Abstract
Combining the latest targeted biologic agents with the most advanced radiation technologies has been an exciting development in the treatment of cancer patients. Stereotactic body radiation therapy (SBRT) is an ablative radiation approach that has become established for the treatment of a variety of malignancies, and it has been increasingly used in combination with biologic agents, including those targeting angiogenesis-specific pathways. Multiple reports have emerged describing unanticipated toxicities arising from the combination of SBRT and angiogenesis-targeting agents, particularly of late luminal gastrointestinal toxicities. In this review, we summarize the literature describing these toxicities, explore the biological mechanism of action of toxicity with the combined use of antiangiogenic therapies, and discuss areas of future research, so that this combination of treatment modalities can continue to be used in broader clinical contexts.
Introduction
Since the recognition of angiogenesis in the 1970s as playing a vital role in tumor growth, a process largely dependent on the vascular endothelial growth factor (VEGF) pathway (1), multiple antiangiogenic agents have been and are currently being studied in clinical trials, and many are now approved for the treatment of colon (2–4), lung (5, 6), brain (7), and hepatocellular carcinoma (8, 9); renal cell carcinoma (10); and thyroid carcinoma (11). Although these events have shown promising antitumor effect, their efficacy when used as monotherapy is limited by adverse effects, acquired resistance (12), and rapid vascular regrowth after removal of anti-VEGF therapy (13). As a result, these agents have been integrated with conventional cancer therapies, including radiation, to enhance antitumor activity.
Serious toxicities from VEGF inhibitors (VEGFIs) were initially unexpected because they were believed to interfere with growth factor signaling pathways in proliferating endothelial cells but not in the nonproliferating endothelial cells of the established vasculature. However, vascular-related side effects have been observed with the clinical development of these agents, including hypertension, hemorrhage, and thromboembolism, and a 1% to 2% risk of gastrointestinal (GI) perforation (14). Although several trials have shown that the addition of conventionally fractionated radiation therapy to antiangiogenic agents is well tolerated (15, 16), there have been reports showing increased luminal GI toxicity with the combination (17–19).
The role of stereotactic body radiation therapy (SBRT), also known as stereotactic ablative radiation therapy (SABR), has similarly become an area of rapid expansion and active investigation. Advances in image guidance, respiratory motion management, and treatment planning and delivery systems have enabled SBRTand have resulted in a shift from the paradigm of fractionation that was established by radiobiologic experiments in the 1920s. SBRT allows the delivery of large doses of radiation, with rapid dose falloff at the periphery of the target that has allowed for a significant reduction in the volume of normal tissue irradiated. SBRT has rapidly gained acceptance in the treatment of lung (20), liver (21), spine (22), kidney (23), and pancreas tumors (24). The indications for SBRT are expanding, particularly in the setting of oligometastatic disease (25).
The safety of SBRT, however, is predicated on avoiding organs at risk in the delivery of high-dose radiation. Toxicity is a concern when the tumor is in close proximity to sensitive GI structures. Dose-dependent GI ulceration and perforation have been reported in patients treated with SBRT for abdominal lesions (24, 26) and lesions of the spine and lung (27, 28). With increased use of both SBRT and anti-VEGF agents, reports have arisen describing unanticipated late luminal GI toxicities when these agents are used in combination, which is particularly alarming given that our understanding of normal organ tolerance with SBRT is still in its infancy. Improved understanding of this potential risk is critical to preserve the safety of both novel treatment modalities and to continue expanding their use in broader clinical contexts. In this review, we summarize the available clinical literature describing these toxicities associated with combined SBRT and anti-VEGF therapy, explore possible biological mechanisms for this potential interaction, and recommend areas of future investigation.
Clinical Reports of GI Toxicity With SBRT and Antiangiogenic Agents
Combining radiosensitizing chemotherapy with conventional radiation therapy has long been used to enhance treatment efficacy, though with increased treatment morbidity (29). Conversely, radiosensitization has not been routinely combined with SBRT, given the high rates of local control with SBRT alone, and from concern for an increased risk of toxicity, given that normal organ radiation tolerance is poorly understood in the setting of large doses per fraction. Given the increasing indications for SBRT and antiangiogenic agents, their combined use may be inevitable. However, data have emerged suggesting increased toxicity risk when SBRT is combined with antiangiogenic therapy.
To review the literature reporting luminal GI toxicity with SBRT, a PubMed search was performed using these terms: VEGF, angiogenic, SBRT, SABR, radiation, radio-surgery, gastrointestinal toxicity, and TKI. The search was limited to articles in the English language. Relevant prospective and retrospective studies that reported ≥grade 3 luminal GI toxicities with the use of SBRT and an anti-VEGF agent were selected and are discussed here and summarized in Table 1. We contacted study authors for any missing data.
Table 1.
Clinical reports of luminal GI toxicity with SBRT and hypofractionated radiation therapy and anti-VEGF agents
| Study | Patient | % of patients treated with anti-VEGF agent and SBRT | Radiation schedule | Anti-VEGF agent | Toxicity | In-field toxicity? |
|---|---|---|---|---|---|---|
| Peters et al (30) | 1 patient with renal cell carcinoma | N/A | 8 Gy in 1 fraction | Sorafenib 400 mg twice daily for 5 weeks before and 3 days after RT | Grade 5 colon perforations, 1 week after SBRT | Yes |
| Lordick et al (31) | 1 patient with renal cell carcinoma | N/A | 28 Gy in 7 fractions | Bevacizumab 10 mg/kg every other week, 4 months after RT | Grade 5 cecal perforation, 3.5 months after SBRT | Yes |
| Stephans et al (32) | 2 patients with lung/liver tumors near esophagus | 29% | 50 Gy in 5 fractions | Not specified; received anti-VEGF agent within 2 months of completing SBRT | ≥Grade 3 esophageal fistula at median time of 8.4 months after SBRT | Yes |
| Barney et al (33) | 1 patient with hepatocellular carcinoma | 35% | 30 Gy in 3 fractions | Sorafenib 400 mg BID, 1.3 months after SBRT | Grade 3 gastric ulcer, 4.6 months after SBRT | Yes |
| 1 patient with colorectal carcinoma | 60 Gy in 5 fractions | Bevacizumab 5 mg/kg every 2 weeks, 0.5 months after SBRT | Grade 4 gastric perforation, 4.5 months after SBRT | Yes | ||
| 1 patient with pancreas cancer | 42 Gy in 5 fractions | Bevacizumab 15 mg/kg every 3 weeks, 7 months after SBRT | Grade 5 duodenal perforation, 10.4 months after SBRT | Yes | ||
| 1 patient with melanoma | 60 Gy in 3 fractions | Bevacizumab 15 mg/kg every 3 weeks, 2 months after SBRT | Grade 4 small bowel perforation, 5.1 months after SBRT | Yes | ||
| 1 patient with renal cell carcinoma | 40 Gy in 5 fractions | Bevacizumab 10 mg/kg every 2 weeks, 16.3 months after SBRT | Grade 3 duodenal ulcer, 17.2 months after SBRT | Yes | ||
| 1 patient with melanoma | 60 Gy in 3 fractions | Bevacizumab 10 mg/kg every 2 weeks, with SBRT | Grade 3 gastric ulcer, 3.1 months after SBRT | Yes | ||
| 1 patient with melanoma | 60 Gy in 5 fractions | Bevacizumab 10 mg/kg every 2 weeks, 2.3 months after SBRT | Grade 4 gastric ulcer, 2.7 months after SBRT | Yes | ||
| Dawson et al (34) | 1 patient with hepatocellular carcinoma | 12.5% | 33 Gy in 6 fractions | Sorafenib 400 mg daily, with SBRT | Grade 4 acute on chronic small bowel obstruction, 27 days after SBRT | Yes |
| 1 patient with hepatocellular carcinoma | 30 Gy in 6 fractions | Sorafenib 400 mg daily, with SBRT | Grade 3 GI bleed, 51 days after SBRT | Yes |
Abbreviations: GI = gastrointestinal; SBRT = stereotactic body radiation therapy; VEGF = vascular endothelial growth factor.
Peters et al (30) reported the case of a woman with renal cell carcinoma who received 8 Gy in 1 fraction for a L3–L5 metastasis 5 weeks after the start of sorafenib. Sorafenib was stopped 2 days before radiation therapy and started again 3 days later. One week after radiation treatment, the patient experienced multiple colon perforations within the radiation field. Biopsy specimens of the colon showed ischemic enteritis with radiation effects and vascular changes with thrombus formation, but no evidence of tumor.
Toxicity has been observed whether radiation is delivered before and after antiangiogenic therapy. A German case series reported 3 patients who experienced bowel adverse events after receiving bevacizumab after a course of radiation therapy (31). Although 2 of the 3 patients had been treated with conventionally fractionated radiation and eventually recovered, 1 patient who underwent hypofractionated radiation treatment (7 × 4 Gy) for a right ileum metastasis 4 months before starting bevacizumab (10 mg/kg every other week) experienced a cecal perforation within the radiation field and ultimately died. This perforation occurred 1 day after his second dose of bevacizumab.
Institutional series have also reported high rates of GI toxicity with the use of VEGF-modulating agents and SBRT. Stephans et al (32) retrospectively reviewed 52 patients with lung and liver tumors near the esophagus treated with SBRT (median dose 50 Gy in 5 fractions) and found 2 patients in whom esophageal fistulas developed, both within the irradiated field (personal communication, Dr Gregory Videtic, 2014). These toxicities occurred at D0.01 cc of 51.5 and 52 Gy, both below the Radiation Therapy Oncology Group (RTOG) 0813 specified maximum esophageal constraint of 52.5 Gy. However, 1 patient exceeded the RTOG 0813 5-cc dose limit of 27.5 Gy, having received 37.3 Gy to 5 cc (the other patient had a D5 cc of 21.5 Gy). Both patients had received adjuvant anti-VEGF agents within 2 months of completing SBRT. No patient in their series had an esophageal fistula in the absence of adjuvant VEGF-modulating agents.
A report from the Mayo Clinic also noted serious bowel injuries in 7 of 20 patients (9%) receiving VEGF-modulating agents within 13 months of completing SBRT (median 50 Gy in 5 fractions) (33). All 7 patients with bowel injuries had begun receiving VEGFI therapy after completing SBRT and were actively receiving a VEGFI at the time of bowel injury. In addition, all toxicities occurred in bowel at the level of the treated lesions and therefore received radiation (personal communication, Kenneth Olivier, 2014). Five patients had started a VEGFI within 3 months of completing SBRT, and the median time from initiation of VEGFI to bowel injury was 3.3 months (range, 0.4–4.0 months). There were no bowel injuries in the other 56 patients not receiving VEGF active agents.
Gastrointestnal toxicity has also been observed in the prospective setting. Dawson et al (34) conducted a phase I study of patients with advanced hepatocellular carcinoma and found serious luminal toxicity with large irradiated volumes and concurrent sorafenib. Sorafenib was delivered 7 days before SBRT, during SBRT, and after SBRT. Two of 3 patients with an effective irradiated liver volume of 30% to 60% treated to 30 Gy and 33 Gy in 6 fractions with concurrent sorafenib (400 mg daily) experienced GI toxicity. All GI toxicities were confirmed or suspected to have occurred within the radiation field (personal communication, Laura Dawson, 2014). Thus, sorafenib was deescalated to 200 mg by mouth daily. In the 3 patients who had a low effective liver volume irradiated (<30%) and received concurrent sorafenib (400 mg daily), none experienced dose-limiting toxicity. Luminal GI toxicity was significantly less in their SBRT-alone experience for hepatocellular carcinoma (35).
Finally, GI toxicity has been reported with fewer hypofractionated schedules. Chi et al (36) reported 3 episodes of upper GI bleeding in 23 patients treated with sunitinib 25 mg daily at least 1 week before radiation (median, 52.5 Gy in 15 fractions), during radiation, and 2 weeks after radiation.
In contrast to those reports, Koukourakis et al (37) reported no increased GI toxicity in 19 patients with locally advanced rectal cancer treated with radiation (34 Gy in 10 fractions) with amifostine (500–1000 mg daily), capecitabine (500 mg/m2 twice daily, 5 days per week), and bevacizumab (2 doses at 5 mg/kg every 2 weeks) followed by surgery 6 weeks after the end of radiation. At a followup time of 21 months, there were no late radiation sequelae. The administration of amifostine, a radio-protectant, confounds the comparison of these data with other reports, and further investigation is needed to determine its role with SBRT.
Mechanism of Action of Toxicity of SBRT and Antiangiogenic Therapy
Acute and late intestinal injury with radiation alone
The small intestine is a highly regenerative organ, renewing every 2 to 5 days (38). Mature and differentiated epithelium cells at the top of the villi are continuously shed into the lumen and are replaced by new cells dividing from stem cells located at the crypt of Lieberkuhn base. In the acute setting, radiation is believed to selectively kill these stem cells, resulting in an insufficient supply of new cells to replace the sloughed villi (39–41).
The mechanism of late effects of radiation is generally considered to be related to fibrosis and endothelial abnormality (42). The postradiation intestine has decreased motility and is more prone to mechanical friction and damages. TGF-β1, which is associated with fibrosis, increases within the first day after irradiation (43) and is still highly expressed months later after >10 Gy irradiation (44). In vitro, radiation also promotes fibroblast differentiation to fibrocytes, which results in increased collagen synthesis (45). Endothelial damage may also be involved in the fibrotic process. Irradiated endothelium loses thrombomodulin, leading to increased levels of thrombin, which promotes fibrosis, and thrombosis (46). Finally, radiation results in a decrease in vascular density, leading to ischemia, telangiectasia, and a predisposition for bleeding (47). Figure 1 shows the chronic radiation changes of the bowel endothelium.
Fig. 1.
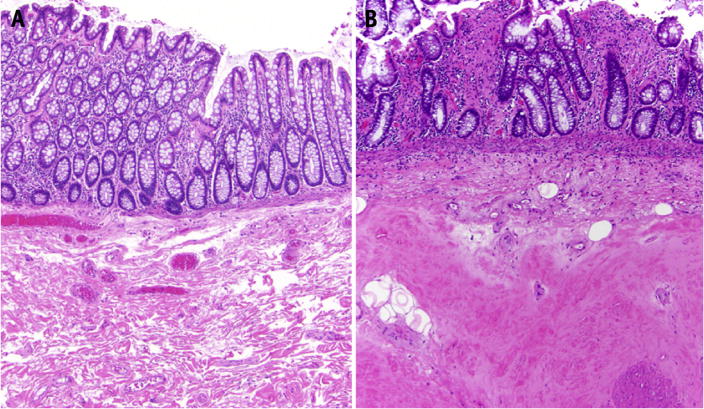
(A) Unirradiated colon showing normal-appearing glands overlying loose fibrovascular submucosa (hematoxylin and eosin, ×40). (B) The colon in this patient with chronic radiation injury demonstrates hyalinized collagen (fibrosis) replacing the submucosa. The overlying mucosa also demonstrates distorted crypt architecture and patchy lamina propria fibrosis (hematoxylin and eosin, ×40).
“New biology” of SBRT?
There is controversy regarding the mechanism of SBRT and whether its improved efficacy is the result of the high biologically effective doses (BEDs) delivered to the tumor or whether there may be a “new biology” leading to enhanced killing of tumor cells (48). Fuks and Kolesnick (49) have suggested that the vascular endothelium of tumors is an important target of high single doses of radiation. They report that high radiation doses (>8–10 Gy but <20 Gy) produce sphingomyelinase-dependent rapid vascular collapse resulting from endothelial apoptosis, leading to greater cytotoxic effect (50). Park et al (51) have also suggested that SBRT induces vascular damage leading to indirect tumor cell death. However, clinical data seem to suggest that the excellent tumor control of SBRT may be simply due to dose escalation not previously achievable with older techniques (52).
Similarly, whether stem cell loss is due to direct radiation killing or secondary to vascular insult from high irradiation dose has been debated. It has been recently suggested that SBRT induces microvascular endothelial apoptosis, resulting in stem cell dysfunction at the crypt base (53). However, the high level of endothelial apoptosis after irradiation has not been consistently observed (54, 55). Selective irradiation of endothelial cells did not cause additional crypt stem cell loss beyond whole body radiation (56), and genetic deletion of proapoptic Bax and Bak1 from endothelial cells did not protect mice from irradiation-induced GI syndrome (57).
As with improved tumor efficacy, increased rates of GI toxicity may also be simply due to the increased dose delivered with SBRT. The loss of stem cells has been shown to be dose dependent (41, 58, 59) and has long been considered the primary cause of acute intestinal toxicity after radiation. Crypt base columnar cells, which are believed to be Lgr5+ (Leucine-rich repeat-containing G-protein coupled receptor + 5) intestinal stem cells, have been found to undergo increased apoptosis at 10 Gy compared with 1 Gy (58). Metcalfe et al (41) further found that genetic ablation of Lgr5+ cells at 2 Gy of irradiation did not lead to an obvious change of crypt villus architecture, but radiation at 6 Gy or higher resulted in about 60% crypt loss and blunting of villi.
The nonhealing “complex wound” in the setting of antiangiogenic therapy
As just described, radiation can result in a complex wound (60), which may be more likely to result from SBRT and higher BEDs, and it may be that antiangiogenic treatment simply inhibits the healing process (Fig. 2).
Fig. 2.
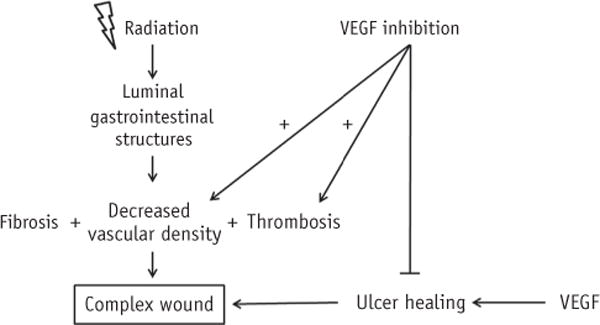
Vascular endothelial growth factor inhibition combined with radiation results in nonhealing complex wound.
The VEGF has been shown to play an important role in GI mucosa and ulcer healing (61), and it may be protective against small bowel injury after total body irradiation (62). Higher levels of VEGF expression correlate with smaller and shallower stress-induced gastric ulcers in murine models (63). Infusion of an anti-VEGF antibody in rats results in delayed healing of gastric erosions (64). Additionally, excessive VEGF inhibition has been shown to result in regression of normal blood vessels and reduced vascular density in the small intestinal villi in the GI tract, leading to impaired delivery of factors necessary for recovery of damaged tissue (65–67). Finally, VEGF inhibition may worsen ischemic injury caused by radiation. Cholesterol emboli syndrome resulting from VEGF inhibition has been hypothesized to cause thromboembolic events in atherosclerotic patients that may result in mesenteric ischemia and GI perforation (68). Meyer et al (69) found that bevacizumab forms an immune complex with VEGF and induces platelet aggregation and degranulation, which may be the mechanism of bevacizumab-associated thrombosis.
Experiments by Mangoni et al (70) support this hypothesis. They showed that anti-VEGF antibody enhances intestinal damage, leading to decreased mucosal surface, shortened villi, and edema and inflammation, as early as 24 hours after total body irradiation with 12 Gy. Damage was worse 74 hours after radiation. They also found that radiation to a preexisting ulcer worsened the injury, which was further exacerbated with concomitant anti-VEGF antibody, resulting in massive necrosis.
Thus, if SBRT is more likely to result in GI mucosal injury, VEGF inhibitors and more severe may prevent normal tissue recovery in the post-SBRT period and thus make SBRT-related toxicity more likely.
Interestingly, cerebral radiation necrosis, which has been associated with elevated levels of VEGF in preclinical models (71) and increased endothelial cell proliferation, vascular permeability, and extracellular edema (72), is treated with bevacizumab (73). The effectiveness of antiangiogenic therapy in the setting of radiation damage again highlights the need for further investigation into the mechanism behind normal tissue response in the setting of these therapies.
Areas of Future Research
The literature reporting luminal GI toxicity with SBRT and antiangiogenic therapy is sparse. Even though there are more data on the use of conventionally fractionated radiation with antiangiogenic therapy, the studies differ widely in the toxicities reported, the site treated, and the radiation dosing and drugs used. Additionally, there is inconsistent reporting of confounding factors such as blood thinners (74, 75) and proton pump inhibitors (76), which may modify the risk of radiation-induced GI toxicity. Finally, the reports of toxicity are subject to reporting bias and thus may reflect a higher incidence of toxicity with the combined use of SBRT and anti-VEGF therapy than in reality. However, our goal in this review is to highlight the potential risks rather than exhaustively review the combined use of these therapies, which is beyond the scope of this review. Based on the available data, we list here our recommendations and questions that are important to investigate further.
Timing of SBRT and antiangiogenic therapy
First, the optimal timing, duration, and dosing of anti-angiogenic therapy when used in combination with SBRT remain unknown. The studies mentioned in this review have used a variety of radiation doses, antiangiogenic agent doses, and treatment schedules, and the toxicity rates have been accordingly variable.
Several investigators have proposed the existence of a vascular normalization window after antiangiogenic therapy, during which peak radiosensitivity occurs and radiation should be given (77, 78). Anti-VEGF agents can normalize tumor vasculature (79), resulting in increased tumor oxygenation (80, 81). Radioresistance has been shown to result from hypoxia and VEGF upregulation in tumor cells (82), which can potentially be overcome by VEGFI therapy (83, 84). Because blood vessels in normal tissues are mature and are generally well oxygenated, their radiosensitivity should not be increased by giving radiation in this window. However, the window of vascular normalization is typically short lived, and a recent review shows that a similar number of studies report increased hypoxia after anti-VEGF therapy (85). Additionally, preclinical tumor models often do not adequately reflect the heterogeneity of human tumors, and applying data from animal models to human cancer should be done with this in mind.
It is also unknown whether there is a “wash-out” period that should be observed between antiangiogenic therapy and radiation. There is some experience with timing bevacizumab with surgery. A retrospective series in patients with colorectal cancer scheduled to undergo hepatic resection showed no increase in rates of adverse events with the administration of preoperative bevacizumab (86). The half-life of bevacizumab is approximately 20 days, and in this study, the median time from last dose of bevacizumab to surgery was 58 days (range, 31–117 days). Thus, a “wash-out” period after which surgery or radiation may be safe to give may be dependent on the half-life of the specific agent. However, inasmuch as toxicity has been reported to occur as long as 17.2 months after SBRT (33), additive toxicity can be seen through and beyond this period of time. Until more data become available, we recommend caution with using SBRT and anti-VEGF agents concurrently or sequentially.
Antiangiogenic agent selection
Multiple agents that target VEGF signaling have been developed, including bevacizumab, a monoclonal antibody against VEGF, and sunitinib and sorafenib, antiangiogenic receptor tyrosine kinase small molecule inhibitors. These agents differ in target receptors, receptor affinity, and toxicity profiles, and additional study is needed to determine whether any particular agent is safer to use with radiation than another. However, bevacizumab appears to be more associated with GI toxicity than are some of the other agents (87), perhaps because of bevacizumab’s formation of immune complexes and induction of vascular inflammation and clotting, as discussed previously.
Dose is another consideration when combining anti-angiogenic therapy with radiation therapy. Willett et al (80) demonstrated that concurrent chemoradiation with bevacizumab at a dosage of 5 mg/kg was well tolerated in rectal cancer patients but reported the development of dose-limiting toxicities of diarrhea and colitis when the bevacizumab dosage was escalated to 10 mg/kg (88).
Optimizing radiation
This review is intended to focus on the potential risk of GI toxicity specifically with SBRT in the setting of anti-angiogenic therapy because of the increased rates of luminal toxicities reported with early SBRT alone (24, 26–28). Investigators at Memorial Sloan-Kettering reported that grade ≥3 esophageal toxicity was extremely rare until the single-fraction esophageal dose to 2.5 cc exceeded 15 Gy, at which point toxicity rose sharply with increasing dose (27). A series from Stanford similarly reported 2 high-grade esophageal toxicities that occurred at single-fraction biological equivalents of 14.2 and 18.1 Gy to 2.0 cc of esophagus (28).
Dose and volume limits for the duodenum have also been reported (89, 90). Dosimetric analysis of our single-fraction SBRT series found the maximum dose to 1 cc of the duodenum ≥23Gy (in a single fraction) to be associated with increased rates of duodenal toxicity (80). Bae et al (90) found V25 > 20 cc and maximum point dose of 35 Gy and 38 Gy (over 3 fractions) predicted for severe gastroduodenal toxicity. Despite these early toxicities, optimization of fractionating and delivery technique and improved understanding of organ volumetric tolerance has allowed SBRT to become safer with more study and longer followup times. Data from Stanford show that GI toxicity is significantly less using a multifraction regimen (25–45 Gy in 3–5 fractions with median BED3, using α/β of 3, of 105.6 Gy; range, 66.7–180.0 Gy) compared with single fraction (all treated with 25 Gy in 1 fraction or BED3 of 233.3 Gy) for pancreas SBRT (91). Patients were typically followed up every 3 months for the first 1 to 2 years and monitored for toxicity. The cumulative incidence of grade 2 or higher GI toxicity with single-fraction SBRT was 26.1% 1 year after treatment. With fractionation, the toxicity rate was reduced to 7.8%, with no compromise in local control.
Dose constraints specific to the setting of antiangiogenic therapy warrant further study because the available data are too heterogeneous and complex to enable the formulation of any specific recommendations at this time. Registries to allow reporting and tracking of toxicities would help accelerate our understanding of radiation tolerance and dosimetric constraints of bowel structures. As investigators continue to report their experiences to determine dose tolerance thresholds, specific attention should be paid to any concurrent biologic agents in their study population and should be taken into account. In the meantime, we recommend multifraction SBRT using the lowest BED that provides high local control and adhering to established normal tissue constraints in the setting of concurrent or possible sequential anti-VEGF therapy.
Better patient selection
It is important to screen and identify patients who may be at higher risk for GI toxicities while using antiangiogenic therapies. Patients with preexisting mucosal lesions and tumors adjacent to mucosal surfaces have been shown to be at higher risk for the development of ulcers and perforations. Involvement of bowel with tumor predisposes to GI toxicity during treatment with bevacizumab, as reported in ovarian cancer patients (92). When bevacizumab treatment was limited to ovarian cancer patients without evidence of bowel involvement on imaging or examination, no GI perforations were reported (93). Patients with larger radiation targets are also at potentially higher risk for toxicity.
Biomarkers for efficacy and GI toxicity
Biomarkers have been proposed to help determine sufficient inhibition of a targeted pathway and predict efficacy, allowing for appropriate modification of treatment dose and schedule. Although these biomarkers have been difficult to identify for angiogenesis pathways, studies have shown that plasma VEGF levels can serve as a surrogate for VEGF receptor blockade (94). Additionally, the development of noninvasive biomarkers of GI toxicity would be useful because the GI tract cannot be inspected easily or safely, especially in the setting of injured tissue.
Conclusion
The increasing use of stereotactic radiation and an expanding array of antiangiogenic agents open the door for exciting new possibilities of combining these therapies. However, the reported GI toxicities summarized here should raise warnings to clinicians of potential interactions that may lead to increased toxicity. Further investigation is needed to confirm this interaction and to enable our understanding of the possible underlying mechanism. How SBRT and these novel targeted agents affect tumor and normal tissue is complex, multifactorial, and still poorly understood. Thus, many additional questions remain.
In the meantime, we caution the use of anti-VEGF therapy and SBRT until further prospective data are available. Although GI toxicity has been observed in a phase I trial of SBRT and sorafenib in hepatocellular carcinoma, this regimen is currently being studied in the phase III RTOG 1112 trial randomizing patients with hepatocellular carcinoma to sorafenib alone versus SBRT followed by sorafenib, albeit with lower radiation and sorafenib doses. Additionally, sorafenib is given sequentially rather than concurrently to further reduce the risk of toxicity. The results of this trial and many others in the future will help clarify the efficacy and the toxicity of combining SBRT and VEGF-modulating agents.
Acknowledgments
B.W.L. receives grant support and a speaker honorarium from Varian.
Footnotes
Conflict of interest: The authors report no other conflict of interest.
References
- 1.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 3.Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 4.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 5.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 9.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 10.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 11.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: A meta-analysis. Lancet Oncol. 2009;10:559–568. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 15.Crane CH, Eng C, Feig BW, et al. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2010;76:824–830. doi: 10.1016/j.ijrobp.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Small W, Jr, Mulcahy MF, Rademaker A, et al. Phase II trial of full-dose gemcitabine and bevacizumab in combination with attenuated three-dimensional conformal radiotherapy in patients with localized pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;80:476–482. doi: 10.1016/j.ijrobp.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 17.Crane CH, Ellis LM, Abbruzzese JL, et al. Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol. 2006;24:1145–1151. doi: 10.1200/JCO.2005.03.6780. [DOI] [PubMed] [Google Scholar]
- 18.Chen SW, Lin LC, Kuo YC, et al. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:1041–1047. doi: 10.1016/j.ijrobp.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2010;28:43–48. doi: 10.1200/JCO.2009.24.7353. [DOI] [PubMed] [Google Scholar]
- 20.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 23.Wersall PJ, Blomgren H, Lax I, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol. 2005;77:88–95. doi: 10.1016/j.radonc.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 25.Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008;112:650–658. doi: 10.1002/cncr.23209. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823–830. doi: 10.1080/02841860600904854. [DOI] [PubMed] [Google Scholar]
- 27.Cox BW, Jackson A, Hunt M, et al. Esophageal toxicity from high-dose, single-fraction paraspinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:e661–e667. doi: 10.1016/j.ijrobp.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abelson JA, Murphy JD, Loo BW, Jr, et al. Esophageal tolerance to high-dose stereotactic ablative radiotherapy. Dis Esophagus. 2012;25:623–629. doi: 10.1111/j.1442-2050.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 29.Bourhis J, Sire C, Graff P, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): An open-label phase 3 randomised trial. Lancet Oncol. 2012;13:145–153. doi: 10.1016/S1470-2045(11)70346-1. [DOI] [PubMed] [Google Scholar]
- 30.Peters NA, Richel DJ, Verhoeff JJ, et al. Bowel perforation after radiotherapy in a patient receiving sorafenib. J Clin Oncol. 2008;26:2405–2406. doi: 10.1200/JCO.2007.15.8451. [DOI] [PubMed] [Google Scholar]
- 31.Lordick F, Geinitz H, Theisen J, et al. Increased risk of ischemic bowel complications during treatment with bevacizumab after pelvic irradiation: Report of three cases. Int J Radiat Oncol Biol Phys. 2006;64:1295–1298. doi: 10.1016/j.ijrobp.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Stephans KL, Djemil T, Diaconu C, et al. Esophageal dose tolerance to hypofractionated stereotactic body radiation therapy: Risk factors for late toxicity. Int J Radiat Oncol Biol Phys. 2014;90:197–202. doi: 10.1016/j.ijrobp.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Barney BM, Markovic SN, Laack NN, et al. Increased bowel toxicity in patients treated with a vascular endothelial growth factor inhibitor (VEGFI) after stereotactic body radiation therapy (SBRT) Int J Radiat Oncol Biol Phys. 2013;87:73–80. doi: 10.1016/j.ijrobp.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Dawson L, Brade A, Cho C, et al. Phase I Study of Sorafenib and SBRT for Advanced Hepatocellular Carcinoma. Paper presented at: American Society for Therapeutic Radiology and Oncology; October 28–31, 2012; Boston, MA. [Google Scholar]
- 35.Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 36.Chi KH, Liao CS, Chang CC, et al. Angiogenic blockade and radiotherapy in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2010;78:188–193. doi: 10.1016/j.ijrobp.2009.07.1725. [DOI] [PubMed] [Google Scholar]
- 37.Koukourakis MI, Giatromanolaki A, Tsoutsou P, et al. Bevacizumab, capecitabine, amifostine, and preoperative hypofractionated accelerated radiotherapy (HypoArc) for rectal cancer: A phase II study. Int J Radiat Oncol Biol Phys. 2011;80:492–498. doi: 10.1016/j.ijrobp.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 38.Tian H, Biehs B, Warming S, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown M. What causes the radiation gastrointestinal syndrome?: Overview. Int J Radiat Oncol Biol Phys. 2008;70:799–800. doi: 10.1016/j.ijrobp.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 41.Metcalfe C, Kljavin NM, Ybarra R, et al. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Brush J, Lipnick SL, Phillips T, et al. Molecular mechanisms of late normal tissue injury. Semin Radiat Oncol. 2007;17:121–130. doi: 10.1016/j.semradonc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Langberg CW, Hauer-Jensen M, Sung CC, et al. Expression of fibrogenic cytokines in rat small intestine after fractionated irradiation. Radiother Oncol. 1994;32:29–36. doi: 10.1016/0167-8140(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 44.Okunieff P, Cornelison T, Mester M, et al. Mechanism and modification of gastrointestinal soft tissue response to radiation: Role of growth factors. Int J Radiat Oncol Biol Phys. 2005;62:273–278. doi: 10.1016/j.ijrobp.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Lara PC, Russell NS, Smolders IJ, et al. Radiation-induced differentiation of human skin fibroblasts: Relationship with cell survival and collagen production. Int J Radiat Biol. 1996;70:683–692. doi: 10.1080/095530096144572. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Zheng H, Ou X, et al. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: Possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol. 2002;160:2063–2072. doi: 10.1016/S0002-9440(10)61156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171–1185. doi: 10.1016/0360-3016(94)00423-I. [DOI] [PubMed] [Google Scholar]
- 48.Brown JM, Koong AC. High-dose single-fraction radiotherapy: Exploiting a new biology? Int J Radiat Oncol Biol Phys. 2008;71:324–325. doi: 10.1016/j.ijrobp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 51.Park HJ, Griffin RJ, Hui S, et al. Radiation-induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) Radiat Res. 2012;177:311–327. doi: 10.1667/rr2773.1. [DOI] [PubMed] [Google Scholar]
- 52.Brown JM, Brenner DJ, Carlson DJ. Dose escalation, not “new biology,” can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:1159–1160. doi: 10.1016/j.ijrobp.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 54.Schuller BW, Rogers AB, Cormier KS, et al. No significant endothelial apoptosis in the radiation-induced gastrointestinal syndrome. Int J Radiat Oncol Biol Phys. 2007;68:205–210. doi: 10.1016/j.ijrobp.2006.12.069. [DOI] [PubMed] [Google Scholar]
- 55.Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123–136. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- 56.Schuller BW, Binns PJ, Riley KJ, et al. Selective irradiation of the vascular endothelium has no effect on the survival of murine intestinal crypt stem cells. Proc Natl Acad Sci U S A. 2006;103:3787–3792. doi: 10.1073/pnas.0600133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirsch DG, Santiago PM, di Tomaso E, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–596. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 59.Yan KS, Chia LA, Li X, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denham JW, Hauer-Jensen M. The radiotherapeutic injuryea complex ’wound’. Radiother Oncol. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 61.Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50(Suppl 1):S24–S33. doi: 10.1007/s10620-005-2803-6. [DOI] [PubMed] [Google Scholar]
- 62.Okunieff P, Mester M, Wang J, et al. In vivo radioprotective effects of angiogenic growth factors on the small bowel of C3H mice. Radiat Res. 1998;150:204–211. [PubMed] [Google Scholar]
- 63.Malara B, Josko J, Tyrpien M, et al. Dynamics of changes in vascular endothelial growth factor (VEGF) expression and angiogenesis in stress-induced gastric ulceration in rats. J Physiol Pharmacol. 2005;56:259–271. [PubMed] [Google Scholar]
- 64.Yoshida M, Wakabayashi G, Ishikawa H, et al. A possible defensive mechanism in the basal region of gastric mucosa and the healing of erosions. Clin Hemorheol Microcirc. 2003;29:301–312. [PubMed] [Google Scholar]
- 65.Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol. 2007;14:1860–1869. doi: 10.1245/s10434-006-9337-9. [DOI] [PubMed] [Google Scholar]
- 66.Kamba T, Tam BY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. A J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 67.Howdieshell TR, Callaway D, Webb WL, et al. Antibody neutralization of vascular endothelial growth factor inhibits wound granulation tissue formation. J Surg Res. 2001;96:173–182. doi: 10.1006/jsre.2001.6089. [DOI] [PubMed] [Google Scholar]
- 68.Mir O, Mouthon L, Alexandre J, et al. Bevacizumab-induced cardiovascular events: A consequence of cholesterol emboli syndrome? J Natl Cancer Inst. 2007;99:85–86. doi: 10.1093/jnci/djk011. [DOI] [PubMed] [Google Scholar]
- 69.Meyer T, Robles-Carrillo L, Robson T, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost. 2009;7:171–181. doi: 10.1111/j.1538-7836.2008.03212.x. [DOI] [PubMed] [Google Scholar]
- 70.Mangoni M, Vozenin MC, Biti G, et al. Normal tissues toxicities triggered by combined anti-angiogenic and radiation therapies: Hurdles might be ahead. Br J Cancer. 2012;107:308–314. doi: 10.1038/bjc.2012.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsao MN, Li YQ, Lu G, et al. Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J Neuropathol Exp Neurol. 1999;58:1051–1060. doi: 10.1097/00005072-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Wong ET, Huberman M, Lu XQ, et al. Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol. 2008;26:5649–5650. doi: 10.1200/JCO.2008.19.1866. [DOI] [PubMed] [Google Scholar]
- 73.Matuschek C, Bolke E, Nawatny J, et al. Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther Onkol. 2011;187:135–139. doi: 10.1007/s00066-010-2184-4. [DOI] [PubMed] [Google Scholar]
- 74.Choe KS, Jani AB, Liauw SL. External beam radiotherapy for prostate cancer patients on anticoagulation therapy: How significant is the bleeding toxicity? Int J Radiat Oncol Biol Phys. 2010;76:755–760. doi: 10.1016/j.ijrobp.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 75.D’Amico AV, Manola J, McMahon E, et al. A prospective evaluation of rectal bleeding after dose-escalated three-dimensional conformal radiation therapy using an intrarectal balloon for prostate gland localization and immobilization. Urology. 2006;67:780–784. doi: 10.1016/j.urology.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 76.Coia LR, Myerson RJ, Tepper JE. Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys. 1995;31:1213–1236. doi: 10.1016/0360-3016(94)00419-L. [DOI] [PubMed] [Google Scholar]
- 77.Dings RP, Loren M, Heun H, et al. Scheduling of radiation with angiogenesis inhibitors anginex and Avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res. 2007;13:3395–3402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meng MB, Jiang XD, Deng L, et al. Enhanced radioresponse with a novel recombinant human endostatin protein via tumor vasculature remodeling: Experimental and clinical evidence. Radiother Oncol. 2013;106:130–137. doi: 10.1016/j.radonc.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 79.Jain RK, Duda DG, Clark JW, et al. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 80.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–680. [PubMed] [Google Scholar]
- 82.Moeller BJ, Cao Y, Li CY, et al. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 83.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 84.Gupta VK, Jaskowiak NT, Beckett MA, et al. Vascular endothelial growth factor enhances endothelial cell survival and tumor radio-resistance. Cancer J. 2002;8:47–54. doi: 10.1097/00130404-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 85.Ostergaard L, Tietze A, Nielsen T, et al. The relationship between tumor blood flow, angiogenesis, tumor hypoxia, and aerobic glycolysis. Cancer Res. 2013;73:5618–5624. doi: 10.1158/0008-5472.CAN-13-0964. [DOI] [PubMed] [Google Scholar]
- 86.Kesmodel SB, Ellis LM, Lin E, et al. Preoperative bevacizumab does not significantly increase postoperative complication rates in patients undergoing hepatic surgery for colorectal cancer liver metastases. J Clin Oncol. 2008;26:5254–5260. doi: 10.1200/JCO.2008.17.7857. [DOI] [PubMed] [Google Scholar]
- 87.Elice F, Rodeghiero F, Falanga A, et al. Thrombosis associated with angiogenesis inhibitors. Best Pract Res Clin Haematol. 2009;22:115–128. doi: 10.1016/j.beha.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Willett CG, Boucher Y, Duda DG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: Continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 89.Murphy JD, Christman-Skieller C, Kim J, et al. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:1420–1426. doi: 10.1016/j.ijrobp.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 90.Bae SH, Kim MS, Cho CK, et al. Predictor of severe gastroduodenal toxicity after stereotactic body radiotherapy for abdominopelvic malignancies. Int J Radiat Oncol Biol Phys. 2012;84:e469–e474. doi: 10.1016/j.ijrobp.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 91.Pollom EL, Alagappan M, Eyben Rv, et al. Single-versus multi-fraction stereotactic body radiation therapy for pancreatic adenocarcinoma: Outcomes and toxicity. Int J Radiat Oncol Biol Phys. 2014;90:918–925. doi: 10.1016/j.ijrobp.2014.06.066. [DOI] [PubMed] [Google Scholar]
- 92.Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 93.Simpkins F, Belinson JL, Rose PG. Avoiding bevacizumab related gastrointestinal toxicity for recurrent ovarian cancer by careful patient screening. Gynecol Oncol. 2007;107:118–123. doi: 10.1016/j.ygyno.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 94.Bocci G, Man S, Green SK, et al. Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res. 2004;64:6616–6625. doi: 10.1158/0008-5472.CAN-04-0401. [DOI] [PubMed] [Google Scholar]


