. At each blood draw, ∼600 microliters of blood were collected via a venous catheter, and blood samples were then centrifuged at 5,000 revolutions/min for 10 min at 4°C. The resulting plasma was stored at −80°C until analysis. The plasma levels of ANG II were determined via ELISA (ANG II; Phoenix Pharmaceuticals, Belmont, CA) using the manufacturer's instructions. Also, plasma levels of IL-6 were determined via ELISA (RAB0312; Sigma Aldrich, St. Louis, MO) using the manufacturer's instructions. Similarly, plasma levels of corticosterone were determined via ELISA (K014; Arbor Assays, Ann Arbor, MI) using the manufacturer's instructions.
Determination of relative abundance of diaphragmatic proteins.
The relative abundance of selected proteins was determined in diaphragm muscle samples via Western blot analysis (see online supplement for methodological details). The proteins of interest included biomarkers of proteolysis, antioxidant enzymes, and oxidative stress. The specific antibodies used to detect diaphragm proteins included: atrogin 1 (AP2041; ECM Bioscience, Versailles, KY); calpain 1 (2556; Cell Signaling, Danvers, MA); superoxide dismutase 1 (SOD1) (11407; Santa Cruz, Santa Cruz, CA); superoxide dismutase 2 (SOD2) (30080; Santa Cruz); glutathione peroxidase 1 (GPX1) (22604; Santa Cruz); and 4-hydroxynonenal (4-HNE) (ab46545; Abcam, Cambridge, MA). The protein abundance of each protein was normalized to α-tubulin (12G10; Developmental Studies Hybridoma Bank, Iowa City, IA), which served as a loading control (23).
Histological Analysis of Diaphragm Myofiber Cross-Sectional Area and Identification of AT1 Receptors in Diaphragm Muscle
Sections from frozen diaphragm muscle samples were transversely sectioned (10 μm thick) using a cryotome (Shandon, Pittsburgh, PA) and stained for dystropin, myosin heavy chain (MHC) I, and MHC type IIa proteins to determine diaphragm fiber cross-sectional area analysis (CSA) as descried previously. CSA was determined using computerized image analysis (Scion software NIH) (17).
Immunohistochemistry was used to visualize the presence of AT1 receptors in diaphragm muscle samples. Briefly, frozen diaphragm samples were cut into 5-μm-thick transverse sections and independently stained for AT1 receptors (Anti-AT1 ATTO 550; Alomone Labs, Jerusalem, Israel). Furthermore, frozen samples of the carotid artery were cut into 5-μm-thick sections and independently stained for AT1 receptors as a positive control for the presence of AT1 receptors. The presence of AT1 receptors in the diaphragm was then visualized using fluorescence microscopy.
Measurement of diaphragmatic contractile function.
To assess diaphragm contractile function, a costal diaphragm muscle strip (∼3 mm wide), including the tendinous attachments at the central tendon and rib cage, was suspended vertically between two lightweight Plexiglas clamps with one end connected to an isometric force transducer within a jacketed tissue bath containing 25°C Krebs-Hensleit solution equilibrated with 95% O2-5% CO2 gas. Muscle performance was measured as previously described (26) (see online supplement for details). To control for differences in muscle strip size, force production was normalized to physiological CSA, and specific force production was compared between the experimental groups.
Assessment of losartan as an antioxidant scavenger.
To determine if losartan or enalapril has the capacity to act directly as an antioxidant scavenger we used the Trolox equivalent antioxidant capacity assay to measure the antioxidant capacity of losartan and enalapril (in vitro) compared with the standard, Trolox (30) (see online supplement for details). Plasma concentrations of the drugs were estimated using pharmacological kinetic computations (41).
Mitochondrial respiration in permeabilized diaphragm fibers.
Mitochondrial respiration was measured polargraphically in a respiration chamber (Hansatech Instruments) maintained at 37°C. The respiratory control ratio (RCR) was calculated by dividing oxygen consumption during state 3 respiration by the oxygen consumption during state 4 respiration (see online supplement for details).
Statistical Analysis
Comparisons between groups were made by a one-way ANOVA, and when appropriate a Tukey Honest Significant Difference test was performed post hoc. Significance was established at P < 0.05. Values are expressed as means ± SE.
RESULTS
Treatment with Losartan or Enalapril Does Not Alter Diaphragm Structure or Function in SB Animals
To determine if losartan or enalapril influenced diaphragmatic physiology and biochemistry independent of prolonged MV, we measured diaphragm fiber CSA, contractile function, and mitochondrial respiration in the diaphragm of SB animals following 12 h of treatment with losartan or enalapril. Our results reveal that, compared with SB animals without drug treatment, independent treatment with either losartan or enalapril had limited influence on: 1) diaphragm fiber CSA; 2) diaphragm contractile properties; or 3) mitochondrial coupling (i.e., mitochondrial respiratory ratio) (Supplemental Figs. 1–3). Furthermore, treatment with these drugs did not alter plasma ANG II levels in SB animals (data not shown). Therefore, all dependent measures in this study were compared with SB animals without drug treatment only.
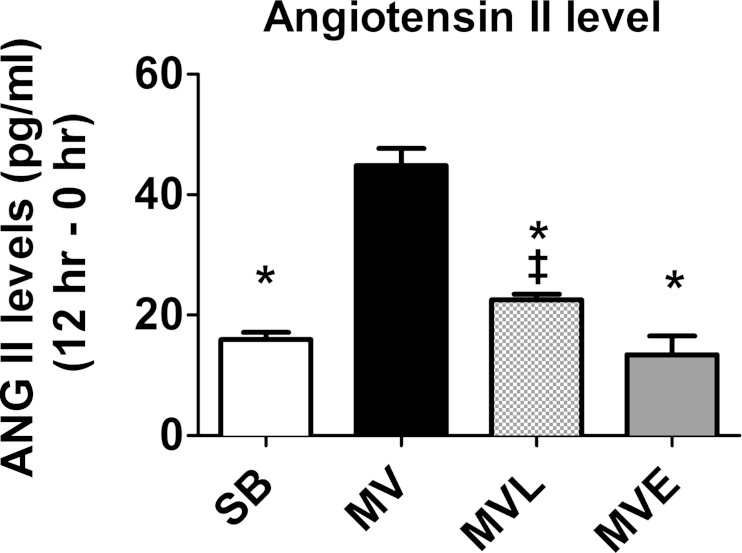
Fig. 1.
Plasma angiotensin II (ANG II) levels were measured before the beginning of mechanical ventilation (MV; 0 h) and following 12 h of MV. The reported plasma ANG II levels are the differences between these two time points. SB, spontaneous breathing; MVL, mechanical ventilation treated with losartan; MVE, mechanical ventilation treated with enalapril. Values are means ± SE. P < 0.05, significantly different vs. MV (*) and MVE (‡).
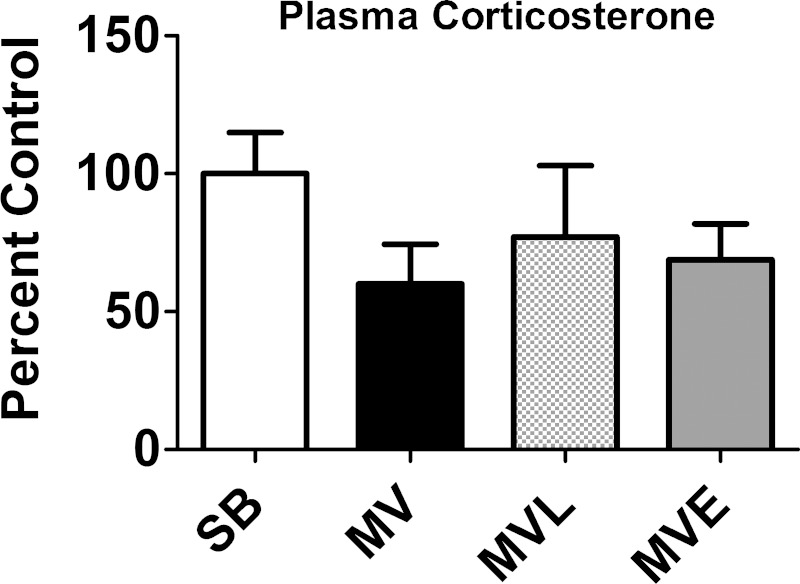
Fig. 3.
Plasma corticosterone levels were measured at the completion of 12 h of prolonged MV. Values are means ± SE. Note that no significant group differences existed in plasma corticosterone levels.
Systemic response to MV.
Because body weight is significantly correlated with diaphragm muscle fiber CSA, our experiments were designed to assure that no differences existed in animal body weights between the experimental groups (Table 1). Furthermore, because the maintenance of blood gas homeostasis is important during experiments involving prolonged MV, animals in the three MV groups were carefully monitored during these experiments to prevent disparities in heart rates, PaO2, PaCO2, and arterial pH (Table 1). Not surprisingly, systolic blood pressure during MV was significantly lower in both the losartan- and enalapril-treated animals compared with animals without drug treatment (Table 1). Importantly, at the completion of 12 h of MV, no visual abnormalities of the lungs or peritoneal cavity were noted, and no evidence of infection existed. These observations indicate that our aseptic surgical technique was successful and that prolonged MV did not result in major lung injury.
Table 1.
Body weight, HR, PaO2, PaCO2, arterial pH, and SBP measurements
| SB | SBL | SBE | MV | MVL | MVE | |
|---|---|---|---|---|---|---|
| Weight, g | 296 ± 4 | 299 ± 7 | 301 ± 6 | 302 ± 6 | 300 ± 5 | 298 ± 7 |
| HR, beats/min | 333 ± 6 | 345 ± 5 | 346 ± 7 | |||
| PaO2, mmHg | 50 ± 4 | 61.5 ± 7 | 68 ± 6 | 70 ± 6 | 65 ± 5 | 65 ± 7 |
| PaCO2, mmHg | 42 ± 4 | 39 ± 7 | 41 ± 6 | 34 ± 6 | 38 ± 5 | 38 ± 7 |
| Arterial pH | 7.4 ± 4 | 7.39 ± 7 | 7.42 ± 6 | 7.47 ± 6 | 7.44 ± 5 | 7.44 ± 7 |
| SBP, mmHg | 104 ± 4 | 84 ± 7 | 87 ± 6 | 106 ± 6 | 77 ± 5* | 86 ± 7* |
Values are means ± SE at the completion of the experiment.
Body weight, heart rate (HR), arterial partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2), arterial pH, and systolic blood pressure (SBP) were measured at 3-h time intervals during both spontaneous breathing (SB) and mechanical ventilation (MV). SBL, spontaneous breathing treated with losartan; SBE, spontaneous breathing treated with enalapril; MVL, mechanical ventilation treated with losartan; MVE, mechanical ventilation treated with enalapril.
Significantly different from SB and MV (P < 0.05).
Plasma ANG II Levels Increase during Prolonged MV
Plasma levels of ANG II significantly increased within the first 3 h of MV and continued to rise during the 12 h of MV (data not shown). Indeed, compared with SB controls, prolonged MV significantly increased plasma ANG II levels, and administration of enalapril prevented this rise (Fig. 1). Although the MV-induced rise in circulating ANG II levels was lower in the losartan-treated animals, plasma ANG II levels remained significantly increased during 12 h of MV in animals treated with losartan compared with SB controls (Fig. 1 and Table 2). The explanation for the lower levels of circulating ANG II in the losartan group compared with MV animals (without drug treatment) is unclear but could be due to the higher levels of saline infusion that were required to maintain arterial blood in the losartan-treated animals.
Table 2.
Plasma angiotensin II levels before starting MV and after 12 h MV
| SB | MV | MVL | MVE | |
|---|---|---|---|---|
| MV (0 h) | 86.3 ± 5* | 105.8 ± 1 | 96.1 ± 5 | 91.2 ± 4* |
| MV (12 h) | 101.2 ± 4* | 153.6 ± 1 | 120.6 ± 3*‡ | 104.6 ± 4* |
Values are means ± SE. Units are pg/ml. P < 0.05, significantly different from 12 h of MV (*) and different vs. MVE (12 h) (‡).
Also, note that small increases in plasma levels of ANG II occurred in the SB animals over the 12-h experimental period. This limited increase in plasma ANG II was likely due to the decreased arterial blood pressure that occurs in animals anesthetized to a surgical plane of anesthesia with pentobarbital sodium.
Plasma Levels of Glucocorticoids and IL-6 Are Not Increased during Prolonged MV
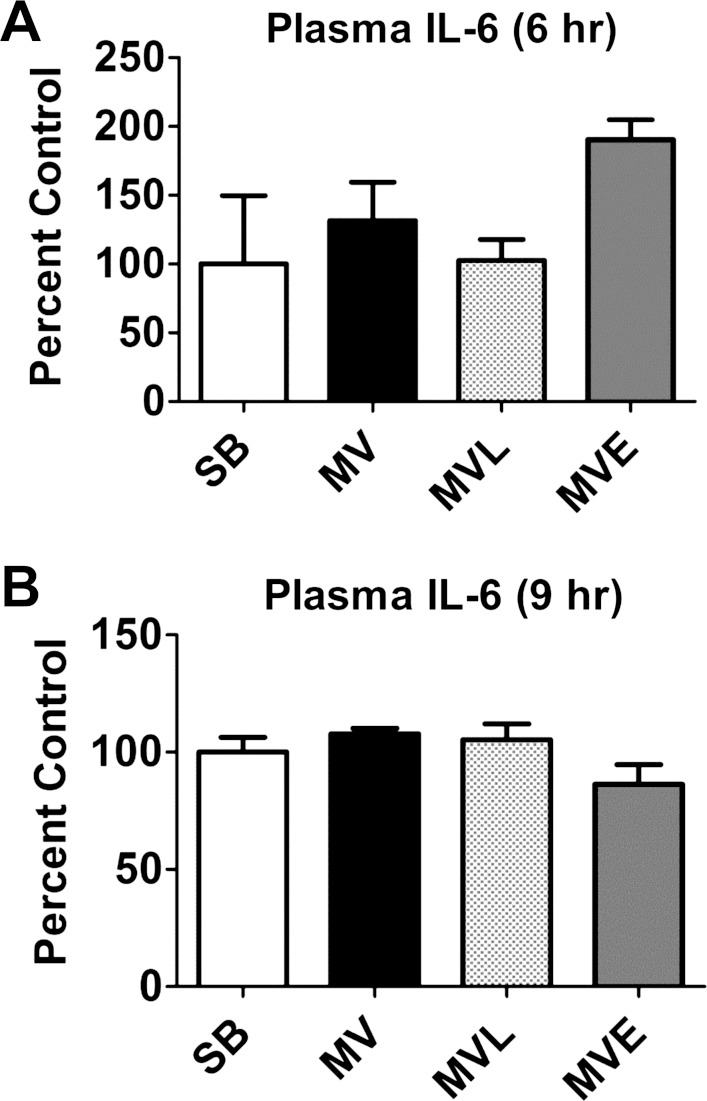
ANG II receptors (AT1 and AT2 class receptors) mediate the majority of ANG II effects in vitro and in vivo (40). However, debate exists as to whether the ANG II-induced atrophy in skeletal muscle is due to the direct effects of ANG II on the muscle fiber or due to a systemic effect of ANG II that results in muscle atrophy (42). Indeed, infusion of ANG II results in increased plasma levels of both IL-6 and glucocorticoids, and high circulating levels of both IL-6 and glucocorticoids can promote muscle atrophy (10, 13, 42, 43). Therefore, we measured the plasma levels of both IL-6 and glucocorticoids in animals from all experimental groups. Our results revealed that, compared with SB animals, plasma levels of IL-6 were not elevated in any of the MV groups following 6 or 9 h of prolonged MV (Fig. 2). Similarly, plasma levels of corticosterone did not differ between SB animals and the three MV experimental groups following 12 h of MV (Fig. 3). Together, these results demonstrate that prolonged MV does not increase plasma levels of IL-6 or corticosterone in the rat.
Fig. 2.
Plasma interleukin-6 (IL-6) levels were measured at the completion of both 6 (A) and 9 (B) h of prolonged MV. Note that plasma IL-6 levels were not measured following 12 h of MV because of inadequate sample availability. Values are means ± SE. Note that no significant group differences existed in plasma IL-6 levels at either time point.
Immunohistochemistry Reveals Presence of AT1 Receptors in Diaphragm Muscle
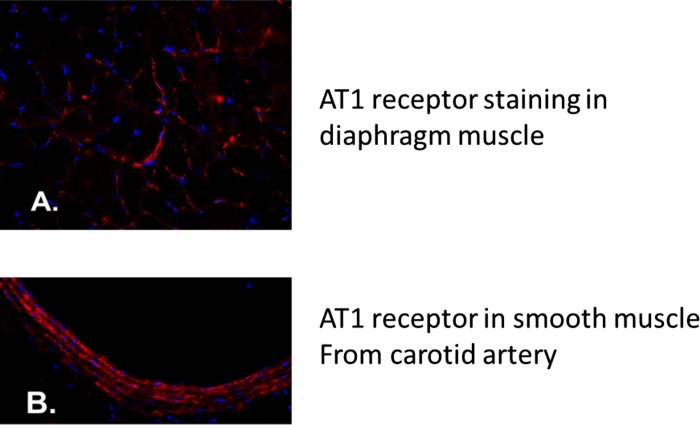
Because increased circulating levels of ANG II were not associated with increased plasma levels of IL-6 or corticosterone, we then determined whether AT1 receptors are present in diaphragm muscle fibers. This is important because whether or not AT1 receptors exist in limb skeletal muscles remains a topic of debate (42), and, to our knowledge, the current experiments are the first to investigate the presence of AT1 receptors in diaphragm skeletal muscle. Our histochemical results reveal the presence of AT1 receptors in diaphragm muscle (Fig. 4A). Note that the carotid artery was used as positive control for AT1 receptors (Fig. 4B).
Fig. 4.
Immunohistochemistry was used to identify the presence of ANG II type 1 (AT1) receptors in rat diaphragm muscle. Blue represents DAPI staining of myonuclei. A: photograph of the presence of AT1 receptors within diaphragm muscle fibers in rat. AT1 receptors are stained in red. B: photograph illustrating the presence of AT1 receptors in smooth muscle of the carotid artery in rat. AT1 receptors are stained in red.
Impact of Losartan and Enalapril on MV-Induced Diaphragmatic Oxidative Stress and Mitochondrial Function
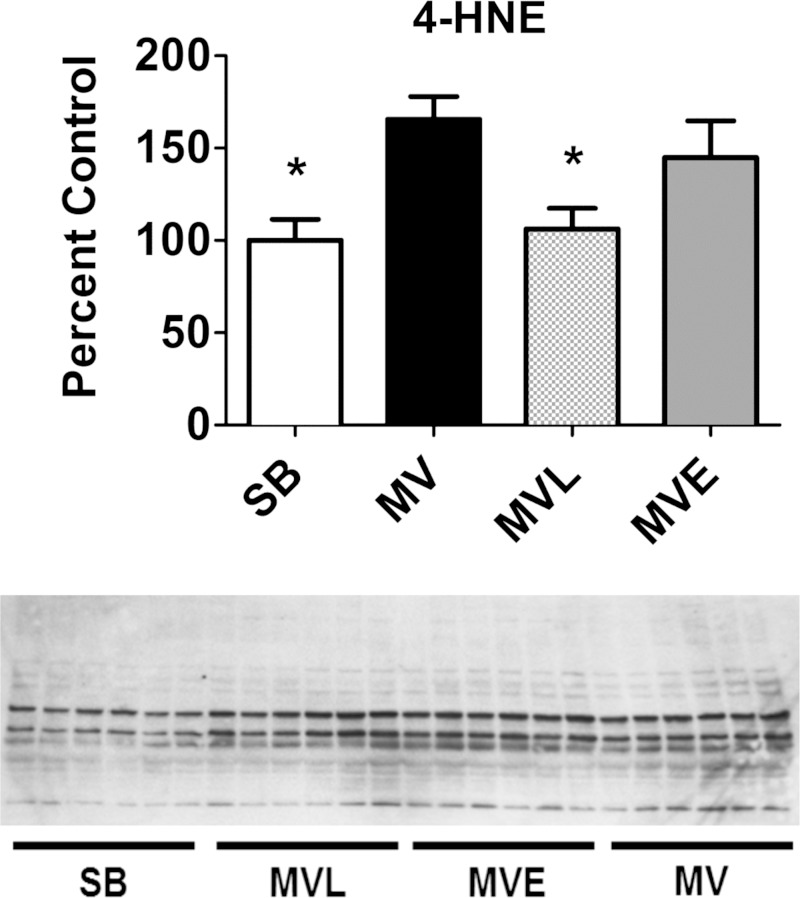
We determined if increased plasma levels of ANG II are required for MV-induced increases in diaphragmatic oxidative stress. Cause and effect was determined by administering the ACE inhibitor enalapril to prevent the MV-induced increase in plasma ANG II levels, and, in another experiment, losartan was administered to block ANG II receptor activation. To establish if MV-induced increases in plasma ANG II levels are required for MV-induced oxidative damage, we measured 4-HNE-conjugated proteins in the diaphragm as a biomarker of lipid peroxidation and oxidative stress. As expected, prolonged MV resulted in a significant increase in the levels of 4-HNE-conjugated proteins in the diaphragm of animals without drug treatment (Fig. 5). However, treatment with the AT1 receptor blocker losartan attenuated the MV-induced accumulation of 4-HNE-modified proteins in the diaphragm (Fig. 5). Compared with SB animals, the mean diaphragmatic 4-HNE levels tended to increase in the MV animals treated with enalapril but did not reach significance.
Fig. 5.
The relative abundance of 4-hydroxynonenal (4-HNE)-modified proteins (index of lipid peroxidation) in the diaphragm were determined via Western blot. Values are means ± SE and normalized to α-tubulin. *Significantly different from MV (P < 0.05).
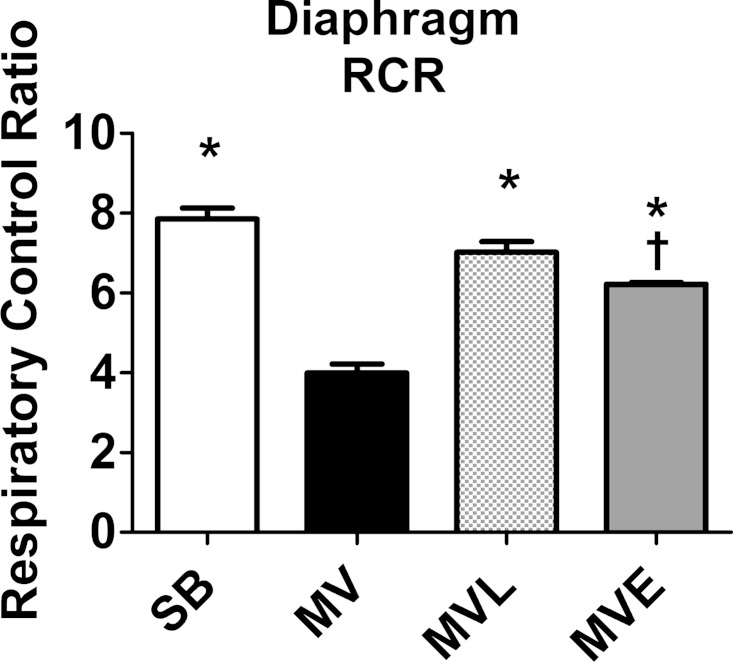
It is established that prolonged MV results in increased mitochondrial ROS production in the diaphragm and mitochondrial dysfunction (15, 23). The mitochondrial RCR is a biomarker of mitochondrial coupling, and decreases in RCR indicate impaired mitochondrial coupling. Our results reveal that 12 h of MV resulted in a significant reduction of the RCR in diaphragm mitochondria. Compared with animals exposed to MV without drug intervention, treatment of animals with losartan attenuated the MV-induced decrease in mitochondrial RCR (Fig. 6). Treatment with enalapril provided partial protection against MV-induced decreases in mitochondrial coupling, since the RCR in diaphragmatic mitochondria from enalapril MV animals was significantly higher than the RCR in MV animals without drug treatment. Nonetheless, the RCR in mitochondria from enalapril-treated MV animals was significantly lower than SB controls.
Fig. 6.
Mitochondrial respiratory control ratio (RCR) measured in mitochondria within permeabilized diaphragm muscle fibers. Values are expressed as means ± SE. P < 0.05, significantly different vs. MV (*) and SB (†).
Treatment with Enalapril or Losartan Does Not Influence Diaphragmatic Antioxidant Capacity
It is feasible that losartan protected against MV-induced oxidative stress in the diaphragm due to blockade of AT1 receptor activation. Indeed, the prevention of MV-induced AT1 receptor activation in the diaphragm could avert the increase in both NADPH oxidase activity and mitochondrial ROS production. Nonetheless, the losartan-induced protection against MV-induced oxidative stress could be due to off-target effects such as losartan functioning as an antioxidant scavenger or losartan increasing the abundance of endogenous antioxidant enzymes in the diaphragm. Therefore, we determined whether losartan acts directly as a radical scavenger, and we also measured the protein abundance of key antioxidant enzymes in the diaphragm. A series of in vitro experiments confirmed that losartan does not directly act as a radical scavenger (Supplemental Fig. 4). Our results also show that treatment with losartan does not significantly increase the relative protein abundance of SOD1, SOD2, or GPX1 in diaphragm muscle fibers (Supplemental Fig. 5). Hence, losartan's protection against MV-induced oxidative stress in the diaphragm is not likely due to an off-target effect of losartan producing increased antioxidant protection in the muscle.
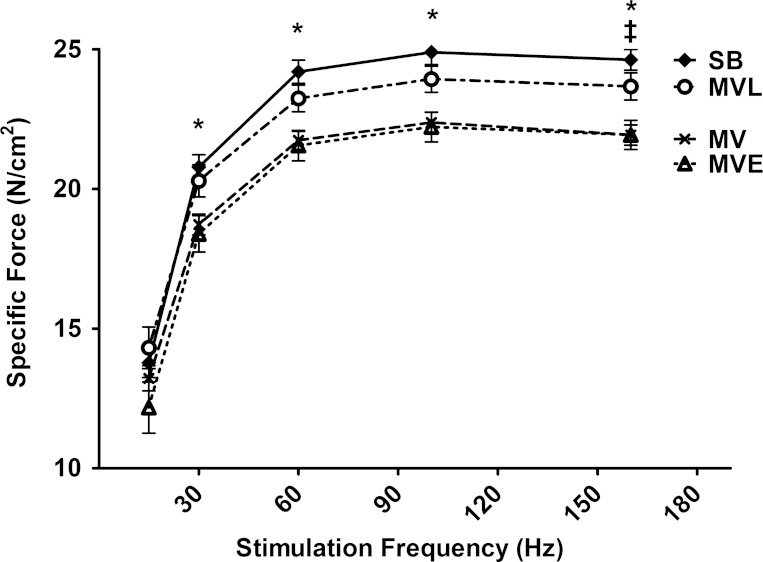
Losartan Protects against MV-Induced Diaphragmatic Contractile Dysfunction
We measured diaphragm contractile properties to determine the role that circulating ANG II and AT1 receptor activation plays in MV-induced diaphragmatic contractile dysfunction. Treatment of MV animals with the ACE inhibitor enalapril did not protect against MV-induced depression of diaphragm-specific force production at any stimulation frequency (Fig. 7). In contrast, treatment with the AT1 receptor antagonist losartan significantly attenuated the MV-induced reduction in diaphragm force production at all stimulation frequencies (Fig. 7).
Fig. 7.
Diaphragm-specific force production as a function of the stimulation frequency (i.e., force-frequency curve) measured in vitro in costal diaphragm muscle strips following 12 h of SB or MV. Values are means ± SE. Note that no significant force differences existed between SB animals and MVL animals at any stimulation frequency. P < 0.05, SB significantly different vs. MV and MVE (*) and MVL significantly different vs. MVE (‡).
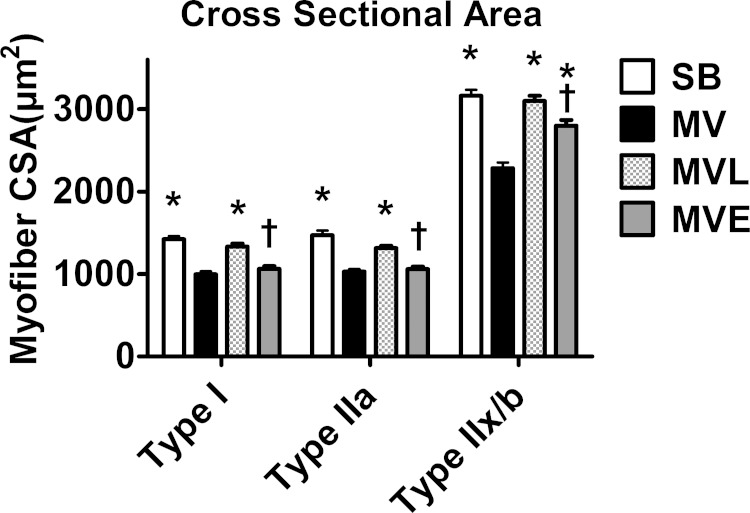
Losartan Protects against MV-Induced Diaphragmatic Atrophy
Another objective of these experiments was to determine if increased circulating levels of ANG II and/or AT1 receptor signaling contribute to MV-induced diaphragm muscle atrophy. We first established that treatment of spontaneously breathing animals with losartan or enalapril does not impact diaphragm fiber CSA. We then determined whether treatment of animals with losartan or enalapril can protect the diaphragm against MV-induced contractile dysfunction. As expected, prolonged MV resulted in significant atrophy of type I, type IIa, and type IIx/b diaphragm myofibers. Importantly, compared with the MV group, treatment of animals with losartan (MVL group) significantly attenuated the MV-induced atrophy in diaphragm type I, type IIa, and type IIx/b fibers (Fig. 8). In contrast, treatment of animals with enalapril did not protect against MV-induced diaphragmatic atrophy (Fig. 8).
Fig. 8.
Diaphragm muscle fiber cross-sectional area. Values are means ± SE. P < 0.05, significantly different vs. MV (*) and significantly different from SB (†).
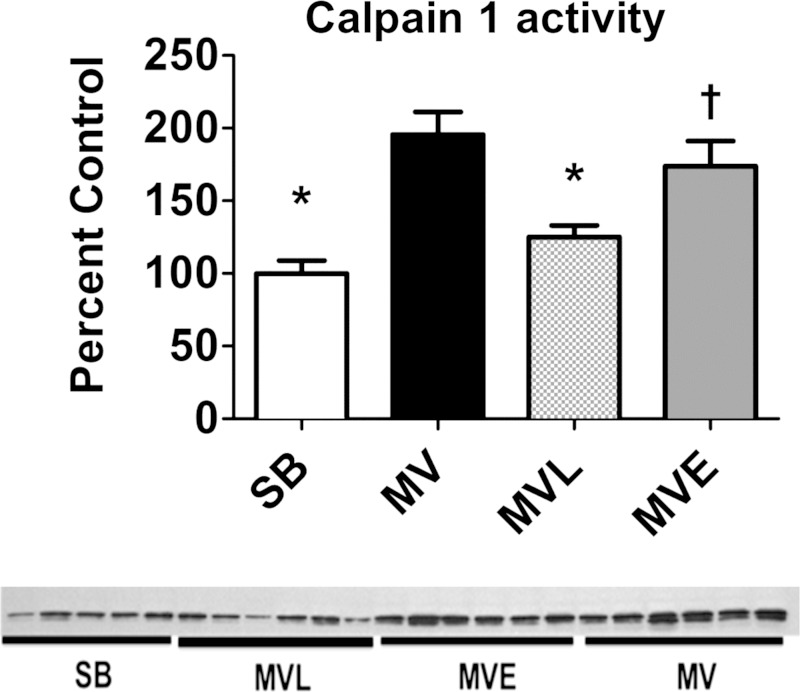
Losartan Protects Against MV-Induced Calpain Activation in the Diaphragm
Activation of the calcium-dependent protease calpain is required for the development of VIDD (16, 22). Importantly, treatment of MV animals with losartan attenuated the MV-induced calpain activation in the diaphragm, whereas enalapril treatment did not prevent MV-induced calpain activation in the diaphragm (Fig. 9).
Fig. 9.
A: calpain 1 activity in diaphragm was determined via Western blotting. B: values are means ± SE. P < 0.05, significantly different vs. MV (*) and significantly different from SB (†).
Losartan Prevents MV-Induced Increases in Muscle-Specific E3 Ligases in the Diaphragm
Although the role that the ubiquitin-proteasome system plays in VIDD remains in question (39), this proteolytic system clearly contributes to myofibrillar protein breakdown during disuse-induced limb skeletal muscle atrophy (24). Specifically, increased expression of the muscle-specific E3 ligases atrogin-1 and MuRF-1 contributes to the ubiquitination of muscle proteins for subsequent breakdown by the 26S-proteosome (24). Our results confirm that MV results in an increased protein expression of both atrogin-1 and MuRF1 in the diaphragm (Supplemental Figs. 6 and 7). Importantly, treatment of MV animals with losartan attenuated MV-induced increases in both atrogin-1 and MuRF1 expression in the diaphragm, whereas treatment of MV animals with enalapril attenuated MV-induced increases in MuRF1 expression only.
DISCUSSION
Overview of Principal Findings
These experiments provide new and important information regarding the role that circulating ANG II and AT1 receptors play in the development of VIDD. Specifically, we tested the hypothesis that increases in plasma ANG II levels are required for MV-induced diaphragmatic oxidative stress and the development of VIDD. Our results do not support this prediction. Indeed, our findings reveal that treatment of animals with the ACE inhibitor enalapril successfully prevented the MV-induced increase in plasma ANG II levels but did not avert MV-induced oxidative stress or protect against VIDD. In contrast, treatment of animals with the AT1 receptor antagonist losartan attenuated both MV-induced diaphragmatic oxidative stress and VIDD. This finding suggests that AT1 receptor activation occurs in the diaphragm in the absence of increased plasma levels of ANG II and that AT1 receptor activation is required for the development of VIDD. A discussion of these key findings follows.
ANG II and Skeletal Muscle Atrophy
Our results show that prolonged MV results in a 50% increase in plasma ANG II levels following 12 h ventilator support (Fig. 1). This is significant because elevated ANG II is sufficient to induce oxidative stress and promote protease activation, resulting in fiber atrophy in both limb and diaphragm skeletal muscles (2, 3, 8, 31–33, 36). However, the specific signaling mechanism(s) that connect ANG II to muscle atrophy remain controversial. Nonetheless, at least three mechanisms could link ANG II with muscle atrophy: 1) IL-6; 2) glucocorticoids; and 3) AT1 receptor signaling. A discussion of each of the mechanisms follows.
IL-6.
Elevated plasma levels of ANG II in animals result in increased hepatic production of IL-6 and serum amyloid A (50). This is important because elevated levels of both IL-6 and serum amyloid A can disrupt insulin/IGF-I signaling in muscle to depress protein synthesis and accelerate proteolysis (50). It is currently unknown if prolonged MV results in increased circulating levels of serum amyloid A. Furthermore, studies investigating the impact of prolonged MV on plasma IL-6 levels are inconsistent, with one report indicating that plasma IL-6 levels are increased during MV (38) and another study reporting that prolonged MV does not increase plasma IL-6 levels (12). In the current study, plasma IL-6 levels were not elevated following 12 h of MV, and, therefore, elevated plasma IL-6 levels do not appear to contribute to VIDD in the current experiments.
Glucocorticoids.
A second potential mechanism that can link ANG II to skeletal muscle atrophy is that infusion of ANG II increases circulating glucocorticoid levels in rodents, and elevated glucocorticoids promote skeletal muscle atrophy by increasing both protease activation and the expression of myostatin (2, 35). Nonetheless, plasma corticosterone levels were not elevated during prolonged MV, and, consequently, high levels of glucocorticoids do not appear to contribute to VIDD in our experiments.
AT1 receptor signaling.
The third and final mechanism that can connect ANG II to muscle atrophy relates to the direct impact of AT1 receptor signaling on the production of ROS in muscle fibers. Indeed, infusion of ANG II results in increased ROS production in rodent skeletal muscles (36). This ANG II-mediated increase in muscle ROS production occurs, at least in part, due to AT1 receptor-mediated increases in both NADPH oxidase activity and elevated mitochondrial ROS production leading to speculation that NADPH oxidase/mitochondrial cross talk exists in skeletal muscle exposed to high levels of ANG II (36, 45, 49). Regardless of the cellular site(s) of ANG II-induced ROS production in skeletal muscles, it is established that oxidative stress contributes to inactivity-induced muscle atrophy by increasing proteolysis, increasing myonuclear apoptosis, and depressing protein synthesis (19, 27, 36). Although administering the ACE inhibitor enalapril to animals successfully prevented the MV-induced increase in ANG II, this treatment did not protect against MV-induced oxidative stress in the diaphragm and VIDD. In contrast, treatment of animals with the AT1 receptor antagonist losartan protected against MV-induced diaphragmatic oxidative stress and VIDD. A discussion of the potential mechanism(s) responsible for losartan's protection against VIDD follows.
Losartan Prevents MV-Induced Oxidative Stress and Protects against VIDD
ACE inhibitors limit the amount of ANG II available for binding to the AT1 receptor, whereas AT1 receptor antagonists prevent the binding of ANG II to the AT1 receptor. Therefore, in theory, both the AT1 receptor antagonist losartan and the ACE inhibitor enalapril should have the same overall effect of diminished AT1 receptor stimulation by its ligand ANG II (11).
Although evidence exists that using AT1 receptor antagonists or ACE inhibitors can attenuate muscle atrophy in some conditions (3, 6, 21), the current study reveals that an ACE inhibitor prevents MV-induced increases in circulating ANG II but does not attenuate VIDD. In contrast, protection against VIDD was achieved by treatment with the AT1 receptor blocker losartan. At least two explanations exist for this finding. First, it is possible that losartan protects against VIDD via an off-target effect that is unrelated to AT1 receptor signaling. Second, losartan could protect against VIDD by acting as an AT1 receptor antagonist and prevent downstream AT1 receptor signaling. A brief discussion of each of these possibilities follows.
Potential off-target effects of losartan.
Given that oxidative stress is a required upstream signal to promote VIDD, a potential off-target effect of losartan is that this molecule acts as an antioxidant to prevent MV-induced oxidative stress in the diaphragm. The structure of losartan has a hydroxyl functional group positioned on a phenolic ring, and, therefore, it is feasible that losartan can act as a radical scavenger to protect against MV-induced oxidative stress in the diaphragm. Nonetheless, in vitro experiments indicate that losartan does not act as an oxidant scavenger at or above the concentrations used in the current experiments (Supplemental Fig. 4). It is also possible that losartan could act as an antioxidant by promoting the expression of endogenous antioxidant enzymes in the diaphragm. However, the protein abundance of key antioxidant enzymes (e.g., SOD1, SOD2, and GPX1) in the diaphragm was not elevated in the diaphragms of animals treated with losartan (Supplemental Fig. 5). Collectively, these results suggest that the losartan-mediated protection against VIDD was not due to losartan acting as an antioxidant. Nonetheless, we cannot completely rule out an unknown “off-target” effect of losartan.
Blockade of AT1 receptor signaling.
Almost every organ system in the body contains AT1 receptors, and, although one report failed to detect AT1 receptors in limb skeletal muscles (50), there is evidence that limb skeletal muscle fibers express AT1 receptors (14, 20, 29). Importantly, with the use of immunohistochemistry, the current experiments confirm the presence of AT1 receptors in diaphragm muscle (Fig. 4). If losartan protects against VIDD by prevention of AT1 receptor activation in the diaphragm, the results from the enalapril treatment experiments indicate that increased plasma ANG II levels are not required for AT1 receptor activation. Indeed, it is established that AT1 receptors can be stimulated via stretch-induced activation, which is independent of ANG II binding to the AT1 receptor (47, 51). Given that diaphragm muscle fibers undergo passive and repetitive length changes during controlled MV, it is feasible that mechanical activation of AT1 receptors occurs in the diaphragm during prolonged MV. In regard to mechanical stretch-induced AT1 receptor activation, it has been reported that an unknown molecule was bound to AT1 receptor during mechanical activation of the receptor (51). This raises the possibility that stretch-induced activation of the AT1 receptor occurs via an unknown ligand that is capable of binding to the receptor and promoting AT1 receptor signaling. Furthermore, this mechanical stretch-induced activation of the AT1 receptor has been shown to activate the Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) signaling pathway (51), and activation of JAK signaling is linked to muscle atrophy. Indeed, new evidence reveals that inhibition of JAK1/JAK3 signaling can protect the diaphragm against MV-induced oxidative stress and VIDD (38). It follows that AT1 receptor activation in the diaphragm could be an upstream mechanism that is required for MV-induced activation of the JAK/STAT3 signaling pathway leading to diaphragmatic oxidative stress and activation of proteolytic systems.
Summary and Conclusions
These experiments do not support the hypothesis that prevention of MV-induced increases in plasma ANG II levels can prevent MV-induced diaphragmatic oxidative stress and protect against VIDD. Indeed, although treatment of animals with the ACE inhibitor enalapril successfully prevented the MV-induced increases in plasma ANG II levels, averting the rise in circulating ANG II levels did not forestall MV-induced oxidative stress in the diaphragm or protect against VIDD. By contrast, treatment with the AT1 receptor antagonist losartan attenuated both MV-induced diaphragmatic oxidative stress and VIDD. Together, these results suggest that, during prolonged MV, diaphragmatic AT1 receptor activation occurs via a mechanism independent of increased plasma levels of ANG II and that AT1 receptor activation plays a required role in MV-induced diaphragmatic oxidative stress and VIDD. Importantly, our findings indicate that the Food and Drug Administration (FDA)-approved drug losartan could be beneficial in protecting humans against VIDD in situations where hemodynamics can be maintained. This is a significant finding because, at present, there is no standard treatment to prevent VIDD, and the pharmacological interventions that have previously been reported to protect against VIDD are not FDA approved.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01-AR-064189.
DISCLOSURES
None of the authors have financial or professional conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: O.S.K., A.S., M.P.W., S.E.H., K.S., A.M., E.T., H.Z.T., N.T., and S.K.P. conception and design of research; O.S.K., A.S., M.P.W., S.E.H., K.S., A.M., E.T., H.Z.T., N.T., and S.K.P. performed experiments; O.S.K., A.S., M.P.W., S.E.H., K.S., A.M., E.T., H.Z.T., N.T., and S.K.P. analyzed data; O.S.K., A.S., M.P.W., S.E.H., K.S., A.M., E.T., H.Z.T., N.T., and S.K.P. interpreted results of experiments; O.S.K., A.S., M.P.W., S.E.H., K.S., A.M., E.T., H.Z.T., N.T., and S.K.P. prepared figures; O.S.K., A.S., M.P.W., S.E.H., K.S., A.M., E.T., H.Z.T., N.T., and S.K.P. drafted manuscript; O.S.K., A.S., M.P.W., S.E.H., K.S., A.M., E.T., H.Z.T., N.T., and S.K.P. edited and revised manuscript; O.S.K., A.S., M.P.W., S.E.H., K.S., A.M., E.T., H.Z.T., N.T., and S.K.P. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Betters JL, Criswell DS, Shanely RA, Van Gammeren D, Falk D, Deruisseau KC, Deering M, Yimlamai T, Powers SK. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med 170: 1179–1184, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Brink M, Price SR, Chrast J, Bailey JL, Anwar A, Mitch WE, Delafontaine P. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology 142: 1489–1496, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Burks TN, Andres-Mateos E, Marx R, Mejias R, Van Erp C, Simmers JL, Walston JD, Ward CW, Cohn RD. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med 3: 82–87, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabello-Verrugio C, Cordova G, Salas JD. Angiotensin II: role in skeletal muscle atrophy. Curr Protein Pept Sci 13: 560–569, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Cabello-Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F. Renin-Angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev 35: 437–463, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Carter CS, Giovannini S, Seo DO, DuPree J, Morgan D, Chung HY, Lees H, Daniels M, Hubbard GB, Lee S, Ikeno Y, Foster TC, Buford TW, Marzetti E. Differential effects of enalapril and losartan on body composition and indices of muscle quality in aged male Fischer 344 × Brown Norway rats. Age (Dordr) 33: 167–183, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cisternas F, Morales MG, Meneses C, Simon F, Brandan E, Abrigo J, Vazquez Y, Cabello-Verrugio C. Angiotensin-(1–7) decreases skeletal muscle atrophy induced by angiotensin II through a Mas receptor-dependent mechanism. Clin Sci (Lond) 128: 307–319, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, Fernandez R, de la Cal MA, Benito S, Tomas R. A comparison of four methods of weaning patients from mechanical ventilation Spanish Lung Failure Collaborative Group. N Engl J Med 332: 345–350, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Hilgers KF, Mann JF. ACE inhibitors versus AT(1) receptor antagonists in patients with chronic renal disease. J Am Soc Nephrol 13: 1100–1108, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Hudson MB, Smuder AJ, Nelson WB, Bruells CS, Levine S, Powers SK. Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med 40: 1254–1260, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287: C834–C843, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Johnston AP, Baker J, De Lisio M, Parise G. Skeletal muscle myoblasts possess a stretch-responsive local angiotensin signalling system. J Renin Angiotensin Aldosterone Syst 12: 75–84, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med 46: 842–850, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maes K, Testelmans D, Powers S, Decramer M, Gayan-Ramirez G. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am J Respir Crit Care Med 175: 1134–1138, 2007. [DOI] [PubMed] [Google Scholar]
- 17.McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol 585: 203–215, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB, Gayan-Ramirez G, Decramer M, DeRuisseau KC, Powers SK. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med 37: 1373–1379, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meneses C, Morales MG, Abrigo J, Simon F, Brandan E, Cabello-Verrugio C. The angiotensin-(1–7)/Mas axis reduces myonuclear apoptosis during recovery from angiotensin II-induced skeletal muscle atrophy in mice. Pflugers Arch 467: 1975–1984, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Morales MG, Vazquez Y, Acuna MJ, Rivera JC, Simon F, Salas JD, Alvarez Ruf J, Brandan E, Cabello-Verrugio C. Angiotensin II-induced pro-fibrotic effects require p38MAPK activity and transforming growth factor beta 1 expression in skeletal muscle cells. Int J Biochem Cell Biol 44: 1993–2002, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Inhibition of the renin-angiotensin system improves physiological outcomes in mice with mild or severe cancer cachexia. Int J Cancer 133: 1234–1246, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Nelson WB, Smuder AJ, Hudson MB, Talbert EE, Powers SK. Cross-talk between the calpain and caspase-3 proteolytic systems in the diaphragm during prolonged mechanical ventilation. Crit Care Med 40: 1857–1863, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 39: 1749–1759, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R337–R344, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Powers SK, Kavazis AN, Levine S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med 37: S347–S353, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D, Cicale M, Dodd SL. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol 92: 1851–1858, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Powers SK, Smuder AJ, Criswell DS. Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxid Redox Signal 15: 2519–2528, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powers SK, Wiggs MP, Sollanek KJ, Smuder AJ. Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol 305: R464–R477, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Qi JS, Minor LK, Smith C, Hu B, Yang J, Andrade-Gordon P, Damiano B. Characterization of functional urotensin II receptors in human skeletal muscle myoblasts: comparison with angiotensin II receptors. Peptides 26: 683–690, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26: 1231–1237, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Rezk BM, Yoshida T, Semprun-Prieto L, Higashi Y, Sukhanov S, Delafontaine P. Angiotensin II infusion induces marked diaphragmatic skeletal muscle atrophy. PLoS One 7: e30276, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell ST, Eley H, Tisdale MJ. Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell Signal 19: 1797–1806, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Sanders PM, Russell ST, Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer 93: 425–434, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sassoon CS, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol 92: 2585–2595, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Schakman O, Kalista S, Barbe C, Loumaye A, Thissen JP. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 45: 2163–2172, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Semprun-Prieto LC, Sukhanov S, Yoshida T, Rezk BM, Gonzalez-Villalobos RA, Vaughn C, Michael Tabony A, Delafontaine P. Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem Biophys Res Commun 409: 217–221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med 166: 1369–1374, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Smith IJ, Godinez GL, Singh BK, McCaughey KM, Alcantara RR, Gururaja T, Ho MS, Nguyen HN, Friera AM, White KA, McLaughlin JR, Hansen D, Romero JM, Baltgalvis KA, Claypool MD, Li W, Lang W, Yam GC, Gelman MS, Ding R, Yung SL, Creger DP, Chen Y, Singh R, Smuder AJ, Wiggs MP, Kwon OS, Sollanek KJ, Powers SK, Masuda ES, Taylor VC, Payan DG, Kinoshita T, Kinsella TM. Inhibition of Janus kinase signaling during controlled mechanical ventilation prevents ventilation-induced diaphragm dysfunction. FASEB J 28: 2790–2803, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smuder AJ, Nelson WB, Hudson MB, Kavazis AN, Powers SK. Inhibition of the ubiquitin-proteasome pathway does not protect against ventilator-induced accelerated proteolysis or atrophy in the diaphragm. Anesthesiology 121: 115–126, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stegbauer J, Coffman TM. New insights into angiotensin receptor actions: from blood pressure to aging. Curr Opin Nephrol Hypertens 20: 84–88, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strauss SDB. Mathematical Modeling of Pharmacokinetic Data. Boca Raton, FL: CRC, 1995. [Google Scholar]
- 42.Sukhanov S, Semprun-Prieto L, Yoshida T, Michael Tabony A, Higashi Y, Galvez S, Delafontaine P. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci 342: 143–147, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tisdale MJ. Loss of skeletal muscle in cancer: biochemical mechanisms. Front Biosci 6: D164–D174, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med 169: 336–341, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 281: 35137–35146, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol 108: 1376–1382, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yasuda N, Miura S, Akazawa H, Tanaka T, Qin Y, Kiya Y, Imaizumi S, Fujino M, Ito K, Zou Y, Fukuhara S, Kunimoto S, Fukuzaki K, Sato T, Ge J, Mochizuki N, Nakaya H, Saku K, Komuro I. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep 9: 179–186, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zergeroglu MA, McKenzie MJ, Shanely RA, Van Gammeren D, DeRuisseau KC, Powers SK. Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol 95: 1116–1124, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Zhang GX, Lu XM, Kimura S, Nishiyama A. Role of mitochondria in angiotensin II-induced reactive oxygen species and mitogen-activated protein kinase activation. Cardiovasc Res 76: 204–212, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, Mitch WE. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol 20: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 6: 499–506, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.