Abstract
Immunohistochemistry (IHC) is an important adjunct in the diagnosis of neoplastic skin diseases. In addition to the many established IHC markers currently in use, new markers continue to emerge, although their general acceptance and routine application requires robust validation. Here, we summarize the most well-established and commonly used biomarkers along with an array of newer ones reported in the past several decades that either demonstrate or hold high clinical promise in the field of cutaneous pathology. We also highlight recent applications of novel IHC markers in melanoma diagnosis including genetic mutation status markers [e.g. BRAF (v-raf murine sarcoma viral oncogene homolog B) and NRAS (neuroblastoma RAS viral oncogene homolog)] and an epigenetic alteration marker (e.g. 5-hydroxymethylcytosine). We specifically focus on the role of IHC in the differential diagnosis of cutaneous lesions that fall under the following categories: melanoma, epidermal tumors with an intraepidermal epitheliomatous pattern, spindle cell lesions of the dermis, small round blue cell tumors of the dermis, and cutaneous adnexal tumors. While IHC is a valuable tool in diagnostic dermatopathology, marker selection and interpretation must be highly informed by clinical context and the histologic differential diagnosis. With rapid progress in our understanding of the genetic and epigenetic mechanisms of tumorigenesis, new IHC markers will continue to emerge in the field of diagnostic dermatopathology.
Key Words: Immunohistochemistry, Skin cancer, Cutaneous neoplasia, Biomarkers
Introduction
While the routine practice of dermatopathology relies predominantly on histologic findings and clinical context, immunohistochemistry (IHC) will remain an important adjunct tool for the diagnosis of difficult cases, tumor staging and identification of genetic variants of therapeutic significance. The utility of IHC is broad across cutaneous neoplasms but becomes particularly powerful when ‘extracutaneous’ lesions, such as metastatic carcinoma, soft tissue neoplasms and hematologic malignancies enter the differential. Overreliance upon or uninformed utilization of biomarkers, however, can be treacherous due to the diagnostic pitfalls they can create. Here, we summarize some of the most commonly used and newly emerged biomarkers for the diagnosis of cutaneous neoplasms.
Staining Patterns of the Common IHC Markers in Normal Skin
Skin is rich in normal internal positive controls for the majority of antigens used as markers of neoplastic lesions, including keratinocytes, melanocytes, Langerhans cells and Merkel cells, as well as vessels, muscle and nerve bundles (table 1; fig. 1, 2, 3) [1]. For example, the cells of secretory coils of eccrine sweat glands are positive for low-molecular-weight cytokeratin, including CK7, cell adhesion molecule 5.2 (CAM5.2), carcinoembryonic antigen (CEA) and epithelial membrane antigen (EMA), while acrosyringeal cells of the intraepidermal portion of sweat glands are positive for high-molecular-weight cytokeratins (HMWCK). Careful attention to positive and negative internal controls is a critical first step in the interpretation of IHC staining.
Table 1.
IHC staining patterns in normal skin
| Antigen | Antibodies | Internal control | Figure |
|---|---|---|---|
| Pan–cytokeratin | AE1/3 (AE1–acidic CK; AE3–basic CK), Pan–K | suprabasal keratinocytes | 1a, b, f |
| HMWCK | 34βE12 (CK1, 5, 10, 14, 15), CK5/6 | suprabasal keratinocytes and adnexal epithelia | 1c, d |
| LMWCK | CAM5.2, CK7 | secretory portion of eccrine and apocrine glands | 1e |
| Muscle markers | SMA, desmin | pilar smooth muscle | 2a–d |
| Eccrine and apocrine glands | CEA, EMA | sweat glands | 2e, f |
| Endothelial cell markers | CD31, CD34, vWF, ERG–1 | endothelial cells in vessels | 3a–d |
| Melanocytic markers | S100, MART–1, MITF, HMB–45, tyrosinase | epidermal basal layer melanocytes | 4 |
| Langerin | CD1a, CD4 | epidermal Langerhans cells | |
| Axon | NPF, S100 | nerve bundle | |
LMWCK = Low–molecular–weight cytokeratin; NPF = neuropeptide F; vWF = von Willebrand factor; Pan–K = pan–keratin.
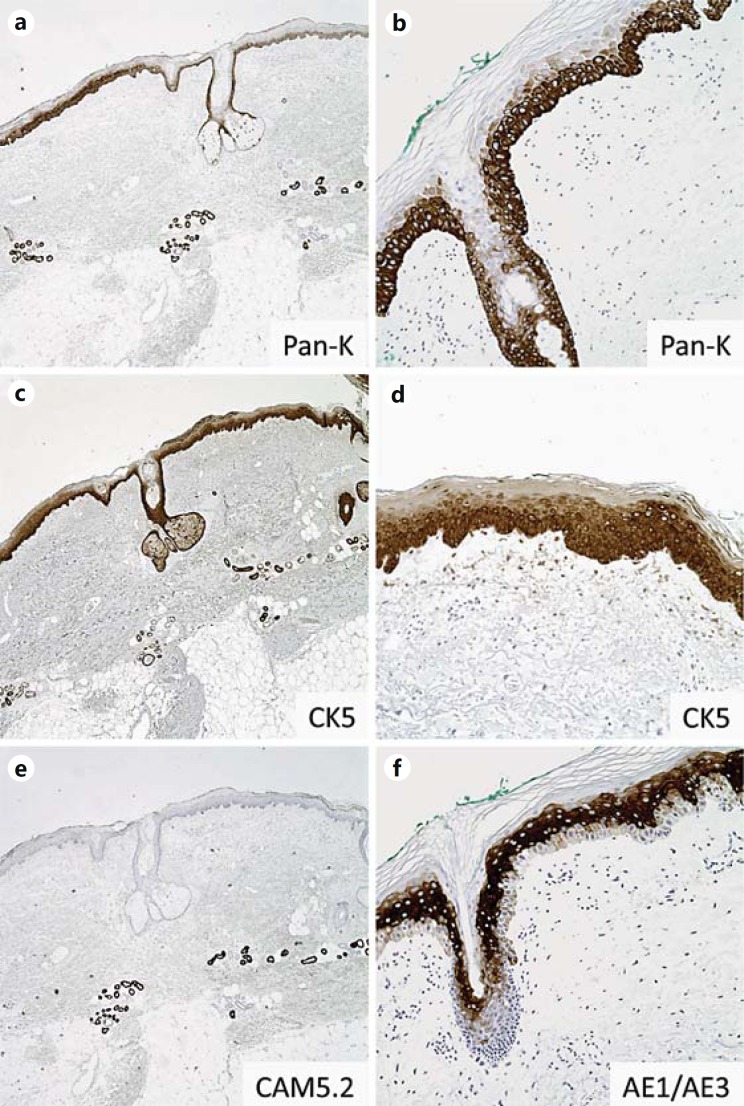
Fig. 1.
Keratin expression in normal skin. a, c, e Staining patterns of pan-keratin (Pan-K), HMWCK (CK5) and low-molecular-weight cytokeratin (LMWCK; CAM5.2) in normal skin. Pan-K (a) and HMWCK CK5 (c) highlight epidermal and follicular epithelium as well as dermal eccrine myoepithelium; e LMWCK CAM5.2 stains eccrine myoepithelium but not the epidermis or follicular epithelium. b, d, f Staining patterns of Pan-K, CK5 and AE1/AE3 in epidermis. Basal layers of the epidermis and hair follicle strongly express Pan-K (b) and HMWCK CK5 (d), while more superficial cell layers show decreased expression with cell maturation. f By contrast, more superficial/mature layers of the epidermis and follicle show strong expression of AE1/AE3, while the basal layers show little expression.
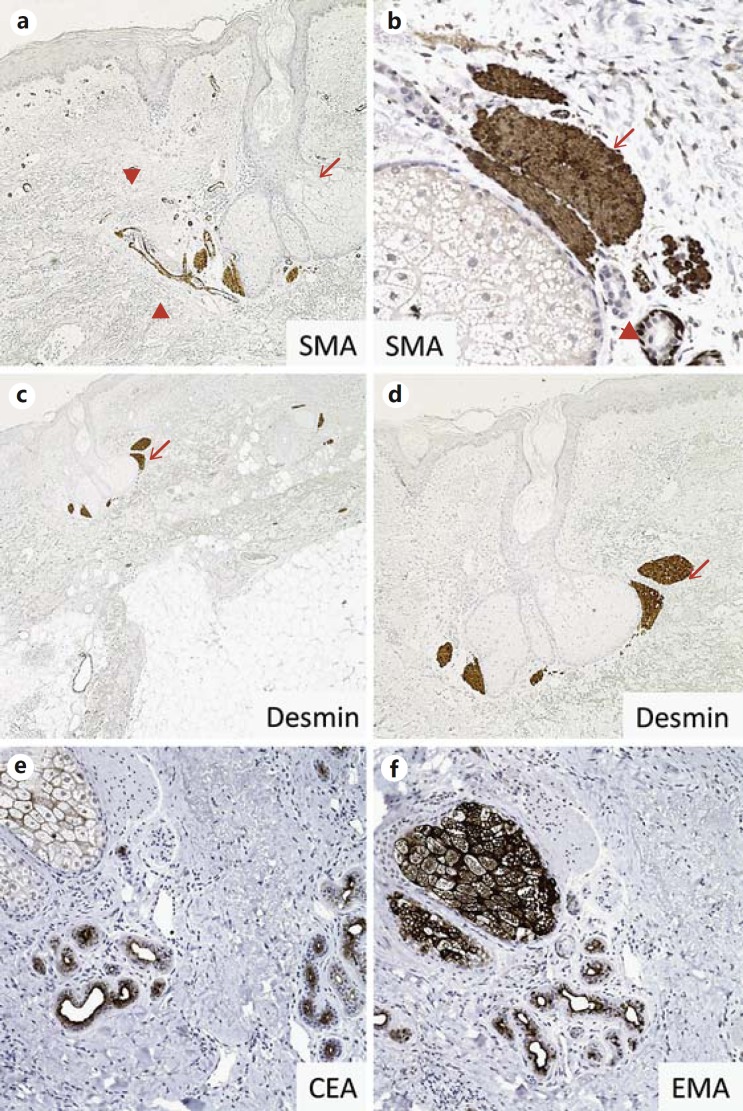
Fig. 2.
Pilosebaceous unit IHC markers in normal skin. a, b SMA is strongly expressed in pilar muscle (arrows), eccrine myoepithelium (arrowheads) and vascular smooth muscle (not shown) in normal skin. c, d Pilar muscle also strongly expresses desmin (arrows). e Adnexal ducts are highlighted by CEA. f EMA immuno- stain highlights the luminal lining of adnexal ducts and sebaceous glands.
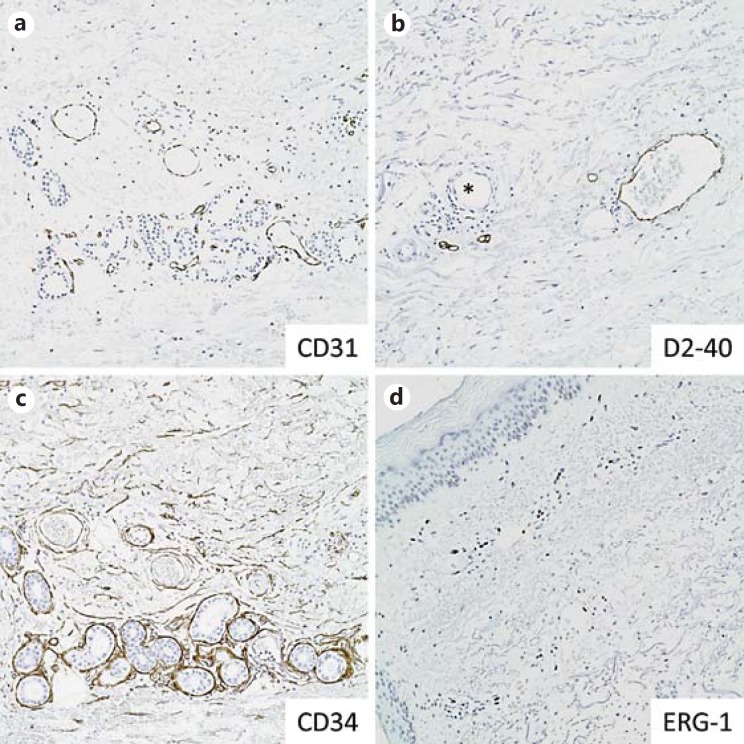
Fig. 3.
Lymphovascular IHC markers in normal skin. a CD31 highlights endothelial cells of dermal vessels. b D2-40 highlights the lining of dermal lymphatic channels but does not stain vascular endothelium (asterisk). c CD34 highlights dermal vascular endothelium and dermal mesenchymal cells. d ERG-1 is a nuclear stain of vascular endothelium.
IHC Markers for Melanoma Diagnosis
Melanoma is the most lethal type of skin malignancy, causing approximately 75% of skin cancer-related deaths worldwide [2]. Optimal therapy and patient prognosis for melanoma critically depend on early detection and accurate diagnosis. Given the tremendous histologic diversity of melanoma, diagnosis can be challenging [3]. Melanocytic lesions are now the second highest source of litigation in surgical pathology, following only lesions of the breast [4,5]. Utilization of a panel of melanocytic IHC markers can be an extremely valuable adjunct to hematoxylin and eosin (H&E) morphology for the diagnosis of melanoma, although what appears to be routine use of such panels by some laboratories for a majority of melanocytic lesions is inappropriate [6,7]. Accordingly, stringent and informed selection of cases that require IHC as a diagnostic adjunct is of utmost importance.
Among all IHC markers, S100 is among the most sensitive for melanocytic lesions, although it lacks specificity. Melanoma antigen recognized by T cells (MART-1), micropthalmia-associated transcription factor (MITF) and/or human melanoma black-45 (HMB-45) are frequently used because they provide increased specificity and reasonable sensitivity for differentiating most conventional melanomas from nonmelanocytic histologic mimics. HMB-45 has additional utility in differentiating benign melanocytic lesions from malignant melanoma as benign lesions (e.g. dermal nevi) tend to show decreased expression with lesion depth/maturation, while melanoma often shows more consistent staining in the deeper component. However, numerous exceptions exist, particularly in the setting of certain nevic variants of melanoma. Thus, interpretation of HMB-45 staining is not always straightforward, and expression (or lack thereof) should never be a single or dominant criterion in the establishment of a benign versus a malignant lesion. IHC is also useful in gauging confluent (contiguous/continuous) junctional growth of intraepidermal melanocytic proliferations or extent of pagetoid spread to help rule in or out a diagnosis of melanoma in situ or an in situ melanoma component of a worrisome compound melanocytic lesion. Although MART-1 is widely used for this purpose, the degree of confluent growth may be overestimated due to positive staining of melanocyte dendritic processes that wrap around keratinocytes and/or as a consequence of false-positive ‘overstaining’ [7,8,9,10] (fig. 4a-d). For these reasons, some prefer staining with the nuclear marker MITF when evaluating possible confluent lentiginous growth of the intraepidermal components of melanocytic lesions [7,8,9,10]. IHC for keratin may also be useful, as it provides the negative image of an intraepidermal melanocytic proliferation [11]. Of note, it is important to be aware of a few reports of melanomas with heterologous differentiation that express keratin, vimentin, EMA or smooth muscle actin (SMA), and such cases may create difficult diagnostic pitfalls, particularly in the setting of metastatic malignancy [3,12,13,14], further emphasizing the value of assessing a complementary panel of well-selected biomarkers when faced with challenging neoplasms of potential melanocytic differentiation.
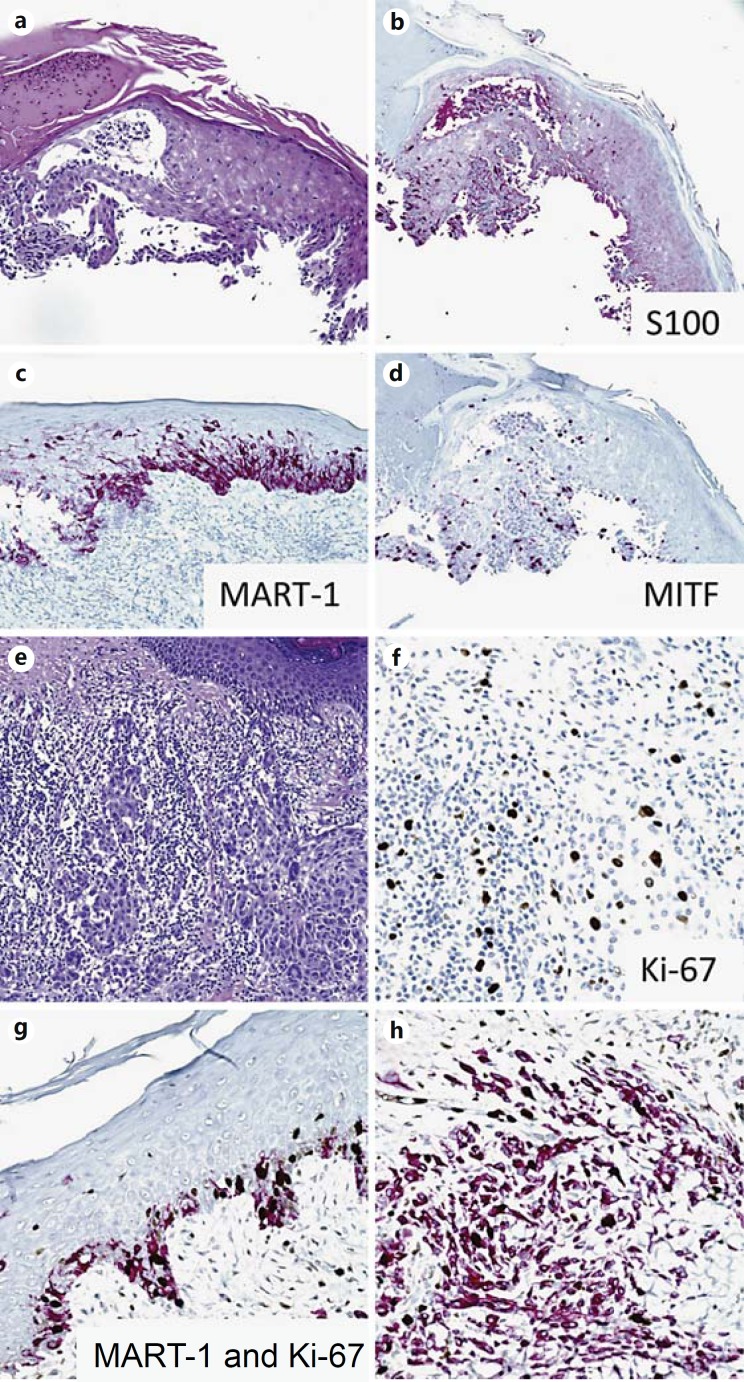
Fig. 4.
Melanocytic markers. Melanoma in situ (a) highlighted by immunostains of S100 (b), MART-1 (c) and MITF (d). e, f Ki-67 immunostain in a melanoma with a brisk tumor infiltrating lymphocytic response, highlighting the difficulty in interpreting lineage of Ki-67-positive cells. g, h Double Ki-67 and MART-1 immunostains of melanoma, highlighting cell proliferation specifically in melanocytic lineages.
Surrogate IHC Markers for Mitoses in Melanoma - Phosphorylated Histone H3 and Ki-67
In the 7th edition of the American Joint Committee on Cancer (AJCC) Staging Manual, mitoses within the dermal/invasive component were added to the tumor staging criteria for primary cutaneous melanoma [15] as their presence is believed to have significant prognostic value. While mitotic counts are a routine part of the practice of pathology, accurate evaluation in skin biopsies and excisions may be challenging due to the small sample size and as a result of histologic mimics, such as apoptotic tumor cells or mitoses in surrounding inflammatory or stromal cells. Studies have demonstrated a potential role for immunohistochemical markers of proliferation in the staging of primary cutaneous melanoma. Phosphorylated histone H3 (pHH3) is a general marker of cell proliferation whose utility in melanocytic lesions remains under active investigation. Histone H3 is phosphorylated during late G2/early M phases of the cell cycle coincident with chromatin condensation and is dephosphorylated between late anaphase and telephase, making pHH3 a useful proxy of cell division. Data suggest that pHH3 staining successfully highlights mitoses in cutaneous melanomas and reduces interobserver variability [16,17]. Co-staining for pHH3 with one or more markers of melanoma, such as MART-1, may further improve accurate enumeration of mitoses restricted to cells of melanocytic lineage and reduce time spent on analysis [16,18]. In these studies, a mitosis was counted if the cell lacked a nuclear membrane and the chromatin was condensed and positive for pHH3. Multiple studies have identified the pHH3 index to be a powerful independent prognostic factor that rivals, and in some cases exceeds, the predictive value of histologic mitotic counts [16,19,20]. Double staining for a melanoma marker and limiting analysis to a mitotic ‘hot spot’, instead of the entire slide or specimen, has the greatest prognostic value [20]. Currently, the pHH3 index has not been introduced into standardized practice guidelines, such as the AJCC, and has not achieved widespread use in routine dermatopathology. For now, pHH3/melanoma marker co-stains serve to increase confidence in staging difficult cases of cutaneous melanomas, particularly when H&E stains are equivocal.
Antigen KI-67 (Ki-67) is a nuclear protein expressed as two isoforms during the G1, S, G2 and M stages of cell division, making it a more sensitive (inclusive) marker of proliferation when compared with histologic mitotic counts or pHH3 staining approaches. Similar to pHH3, the Ki-67 index of cutaneous melanomas is a powerful, statistically significant, independent prognostic factor [20,21]. Going forward, standardized Ki-67 indices would need to be established for melanoma by consensus prior to widespread use in staging. In melanomas with a brisk tumor-infiltrating lymphocyte response, single labeling with Ki-67 may lead to overestimation of the proliferative index due to the high density of mitotically active inflammatory cells (fig. 4e, f). In such cases, a Ki-67 and MART-1 double staining is recommended to clarify the histogenesis of proliferating cells (fig. 4g, h). Additionally, a recent report indicates that the mixed variant of desmoplastic melanoma (e.g. lesions also containing sarcomatoid or epithelioid regions) has a higher proliferative index based on Ki-67 IHC compared with the pure variant [22,23]. Studies with larger numbers of cases and a broader spectrum of melanocytic lesions to include nevi (especially traumatized and irritated lesions), Spitzoid lesions and borderline melanocytic lesions are needed to firmly establish the diagnostic and prognostic value of Ki-67 staining in melanocytic neoplasms.
SRY (Sex-Determining Region Y)-Box 10
SRY (sex-determining region Y)-box 10 (SOX10), a member of the Sry HMG box (Sox) family of transcription factors, is essential for maintenance of neural crest progenitor cells and their subsequent differentiation down melanocytic and glial lineages during embryogenesis [24]. Multiple studies have demonstrated that SOX10 is a highly sensitive immunohistochemical marker of both benign and malignant melanocytic lesions, making it a useful cell lineage indicator in the appropriate clinical context. Nearly 100% of primary melanomas display strong nuclear staining for SOX10 [25,26,27]. While SOX10 shows similarly high sensitivity for metastatic melanoma, staining intensity and the percentage of positive nuclei may be lower compared with benign melanocytic lesions and primary melanomas [25]. As with most IHC biomarkers, SOX10 should not be used in isolation for the diagnosis of metastatic lesions, particularly in the setting of unknown primary tumors, given that a subset of breast and salivary gland carcinomas stain positively for SOX10 [28,29,30], and expression has yet to be defined in many carcinomas. One area, however, where SOX10 may have particular utility is in differentiating desmoplastic melanoma from a variety of histologic mimics including fibrohistiocytic lesions, mesenchymal neoplasms, ‘spindled’ carcinomas and cellular excisional scars. Desmoplastic melanoma often does not stain for proteins considered to have higher specificity for conventional melanoma, such as MART-1, MITF and HMB-45. S100 is a highly sensitive marker of desmoplastic melanoma, but is also expressed by a number of mimics, including excisional scars, limiting its diagnostic utility. SOX10, however, is strongly expressed in 100% of desmoplastic melanomas [25,26,27,31], but not in the majority of spindle cell mimics, including a variety of sarcomas, spindle cell squamous cell carcinoma (SCC), fibrohistiocytic lesions such as Rosai Dorfman, atypical fibroxanthoma (AFX), dermatofibroma, Langerhans cell histiocytosis (LCH), juvenile xanthogranuloma, cellular neurothekeoma, reticulohistiocytoma, and the most frequently encountered (and perhaps treacherous mimic) cellular scar [25,26,31]. Thus, SOX10 may be particularly useful in evaluating residual desmoplastic melanoma and assessing its proximity to margins in reexcision specimens. Of note, SOX10 is expressed in 100% of clear cell sarcomas, 100% of neurofibromas and schwannomas and approximately 50% of malignant peripheral nerve sheath tumors (MPNST). As such, SOX10 should not be utilized to differentiate these entities from melanoma. However, in most instances, histologic features alone should suffice (the diagnosis of spindled cell dermal tumors is further discussed in a subsequent section). In addition to positivity for SOX10, clear cell sarcomas frequently show at least partial positivity for other melanocytic markers including S100, MITF, Melan A and HMB-45 [32,33]. Unlike melanoma, however, approximately 75% of clear cell sarcomas harbor a t(12;22)(q13;q12) that creates an EWS-ATF1 fusion protein known to activate the MITF promoter in a SOX10-dependent manner [34]. PCR-based methods and fluorescent in situ hybridization may be used to detect this chromosomal translocation [35], which has never been described to occur in melanoma.
Genetic and Epigenetic IHC Markers with Potential Clinical Implications
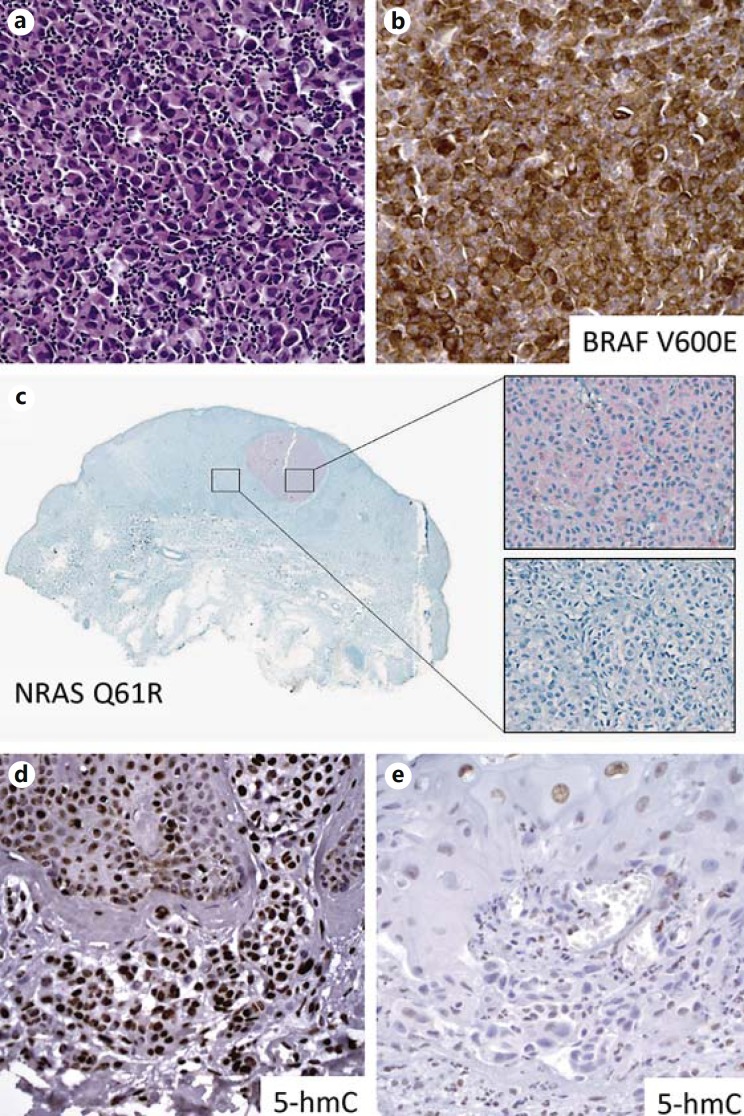
The recent rapid progress in our understanding of the genetic and epigenetic mechanisms of melanomagenesis has heralded a new set of biomarkers of potential clinical importance. Recent studies demonstrate that IHC is a practical and easy method to detect the genetic mutational status of BRAF V600E (fig. 5a, b) and NRAS Q16R (fig. 5c) in melanoma. Genetic mutations in v-raf murine sarcoma viral oncogene homolog B (BRAF) and neuroblastoma RAS viral oncogene homolog (NRAS) are present in approximately 50 and 20% of melanomas, respectively [36,37], and result in constitutive activation of MAPK pathways. The RAS-RAF-MAPK pathway has, in fact, proven to be a key therapeutic target for the treatment of metastatic melanoma, as multiple inhibitors either have achieved FDA approval, or are under investigation in clinical trials (e.g. BRAF inhibitor vemurafenib, MEK inhibitors). As such, accurate and rapid tests to assay mutational status are essential. Currently, molecular genetic testing is the preferred assay to determine BRAF inhibitor eligibility due to its superior sensitivity. Recent studies have shown that IHC for BRAF V600E and NRAS Q16R has excellent specificity that is highly concordant with molecular testing (e.g. PCR, pyrosequencing or next-generation sequencing) [38,39,40,41]. Other point mutations of BRAF (V600K, V600Q, and V600R) or NRAS (Q61K, Q61L, Q61H) melanomas do not stain positively with BRAF VE1 and NRAS Q61R antibodies [39,41], respectively. In addition, studies indicate that germline mutation of BRAC1-associated protein (BAP-1) is associated with multiple cutaneous Spitzoid melanocytic neoplasms, as well as frequent somatic mutations in uveal melanoma [42,43,44]. The loss of BAP-1 in BAP-1 mutant melanocytic lesions can be detected by IHC [42,43,44].
Fig. 5.
IHC markers for genetic mutations and epigenetic alteration in melanoma. a, b Cytoplasmic positivity of BRAFV 600E immunostains highlights the melanoma cells with BRAF V600E mutation. c Intratumoral heterogeneity shown by NRAS (Q61R) immunostains correlates with the different NRAS Q16R mutational status confirmed by high-resolution melting analysis and sequencing of NRAS exon 3. Reprinted from Modern Pathology with permission from Nature Publishing Group. IHC for epigenetic DNA hydroxymethylation mark (5-hmC) shows significant loss of 5-hmC positivity in metastatic melanoma (e) compared with strong staining intensity in nevus (d).
In addition to genomic mutations, recent data demonstrate that epigenetic alterations play important roles in melanoma pathogenesis. Our group reported that detection by IHC of the loss of the DNA hydroxymethylation mark, 5-hydroxymethylcytosine (5-hmC), is a putative biomarker with diagnostic and prognostic value in melanoma [45] (fig. 5d, e). Additional studies by our group and others have demonstrated clinical application of immunohistochemical evaluation of 5-hmC, particularly with respect to differentiating micrometastases in sentinel lymph nodes from dermal nevic nests, and in the evaluation of melanocytic dysplasia [45,46,47,48,49].
Other markers under active investigation for their utility in the diagnosis and staging of melanoma include RUNX3 [50,51], MAP-2 [52], nucleolin protein [53], KIT and p16 [54]. We also have noted that melanomas express the embryonic stem cell transcription factor SOX2 [55]. While not lineage specific, its expression appears to correlate with a more invasive phenotype [56], and IHC may be practically useful, when used in combination with detection of the intermediate filament protein nestin in verifying nodal melanoma metastases [57].
IHC Biomarkers for Prediction of Response to Melanoma Therapy
Given the recent progress of melanoma immunotherapy with regard to the blockade of immune checkpoints [e.g. CTLA-4 Ig and programmed cell death (PD-1) and programmed cell death ligand 1 (PD-L1)], there is urgent need to develop biomarkers to predict treatment responses. A recent study showed that PD-L1 IHC highlighted metastatic melanoma cells with membrane positivity in 30/81 (37%) of patients [58]. By multivariate analysis, Breslow thickness and PD-L1 membrane positivity were independent risk factors for melanoma-specific death [58]. However, interpretation of PD-1 and PD-L1 IHC stains requires caution given the expression of these epitopes on immune cells within the tumor microenvironment [59,60]. Nonetheless, the ability to profile expression of potentially targetable immune checkpoints in melanoma tissue as a means of stratifying patients who may benefit most from specific therapies holds significant promise for personalized melanoma therapies. IHC to assess induction of metastasis infiltrating lymphocytes may also be used as one potential measure of immunotherapeutic treatment efficacy, as has recently been shown in a patient cohort treated with bevacizumab (VEGF inhibitor) and ipilimumab (CTLA-4 blocker) [61]. The importance of biomarker validation prior to clinical application has recently been emphasized in reference to a prominent report, based largely on in vitro data, implicating detection of hepatocyte growth factor in melanoma stroma as a means of predicting response to RAF inhibitors [62]. Detailed examination of this possibility using a large cohort and meticulously controlled reagents, however, failed to support this claim [63], further emphasizing the necessity for rigorous validation testing of any new biomarker that may potentially be employed in the care of patients.
Epidermal Tumors with Intraepidermal Epitheliomatous Pattern
The differential diagnosis of intraepithelial lesions can be difficult given their frequent overlap in architectural and cytomorphologic patterns. Moreover, diagnostic pitfalls can be compounded by superficial and fragmented biopsies that only show part of the lesion. SCC, extramammary Paget's disease (EMPD), malignant melanoma, Merkel cell carcinoma (MCC), LCH and adnexal carcinoma (sebaceous, eccrine, and apocrine) can all exhibit pagetoid and nested patterns of intraepidermal growth in the presence or absence of an invasive/dermal component. And while most cases of these entities can be diagnosed by routine histology, IHC can be of considerable assistance in the diagnosis of problematic lesions, thus avoiding diagnostic pitfalls that may impact on patient care [64].
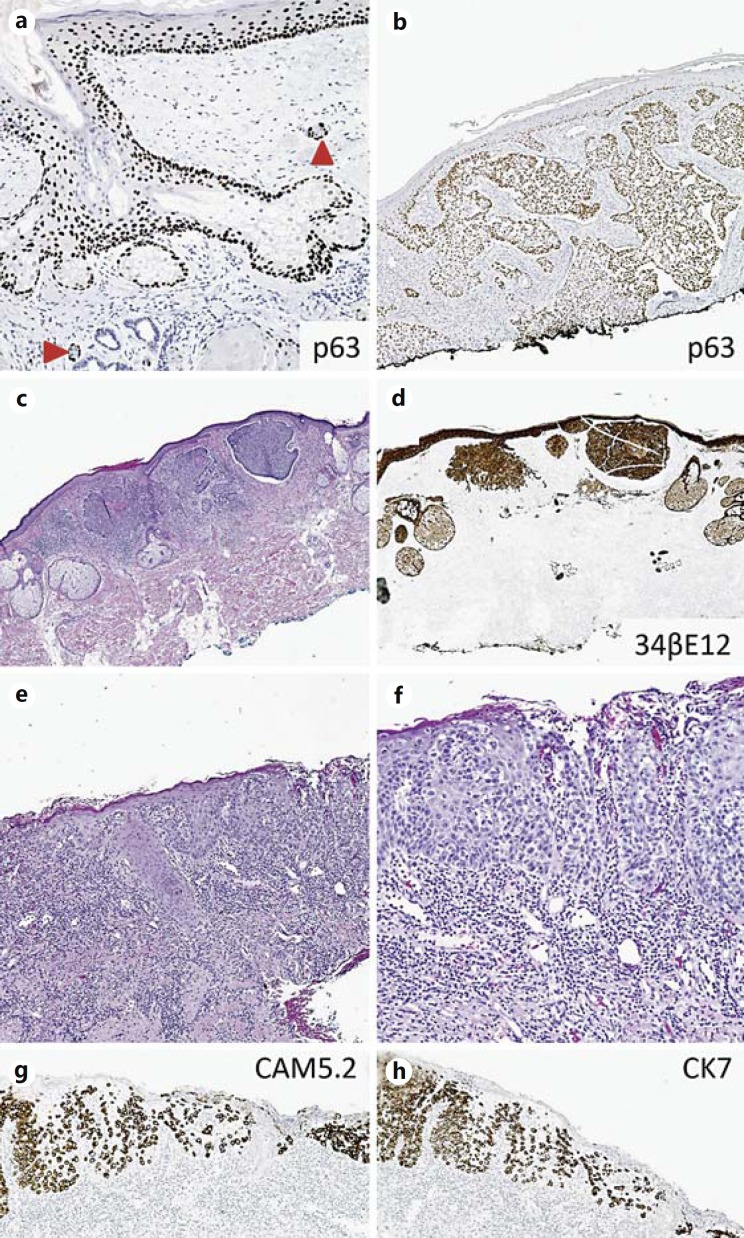
In situ SCC and melanoma, as well as EMPD are among the most frequently encountered entities demonstrating pagetoid growth patterns [65,66,67,68], and both occasionally will closely mimic pagetoid radial growth in melanoma. Historically, immunohistochemical positivity for CK7 and Cam5.2 has been utilized to favor a diagnosis of EMPD over SCC, and it also assists in excluding pagetoid radial growth phase melanoma. However, while invasive SCC does not frequently express these markers, recent data suggest pagetoid/‘Bowenoid’ SSCIS is frequently positive for CK7 and CAM5.2 [66,69,70]. p63, encoded on chromosome 3q27-28, is a p53 homolog required for epidermal development and stem cell maintenance [71,72]. Due to its nuclear pattern of positivity, many regard this stain as easier to interpret, compared with cytokeratin reactivity. p63 is strongly expressed in normal basal and suprabasal epidermal layers and basal cells of sebaceous and sweat glands [73], and is not expressed in terminally differentiated cells (fig. 6a). Thus, the majority of SCC and basal cell carcinoma (BCC) express p63 immunostains (fig. 6b-d; tables 2, 3), including intraepidermal epitheliomatous variants, while staining is generally negative for EMPD [73,74,75,76,77]. On the other hand, Ber-EP4 is a highly sensitive marker of EMPD and BCC that is not expressed by pagetoid/Bowenoid SCC or melanoma [66,78] (fig. 6c, d). Therefore, a panel of Ber-EP4, p63, and a specific melanoma marker such as MART-1 would be effective in differentiating these entities (table 2; fig. 6).
Fig. 6.
IHC markers for epidermal tumors with intraepitheliomatous pattern. a, b p63 expression in normal skin and squamous cell carcinoma. The epidermis, hair follicle and basal layers of the sebaceous unit express p63, with the strongest expression present towards the basal-most layers. p63 highlights myoepithelial cells of the eccrine units/ducts (arrowheads). Squamous cell carcinoma intensely expresses p63. c, d IHC for HMWCK 34βE12 highlights basal cell carcinoma. e, f HE sections of EMPD show Paget tumor cells in epidermis and extend into dermis. LMWCK immunostains CAM5.2 (g) and CK7 (h) highlight tumor cells in EMPD.
Table 2.
IHC panels for epidermal tumors with intraepitheliomatous patterns
| MMIS | SCCIS | MCC | Seb CIS | LCH | EMPD | BCC | |
|---|---|---|---|---|---|---|---|
| Screening | S100 | p63, EMA | CK20 | EMA, AR, adipophilin | CD1a, langerin | LMWCK | Ber–EP4 |
| EMA | 5% | 79–96% | 100% | 91–100% | 0% | 100% | 0–6% |
| [161] | [66, 83, 170, 171] | [81] | [83, 85, 171] | [93] | [172] | [83, 170, 171] | |
| Androgen R | 0% | 0–10% | – | 83–100% | – | 52–80% | 33–60% |
| [173] | [83, 84] | [83, 84, 85] | [86, 87] | [83, 85] | |||
| Ber-EP4 | 0% | 0–2% | 75% | 6% | – | 100% | 80–100% |
| [78] | [66, 83, 170] | [79] | [83] | [66, 78] | [83, 170, 174] | ||
| CEA | – | 20–30% | 0% | 42% | – | 100% | 0–20% |
| [83, 170] | [175] | [83] | [172] | [83, 170, 176] | |||
| Adipophilin | – | 0–10% | 0% | 92–97% | – | – | 0% |
| [83, 88] | [89] | [83, 88, 89] | [83, 88] | ||||
| CD1a | 0% | Negative | – | – | 94–100% | – | – |
| [91, 177] | [178, 179] | [180] | |||||
| Langerin | – | – | – | – | 99–100% | – | – |
| [92, 180] | |||||||
| CK7 | 0% | 0–40% | 50% | 75% | – | 100% | 0–70% |
| [66] | [174] | [79] | [83] | [66, 181] | [174, 176, 182] | ||
| CK20 | 0% | 0% | 83–100% | 0% | – | 0% | 0% |
| [82] | [183] | [80, 81, 82, 170] | [83] | [181] | [176, 182] | ||
| Cam5.2 | 0–10% | 0–10% | 100% | 73% | – | 100% | 20–95% |
| [66, 161] | [66, 171, 174] | [79, 184] | [171] | [66, 69] | [170, 171, 174, 176] | ||
| p63 | 0% | 87–100% | 33% | 100% | – | 0–17% | 100% |
| [75] | [73, 74] | [185] | [186, 187] | [76, 77] | [73] | ||
| S100A | 100% | 0% | 0–17% | N/A | 93–99% | 0% | 0% |
| [82] | [188] | [82, 175] | [93, 180] | [189, 190, 191] | [188] | ||
| MART–1 | 94% | 0% | – | – | – | – | 0% |
| [188] | [188] | [188] | |||||
| HMB 45 | 100% | 0% | 0% | – | – | 0% | 0% |
| [78, 82] | [188] | [82] | [78] | [188] | |||
MMIS = Malignant melanoma in situ. Numbers in square brackets indicate references.
Table 3.
IHC panels for spindle cell neoplasms
| Desmoplastic melanoma | SCC(PD/ sarcomatoid) | AFX | Angiosarcoma | Kaposi's sarcoma | |
|---|---|---|---|---|---|
| Screening | S100 | p63 | CD10 | CD34 | HHV–8 |
| S100 | 83–100% | 0% | 0% | 6% | 0% |
| [26, 27, 192, 193] | [192] | [104, 192, 194, 195] | [108] | [196] | |
| HMB45 | 0% | 0% | 0% | 0% | – |
| [26, 197] | [198] | [194, 195] | [108, 109] | ||
| SOX10 | 100% | 0% | 0% | N/A | – |
| [25, 26, 27, 31] | [31] | [25, 31] | |||
| AE1/AE3 | 0% | 67–100% | 0% | 3–45% | – |
| [192] | [105, 192, 199, 200] | [105, 192, 194, 200] | [108, 113] | ||
| Pan–K | 0% | 40% | 2% | 35% | – |
| [201] | [201] | [201] | [108] | ||
| p63 | 5% | 70–100% | 0–20% | 0–22% | 0% |
| [70, 102] | [102, 105, 199, 200, 201, 202] | [102, 105, 195, 200, 201, 202] | [70] | [70] | |
| CD10 | 0–44% | 14–50% | 83–100% | – | – |
| [102, 104, 192, 201] | [102, 192, 201] | [102, 104, 192, 201, 203] | |||
| CD34 | See text | – | 0% | 21–74% | 89–100% |
| [204, 205, 206] | [195] | [108, 110, 111] | [120, 207] | ||
| ERG | – | 0% | 0% | 100% | 92–100% |
| [113] | [113, 195] | [113] | [208, 209] | ||
| CD31 | – | – | Up to 43% | 33–100% | HIV: 100% [207] |
| [195] | [108, 109, 110, 111, 112] | Non–HIV: 83% [207] | |||
| Not specified: 84% [120] | |||||
| MYC | 5% | – | – | Secondary: 100% | 43–54% |
| [210] | Primary: 0–45% [114, 115, 116, 117] | [211, 212] | |||
| HHV–8 | – | 0% | – | 0% | 95–100% |
| [198] | [118, 213, 214] | [118, 119, 120] | |||
Numbers in square brackets indicate references. Pan–K = Pan–keratin.
Epidermotropic/in situ MCC is a rare entity that frequently expresses Ber-EP4 [79], making this marker a poor choice to differentiate it from EMPD, although cytomorphologic features of these two entities are quite distinct from one another. Amongst intraepidermal lesions, CK20 is the most specific marker of MCC, and this can be added to the panel of diagnostic markers in the appropriate clinical setting or in difficult cases [80,81,82]. We have seen cases where squamous cell carcinoma in situ (SCCIS) and MCC in situ coexist, a rare and diagnostically challenging event that is easily resolved by the use of appropriate immunohistochemical panels.
Sebaceous carcinomas (SC) can have cytomorphologic and architectural overlap with SCC, BCC, melanoma and their variants. SC, when invasive, is a more aggressive tumor than BCC and most SCC, although surgical management can be curative, making accurate diagnosis of high clinical importance. Androgen receptor (AR) is a highly sensitive marker of SC that is useful for differentiating SC from SCC or melanoma. BCCs and EMPD [83,84,85], however, frequently express AR, making it a poor choice to differentiate SC with a similar intraepithelial pattern from these entities [83,85,86,87]. More recent studies have identified adipophilin as a sensitive marker of SC, with high specificity among intraepithelial lesions, although expression in melanoma and EMPD has not been described [83,88,89]. Adipophilin is expressed in a membranous and vesicular pattern due to its association with membrane-bound and vesicle-laden lipids. A granular pattern of staining, sometimes present in other intraepidermal carcinomas like SCC, is not considered positive. Clear cell variants of SCC and BCC are negative for adipophilin, supporting the utility of this marker in differentiating intraepidermal carcinomas with clear cell features. The luminal cells recapitulating ductal differentiation in sweat gland neoplasms can be highlighted by CEA and/or EMA IHC stains [90], which can be helpful to distinguish the in situ component of eccrine, apocrine, and sebaceous neoplasms from EMPD or SCCIS.
Because LCH in the adult is rarely limited to the skin, it is frequently overlooked in the differential diagnosis of intraepidermal lesions. Case reports describe instances in which LCH was mistaken for malignant melanoma due to an epithelioid cytomorphology and positivity for S100 [91], and we have also seen a case of Langerhans cell proliferation within the epidermis associated with a delayed hypersensitivity reaction (so-called Langerhans cell granulomas) mistaken for Pautrier microabscesses of mycosis fungoides, and when on nipple skin, for mammary Paget disease. Both CD1a and langerin are sensitive markers of LCH, and their use should be considered in the diagnosis of intraepidermal lesions. CD1a appears to have greater sensitivity for LCH among histiocytoses, and langerin, which correlates with the expression of Birbeck granules required to differentiate a Langerhans cell from an indeterminate cell, is of greater specificity [92,93]. A panel approach is recommended that includes appropriate markers of epithelial and melanocytic lineage, when the differential diagnosis includes SCCIS, EMPD, and/or Pagetoid melanoma. Because both LCH cells and malignant T cells may express CD4, pan T cell markers (e.g. CD3) should be employed when the differential diagnosis includes mycosis fungoides.
Spindle Cell Tumors in the Dermis
The differential diagnosis for spindle cell lesions in the dermis is quite broad, making IHC invaluable for classifying the many entities that may have overlapping histomorphologies. Here, we present markers used to diagnose some of the most commonly encountered tumors affecting the skin, including desmoplastic melanoma, poorly differentiated/sarcomatoid SCC, AFX, angiosarcoma (AS), and Kaposi's sarcoma (KS). Less common spindled cell tumors involving the dermis include cellular neurothekeoma with spindle cell differentiation, amelanotic cellular blue nevus [26,94], cutaneous leiomyosarcoma/atypical intradermal smooth muscle neoplasms [95] and pleomorphic liposarcoma. In addition, exuberant formation of scar tissue is also common in the differential diagnosis, and it is worth noting that both desmoplastic melanoma and cellular scar tissue may contain cells that are immunoreactive for S100 [26,96,97], and neither may stain with antibodies that generally are associated with melanocytic lineage, such as HMB-45 [26]. Markers of high utility in the accurate diagnosis of dermal spindle cell tumors (table 3), when used in the proper clinical context and in the form of well-selected panels, include keratins (pan-keratin, HMWCK), p63, melanocytic markers (S100, MART-1, and HMB-45), desmin, SMA, and endothelial markers (CD31 and CD34).
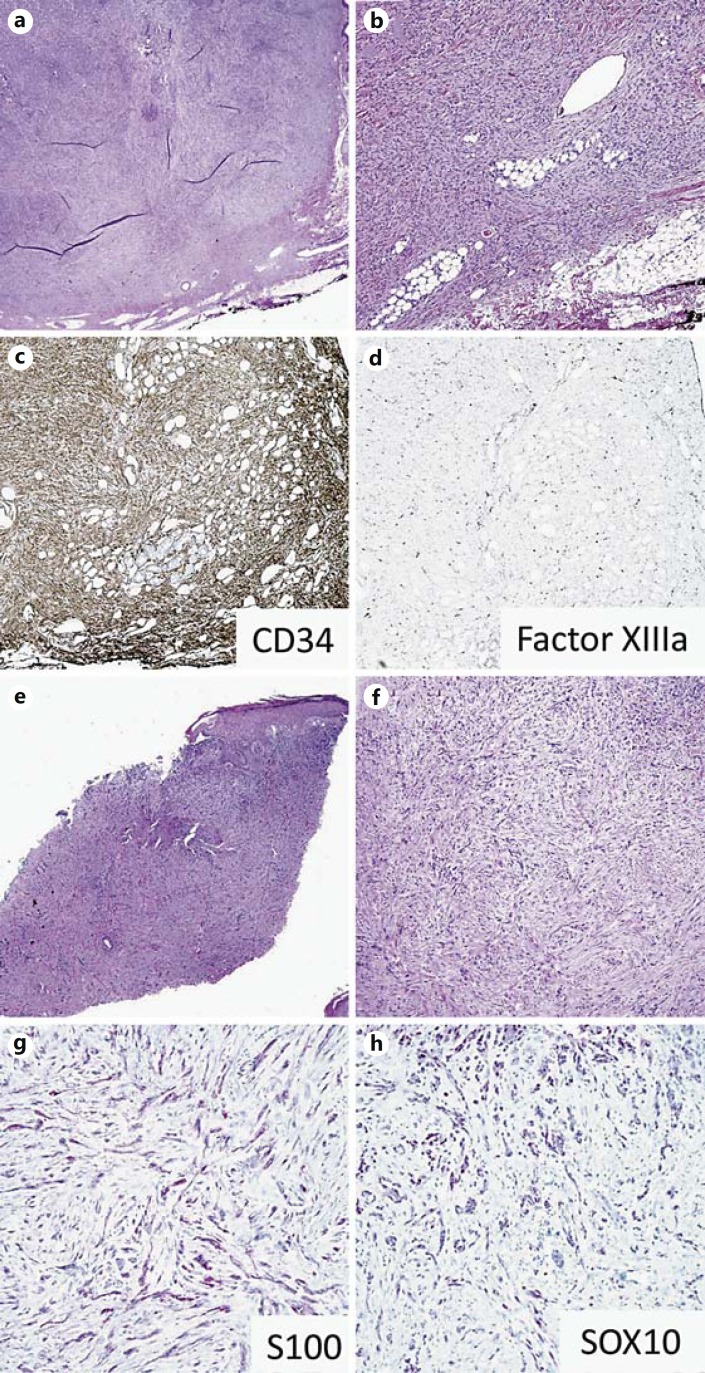
Dermatofibroma is one of the most frequently encountered spindle cell lesions of the dermis, and when cellular, deep, or incompletely sampled, may pose vexing diagnostic challenges. CD34 is helpful in distinguishing dermatofibrosarcoma protuberans (DFSP) from DF and its variants. While DFSP often shows diffuse, strong positivity for CD34 [98,99], DF may have focal staining at the periphery and is positive for factor XIIIa (fig. 7a-d) [99]. However, it is the CD34 negativity that is most helpful in establishing a diagnosis of an unusual or worrisome dermatofibroma because FXIIIa expression lacks specificity (and may even be expressed by stromal cells in desmoplastic melanoma [100], making overinterpretation based on its expression potentially hazardous).
Fig. 7.
IHC markers for dermal spindle cell neoplasms. a-d DFSP tumor cells are positive for CD34 (c) and negative for factor XIIIa (d). e-h Desmoplastic melanoma cells are positive for S100 (g) and SOX10 (h).
In the absence of an intraepidermal component, desmoplastic melanoma can be easily overlooked or mistaken for biopsy/excisional scar, making it a frequent cause of melanoma-related litigation. The utility of SOX10 in diagnosing desmoplastic melanoma due to the paucity of sensitive and specific markers is discussed elsewhere in this article (fig. 7e-h). Although MPNST, schwannomas, and neurofibromas are positive for SOX10, data published to date suggest that most other soft tissue and dermal spindle cell tumors, including spindle cell SCC, AFX, and DFSP are negative for SOX10 [31,101]. Expression of SOX10 in AS and KS remains undescribed.
Immunohistochemical studies for p63 are widely regarded as useful in confirming squamous differentiation, with particular utility in the diagnostic differentiation of poorly differentiated/spindle cell variants of invasive SCC from AFX and melanoma, as well as from some mesenchymal neoplasms [70]. However, a comprehensive analysis of p63 expression in soft tissue neoplasms demonstrated a total lack of immunoreactivity in AS, lipomatous neoplasms, DFSP, solitary fibrous tumor, schwannoma, neurofibroma, and leiomyosarcoma [70]. Nuclear p63 reactivity was found in a subset of soft tissue neoplasms including myoepithelioma, cellular neurothekeoma, soft tissue perineurioma, Ewing's sarcoma/peripheral neuroectodermal tumor, diffuse-type giant cell tumor, and giant cell tumor of soft parts. Infrequent, weak, or focal p63 positivity was observed in low-grade fibromyxoid sarcoma, MPNST, extraskeletal myxoid chondrosarcoma, myxofibrosarcoma, proximal-type epithelioid sarcoma, synovial sarcoma, embryonal rhabdomyosarcoma, desmoplastic small round cell tumor (DSRCT), AFX, and spindle cell melanoma.
When a differential diagnosis includes melanoma, sarcomatoid SCC, and AFX, a panel of S100 and/or SOX10, cytokeratins, and p63 are useful. Within the context of this differential diagnosis, S100 is very sensitive and specific for melanoma (but not specific when other soft tissue entities enter the diagnosis). While some spindle SCCs are negative for cytokeratins, the vast majority retain expression of p63. AFX is a diagnosis of exclusion based on strict histologic criteria and negativity for keratin(s), p63, and melanocytic markers. As previously indicated, a small number of studies suggest weak focal p63 staining in AFX and melanoma [102,103], although this is in sharp contrast to strong, diffuse staining seen in most SCCs. Early studies suggested that CD10 may be a sensitive marker of AFX [102,104]; however, this marker lacks specificity and it is not recommended for inclusion in the panel listed above [105,106]. Of note, KS does not express S100 or p63, although a small percentage of AS may show weak/focal positivity.
KS and AS are occasionally encountered cutaneous soft tissue malignancies [107] that may display diagnostically ambiguous spindled morphology and frequently express a variety of vascular markers including CD31, CD34, and erythroblast transformation-specific related gene (ERG). While these markers are not specific for these tumors, they may be helpful in narrowing the lineage differential among spindled cell dermal lesions. A wide range of sensitivities of CD31 and CD34 have been reported for AS; this likely relates to studies combining data for both radiation-induced and primary tumors from a variety of sites [108,109,110,111,112]. Limited data suggest ERG is a highly sensitive marker of both primary (non-radiation-associated) and radiation-associated AS [113]. MYC is a highly sensitive marker of radiation-associated AS that can effectively differentiate it from a primary (non-radiation-associated) AS and from atypical postradiation vascular proliferations [114,115,116,117]. KS frequently expresses ERG (92-100%) and may also express MYC; however, it may be differentiated from AS, and from all other spindle cell and vascular neoplasms for that matter, based upon HHV-8 positivity, which is generally expressed in patch and plaque lesions, and is 100% specific, among solid tumors (HHV-8 positivity is also associated with some forms of lymphoma and Castleman's disease) [118,119,120]. There are limited data regarding the expression of vascular markers and MYC in SCC, AFX and melanoma, save for rare CD34 positivity in melanoma. However, it must be recalled that tumors showing the phenomenon of vasculogenic mimicry, including melanoma, may contain tumor cells that transcribe proteins encoded by certain aberrantly expresses endothelial genes, such as CD144 (VE cacherin) and TIE-1, but not CD31 [121,122]. Indeed, multiple studies have demonstrated CD34 expression in desmoplastic melanoma, although in >90% of cases, <30% of tumor cells were positive.
Cutaneous Adnexal Tumors
Sclerosing adnexal tumors, particularly microcystic adnexal carcinoma (MAC), desmoplastic trichoepithelioma (DTE) and morpheaform BCC (mBCC), pose frequent diagnostic challenges due to their overlapping histologic characteristics. Differences in tumor behavior (locally aggressive vs. benign) and management (wide local excision vs. conservative management) make accurate diagnosis essential, especially given the predilection of these tumors for head and neck regions where over- or undertreatment can have devastating consequences. Further, other entities, such as cutaneous SCC and metastatic carcinomas with a marked desmoplastic stromal response, enter into the differential diagnosis, with a diagnosis of the latter having critical implications for prognosis and management.
To date no immunohistochemical markers of sclerosing adnexal tumors have been described to provide sufficient sensitivity and specificity for diagnosis when the differential diagnosis is broad. Therefore, when diagnosing sclerosing adnexal tumors, or their mimics, it is best to narrow the differential diagnosis based on histologic and clinical features prior to enlisting the use of markers. Further, data regarding the utility of these markers have been inconsistent across studies. This may be partially due to instances of misdiagnosis, although it more likely relates to differences in the interpretation of ‘positivity’ versus ‘negativity’ due to variations in staining patterns, degree (percentage of labeled cells), and intensity amongst different tumor types and within a single tumor (table 4).
Table 4.
IHC panels for adnexal neoplasms
| MAC | DTE | mBCC | SCC | |
|---|---|---|---|---|
| CEA | 44–58% | 0% | 0% | 0–30% |
| [215, 216] | [215, 216] | [216] | [170, 215] | |
| D2–40 | – | 64% | 6% | 100% |
| [124] | [124] | [187] | ||
| Ber–EP4 | 0–38% | 57–75% | 100% | 0% |
| [135, 216, 217] | [135, 216, 217] | [123, 135, 216, 217] | [170] | |
| EMA | 58% | 60% | 0% | 95% |
| [215] | [215] | [123] | [170] | |
| 34bE12 | – | – | – | 100% [170] |
| CK7 | 100% | 0% | 0% | 22–40% |
| [123] | [123] | [123] | [174, 218] | |
| Cam5.2 | – | – | – | 0% |
| [174] | ||||
| CK5/6 | 100% | 100% | 100% | 70% |
| [125] | [219] | [219] | [220] | |
| CK20 | – | 100% [131, 132, 133] | 0–3% [132, 133] | – |
| (intratumoral Merkel cells) | (intratumoral Merkel cells) | |||
| PHLDA1 | 40% | 88–100% | 0% | – |
| [135] | [134, 135, 136] | [134, 135, 136] | ||
| Androgen R | 0% | 0–13% | 65–100% | 0–10% |
| [125] | [131, 132] | [131, 132] | [83, 85] | |
| p63 | 100% | 100% | 100% | 99–100% |
| [75, 125, 126] | [126] | [126] | [75, 187, 221] | |
| Bcl–2 | 99–100% | 0–89% | 98–100% | 10–67% |
| [123, 124] | [123, 131] | [123, 124 127, 128] | [127, 129, 130] | |
| CK15 | 10–92% | 94–100% | 0–64% | 0–3% |
| [135, 216] | [124, 135, 216] | [124, 135, 216] | [216] | |
| CK17 | 100% | 94–100% | 100% | – |
| [125, 222] | [222] | [223] | ||
| CK19 | 45–89% | 10–11% | 35% | 17% |
| [135, 222] | [135, 222] | [135] | [224] | |
Numbers in square brackets indicate references.
A number of markers may aid in the distinction of MAC from other sclerosing lesions based on the staining pattern instead of straightforward positivity/negativity. Bcl-2 and CK7 are expressed in the central areas of individual MAC tumor nests, while peripheral cells are negative for these markers and positive for p63 [75,123,124,125,126]. DTE, mBCC, and SCC do not display this pattern of staining and instead show either diffuse, patchy, or limited expression of these markers [127,128,129,130]. Of note, the pattern of peripheral p63 expression in MAC is more prominent with increasing tumor depth, and accordingly, the utility of this marker may be limited in superficial biopsies.
AR expression is highly specific for BCC within this differential diagnosis, but the reported sensitivity for morpheaform variants ranges from 65 to 100% [131,132]. Thus, a negative result cannot rule out mBCC. One study suggests that AR in combination with CK20 may have utility in differentiating mBCC from DTE, as CK20-positive Merkel cells frequently infiltrate DTE but are absent in mBCC [133]. However, we are unaware of the regular or reproducible utility of this panel in routine practice. Until recently, Ber-EP4 was accepted as a reliable marker for differentiating mBCC, which is nearly always positive, from MAC, which were putatively negative. More recent data, however, suggest that up to 38% of MACs are positive for Ber-EP4, calling into question the utility of this marker in differentiating these two morphologically similar entities. Pleckstrin homology-like domain, family A, member 1 (PHLDA1), a protein highly expressed in the hair follicle, is a more recently described marker of DTE that is not expressed in BCC, making it useful for differentiating these two entities [134,135,136]. Of note, up to 40% of MACs express PHLDA1, and expression in cutaneous SCC has not been described, making interpretation difficult, if not impossible, when the differential diagnosis includes these entities. Further, mBCC may express PHLDA1 near sites involved by ulceration, making interpretation challenging in superficial biopsies showing this feature. There has been increasing interest in the use of stem cell keratins (CK15 [137,138], CK17 [125] and CK19 [139]) for the diagnosis of sclerosing adnexal tumors, although the data remain limited and variable, and thus these markers should be used with caution.
Finally, metastatic carcinomas may histologically mimic sclerosing adnexal tumors, and the expression of IHC markers, especially keratins, in the former, varies greatly depending on tumor type (i.e. primary site/histogenesis) and degree of differentiation, creating potential diagnostic pitfalls in the diagnosis of cutaneous carcinomas. For example, cutaneous neoplasms with eccrine differentiation are often positive for estrogen receptor (ER) and/or progesterone receptor (PR) [140], and IHC is performed to predict response to hormonal therapy. The differential diagnosis of cutaneous eccrine neoplasms and metastatic breast cancer largely requires clinical and radiological correlation, since both can be positive for ER and PR [141]. Therefore, interpretation of IHC marker expression in cutaneous adnexal neoplasms should be guided by clinical and histologic features to avoid diagnostic pitfalls that could result in clinical harm.
Cutaneous Small Blue Cell Neoplasms
The differential diagnosis for small round blue cell tumors of the skin and subcutis includes primary cutaneous neoplasms (e.g. small cell variants of melanoma and adnexal carcinomas, as well as MCC), certain soft tissue neoplasms, metastatic carcinoma - particularly small cell lung carcinoma, and lymphoma. Prior to utilizing IHC to aid in the diagnosis of these entities, a differential diagnosis based on patient age, lesion location, cancer history and imaging must be formulated, given the significant overlap in marker expression within this category (table 5).
Table 5.
IHC panels for small round blue cell neoplasms
| Ewing's sarcoma/ PNET | Merkel cell | Melanoma | Metastatic lung SCC | DSRCT | |
|---|---|---|---|---|---|
| Keratin | 0–50% | 80–100% | 0–10% | 100% | 69–100% |
| [146, 149, 160, 225] | [79, 147, 226, 227] | [154, 161, 162, 163, 228] | [226] | [31, 32, 153, 159] | |
| CK20 | 0% | 75–100% | 0% | 0% | 0% |
| [146] | [79, 82, 168, 226, 227, 229] | [82, 228] | [80] | [159] | |
| EMA | 16% | 95% | 0–7% | – | 90–96% |
| [160] | [168] | [154, 161, 162, 163] | [151, 152, 159] | ||
| S100 | 38% | 17–22% | 95–100% | – | 0–13% |
| [3] | [79, 82] | [82, 154, 161, 228] | [151, 152] | ||
| LCA | 0% | 0% | – | – | – |
| [230] | [79] | ||||
| Synapto 23–25% | 75–96% | – | 100% | 15–75% | |
| [3, 160] | [79, 168, 226, 227] | [226] | [151, 152, 159] | ||
| NSE | 36–88% | 100% | 30% | 58–100% | 72% |
| [3] | [82] | [82] | [231, 232, 233] | [159] | |
| PAX5 | 0% | 67–94% | 0% | 0–73% | – |
| [234] | [226, 235, 236] | [235, 237] | [226, 236] | ||
| CD99 | 91–100% | 55–100% | 57–60% | – | 0–57% |
| (MIC2) | [3, 142, 143, 144, 145, 146, 147, 148, 149] | [168, 229] | [238, 239] | [145, 151, 152, 153, 154] | |
| FLI–1 | 71–100% | 90–100% | 50–60% | – | 0–87% |
| [147, 148, 149, 150] | [155, 168] | [155, 239] | [150, 155] | ||
| TTF–1 | – | 0–12% | 0% | 90–100% | – |
| [80, 147, 226, 227] | [228] | [80, 169, 226] | |||
| NKX2.2 | 93% [157] | 0% | 33% | 25% | 0% |
| [157] | [157] | [157] | [157] | ||
Numbers in square brackets indicate references.
Within the pediatric and young adult populations, small cell melanoma, Ewing's sarcoma/primitive neuroectodermal tumor (PNET), DSRCT, and rhabdomyosarcoma are frequently in the differential diagnosis of small round blue cell tumors that may be present in the skin and subcutis. Unlike desmoplastic melanoma, a substantial portion of most other morphologic variants of melanoma retain expression of the more specific lineage markers MART-1, MITF, and HMB-45, making them useful in view of the lack of specificity of S100 when small cell variants of melanoma are suspected in the differential diagnosis of small round blue cell tumor. CD99 [142,143,144,145,146,147,148,149] and Friend leukemia virus integration 1 (FLI-1) [147,148,149,150] are the most sensitive markers of Ewing's sarcoma/PNET, although a substantial portion of melanomas, and by some reports, DSRCT, may express one or both of these markers [145,150,151,152,153,154,155]. Further, lymphoblastic lymphoma, a hematopoietic malignancy observed in young adults, expresses CD99 and is frequently negative for leukocyte common antigen (LCA). Molecular analysis to confirm the presence of an EWS-FLI-1 fusion or the associated t(11;22)(q24;q12) translocation is more definitive, although a number of histologically distinct tumors are now known to be associated with this translocation, and not all Ewing's sarcoma/PNET are positive [156]. Of note, NK2 homeobox 2 (NKX2.2) is a recently described sensitive marker of Ewing's sarcoma/PNET with greater specificity compared with CD99 and FLI-1 [157]. Most DSRCTs express EMA [152,158,159], while Ewing's sarcoma/PNET and melanoma are generally negative [154,160,161,162,163], making this a useful marker in a narrowed differential diagnosis. Finally, when rhabdomyosarcoma enters the differential diagnosis, particularly in slightly younger patient populations, myogenin and myogenic differentiation 1 are sensitive markers of this tumor, although other malignancies with rhabdoid features may also express these markers [164,165,166]. In particular, loss of SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B, member 1 (INI-1) expression can aid in distinguishing malignant rhabdoid tumor from rhabdomyosarcoma [167].
Among older patient populations, MCC, metastatic carcinoma, lymphoma and melanoma are in the differential diagnosis of cutaneous small round blue cell tumors. Although MCC and metastatic small cell carcinoma both express neuroendocrine markers [79,168], they may be differentiated in most instances based on paranuclear dot-like expression of CK20 in MCC [79,80,82,168] and TTF-1 expression in metastatic SCLC [80,169]. While lymphomas may have a variety of immunohistochemical profiles, most cases are distinguishable from other small round blue cell tumors based upon their expression of LCA. Of note, there are reports of lymphomas that have lost expression of LCA, again signifying the importance of utilizing a multimarker panel. Further, PAX5 (BSAP) and terminal deoxynucleotidyl transferase (TdT), two markers of some variants of lymphoma, are expressed in a portion of MCCs and thus represent a potential diagnostic pitfall [226,227].
Finally, although rare, some sweat gland tumors may enter this differential diagnosis. While the immunophenotype among different variants of sweat gland tumors is variable, many express p63 [125], while melanoma, small cell carcinoma and soft tissue neoplasms typically do not. Of note, studies suggest some sweat gland tumors may express PAX5 [125], creating a diagnostic dilemma if lymphoma or MCC are in the differential diagnosis.
Conclusion
Herein, we have summarized the existing and more recent literature concerning the use of IHC markers in the diagnosis of cutaneous neoplasms, with specific emphasis on melanoma, epidermal tumors with intraepitheliomatous growth patterns, dermal spindle cell neoplasms, sclerosing adnexal tumors, and dermal small round blue cell tumors. IHC markers will continue to emerge in the field of diagnostic dermatopathology, especially as a result of recent rapid progress in our understanding of the genetic and epigenetic mechanisms of tumorigenesis. However, IHC must always be interpreted within an informed clinical context, a narrowed histologic differential diagnosis, a complementary panel of probes, and rigorous antibody and tissue controls to exclude false-positive and -negative interpretations.
References
- 1.Lian CG, Murphy GF. Histology of the skin. In: Elder DE, et al., editors. Atlas and Synopsis of Lever's Histopathology of the Skin. Philadelphia: Lippincott Williams & Wilkins; 2014. p. 1544. [Google Scholar]
- 2.Lian CG, et al. Skin cancer. In: Stewart BW, Wild CP, editors. World Cancer Report 2014. Lyon: International Agency for Research on Cancer; 2014. [PubMed] [Google Scholar]
- 3.Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387–402. doi: 10.1046/j.1365-2559.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- 4.Troxel DB, Sabella JD. Problem areas in pathology practice. Uncovered by a review of malpractice claims. Am J Surg Pathol. 1994;18:821–831. doi: 10.1097/00000478-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Singh H, Sethi S, Raber M, Petersen LA. Errors in cancer diagnosis: current understanding and future directions. J Clin Oncol. 2007;25:5009–5018. doi: 10.1200/JCO.2007.13.2142. [DOI] [PubMed] [Google Scholar]
- 6.Viray H, Bradley WR, Schalper KA, Rimm DL, Gould Rothberg BE. Marginal and joint distributions of S100, HMB-45, and Melan-A across a large series of cutaneous melanomas. Arch Pathol Lab Med. 2013;137:1063–1073. doi: 10.5858/arpa.2012-0284-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein D, Leininger J, Hamby C, Safai B. Diagnostic and prognostic biomarkers in melanoma. J Clin Aesthet Dermatol. 2014;7:13–24. [PMC free article] [PubMed] [Google Scholar]
- 8.Maize JC, Jr, Resneck JS, Jr, Shapiro PE, McCalmont TH, LeBoit PE. Ducking stray ‘magic bullets’: a Melan-A alert. Am J Dermatopathol. 2003;25:162–165. doi: 10.1097/00000372-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Beltraminelli H, Shabrawi-Caelen LE, Kerl H, Cerroni L. Melan-a-positive ‘pseudomelanocytic nests’: a pitfall in the histopathologic and immunohistochemical diagnosis of pigmented lesions on sun-damaged skin. Am J Dermatopathol. 2009;31:305–308. doi: 10.1097/DAD.0b013e31819d3769. [DOI] [PubMed] [Google Scholar]
- 10.Nybakken GE, Sargen M, Abraham R, Zhang PJ, Ming M, Xu X. MITF accurately highlights epidermal melanocytes in atypical intraepidermal melanocytic proliferations. Am J Dermatopathol. 2013;35:25–29. doi: 10.1097/DAD.0b013e31825666c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean NR, Brennan J, Haynes J, Goddard C, Cooter RD. Immunohistochemical labeling of normal melanocytes. Appl Immunohistochem Mol Morphol. 2002;10:199–204. doi: 10.1097/00129039-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119–129. doi: 10.1111/j.1365-2559.2007.02823.x. [DOI] [PubMed] [Google Scholar]
- 13.Jalas JR, Vemula S, Bezrookove V, Leboit PE, Simko JP, Bastian BC. Metastatic melanoma with striking adenocarcinomatous differentiation illustrating phenotypic plasticity in melanoma. Am J Surg Pathol. 2011;35:1413–1418. doi: 10.1097/PAS.0b013e31822280d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morimoto A, Asai J, Wakabayashi Y, Komori S, Hanada K, Takenaka H, Konishi E, Katoh N. Malignant melanoma with probable smooth muscle differentiation. Case Rep Dermatol. 2014;6:16–19. doi: 10.1159/000358375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tetzlaff MT, Curry JL, Ivan D, Wang WL, Torres-Cabala CA, Bassett RL, Valencia KM, McLemore MS, Ross MI, Prieto VG. Immunodetection of phosphohistone H3 as a surrogate of mitotic figure count and clinical outcome in cutaneous melanoma. Mod Pathol. 2013;26:1153–1160. doi: 10.1038/modpathol.2013.59. [DOI] [PubMed] [Google Scholar]
- 17.Schimming TT, Grabellus F, Roner M, Pechlivanis S, Sucker A, Bielefeld N, Moll I, Schadendorf D, Hillen U. pHH3 immunostaining improves interobserver agreement of mitotic index in thin melanomas. Am J Dermatopathol. 2012;34:266–269. doi: 10.1097/DAD.0b013e31823135a3. [DOI] [PubMed] [Google Scholar]
- 18.Ikenberg K, Pfaltz M, Rakozy C, Kempf W. Immunohistochemical dual staining as an adjunct in assessment of mitotic activity in melanoma. J Cutan Pathol. 2012;39:324–330. doi: 10.1111/j.1600-0560.2011.01858.x. [DOI] [PubMed] [Google Scholar]
- 19.Ladstein RG, Bachmann IM, Straume O, Akslen LA. Ki-67 expression is superior to mitotic count and novel proliferation markers PHH3, MCM4 and mitosin as a prognostic factor in thick cutaneous melanoma. BMC Cancer. 2010;10:140. doi: 10.1186/1471-2407-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen PS, Riber-Hansen R, Jensen TO, Schmidt H, Steiniche T. Proliferation indices of phosphohistone H3 and Ki67: strong prognostic markers in a consecutive cohort with stage I/II melanoma. Mod Pathol. 2013;26:404–413. doi: 10.1038/modpathol.2012.188. [DOI] [PubMed] [Google Scholar]
- 21.Ladstein RG, Bachmann IM, Straume O, Akslen LA. Prognostic importance of the mitotic marker phosphohistone H3 in cutaneous nodular melanoma. J Invest Dermatol. 2012;132:1247–1252. doi: 10.1038/jid.2011.464. [DOI] [PubMed] [Google Scholar]
- 22.Miller DD, Emley A, Yang S, Richards JE, Lee JE, Deng A, Hoang MP, Mahalingam M. Mixed versus pure variants of desmoplastic melanoma: a genetic and immunohistochemical appraisal. Mod Pathol. 2012;25:505–515. doi: 10.1038/modpathol.2011.196. [DOI] [PubMed] [Google Scholar]
- 23.Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433–444. doi: 10.1111/j.1600-0560.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 24.Harris ML, Baxter LL, Loftus SK, Pavan WJ. Sox proteins in melanocyte development and melanoma. Pigment Cell Melanoma Res. 2010;23:496–513. doi: 10.1111/j.1755-148X.2010.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin J, Vincent JG, Cuda JD, Xu H, Kang S, Kim J, Taube JM. Sox10 is expressed in primary melanocytic neoplasms of various histologies but not in fibrohistiocytic proliferations and histiocytoses. J Am Acad Dermatol. 2012;67:717–726. doi: 10.1016/j.jaad.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Herberth FI, Karamchandani J, Kim J, Dadras SS. SOX10 immunostaining distinguishes desmoplastic melanoma from excision scar. J Cutan Pathol. 2010;37:944–952. doi: 10.1111/j.1600-0560.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- 27.Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008;32:1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 28.Cimino-Mathews A, Subhawong AP, Elwood H, Warzecha HN, Sharma R, Park BH, Taube JM, Illei PB, Argani P. Neural crest transcription factor Sox10 is preferentially expressed in triple-negative and metaplastic breast carcinomas. Hum Pathol. 2013;44:959–965. doi: 10.1016/j.humpath.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtomo R, Mori T, Shibata S, Tsuta K, Maeshima AM, Akazawa C, Watabe Y, Honda K, Yamada T, Yoshimoto S, Asai M, Okano H, Kanai Y, Tsuda H. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol. 2013;26:1041–1050. doi: 10.1038/modpathol.2013.54. [DOI] [PubMed] [Google Scholar]
- 30.Ivanov SV, Panaccione A, Nonaka D, Prasad ML, Boyd KL, Brown B, Guo Y, Sewell A, Yarbrough WG. Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br J Cancer. 2013;109:444–451. doi: 10.1038/bjc.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palla B, Su A, Binder S, Dry S. SOX10 expression distinguishes desmoplastic melanoma from its histologic mimics. Am J Dermatopathol. 2013;35:576–581. doi: 10.1097/DAD.0b013e31827a0b98. [DOI] [PubMed] [Google Scholar]
- 32.Hisaoka M, Ishida T, Kuo TT, Matsuyama A, Imamura T, Nishida K, Kuroda H, Inayama Y, Oshiro H, Kobayashi H, Nakajima T, Fukuda T, Ae K, Hashimoto H. Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am J Surg Pathol. 2008;32:452–460. doi: 10.1097/PAS.0b013e31814b18fb. [DOI] [PubMed] [Google Scholar]
- 33.Hantschke M, Mentzel T, Rutten A, Palmedo G, Calonje E, Lazar AJ, Kutzner H. Cutaneous clear cell sarcoma: a clinicopathologic, immunohistochemical, and molecular analysis of 12 cases emphasizing its distinction from dermal melanoma. Am J Surg Pathol. 2010;34:216–222. doi: 10.1097/PAS.0b013e3181c7d8b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis IJ, Kim JJ, Ozsolak F, Widlund HR, Rozenblatt-Rosen O, Granter SR, Du J, Fletcher JA, Denny CT, Lessnick SL, Linehan WM, Kung AL, Fisher DE. Oncogenic MITF dysregulation in clear cell sarcoma: defining the MiT family of human cancers. Cancer Cell. 2006;9:473–484. doi: 10.1016/j.ccr.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 35.Wang WL, Mayordomo E, Zhang W, Hernandez VS, Tuvin D, Garcia L, Lev DC, Lazar AJ, Lopez-Terrada D. Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts) Mod Pathol. 2009;22:1201–1209. doi: 10.1038/modpathol.2009.85. [DOI] [PubMed] [Google Scholar]
- 36.Hodis E, Watson IR, Kryukov GV, Arold ST, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edlundh-Rose E, Egyhazi S, Omholt K, Mansson-Brahme E, Platz A, Hansson J, Lundeberg J. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res. 2006;16:471–478. doi: 10.1097/01.cmr.0000232300.22032.86. [DOI] [PubMed] [Google Scholar]
- 38.Fisher KE, Cohen C, Siddiqui MT, Palma JF, Lipford EH, 3rd, Longshore JW. Accurate detection of BRAF p.V600E mutations in challenging melanoma specimens requires stringent immunohistochemistry scoring criteria or sensitive molecular assays. Hum Pathol. 2014;45:2281–2293. doi: 10.1016/j.humpath.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Pearlstein MV, Zedek DC, Ollila DW, Treece A, Gulley ML, Groben PA, Thomas NE. Validation of the VE1 immunostain for the BRAF V600E mutation in melanoma. J Cutan Pathol. 2014;41:724–732. doi: 10.1111/cup.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Just PA, Audebourg A, Pasmant E, Clauser E, Carlotti A, Laurent S, Avril MF, Vacher-Lavenu MC, Vidaud M, Terris B. Immunohistochemistry versus next-generation sequencing for the routine detection of BRAF V600E mutation in melanomas. Hum Pathol. 2014;45:1983–1984. doi: 10.1016/j.humpath.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Massi D, Simi L, Sensi E, Baroni G, Xue G, Scatena C, Caldarella A, Pinzani P, Fontanini G, Carobbio A, Urso C, Mandala M. Immunohistochemistry is highly sensitive and specific for the detection of NRASQ61R mutation in melanoma. Mod Pathol. 2014 doi: 10.1038/modpathol.2014.137. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42.Murali R, Wiesner T, Scolyer RA. Tumours associated with BAP1 mutations. Pathology. 2013;45:116–126. doi: 10.1097/PAT.0b013e32835d0efb. [DOI] [PubMed] [Google Scholar]
- 43.van Essen TH, van Pelt SI, Versluis M, Bronkhorst IH, van Duinen SG, Marinkovic M, Kroes WG, Ruivenkamp CA, de Klein A, Kilic E, Harbour JW, Luyten GP, van der Velden PA, Verdijk RM, Jager MJ. Prognostic parameters in uveal melanoma and their association with BAP1 expression. Br J Ophthalmol. 2014;98:1738–1743. doi: 10.1136/bjophthalmol-2014-305047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiesner T, Murali R, Fried I, Cerroni L, Busam K, Kutzner H, Bastian BC. A distinct subset of atypical Spitz tumors is characterized by BRAF mutation and loss of BAP1 expression. Am J Surg Pathol. 2012;36:818–830. doi: 10.1097/PAS.0b013e3182498be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lian CG, Xu Y, Ceol C, Wu F, et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150:1135–1146. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larson AR, Dresser KA, Zhan Q, Lezcano C, Woda BA, Yosufi B, Thompson JF, Scolyer RA, Mihm MC, Jr, Shi YG, Murphy GF, Lian CG. Loss of 5-hydroxymethylcytosine correlates with increasing morphologic dysplasia in melanocytic tumors. Mod Pathol. 2014;27:936–944. doi: 10.1038/modpathol.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gambichler T, Sand M, Skrygan M. Loss of 5-hydroxymethylcytosine and ten-eleven translocation 2 protein expression in malignant melanoma. Melanoma Res. 2013;23:218–220. doi: 10.1097/CMR.0b013e32835f9bd4. [DOI] [PubMed] [Google Scholar]
- 48.Uchiyama R, Uhara H, Uchiyama A, Ogawa E, Takazawa Y, Ashida A, Koga H, Hayashi K, Kiniwa Y, Okuyama R. 5-Hydroxymethylcytosine as a useful marker to differentiate between malignant melanomas and benign melanocytic nevi. J Dermatol Sci. 2014;73:161–163. doi: 10.1016/j.jdermsci.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Lee JJ, Granter SR, Laga AC, Saavedra AP, Zhan Q, Guo W, Xu S, Murphy GF, Lian CG. 5-Hydroxymethylcytosine expression in metastatic melanoma versus nodal nevus in sentinel lymph node biopsies. Mod Pathol. 2015;28:218–229. doi: 10.1038/modpathol.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han H, Cortez CC, Yang X, Nichols PW, Jones PA, Liang G. DNA methylation directly silences genes with non-CpG island promoters and establishes a nucleosome occupied promoter. Hum Mol Genet. 2011;20:4299–4310. doi: 10.1093/hmg/ddr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, Chen G, Cheng Y, Martinka M, Li G. Prognostic significance of RUNX3 expression in human melanoma. Cancer. 2011;117:2719–2727. doi: 10.1002/cncr.25838. [DOI] [PubMed] [Google Scholar]
- 52.Gambichler T, Rotterdam S, Radkowski K, Altmeyer P, Kreuter A. Differential expression of microtubule-associated protein 2 in melanocytic skin lesions. Am J Clin Pathol. 2009;131:710–714. doi: 10.1309/AJCPR84ULYVMNJHG. [DOI] [PubMed] [Google Scholar]
- 53.Mourmouras V, Cevenini G, Cosci E, Epistolato MC, Biagioli M, Barbagli L, Luzi P, Mannucci S, Miracco C. Nucleolin protein expression in cutaneous melanocytic lesions. J Cutan Pathol. 2009;36:637–646. doi: 10.1111/j.1600-0560.2008.01126.x. [DOI] [PubMed] [Google Scholar]
- 54.Lade-Keller J, Riber-Hansen R, Guldberg P, Schmidt H, Hamilton-Dutoit SJ, Steiniche T. Immunohistochemical analysis of molecular drivers in melanoma identifies p16 as an independent prognostic biomarker. J Clin Pathol. 2014;67:520–528. doi: 10.1136/jclinpath-2013-202127. [DOI] [PubMed] [Google Scholar]
- 55.Laga AC, Zhan Q, Weishaupt C, Ma J, Frank MH, Murphy GF. SOX2 and nestin expression in human melanoma: an immunohistochemical and experimental study. Exp Dermatol. 2011;20:339–345. doi: 10.1111/j.1600-0625.2011.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Girouard SD, Laga AC, Mihm MC, Scolyer RA, Thompson JF, Zhan Q, Widlund HR, Lee CW, Murphy GF. SOX2 contributes to melanoma cell invasion. Lab Invest. 2012;92:362–370. doi: 10.1038/labinvest.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen PL, Chen WS, Li J, Lind AC, Lu D. Diagnostic utility of neural stem and progenitor cell markers nestin and SOX2 in distinguishing nodal melanocytic nevi from metastatic melanomas. Mod Pathol. 2013;26:44–53. doi: 10.1038/modpathol.2012.132. [DOI] [PubMed] [Google Scholar]
- 58.Massi D, Brusa D, Merelli B, Ciano M, Audrito V, Serra S, Buonincontri R, Baroni G, Nassini R, Minocci D, Cattaneo L, Tamborini E, Carobbio A, Rulli E, Deaglio S, Mandala M. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol. 2014;25:2433–2442. doi: 10.1093/annonc/mdu452. [DOI] [PubMed] [Google Scholar]
- 59.Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27:39–46. doi: 10.1093/intimm/dxu095. [DOI] [PubMed] [Google Scholar]
- 60.Berghoff AS, Ricken G, Widhalm G, Rajky O, Dieckmann K, Birner P, Bartsch R, Holler C, Preusser M. Tumour-infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology. 2015;66:289–299. doi: 10.1111/his.12537. [DOI] [PubMed] [Google Scholar]
- 61.Hodi FS, Lawrence D, Lezcano C, Wu X, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lezcano C, Lee CW, Larson AR, Menzies AM, Kefford RF, Thompson JF, Mihm MC, Jr, Ogino S, Long GV, Scolyer RA, Murphy GF. Evaluation of stromal HGF immunoreactivity as a biomarker for melanoma response to RAF inhibitors. Mod Pathol. 2014;27:1193–1202. doi: 10.1038/modpathol.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alhumaidi A. Practical immunohistochemistry of epithelial skin tumor. Ind J Dermatol Venereol Leprol. 2012;78:698–708. doi: 10.4103/0378-6323.102359. [DOI] [PubMed] [Google Scholar]
- 65.Du X, Yin X, Zhou N, Zhang G, Shi H, Cao S. Extramammary Paget's disease mimicking acantholytic squamous cell carcinoma in situ: a case report. J Cutan Pathol. 2010;37:683–686. doi: 10.1111/j.1600-0560.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 66.Lau J, Kohler S. Keratin profile of intraepidermal cells in Paget's disease, extramammary Paget's disease, and pagetoid squamous cell carcinoma in situ. J Cutan Pathol. 2003;30:449–454. doi: 10.1034/j.1600-0560.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 67.Hida T, Yoneta A, Nishizaka T, Ohmura T, Suzuki Y, Kameshima H, Yamashita T. Pigmented mammary Paget's disease mimicking melanoma: report of three cases. Eur J Dermatol. 2012;22:121–124. doi: 10.1684/ejd.2011.1580. [DOI] [PubMed] [Google Scholar]
- 68.Hilliard NJ, Huang C, Andea A. Pigmented extramammary Paget's disease of the axilla mimicking melanoma: case report and review of the literature. J Cutan Pathol. 2009;36:995–1000. doi: 10.1111/j.1600-0560.2009.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raju RR, Goldblum JR, Hart WR. Pagetoid squamous cell carcinoma in situ (pagetoid Bowen's disease) of the external genitalia. Int J Gynecol Pathol. 2003;22:127–135. doi: 10.1097/00004347-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Jo VY, Fletcher CD. p63 immunohistochemical staining is limited in soft tissue tumors. Am J Clin Pathol. 2011;136:762–766. doi: 10.1309/AJCPXNUC7JZSKWEU. [DOI] [PubMed] [Google Scholar]
- 71.Yao JY, Chen JK. Roles of p63 in epidermal development and tumorigenesis. Biomed J. 2012;35:457–463. doi: 10.4103/2319-4170.104410. [DOI] [PubMed] [Google Scholar]
- 72.Orzol P, Nekulova M, Vojtesek B, Holcakova J. P63 - an important player in epidermal and tumour development. Klin Onkol. 2012;25(suppl 2):2S11–2S15. [PubMed] [Google Scholar]
- 73.Reis-Filho JS, Torio B, Albergaria A, Schmitt FC. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29:517–523. doi: 10.1034/j.1600-0560.2002.290902.x. [DOI] [PubMed] [Google Scholar]
- 74.Park HR, Min SK, Cho HD, Kim KH, Shin HS, Park YE. Expression profiles of p63, p53, survivin, and hTERT in skin tumors. J Cutan Pathol. 2004;31:544–549. doi: 10.1111/j.0303-6987.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 75.Sakiz D, Turkmenoglu TT, Kabukcuoglu F. The expression of p63 and p53 in keratoacanthoma and intraepidermal and invasive neoplasms of the skin. Pathol Res Pract. 2009;205:589–594. doi: 10.1016/j.prp.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 76.Memezawa A, Okuyama R, Tagami H, Aiba S. p63 constitutes a useful histochemical marker for differentiation of pagetoid Bowen's disease from extramammary Paget's disease. Acta Derm Venereol. 2008;88:619–620. doi: 10.2340/00015555-0512. [DOI] [PubMed] [Google Scholar]
- 77.Chen S, Moroi Y, Urabe K, Takeuchi S, Kido M, Hayashida S, Uchi H, Uenotsuchi T, Tu Y, Furue M. Differential expression of two new members of the p53 family, p63 and p73, in extramammary Paget's disease. Clin Exp Dermatol. 2008;33:634–640. doi: 10.1111/j.1365-2230.2008.02851.x. [DOI] [PubMed] [Google Scholar]
- 78.Sellheyer K, Krahl D. Ber-EP4 enhances the differential diagnostic accuracy of cytokeratin 7 in pagetoid cutaneous neoplasms. J Cutan Pathol. 2008;35:366–372. doi: 10.1111/j.1600-0560.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 79.Acebo E, Vidaurrazaga N, Varas C, Burgos-Bretones JJ, Diaz-Perez JL. Merkel cell carcinoma: a clinicopathological study of 11 cases. J Eur Acad Dermatol Venereol. 2005;19:546–551. doi: 10.1111/j.1468-3083.2005.01224.x. [DOI] [PubMed] [Google Scholar]
- 80.Leech SN, Kolar AJ, Barrett PD, Sinclair SA, Leonard N. Merkel cell carcinoma can be distinguished from metastatic small cell carcinoma using antibodies to cytokeratin 20 and thyroid transcription factor 1. J Clin Pathol. 2001;54:727–729. doi: 10.1136/jcp.54.9.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D'Agostino M, Cinelli C, Willard R, Hofmann J, Jellinek N, Robinson-Bostom L. Epidermotropic Merkel cell carcinoma: a case series with histopathologic examination. J Am Acad Dermatol. 2010;62:463–468. doi: 10.1016/j.jaad.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 82.Kontochristopoulos GJ, Stavropoulos PG, Krasagakis K, Goerdt S, Zouboulis CC. Differentiation between Merkel cell carcinoma and malignant melanoma: an immunohistochemical study. Dermatology. 2000;201:123–126. doi: 10.1159/000018454. [DOI] [PubMed] [Google Scholar]
- 83.Ansai S, Takeichi H, Arase S, Kawana S, Kimura T. Sebaceous carcinoma: an immunohistochemical reappraisal. Am J Dermatopathol. 2011;33:579–587. doi: 10.1097/DAD.0b013e31820a2027. [DOI] [PubMed] [Google Scholar]
- 84.Asadi-Amoli F, Khoshnevis F, Haeri H, Jahanzad I, Pazira R, Shahsiah R. Comparative examination of androgen receptor reactivity for differential diagnosis of sebaceous carcinoma from squamous cell and basal cell carcinoma. Am J Clin Pathol. 2010;134:22–26. doi: 10.1309/AJCP89LYTPNVOBAP. [DOI] [PubMed] [Google Scholar]
- 85.Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426–431. doi: 10.1097/00000372-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 86.Inoguchi N, Matsumura Y, Kanazawa N, Morita K, Tachibana T, Sakurai T, Utani A, Miyachi Y. Expression of prostate-specific antigen and androgen receptor in extramammary Paget's disease and carcinoma. Clin Exp Dermatol. 2007;32:91–94. doi: 10.1111/j.1365-2230.2006.02304.x. [DOI] [PubMed] [Google Scholar]
- 87.Liegl B, Horn LC, Moinfar F. Androgen receptors are frequently expressed in mammary and extramammary Paget's disease. Mod Pathol. 2005;18:1283–1288. doi: 10.1038/modpathol.3800437. [DOI] [PubMed] [Google Scholar]
- 88.Ostler DA, Prieto VG, Reed JA, Deavers MT, Lazar AJ, Ivan D. Adipophilin expression in sebaceous tumors and other cutaneous lesions with clear cell histology: an immunohistochemical study of 117 cases. Mod Pathol. 2010;23:567–573. doi: 10.1038/modpathol.2010.1. [DOI] [PubMed] [Google Scholar]
- 89.Muthusamy K, Halbert G, Roberts F. Immunohistochemical staining for adipophilin, perilipin and TIP47. J Clin Pathol. 2006;59:1166–1170. doi: 10.1136/jcp.2005.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robson A, Greene J, Ansari N, Kim B, Seed PT, McKee PH, Calonje E. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710–720. doi: 10.1097/00000478-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 91.Billings TL, Barr R, Dyson S. Langerhans cell histiocytosis mimicking malignant melanoma: a diagnostic pitfall. Am J Dermatopathol. 2008;30:497–499. doi: 10.1097/DAD.0b013e3181812b88. [DOI] [PubMed] [Google Scholar]
- 92.Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of Langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615–619. doi: 10.1097/PAS.0b013e31815b212b. [DOI] [PubMed] [Google Scholar]
- 93.Azumi N, Sheibani K, Swartz WG, Stroup RM, Rappaport H. Antigenic phenotype of Langerhans cell histiocytosis: an immunohistochemical study demonstrating the value of LN-2, LN-3, and vimentin. Hum Pathol. 1988;19:1376–1382. doi: 10.1016/s0046-8177(88)80229-6. [DOI] [PubMed] [Google Scholar]
- 94.Zembowicz A, Granter SR, McKee PH, Mihm MC. Amelanotic cellular blue nevus: a hypopigmented variant of the cellular blue nevus: clinicopathologic analysis of 20 cases. Am J Surg Pathol. 2002;26:1493–1500. doi: 10.1097/00000478-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 95.Kraft S, Fletcher CD. Atypical intradermal smooth muscle neoplasms: clinicopathologic analysis of 84 cases and a reappraisal of cutaneous ‘leiomyosarcoma’. Am J Surg Pathol. 2011;35:599–607. doi: 10.1097/PAS.0b013e31820e6093. [DOI] [PubMed] [Google Scholar]
- 96.Robson A, Allen P, Hollowood K. S100 expression in cutaneous scars: a potential diagnostic pitfall in the diagnosis of desmoplastic melanoma. Histopathology. 2001;38:135–140. doi: 10.1046/j.1365-2559.2001.01066.x. [DOI] [PubMed] [Google Scholar]
- 97.Chorny JA, Barr RJ. S100-positive spindle cells in scars: a diagnostic pitfall in the re-excision of desmoplastic melanoma. Am J Dermatopathol. 2002;24:309–312. doi: 10.1097/00000372-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 98.Mentzel T, Beham A, Katenkamp D, Dei Tos AP, Fletcher CD. Fibrosarcomatous (‘high-grade’) dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576–587. doi: 10.1097/00000478-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 99.Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathol. 1997;19:147–153. doi: 10.1097/00000372-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 100.Carlson JA, Dickersin GR, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. A clinicopathologic analysis of 28 cases. Cancer. 1995;75:478–494. doi: 10.1002/1097-0142(19950115)75:2<478::aid-cncr2820750211>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 101.Karamchandani JR, Nielsen TO, van de Rijn M, West RB. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol. 2012;20:445–450. doi: 10.1097/PAI.0b013e318244ff4b. [DOI] [PubMed] [Google Scholar]
- 102.Kanner WA, Brill LB, 2nd, Patterson JW, Wick MR. CD10, p63 and CD99 expression in the differential diagnosis of atypical fibroxanthoma, spindle cell squamous cell carcinoma and desmoplastic melanoma. J Cutan Pathol. 2010;37:744–750. doi: 10.1111/j.1600-0560.2010.01534.x. [DOI] [PubMed] [Google Scholar]
- 103.Matin RN, Chikh A, Chong SL, Mesher D, Graf M, Sanza P, Senatore V, Scatolini M, Moretti F, Leigh IM, Proby CM, Costanzo A, Chiorino G, Cerio R, Harwood CA, Bergamaschi D. p63 is an alternative p53 repressor in melanoma that confers chemoresistance and a poor prognosis. J Exp Med. 2013;210:581–603. doi: 10.1084/jem.20121439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Feraudy S, Mar N, McCalmont TH. Evaluation of CD10 and procollagen 1 expression in atypical fibroxanthoma and dermatofibroma. Am J Surg Pathol. 2008;32:1111–1122. doi: 10.1097/PAS.0b013e31816b8fce. [DOI] [PubMed] [Google Scholar]
- 105.Hall JM, Saenger JS, Fadare O. Diagnostic utility of P63 and CD10 in distinguishing cutaneous spindle cell/sarcomatoid squamous cell carcinomas and atypical fibroxanthomas. Int J Clin Exp Pathol. 2008;1:524–530. [PMC free article] [PubMed] [Google Scholar]
- 106.Wieland CN, Dyck R, Weenig RH, Comfere NI. The role of CD10 in distinguishing atypical fibroxanthoma from sarcomatoid (spindle cell) squamous cell carcinoma. J Cutan Pathol. 2011;38:884–888. doi: 10.1111/j.1600-0560.2011.01768.x. [DOI] [PubMed] [Google Scholar]
- 107.Rouhani P, Fletcher CD, Devesa SS, Toro JR. Cutaneous soft tissue sarcoma incidence patterns in the US: an analysis of 12,114 cases. Cancer. 2008;113:616–627. doi: 10.1002/cncr.23571. [DOI] [PubMed] [Google Scholar]
- 108.Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683–697. doi: 10.1097/00000478-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 109.Ohsawa M, Naka N, Tomita Y, Kawamori D, Kanno H, Aozasa K. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75:2867–2874. doi: 10.1002/1097-0142(19950615)75:12<2867::aid-cncr2820751212>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 110.Donghi D, Kerl K, Dummer R, Schoenewolf N, Cozzio A. Cutaneous angiosarcoma: own experience over 13 years. Clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230–1234. doi: 10.1111/j.1468-3083.2010.03624.x. [DOI] [PubMed] [Google Scholar]
- 111.Orchard GE, Zelger B, Jones EW, Jones RR. An immunocytochemical assessment of 19 cases of cutaneous angiosarcoma. Histopathology. 1996;28:235–240. doi: 10.1046/j.1365-2559.1996.d01-411.x. [DOI] [PubMed] [Google Scholar]
- 112.DeYoung BR, Swanson PE, Argenyi ZB, Ritter JH, Fitzgibbon JF, Stahl DJ, Hoover W, Wick MR. CD31 immunoreactivity in mesenchymal neoplasms of the skin and subcutis: report of 145 cases and review of putative immunohistologic markers of endothelial differentiation. J Cutan Pathol. 1995;22:215–222. doi: 10.1111/j.1600-0560.1995.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 113.McKay KM, Doyle LA, Lazar AJ, Hornick JL. Expression of ERG, an Ets family transcription factor, distinguishes cutaneous angiosarcoma from histological mimics. Histopathology. 2012;61:989–991. doi: 10.1111/j.1365-2559.2012.04286.x. [DOI] [PubMed] [Google Scholar]
- 114.Fernandez AP, Sun Y, Tubbs RR, Goldblum JR, Billings SD. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. 2012;39:234–242. doi: 10.1111/j.1600-0560.2011.01843.x. [DOI] [PubMed] [Google Scholar]
- 115.Ginter PS, Mosquera JM, Macdonald TY, D'Alfonso TM, Rubin MA, Shin SJ. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014;45:709–716. doi: 10.1016/j.humpath.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 116.Guo T, Zhang L, Chang NE, Singer S, Maki RG, Antonescu CR. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer. 2011;50:25–33. doi: 10.1002/gcc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mentzel T, Schildhaus HU, Palmedo G, Buttner R, Kutzner H. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75–85. doi: 10.1038/modpathol.2011.134. [DOI] [PubMed] [Google Scholar]
- 118.Robin YM, Guillou L, Michels JJ, Coindre JM. Human herpesvirus 8 immunostaining: a sensitive and specific method for diagnosing Kaposi sarcoma in paraffin-embedded sections. Am J Clin Pathol. 2004;121:330–334. doi: 10.1309/96U1-6LRR-AN5H-WWVE. [DOI] [PubMed] [Google Scholar]
- 119.Pereira PF, Cuzzi T, Galhardo MC. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An Bras Dermatol. 2013;88:243–246. doi: 10.1590/S0365-05962013000200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wada DA, Perkins SL, Tripp S, Coffin CM, Florell SR. Human herpesvirus 8 and iron staining are useful in differentiating Kaposi sarcoma from interstitial granuloma annulare. Am J Clin Pathol. 2007;127:263–270. doi: 10.1309/GMH9CENH4909AWVB. [DOI] [PubMed] [Google Scholar]
- 121.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 122.Lezcano C, Kleffel S, Lee N, Larson AR, Zhan Q, DoRosario A, Wang LC, Schatton T, Murphy GF. Merkel cell carcinoma expresses vasculogenic mimicry: demonstration in patients and experimental manipulation in xenografts. Lab Invest. 2014;94:1092–1102. doi: 10.1038/labinvest.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith KJ, Williams J, Corbett D, Skelton H. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464–471. doi: 10.1097/00000478-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 124.Tebcherani AJ, de Andrade HF, Jr, Sotto MN. Diagnostic utility of immunohistochemistry in distinguishing trichoepithelioma and basal cell carcinoma: evaluation using tissue microarray samples. Mod Pathol. 2012;25:1345–1353. doi: 10.1038/modpathol.2012.96. [DOI] [PubMed] [Google Scholar]
- 125.Rollins-Raval M, Chivukula M, Tseng GC, Jukic D, Dabbs DJ. An immunohistochemical panel to differentiate metastatic breast carcinoma to skin from primary sweat gland carcinomas with a review of the literature. Arch Pathol Lab Med. 2011;135:975–983. doi: 10.5858/2009-0445-OAR2. [DOI] [PubMed] [Google Scholar]
- 126.Vidal CI, Goldberg M, Burstein DE, Emanuel HJ, Emanuel PO. p63 immunohistochemistry is a useful adjunct in distinguishing sclerosing cutaneous tumors. Am J Dermatopathol. 2010;32:257–261. doi: 10.1097/DAD.0b013e3181b7fc76. [DOI] [PubMed] [Google Scholar]
- 127.Morales-Ducret CR, van de Rijn M, LeBrun DP, Smoller BR. bcl-2 expression in primary malignancies of the skin. Arch Dermatol. 1995;131:909–912. [PubMed] [Google Scholar]
- 128.Mateoiu C, Pirici A, Bogdan F. Immunohistochemical nuclear staining for p53, PCNA, Ki-67 and bcl-2 in different histologic variants of basal cell carcinoma. Rom J Morphol Embryol. 2011;52:315–319. [PubMed] [Google Scholar]
- 129.Stanimirovic A, Cupic H, Bosnjak B, Tomas D, Kruslin B, Belicza M. TP53, Bcl-2 and growth hormone receptor expression in cutaneous squamous cell carcinoma. Acta Dermatovenerol Croat. 2005;13:201–205. [PubMed] [Google Scholar]
- 130.Abu Juba B, Sovrea A, Crisan D, Melincovici C, Coneac A, Badea M, Crisan M. Apoptotic markers in photoinduced cutaneous carcinoma. Rom J Morphol Embryol. 2013;54:741–747. [PubMed] [Google Scholar]
- 131.Costache M, Bresch M, Boer A. Desmoplastic trichoepithelioma versus morphoeic basal cell carcinoma: a critical reappraisal of histomorphological and immunohistochemical criteria for differentiation. Histopathology. 2008;52:865–876. doi: 10.1111/j.1365-2559.2008.03036.x. [DOI] [PubMed] [Google Scholar]
- 132.Katona TM, Perkins SM, Billings SD. Does the panel of cytokeratin 20 and androgen receptor antibodies differentiate desmoplastic trichoepithelioma from morpheaform/infiltrative basal cell carcinoma? J Cutan Pathol. 2008;35:174–179. doi: 10.1111/j.1600-0560.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 133.Hartschuh W, Schulz T. Merkel cells are integral constituents of desmoplastic trichoepithelioma: an immunohistochemical and electron microscopic study. J Cutan Pathol. 1995;22:413–421. doi: 10.1111/j.1600-0560.1995.tb00756.x. [DOI] [PubMed] [Google Scholar]
- 134.Sellheyer K, Krahl D. PHLDA1 (TDAG51) is a follicular stem cell marker and differentiates between morphoeic basal cell carcinoma and desmoplastic trichoepithelioma. Br J Dermatol. 2011;164:141–147. doi: 10.1111/j.1365-2133.2010.10045.x. [DOI] [PubMed] [Google Scholar]
- 135.Sellheyer K, Nelson P, Kutzner H, Patel RM. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363–370. doi: 10.1111/cup.12085. [DOI] [PubMed] [Google Scholar]
- 136.Yeh I, McCalmont TH, LeBoit PE. Differential expression of PHLDA1 (TDAG51) in basal cell carcinoma and trichoepithelioma. Br J Dermatol. 2012;167:1106–1110. doi: 10.1111/j.1365-2133.2012.11165.x. [DOI] [PubMed] [Google Scholar]
- 137.Mahalingam M, Nguyen LP, Richards JE, Muzikansky A, Hoang MP. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol. 2010;23:713–719. doi: 10.1038/modpathol.2010.46. [DOI] [PubMed] [Google Scholar]
- 138.Mahalingam M, Srivastava A, Hoang MP. Expression of stem-cell markers (cytokeratin 15 and nestin) in primary adnexal neoplasms-clues to etiopathogenesis. Am J Dermatopathol. 2010;32:774–779. doi: 10.1097/DAD.0b013e3181dafd8c. [DOI] [PubMed] [Google Scholar]
- 139.Fernandez-Flores A. Immunohistochemical and morphologic evaluation of primary cutaneous apocrine carcinomas and cutaneous metastases from ductal breast carcinoma. Rom J Morphol Embryol. 2012;53:879–892. [PubMed] [Google Scholar]
- 140.Busam KJ, Tan LK, Granter SR, Kohler S, Junkins-Hopkins J, Berwick M, Rosen PP. Epidermal growth factor, estrogen, and progesterone receptor expression in primary sweat gland carcinomas and primary and metastatic mammary carcinomas. Mod Pathol. 1999;12:786–793. [PubMed] [Google Scholar]
- 141.Wallace ML, Longacre TA, Smoller BR. Estrogen and progesterone receptors and anti-gross cystic disease fluid protein 15 (BRST-2) fail to distinguish metastatic breast carcinoma from eccrine neoplasms. Mod Pathol. 1995;8:897–901. [PubMed] [Google Scholar]
- 142.Lee CS, Southey MC, Waters K, Kannourakis G, Georgiou T, Armes JE, Chow CW, Venter DJ. EWS/FLI-1 fusion transcript detection and MIC2 immunohistochemical staining in the diagnosis of Ewing's sarcoma. Pediatr Pathol Lab Med. 1996;16:379–392. doi: 10.1080/15513819609168678. [DOI] [PubMed] [Google Scholar]
- 143.Hasegawa SL, Davison JM, Rutten A, Fletcher JA, Fletcher CD. Primary cutaneous Ewing's sarcoma: immunophenotypic and molecular cytogenetic evaluation of five cases. Am J Surg Pathol. 1998;22:310–318. doi: 10.1097/00000478-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 144.Ramani P, Rampling D, Link M. Immunocytochemical study of 12E7 in small round-cell tumours of childhood: an assessment of its sensitivity and specificity. Histopathology. 1993;23:557–561. doi: 10.1111/j.1365-2559.1993.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 145.Fellinger EJ, Garin-Chesa P, Triche TJ, Huvos AG, Rettig WJ. Immunohistochemical analysis of Ewing's sarcoma cell surface antigen p30/32MIC2. Am J Pathol. 1991;139:317–325. [PMC free article] [PubMed] [Google Scholar]
- 146.Machado I, Llombart B, Calabuig-Farinas S, Llombart-Bosch A. Superficial Ewing's sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636–643. doi: 10.1111/j.1600-0560.2011.01705.x. [DOI] [PubMed] [Google Scholar]
- 147.Llombart-Bosch A, Machado I, Navarro S, Bertoni F, Bacchini P, Alberghini M, Karzeladze A, Savelov N, Petrov S, Alvarado-Cabrero I, Mihaila D, Terrier P, Lopez-Guerrero JA, Picci P. Histological heterogeneity of Ewing's sarcoma/PNET: an immunohistochemical analysis of 415 genetically confirmed cases with clinical support. Virchows Arch. 2009;455:397–411. doi: 10.1007/s00428-009-0842-7. [DOI] [PubMed] [Google Scholar]
- 148.Mhawech-Fauceglia P, Herrmann F, Penetrante R, Beck A, Sait S, Block AM, Odunsi K, Fisher J, Balos L, Cheney RT. Diagnostic utility of FLI-1 monoclonal antibody and dual-colour, break-apart probe fluorescence in situ (FISH) analysis in Ewing's sarcoma/primitive neuroectodermal tumour (EWS/PNET). A comparative study with CD99 and FLI-1 polyclonal antibodies. Histopathology. 2006;49:569–575. doi: 10.1111/j.1365-2559.2006.02535.x. [DOI] [PubMed] [Google Scholar]
- 149.Folpe AL, Goldblum JR, Rubin BP, Shehata BM, Liu W, Dei Tos AP, Weiss SW. Morphologic and immunophenotypic diversity in Ewing family tumors: a study of 66 genetically confirmed cases. Am J Surg Pathol. 2005;29:1025–1033. [PubMed] [Google Scholar]
- 150.Folpe AL, Hill CE, Parham DM, O'Shea PA, Weiss SW. Immunohistochemical detection of FLI-1 protein expression: a study of 132 round cell tumors with emphasis on CD99-positive mimics of Ewing's sarcoma/primitive neuroectodermal tumor. Am J Surg Pathol. 2000;24:1657–1662. doi: 10.1097/00000478-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 151.Rekhi B, Ahmed S, Basak R, Qureshi SS, Desai SS, Ramadwar M, Desai SB, Kurkure P, Jambhekar NA. Desmoplastic small round cell tumor - clinicopathological spectrum, including unusual features and immunohistochemical analysis of 45 tumors diagnosed at a tertiary cancer referral centre, with molecular results t(11; 22) (p13; q12) (EWS-WT1) in select cases. Pathol Oncol Res. 2012;18:917–927. doi: 10.1007/s12253-012-9522-z. [DOI] [PubMed] [Google Scholar]
- 152.Saab R, Khoury JD, Krasin M, Davidoff AM, Navid F. Desmoplastic small round cell tumor in childhood: the St. Jude Children's Research Hospital experience. Pediatr Blood Cancer. 2007;49:274–279. doi: 10.1002/pbc.20893. [DOI] [PubMed] [Google Scholar]
- 153.Lae ME, Roche PC, Jin L, Lloyd RV, Nascimento AG. Desmoplastic small round cell tumor: a clinicopathologic, immunohistochemical, and molecular study of 32 tumors. Am J Surg Pathol. 2002;26:823–835. doi: 10.1097/00000478-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 154.Drier JK, Swanson PE, Cherwitz DL, Wick MR. S100 protein immunoreactivity in poorly differentiated carcinomas. Immunohistochemical comparison with malignant melanoma. Arch Pathol Lab Med. 1987;111:447–452. [PubMed] [Google Scholar]
- 155.Rossi S, Orvieto E, Furlanetto A, Laurino L, Ninfo V, Dei Tos AP. Utility of the immunohistochemical detection of FLI-1 expression in round cell and vascular neoplasm using a monoclonal antibody. Mod Pathol. 2004;17:547–552. doi: 10.1038/modpathol.3800065. [DOI] [PubMed] [Google Scholar]
- 156.Cantile M, Marra L, Franco R, Ascierto P, Liguori G, De Chiara A, Botti G. Molecular detection and targeting of EWSR1 fusion transcripts in soft tissue tumors. Med Oncol. 2013;30:412. doi: 10.1007/s12032-012-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yoshida A, Sekine S, Tsuta K, Fukayama M, Furuta K, Tsuda H. NKX2.2 is a useful immunohistochemical marker for Ewing sarcoma. Am J Surg Pathol. 2012;36:993–999. doi: 10.1097/PAS.0b013e31824ee43c. [DOI] [PubMed] [Google Scholar]
- 158.Rekhi B, Gorad BD, Chinoy RF. Proximal-type epithelioid sarcoma - a rare, aggressive subtype of epithelioid sarcoma presenting as a recurrent perineal mass in a middle-aged male. World J Surg Oncol. 2007;5:28. doi: 10.1186/1477-7819-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ordonez NG. Desmoplastic small round cell tumor. I. A histopathologic study of 39 cases with emphasis on unusual histological patterns. Am J Surg Pathol. 1998;22:1303–1313. doi: 10.1097/00000478-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 160.Ozdemirli M, Fanburg-Smith JC, Hartmann DP, Azumi N, Miettinen M. Differentiating lymphoblastic lymphoma and Ewing's sarcoma: lymphocyte markers and gene rearrangement. Mod Pathol. 2001;14:1175–1182. doi: 10.1038/modpathol.3880455. [DOI] [PubMed] [Google Scholar]
- 161.Kim YC, Lee MG, Choe SW, Lee MC, Chung HG, Cho SH. Acral lentiginous melanoma: an immunohistochemical study of 20 cases. Int J Dermatol. 2003;42:123–129. doi: 10.1046/j.1365-4362.2003.01583.x. [DOI] [PubMed] [Google Scholar]
- 162.Ben-Izhak O, Stark P, Levy R, Bergman R, Lichtig C. Epithelial markers in malignant melanoma. A study of primary lesions and their metastases. Am J Dermatopathol. 1994;16:241–246. doi: 10.1097/00000372-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 163.Lewis JS, Ritter JH, El-Mofty S. Alternative epithelial markers in sarcomatoid carcinomas of the head and neck, lung, and bladder-p63, MOC-31, and TTF-1. Mod Pathol. 2005;18:1471–1481. doi: 10.1038/modpathol.3800451. [DOI] [PubMed] [Google Scholar]
- 164.Wang NP, Marx J, McNutt MA, Rutledge JC, Gown AM. Expression of myogenic regulatory proteins (myogenin and MyoD1) in small blue round cell tumors of childhood. Am J Pathol. 1995;147:1799–1810. [PMC free article] [PubMed] [Google Scholar]
- 165.Cui S, Hano H, Harada T, Takai S, Masui F, Ushigome S. Evaluation of new monoclonal anti-MyoD1 and anti-myogenin antibodies for the diagnosis of rhabdomyosarcoma. Pathol Int. 1999;49:62–68. doi: 10.1046/j.1440-1827.1999.00825.x. [DOI] [PubMed] [Google Scholar]
- 166.Cessna MH, Zhou H, Perkins SL, Tripp SR, Layfield L, Daines C, Coffin CM. Are myogenin and myoD1 expression specific for rhabdomyosarcoma? A study of 150 cases, with emphasis on spindle cell mimics. Am J Surg Pathol. 2001;25:1150–1157. doi: 10.1097/00000478-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 167.Hoot AC, Russo P, Judkins AR, Perlman EJ, Biegel JA. Immunohistochemical analysis of hSNF5/INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am J Surg Pathol. 2004;28:1485–1491. doi: 10.1097/01.pas.0000141390.14548.34. [DOI] [PubMed] [Google Scholar]
- 168.Llombart B, Monteagudo C, Lopez-Guerrero JA, Carda C, Jorda E, Sanmartin O, Almenar S, Molina I, Martin JM, Llombart-Bosch A. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology. 2005;46:622–634. doi: 10.1111/j.1365-2559.2005.02158.x. [DOI] [PubMed] [Google Scholar]
- 169.Lin X, Saad RS, Luckasevic TM, Silverman JF, Liu Y. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407–414. doi: 10.1097/01.pai.0000210416.53493.0f. [DOI] [PubMed] [Google Scholar]
- 170.Beer TW, Shepherd P, Theaker JM. Ber EP4 and epithelial membrane antigen aid distinction of basal cell, squamous cell and basosquamous carcinomas of the skin. Histopathology. 2000;37:218–223. doi: 10.1046/j.1365-2559.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 171.Sinard JH. Immunohistochemical distinction of ocular sebaceous carcinoma from basal cell and squamous cell carcinoma. Arch Ophthalmol. 1999;117:776–783. doi: 10.1001/archopht.117.6.776. [DOI] [PubMed] [Google Scholar]
- 172.Mori O, Hachisuka H, Sasai Y. Immunohistochemical demonstration of epithelial membrane antigen (EMA), carcinoembryonic antigen (CEA), and keratin on mammary and extramammary Paget's disease. Acta Histochem. 1989;85:93–100. doi: 10.1016/S0065-1281(89)80104-7. [DOI] [PubMed] [Google Scholar]
- 173.Zhou JH, Kim KB, Myers JN, Fox PS, Ning J, Bassett RL, Hasanein H, Prieto VG. Immunohistochemical expression of hormone receptors in melanoma of pregnant women, nonpregnant women, and men. Am J Dermatopathol. 2014;36:74–79. doi: 10.1097/DAD.0b013e3182914c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Clarke LE, Conway AB, Warner NM, Barnwell PN, Sceppa J, Helm KF. Expression of CK7, Cam 5.2 and Ber-Ep4 in cutaneous squamous cell carcinoma. J Cutan Pathol. 2013;40:646–650. doi: 10.1111/cup.12135. [DOI] [PubMed] [Google Scholar]
- 175.Layfield L, Ulich T, Liao S, Barr R, Cheng L, Lewin KL. Neuroendocrine carcinoma of the skin: an immunohistochemical study of tumor markers and neuroendocrine products. J Cutan Pathol. 1986;13:268–273. doi: 10.1111/j.1600-0560.1986.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 176.Poniecka AW, Alexis JB. An immunohistochemical study of basal cell carcinoma and trichoepithelioma. Am J Dermatopathol. 1999;21:332–336. doi: 10.1097/00000372-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 177.Ahrens WA, Folpe AL. CD1a immunopositivity in perivascular epithelioid cell neoplasms: true expression or technical artifact? A streptavidin-biotin and polymer-based detection system immunohistochemical study of perivascular epithelioid cell neoplasms and their morphologic mimics. Hum Pathol. 2011;42:369–374. doi: 10.1016/j.humpath.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 178.Galan A, Ko CJ. Langerhans cells in squamous cell carcinoma vs. pseudoepitheliomatous hyperplasia of the skin. J Cutan Pathol. 2007;34:950–952. doi: 10.1111/j.1600-0560.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 179.Ko CJ, Kim J, Phan J, Binder SW. Bcl-2-positive epidermal dendritic cells in inverted follicular keratoses but not squamous cell carcinomas or seborrheic keratosis. J Cutan Pathol. 2006;33:498–501. doi: 10.1111/j.1600-0560.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 180.Xu X, Chu AY, Pasha TL, Elder DE, Zhang PJ. Immunoprofile of MITF, tyrosinase, melan-A, and MAGE-1 in HMB45-negative melanomas. Am J Surg Pathol. 2002;26:82–87. doi: 10.1097/00000478-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 181.Ohnishi T, Watanabe S. The use of cytokeratins 7 and 20 in the diagnosis of primary and secondary extramammary Paget's disease. Br J Dermatol. 2000;142:243–247. doi: 10.1046/j.1365-2133.2000.03291.x. [DOI] [PubMed] [Google Scholar]
- 182.Schirren CG, Rutten A, Kaudewitz P, Diaz C, McClain S, Burgdorf WH. Trichoblastoma and basal cell carcinoma are neoplasms with follicular differentiation sharing the same profile of cytokeratin intermediate filaments. Am J Dermatopathol. 1997;19:341–350. doi: 10.1097/00000372-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 183.Mai KT, Alhalouly T, Landry D, Stinson WA, Perkins DG, Yazdi HM. Pagetoid variant of actinic keratosis with or without squamous cell carcinoma of sun-exposed skin: a lesion simulating extramammary Paget's disease. Histopathology. 2002;41:331–336. doi: 10.1046/j.1365-2559.2002.01523.x. [DOI] [PubMed] [Google Scholar]
- 184.Johansson L, Tennvall J, Akerman M. Immunohistochemical examination of 25 cases of Merkel cell carcinoma: a comparison with small cell carcinoma of the lung and oesophagus, and a review of the literature. APMIS. 1990;98:741–752. doi: 10.1111/j.1699-0463.1990.tb04995.x. [DOI] [PubMed] [Google Scholar]
- 185.Stetsenko GY, Malekirad J, Paulson KG, Iyer JG, Thibodeau RM, Nagase K, Schmidt M, Storer BE, Argenyi ZB, Nghiem P. p63 expression in Merkel cell carcinoma predicts poorer survival yet may have limited clinical utility. Am J Clin Pathol. 2013;140:838–844. doi: 10.1309/AJCPE4PK6CTBNQJY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Qureshi HS, Ormsby AH, Lee MW, Zarbo RJ, Ma CK. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145–152. doi: 10.1111/j.0303-6987.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 187.Plaza JA, Ortega PF, Stockman DL, Suster S. Value of p63 and podoplanin (D2-40) immunoreactivity in the distinction between primary cutaneous tumors and adenocarcinomas metastatic to the skin: a clinicopathologic and immunohistochemical study of 79 cases. J Cutan Pathol. 2010;37:403–410. doi: 10.1111/j.1600-0560.2010.01517.x. [DOI] [PubMed] [Google Scholar]
- 188.Orchard G. Evaluation of melanocytic neoplasms: application of a pan-melanoma antibody cocktail. Br J Biomed Sci. 2002;59:196–202. doi: 10.1080/09674845.2002.11783659. [DOI] [PubMed] [Google Scholar]
- 189.Rosen L, Amazon K, Frank B. Bowen's disease, Paget's disease, and malignant melanoma in situ. South Med J. 1986;79:410–413. doi: 10.1097/00007611-198604000-00005. [DOI] [PubMed] [Google Scholar]
- 190.Ordonez NG, Awalt H, Mackay B. Mammary and extramammary Paget's disease. An immunocytochemical and ultrastructural study. Cancer. 1987;59:1173–1183. doi: 10.1002/1097-0142(19870315)59:6<1173::aid-cncr2820590624>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 191.Glasgow BJ, Wen DR, Al-Jitawi S, Cochran AJ. Antibody to S-100 protein aids the separation of pagetoid melanoma from mammary and extramammary Paget's disease. J Cutan Pathol. 1987;14:223–226. doi: 10.1111/j.1600-0560.1987.tb01337.x. [DOI] [PubMed] [Google Scholar]
- 192.Hultgren TL, DiMaio DJ. Immunohistochemical staining of CD10 in atypical fibroxanthomas. J Cutan Pathol. 2007;34:415–419. doi: 10.1111/j.1600-0560.2006.00635.x. [DOI] [PubMed] [Google Scholar]
- 193.Anstey A, Cerio R, Ramnarain N, Orchard G, Smith N, Jones EW. Desmoplastic malignant melanoma. An immunocytochemical study of 25 cases. Am J Dermatopathol. 1994;16:14–22. doi: 10.1097/00000372-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 194.Beer TW, Drury P, Heenan PJ. Atypical fibroxanthoma: a histological and immunohistochemical review of 171 cases. Am J Dermatopathol. 2010;32:533–540. doi: 10.1097/DAD.0b013e3181c80b97. [DOI] [PubMed] [Google Scholar]
- 195.Thum C, Husain EA, Mulholland K, Hornick JL, Brenn T. Atypical fibroxanthoma with pseudoangiomatous features: a histological and immunohistochemical mimic of cutaneous angiosarcoma. Ann Diagn Pathol. 2013;17:502–507. doi: 10.1016/j.anndiagpath.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 196.Nickoloff BJ, Griffiths CE. The spindle-shaped cells in cutaneous Kaposi's sarcoma. Histologic simulators include factor XIIIa dermal dendrocytes. Am J Pathol. 1989;135:793–800. [PMC free article] [PubMed] [Google Scholar]
- 197.Longacre TA, Egbert BM, Rouse RV. Desmoplastic and spindle-cell malignant melanoma. An immunohistochemical study. Am J Surg Pathol. 1996;20:1489–1500. doi: 10.1097/00000478-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 198.Iyer PV, Leong AS. Poorly differentiated squamous cell carcinomas of the skin can express vimentin. J Cutan Pathol. 1992;19:34–39. doi: 10.1111/j.1600-0560.1992.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 199.Morgan MB, Purohit C, Anglin TR. Immunohistochemical distinction of cutaneous spindle cell carcinoma. Am J Dermatopathol. 2008;30:228–232. doi: 10.1097/DAD.0b013e31816de820. [DOI] [PubMed] [Google Scholar]
- 200.Gleason BC, Calder KB, Cibull TL, Thomas AB, Billings SD, Morgan MB, Hiatt KM, Smoller BR. Utility of p63 in the differential diagnosis of atypical fibroxanthoma and spindle cell squamous cell carcinoma. J Cutan Pathol. 2009;36:543–547. doi: 10.1111/j.1600-0560.2008.01099.x. [DOI] [PubMed] [Google Scholar]
- 201.Buonaccorsi JN, Plaza JA. Role of CD10, wide-spectrum keratin, p63, and podoplanin in the distinction of epithelioid and spindle cell tumors of the skin: an immunohistochemical study of 81 cases. Am J Dermatopathol. 2012;34:404–411. doi: 10.1097/DAD.0b013e318236b17f. [DOI] [PubMed] [Google Scholar]
- 202.Dotto JE, Glusac EJ. p63 is a useful marker for cutaneous spindle cell squamous cell carcinoma. J Cutan Pathol. 2006;33:413–417. doi: 10.1111/j.0303-6987.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 203.Clarke LE, Frauenhoffer E, Fox E, Neves R, Bruggeman RD, Helm KF. CD10-positive myxofibrosarcomas: a pitfall in the differential diagnosis of atypical fibroxanthoma. J Cutan Pathol. 2010;37:737–743. doi: 10.1111/j.1600-0560.2010.01532.x. [DOI] [PubMed] [Google Scholar]
- 204.Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625–630. doi: 10.1111/j.1600-0560.2011.01700.x. [DOI] [PubMed] [Google Scholar]
- 205.Hoang MP, Selim MA, Bentley RC, Burchette JL, Shea CR. CD34 expression in desmoplastic melanoma. J Cutan Pathol. 2001;28:508–512. doi: 10.1034/j.1600-0560.2001.281003.x. [DOI] [PubMed] [Google Scholar]
- 206.Husain S, Silvers DN. Fingerprint CD34 immunopositivity to distinguish neurofibroma from an early/paucicellular desmoplastic melanoma can be misleading. J Cutan Pathol. 2013;40:985–987. doi: 10.1111/cup.12206. [DOI] [PubMed] [Google Scholar]
- 207.Rosado FG, Itani DM, Coffin CM, Cates JM. Utility of immunohistochemical staining with FLI1, D2-40, CD31, and CD34 in the diagnosis of acquired immunodeficiency syndrome-related and non-acquired immunodeficiency syndrome-related Kaposi sarcoma. Arch Pathol Lab Med. 2012;136:301–304. doi: 10.5858/arpa.2011-0213-OA. [DOI] [PubMed] [Google Scholar]
- 208.Miettinen M, Wang ZF, Paetau A, Tan SH, Dobi A, Srivastava S, Sesterhenn I. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol. 2011;35:432–441. doi: 10.1097/PAS.0b013e318206b67b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Minner S, Luebke AM, Kluth M, Bokemeyer C, Janicke F, Izbicki J, Schlomm T, Sauter G, Wilczak W. High level of Ets-related gene expression has high specificity for prostate cancer: a tissue microarray study of 11 483 cancers. Histopathology. 2012;61:445–453. doi: 10.1111/j.1365-2559.2012.04240.x. [DOI] [PubMed] [Google Scholar]
- 210.Weissinger SE, Keil P, Silvers DN, Klaus BM, Moller P, Horst BA, Lennerz JK. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524–534. doi: 10.1038/modpathol.2013.162. [DOI] [PubMed] [Google Scholar]
- 211.Spandidos DA, Kaloterakis A, Yiagnisis M, Varatsos A, Field JK. Ras, C-myc and C-erbB-2 oncoprotein expression in non-AIDS Mediterranean Kaposi's sarcoma. Anticancer Res. 1990;10:1619–1625. [PubMed] [Google Scholar]
- 212.Feller JK, Mahalingam M. c-myc and cutaneous vascular neoplasms. Am J Dermatopathol. 2013;35:364–369. doi: 10.1097/DAD.0b013e31827aad83. [DOI] [PubMed] [Google Scholar]
- 213.Schmid H, Zietz C. Human herpesvirus 8 and angiosarcoma: analysis of 40 cases and review of the literature. Pathology. 2005;37:284–287. doi: 10.1080/00313020500169495. [DOI] [PubMed] [Google Scholar]
- 214.Cheuk W, Wong KO, Wong CS, Dinkel JE, Ben-Dor D, Chan JK. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335–342. doi: 10.1309/B8TC-0LBV-H8XY-5MFV. [DOI] [PubMed] [Google Scholar]
- 215.Wick MR, Cooper PH, Swanson PE, Kaye VN, Sun TT. Microcystic adnexal carcinoma. An immunohistochemical comparison with other cutaneous appendage tumors. Arch Dermatol. 1990;126:189–194. doi: 10.1001/archderm.126.2.189. [DOI] [PubMed] [Google Scholar]
- 216.Hoang MP, Dresser KA, Kapur P, High WA, Mahalingam M. Microcystic adnexal carcinoma: an immunohistochemical reappraisal. Mod Pathol. 2008;21:178–185. doi: 10.1038/modpathol.3801000. [DOI] [PubMed] [Google Scholar]
- 217.Krahl D, Sellheyer K. Monoclonal antibody Ber-EP4 reliably discriminates between microcystic adnexal carcinoma and basal cell carcinoma. J Cutan Pathol. 2007;34:782–787. doi: 10.1111/j.1600-0560.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 218.Pulitzer M, Desman G, Busam KJ. CK7 expression in primary cutaneous squamous cell carcinoma. J Cutan Pathol. 2010;37:966–972. doi: 10.1111/j.1600-0560.2010.01562.x. [DOI] [PubMed] [Google Scholar]
- 219.Plumb SJ, Argenyi ZB, Stone MS, De Young BR. Cytokeratin 5/6 immunostaining in cutaneous adnexal neoplasms and metastatic adenocarcinoma. Am J Dermatopathol. 2004;26:447–451. doi: 10.1097/00000372-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 220.Sigel JE, Skacel M, Bergfeld WF, House NS, Rabkin MS, Goldblum JR. The utility of cytokeratin 5/6 in the recognition of cutaneous spindle cell squamous cell carcinoma. J Cutan Pathol. 2001;28:520–524. doi: 10.1034/j.1600-0560.2001.281005.x. [DOI] [PubMed] [Google Scholar]
- 221.Abbas O, Richards JE, Yaar R, Mahalingam M. Stem cell markers (cytokeratin 15, cytokeratin 19 and p63) in in situ and invasive cutaneous epithelial lesions. Mod Pathol. 2011;24:90–97. doi: 10.1038/modpathol.2010.180. [DOI] [PubMed] [Google Scholar]
- 222.Tse JY, Nguyen AT, Le LP, Hoang MP. Microcystic adnexal carcinoma versus desmoplastic trichoepithelioma: a comparative study. Am J Dermatopathol. 2013;35:50–55. doi: 10.1097/DAD.0b013e31825988df. [DOI] [PubMed] [Google Scholar]
- 223.Anderson-Dockter H, Clark T, Iwamoto S, Lu M, Fiore D, Falanga JK, Falanga V. Diagnostic utility of cytokeratin 17 immunostaining in morpheaform basal cell carcinoma and for facilitating the detection of tumor cells at the surgical margins. Dermatol Surg. 2012;38:1357–1366. doi: 10.1111/j.1524-4725.2012.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Abbas O, Bhawan J. Expression of stem cell markers nestin and cytokeratin 15 and 19 in cutaneous malignancies. J Eur Acad Dermatol Venereol. 2011;25:311–316. doi: 10.1111/j.1468-3083.2010.03791.x. [DOI] [PubMed] [Google Scholar]
- 225.Collini P, Sampietro G, Bertulli R, Casali PG, Luksch R, Mezzelani A, Sozzi G, Pilotti S. Cytokeratin immunoreactivity in 41 cases of ES/PNET confirmed by molecular diagnostic studies. Am J Surg Pathol. 2001;25:273–274. doi: 10.1097/00000478-200102000-00022. [DOI] [PubMed] [Google Scholar]
- 226.Kolhe R, Reid MD, Lee JR, Cohen C, Ramalingam P. Immunohistochemical expression of PAX5 and TdT by Merkel cell carcinoma and pulmonary small cell carcinoma: a potential diagnostic pitfall but useful discriminatory marker. Int J Clin Exp Pathol. 2013;6:142–147. [PMC free article] [PubMed] [Google Scholar]
- 227.Buresh CJ, Oliai BR, Miller RT. Reactivity with TdT in Merkel cell carcinoma: a potential diagnostic pitfall. Am J Clin Pathol. 2008;129:894–898. doi: 10.1309/R494HQ9VRDJWDY30. [DOI] [PubMed] [Google Scholar]
- 228.Drlicek M, Bodenteich A, Urbanits S, Grisold W. Immunohistochemical panel of antibodies in the diagnosis of brain metastases of the unknown primary. Pathol Res Pract. 2004;200:727–734. doi: 10.1016/j.prp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 229.Rajagopalan A, Browning D, Salama S. CD99 expression in Merkel cell carcinoma: a case series with an unusual paranuclear dot-like staining pattern. J Cutan Pathol. 2013;40:19–24. doi: 10.1111/cup.12049. [DOI] [PubMed] [Google Scholar]
- 230.Pilkington GR, Pallesen G. Phenotypic characterization of non-haemopoietic small cell tumours of childhood with monoclonal antibodies to leucocytes, epithelial cells and cytoskeletal proteins. Histopathology. 1989;14:347–357. doi: 10.1111/j.1365-2559.1989.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 231.Sheppard MN, Corrin B, Bennett MH, Marangos PJ, Bloom SR, Polak JM. Immunocytochemical localization of neuron specific enolase in small cell carcinomas and carcinoid tumours of the lung. Histopathology. 1984;8:171–181. doi: 10.1111/j.1365-2559.1984.tb02334.x. [DOI] [PubMed] [Google Scholar]
- 232.Bergh J, Esscher T, Steinholtz L, Nilsson K, Pahlman S. Immunocytochemical demonstration of neuron-specific enolase (NSE) in human lung cancers. Am J Clin Pathol. 1985;84:1–7. doi: 10.1093/ajcp/84.1.1. [DOI] [PubMed] [Google Scholar]
- 233.Reeve JG, Stewart J, Watson JV, Wulfrank D, Twentyman PR, Bleehen NM. Neuron specific enolase expression in carcinoma of the lung. Br J Cancer. 1986;53:519–528. doi: 10.1038/bjc.1986.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sullivan LM, Atkins KA, LeGallo RD. PAX immunoreactivity identifies alveolar rhabdomyosarcoma. Am J Surg Pathol. 2009;33:775–780. doi: 10.1097/PAS.0b013e318191614f. [DOI] [PubMed] [Google Scholar]
- 235.Mhawech-Fauceglia P, Saxena R, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, Penetrante R. Pax-5 immunoexpression in various types of benign and malignant tumours: a high-throughput tissue microarray analysis. J Clin Pathol. 2007;60:709–714. doi: 10.1136/jcp.2006.039917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dong HY, Liu W, Cohen P, Mahle CE, Zhang W. B-cell specific activation protein encoded by the PAX-5 gene is commonly expressed in Merkel cell carcinoma and small cell carcinomas. Am J Surg Pathol. 2005;29:687–692. doi: 10.1097/01.pas.0000155162.33044.4f. [DOI] [PubMed] [Google Scholar]
- 237.Desouki MM, Post GR, Cherry D, Lazarchick J. PAX-5: a valuable immunohistochemical marker in the differential diagnosis of lymphoid neoplasms. Clin Med Res. 2010;8:84–88. doi: 10.3121/cmr.2010.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wilkerson AE, Glasgow MA, Hiatt KM. Immunoreactivity of CD99 in invasive malignant melanoma. J Cutan Pathol. 2006;33:663–666. doi: 10.1111/j.1600-0560.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- 239.Torlakovic EE, Slipicevic A, Florenes VA, Chibbar R, DeCoteau JF, Bilalovic N. Fli-1 expression in malignant melanoma. Histol Histopathol. 2008;23:1309–1314. doi: 10.14670/HH-23.1309. [DOI] [PubMed] [Google Scholar]