Abstract
Obstructive sleep apnea (OSA) is one of the most common causes of hypertension in western societies. OSA causes chronic intermittent hypoxia (CIH) in specialized O2-sensing glomus cells of the carotid body. CIH generates increased reactive oxygen species (ROS) that trigger a feedforward mechanism in which increased intracellular calcium levels ([Ca2+]i) trigger increased HIF-1α synthesis and increased HIF-2α degradation. As a result, the normal homeostatic balance between HIF-1α-dependent prooxidant and HIF-2α-dependent antioxidant enzymes is disrupted, leading to further increases in ROS. Carotid body sensory nerves project to the nucleus tractus solitarii, from which the information is relayed via interneurons to the rostral ventrolateral medulla in the brain stem, which sends sympathetic neurons to the adrenal medulla to stimulate the release of epinephrine and norepinephrine, catecholamines that increase blood pressure. At each synapse, neurotransmitters trigger increased [Ca2+]i, HIF-1α:HIF-2α, and Nox2:Sod2 activity that generates increased ROS levels. These responses are not observed in other regions of the brain stem that do not receive input from the carotid body or signal to the sympathetic nervous system. Thus sympathetic nervous system homeostasis is dependent on a balance between HIF-1α and HIF-2α, disruption of which results in hypertension in OSA patients.
Keywords: hypoxia-inducible factors, NADPH oxidase, superoxide dismutase, oxidative stress
patients with obstructive sleep apnea (OSA) experience episodes of upper airway collapse during sleep that result in periods of hypoxemia (usually 15-30 s in duration) leading to arousal, clearing of the airway, and reoxygenation. These episodes of hypoxemia and reoxygenation are repeated dozens of times each night. Several large population-based studies have demonstrated that OSA is associated with an increased incidence of hypertension (11, 24). OSA is the most common secondary cause of resistant hypertension (12). Among OSA patients the use of continuous positive airway pressure (CPAP) during sleep was associated with a reduced risk of hypertension compared with untreated OSA patients, but the incidence of hypertension was still higher than in control subjects (9).
Experimental Models
Considerable progress has been made in understanding the pathogenesis of hypertension in OSA patients through the use of animal models, which have demonstrated that exposure of rodents to repetitive, brief episodes of hypoxia and reoxygenation is sufficient to induce hypertension. Thus, although OSA patients experience both chronic intermittent hypoxia (CIH) and chronic intermittent hypercapnea, CIH (in the absence of hypercapnea) is a sufficient pathophysiological stimulus to cause hypertension (2, 3) and coexposure to both hypoxia and hypercapnia has no additional effect (8). Remarkably, the duration of the reoxygenation phase is more important than the duration of the hypoxia phase (26), and protocols in which the reoxygenation phase is longer than the hypoxia phase are more effective in eliciting hypertension than protocols with hypoxia and reoxygenation phases of equal duration (17).
Neural Circuitry Activated by Chronic Intermittent Hypoxia
Bilateral transection of the carotid sinus nerve completely prevented the hypertension that was observed in sham-operated rats subjected to CIH for 35 days, demonstrating the essential role of carotid body chemoreceptors (2). The glomus cell chemoreceptors send signals via the carotid sinus nerve to the nucleus tractus solitarii (nTS), from which interneurons project to the rostral ventrolateral medulla (RVLM) in the brain stem (7, 22), from which efferent signals are sent via spinal nerves that synapse in sympathetic ganglia and then are transmitted via the adrenal sympathetic nerve (a branch of the splanchnic nerve), leading to stimulation of the adrenal medulla to secrete catecholamines (epinephrine and norepinephrine), which increase blood pressure (BP) (Fig. 1). Adrenal demedullation blocks the increase in BP caused by CIH (1).
Fig. 1.
Neural circuitry by which chronic intermittent hypoxia increases catecholamine secretion to induce hypertension. Experimental manipulations that are discussed in the text [carotid body ablation (CBA), adrenal sympathetic nerve (ASN) ablation (ASA), and atropine (ATR)] are shown by vertical bars. CSN, carotid sinus nerve; IN, interneuron; SC, spinal cord.
Hypoxia-Inducible Factors Mediate Transcriptional Responses to Continuous Hypoxia
The hypoxia-inducible factors HIF-1 and HIF-2 control cellular and systemic responses to continuous hypoxia (18). Hypoxia-inducible factors are heterodimeric transcription factors composed of an O2-regulated HIF-1α or HIF-2α subunit and a constitutively expressed HIF-1β subunit. Whereas HIF-1α and HIF-1β are found in all metazoans, HIF-2α is only found in vertebrates, in which increase in body mass necessitated the evolution of complex cardiovascular and respiratory systems in order to ensure adequate tissue O2 delivery (21). Homozygosity for a null allele at the locus encoding HIF-1α or HIF-2α results in embryonic or postnatal lethality, respectively (4, 20).
Heterozygous HIF-1α-null (Hif1a+/−) mice develop normally and are indistinguishable from wild-type (WT) littermates under normoxic conditions but have impaired responses to hypoxia and ischemia (25). Carotid bodies isolated from Hif1a+/− mice do not respond to acute hypoxia, although the response to cyanide or hypercarbia remains intact and histology of the carotid body is normal, indicating a specific defect in the ability to sense or respond to hypoxia (6). In contrast, carotid bodies isolated from heterozygous HIF-2α-null (Epas1+/−) mice manifest exaggerated responses to acute hypoxia (13).
Molecular Circuitry Activated by Chronic Intermittent Hypoxia in the Carotid Body
To model CIH experienced by patients with OSA, Hif1a+/− and WT littermate mice were placed in an environmental chamber in which the O2 concentration was rapidly dropped from 21% to 5% for 15 s and then rapidly increased to 21% for 5 min, and this hypoxia-reoxygenation cycle was repeated for 8 h/day over 10 days, which caused an increase in mean BP from ∼110 to >140 mmHg in WT mice but no significant BP increase in Hif1a+/− littermates (15). The increase in BP was associated with a significant increase in plasma catecholamine levels in WT mice subjected to CIH but not in Hif1a+/− littermates (15). Whereas Hif1a+/− mice with partial HIF-1α deficiency fail to develop hypertension in response to CIH, Epas1+/− mice with partial HIF-2α deficiency manifest hypertension even under normoxic conditions (13).
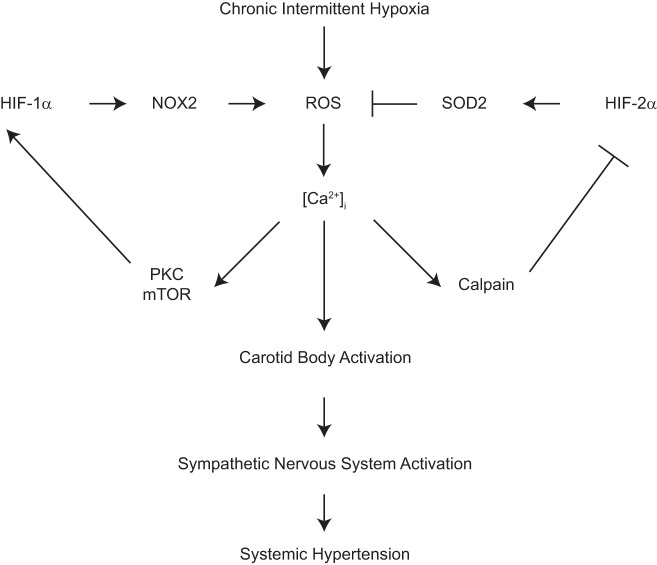
Analysis of WT mice exposed to CIH revealed that HIF-1α levels increased in the carotid body (15) whereas HIF-2α levels decreased (10). The cycles of hypoxia and reoxygenation during CIH cause an increase in reactive oxygen species (ROS) (16). ROS generated during CIH increased intracellular calcium levels [Ca2+]i, leading to protein kinase C (PKC)-dependent induction of mammalian target of rapamycin (mTOR) activity that increased HIF-1α expression (15, 28), which led to increased expression of the Nox2 gene encoding the prooxidant enzyme NADPH oxidase (27). ROS generated by Nox2 have been shown to interfere with the normal function of mitochondrial electron transport complex I, resulting in a further increase in ROS generation (5). The ROS-dependent increase in [Ca2+]i also activated calpains, which are calcium-dependent proteases that mediate the degradation of HIF-2α, thereby preventing it from activating transcription of the Sod2 gene, which encodes a mitochondrial isoform of the antioxidant enzyme superoxide dismutase (10).
Not only did HIF-1α and HIF-2α have opposing effects on the cellular redox state, they actively antagonized each other: In Epas1+/− mice decreased Sod2 activity resulted in a prooxidant state that induced HIF-1α and Nox2 expression under normoxic conditions, whereas in Hif1a+/− mice decreased Nox2 expression and a reduced redox state blocked the degradation of HIF-2α, leading to increased Sod2 expression (28). Most remarkably, Epas1+/−;Hif1a+/− compound heterozygous mice, which are partially deficient for both HIF-1α and HIF-2α, had completely normal BP and carotid body responses to hypoxia (29), demonstrating that it is the ratio of HIF-1α to HIF-2α that determines the set point of carotid body reactivity, thereby controlling sympathetic activation and BP (Fig. 2).
Fig. 2.
Molecular mechanisms leading to activation of glomus cells in the carotid body in response to chronic intermittent hypoxia. HIF, hypoxia-inducible factor; NOX, NADPH oxidase; ROS, reactive oxygen species; SOD, superoxide dismutase; [Ca2+]i, intracellular calcium ion concentration; PKC, protein kinase C; mTOR, mammalian target of rapamycin.
Intermittent Hypoxia Is Transduced to Intermittent Neural Activity
In addition to the remarkable finding that CIH increased HIF-1α and Nox2 expression, decreased HIF-2α and Sod2 expression, and increased oxidative stress in glomus cells of the carotid body, the same changes were observed in neurons of the nTS and RVLM, but not in nearby brain stem regions that do not receive input from the carotid body, as well as in chromaffin cells of the adrenal medulla (14). These findings suggested either that cells in the nTS, RVLM, and adrenal medulla directly sense and respond to CIH in a manner similar to the carotid body or that neural activity induces the same response in these cells that is induced by CIH in the carotid body.
To test whether carotid body neural activity is required for downstream effects, rats were subjected to carotid body ablation. CIH induced increased HIF-1α protein and Nox2 activity and decreased HIF-2α protein and Sod2 activity in nTS, RVLM, and adrenal medulla of control rats but not in rats subjected to carotid body ablation (14). CIH induced increased adrenal sympathetic nerve activity in response to a subsequent episode of acute hypoxia, and this response was lost in rats subjected to carotid body ablation; responses in the adrenal medulla were also lost in rats subjected to adrenal sympathetic nerve ablation (14).
In the adrenal medulla, sympathetic nerves release acetylcholine, which binds to muscarinic or nicotinic acetylcholine receptors on the surface of the catecholamine-secreting chromaffin cells. Treatment of rats with atropine during a 10-day exposure to CIH had no effect on carotid body or adrenal sympathetic nerve activity but blocked the response of the adrenal medulla to CIH, indicating that muscarinic acetylcholine receptors mediate the effects of the sympathetic nervous system on chromaffin cells of the adrenal medulla (14).
To further analyze the response of chromaffin cells to sympathetic activation, the rat PC12 pheochromocytoma cell line, which was originally established from a tumor of rat adrenal medullary chromaffin cells, was studied. Repetitive, intermittent application of muscarine (5-min treatment followed by 10-min washout for 3 cycles) was sufficient to increase HIF-1α and decrease HIF-2α protein levels, and the effect was blocked by atropine. In contrast, repetitive, intermittent application of nicotine, or constant application of muscarine or nicotine, had no effect (14). Repetitive, intermittent muscarine application increased [Ca2+]i and mTOR activity, and these responses, which were blocked by atropine, were required for induction of HIF-1α protein expression because muscarine did not induce HIF-1α expression in the presence of the calcium chelator BAPTA-AM or the mTOR inhibitor rapamycin. Repetitive, intermittent muscarine treatment of PC12 cells also increased calpain activity, and the calpain inhibitor Ac-LLM-CHO blocked HIF-2α degradation that was induced by muscarine treatment (14). Consistent with the PC12 studies, analysis of adrenal medulla from rats subjected to CIH revealed increased activity of mTOR and calpains, which provide a basis for increased HIF-1α and decreased HIF-2α levels, respectively, and these effects were blocked by carotid body ablation, adrenal sympathetic nerve ablation, or atropine treatment (14).
As expected, the increases in plasma catecholamines and BP that are induced by CIH were not observed in rats that were subjected to carotid body ablation or adrenal sympathetic nerve ablation. These results demonstrate that the response of the adrenal medulla is dependent of transmission of neural signals generated in the carotid body in response to CIH. Remarkably, unlike adrenal medulla from control rats, which is unresponsive, sections of adrenal medulla from CIH-exposed rats increased catecholamine secretion when subjected to hypoxia ex vivo (14). It is also interesting that in PC12 cells increased HIF-1α → Nox2 and decreased HIF-2α → Sod2 signaling can be induced by either intermittent hypoxia (10, 14) or repetitive, intermittent muscarine application (14), highlighting the interconversion of ROS signals and neural activity that is mediated by changes in the ratio of HIF-1α to HIF-2α.
Summary
Studies in rodents indicate that the relative steady-state levels of HIF-1α and HIF-2α protein in O2-sensing glomus cells of the carotid body determine the set point of sympathetic nervous system activity and BP in healthy individuals. In patients with OSA, CIH increases ROS, which trigger increased [Ca2+]i, leading to increased PKC-dependent mTOR activity and increased calpain activity, which cause increased HIF-1α and decreased HIF-2α levels, leading to increased Nox2 and decreased Sod2 activity that together further increase ROS in the carotid body in a feedforward mechanism (Fig. 2). Increased [Ca2+]i also results in the depolarization of glomus cells, leading to increased activity of neurons that project to the nTS and from there to the RVLM in the brain stem, where they synapse with sympathetic neurons that project via the spinal cord to sympathetic ganglia, where they synapse with neurons that project via the adrenal sympathetic nerve to activate muscarinic receptors on chromaffin cells in the adrenal medulla and stimulate their release into the circulation of epinephrine and norepinephrine, which are catecholamines that cause vasoconstriction and thereby increase BP (Fig. 1). Clinical studies have also demonstrated impaired endothelium-dependent vasodilation in OSA patients (19), but it is not known whether this defect is a direct effect of CIH on endothelial cells or a secondary effect of increased circulating catecholamines.
Whereas glomus cells in the carotid body respond directly to ROS resulting from CIH, neurons in the nTS, RVLM, and adrenal medulla depolarize in response to neurotransmitters and the resulting increased [Ca2+]i triggers an increase in HIF-1α:HIF-2α expression and Nox2:Sod2 activity that results in ROS generation as well as signal transmission that ultimately results in catecholamine release and hypertension (Fig. 2). Thus sympathetic nervous system homeostasis is dependent on a balance between HIF-1α and HIF-2α levels, the disruption of which results in oxidative stress and hypertension. These studies have delineated the molecular and cellular mechanisms that underlie the most common cause of treatment-resistant hypertension in western society. Drugs that inhibit HIF-1α but not HIF-2α, such as ganetespib, which is currently being evaluated in clinical trials as an anticancer drug (23), may be of therapeutic benefit to hypertensive OSA patients who do not respond to CPAP or standard antihypertensive agents.
Finally, independent of OSA, the balance between HIF-1α and HIF-2α establishes a redox balance in the carotid body, which in turn establishes a set point for sympathetic nervous system activity, which is a major determinant of BP. This fundamental physiological role of antagonism between HIF-1α and HIF-2α in the carotid body is highlighted by the phenotype of Epas1+/− mice, in which reduced HIF-2α expression leads to oxidative stress and increased HIF-1α expression in the carotid body and adrenal medulla that causes hypertension under normal ambient environmental conditions. Other genetic or environmental factors that alter the balance between HIF-1α and HIF-2α in the carotid body may also result in hypertension.
GRANTS
The authors' work on CIH is supported by National Heart, Lung, and Blood Institute Grants P01-HL-90554 and UH2-HL-123610 to N. R. Prabhakar. G. L. Semenza is the C. Michael Armstrong Professor at the Johns Hopkins University School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.L.S. and N.R.P. conception and design of research; G.L.S. and N.R.P. analyzed data; G.L.S. and N.R.P. interpreted results of experiments; G.L.S. and N.R.P. prepared figures; G.L.S. and N.R.P. drafted manuscript; G.L.S. and N.R.P. edited and revised manuscript; G.L.S. and N.R.P. approved final version of manuscript.
REFERENCES
- 1.Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol 83: 95–101, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher EC, Lesske J, Behm R, Miller CC 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol 72: 1978–1984, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher EC, Lesske J, Qian W, Miller CC 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19: 555–561, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev 12: 149–162, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR. NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal 14: 533–542, 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilator responses to chronic hypoxia in mice deficient for hypoxia-inducible factor 1α. Proc Natl Acad Sci USA 99: 821–826, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci 27: 4663–4673, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia—influence of chemoreceptors and sympathetic nervous system. J Hypertens 15: 1593–1603, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbé F, Vicente E, Wei Y, Nieto FJ, Jelic S. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 307: 2169–2176, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2α via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci USA 106: 1199–1204, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283: 1829–1836, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 58: 811–817, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Peng YJ, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 2α (HIF-2α) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci USA 108: 3065–3070, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng YJ, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL, Prabhakar NR. Regulation of hypoxia-inducible factor α isoforms and redox state by carotid body neural activity in rats. J Physiol 592: 3841–3858, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol 90: 1986–1994, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Prabhakar NR, Peng YJ, Kumar GK, Nanduri J. Peripheral chemoreception and arterial pressure responses to intermittent hypoxia. Compr Physiol 5: 561–577, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 92: 967–1003, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichmuth KJ, Dopp JM, Barczi SR, Skatrud JB, Wojdyla P, Hayes D Jr, Morgan BJ. Impaired vascular regulation in patients with obstructive sleep apnea: effects of continuous positive airway pressure treatment. Am J Respir Crit Care Med 180: 1143–1150, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet 35: 331–340, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic intermittent hypoxia: a model of sympathetic activation in the rat. Respir Physiol 121: 173–184, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Xiang L, Gilkes DM, Chaturvedi P, Luo W, Hu H, Takano N, Liang H, Semenza GL. Ganetespib blocks HIF-1 activity and inhibits tumor growth, vascularization, stem cell maintenance, invasion, and metastasis in orthotopic mouse models of triple-negative breast cancer. J Mol Med (Berl) 92: 151–164, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med 157: 1746–1752, 1997. [PubMed] [Google Scholar]
- 25.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest 103: 691–696, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan G, Adhikary G, McCormick AA, Holcroft JJ, Kumar GK, Prabhakar NR. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol 557: 773–783, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol 226: 2925–2933, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1α expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217: 674–685, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan G, Peng YJ, Reddy VD, Makarenko VV, Nanduri J, Khan SA, Garcia JA, Kumar GK, Semenza GL, Prabhakar NR. Mutual antagonism between hypoxia-inducible factors 1α and 2α regulates oxygen sensing and cardio-respiratory homeostasis. Proc Natl Acad Sci USA 110: E1788–E1796, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]