Abstract
Ventricular chambers are essential for the rhythmic contraction and relaxation occurring in every heartbeat throughout life. Congenital abnormalities in ventricular chamber formation cause severe human heart defects. How the early trabecular meshwork of myocardial fibres forms and subsequently develops into mature chambers is poorly understood. We show that Notch signalling first connects chamber endocardium and myocardium to sustain trabeculation, and later coordinates ventricular patterning and compaction with coronary vessel development to generate the mature chamber, through a temporal sequence of ligand signalling determined by the glycosyltransferase manic fringe (MFng). Early endocardial expression of MFng promotes Dll4–Notch1 signalling, which induces trabeculation in the developing ventricle. Ventricular maturation and compaction require MFng and Dll4 downregulation in the endocardium, which allows myocardial Jag1 and Jag2 signalling to Notch1 in this tissue. Perturbation of this signalling equilibrium severely disrupts heart chamber formation. Our results open a new research avenue into the pathogenesis of cardiomyopathies.
Ventricular chamber development begins with the formation of trabeculae, a mesh of endocardium-lined luminal cardiomyocyte projections1,2. Trabeculae extend radially and longitudinally, forming a network that comprises most of the myocardial mass, increasing myocardial surface area for gas exchange1. Subsequent remodelling and compaction thickens the trabeculae radially until they are indistinguishable from the myocardial wall. Simultaneously, epicardium-derived cells3 invade the outer compact myocardium and together with ventricular endocardial cells4 contribute to the coronary vasculature that will support the final growth phase and nourish the adult myocardium5. Defective trabecular compaction manifests as left ventricular non-compaction (LVNC) cardiomyopathy, characterized by prominent trabeculations separated by deep intertrabecular recesses in a thin ventricular wall6,7. LVNC is a genetic cardiomyopathy8 caused by mutations in genes encoding sarcomeric, cytoskeletal and nuclear-membrane proteins, and can manifest as depressed systolic function or as serious complications including systemic embolism, malignant arrhythmias, heart failure and sudden death9,10. LVNC is generally transmitted as an autosomal dominant trait, but the underlying disease mechanisms are poorly understood7,11.
Inactivation of the Notch1 receptor or its effector RBPJK disrupts trabeculation owing to impaired trabecular endocardium and myocardium differentiation and reduced ventricular cardiomyocyte proliferation12. Moreover, targeted inactivation of the Notch pathway modulator mind bomb1 (Mib1) in murine myocardium disrupts ventricular chamber maturation leading to LVNC. Mib1 is an E3-ubiquitin ligase that ubiquitylates Notch ligands, triggering their endocytosis and the transendocytosis of the Notch extracellular domain13, this last event being essential for generation and release of the Notch intracellular domain14 (NICD). Inactivating mutations in human MIB1 have been identified in patients with autosomal dominant familial LVNC (ref. 15). Thus, the NOTCH pathway plays central roles in ventricular chamber development and LVNC (refs 12,15), but the signalling elements and underlying regulatory circuitries involved are unresolved.
Endocardial Dll4 and myocardial Jag1 are candidate Notch ligands in the early mouse heart
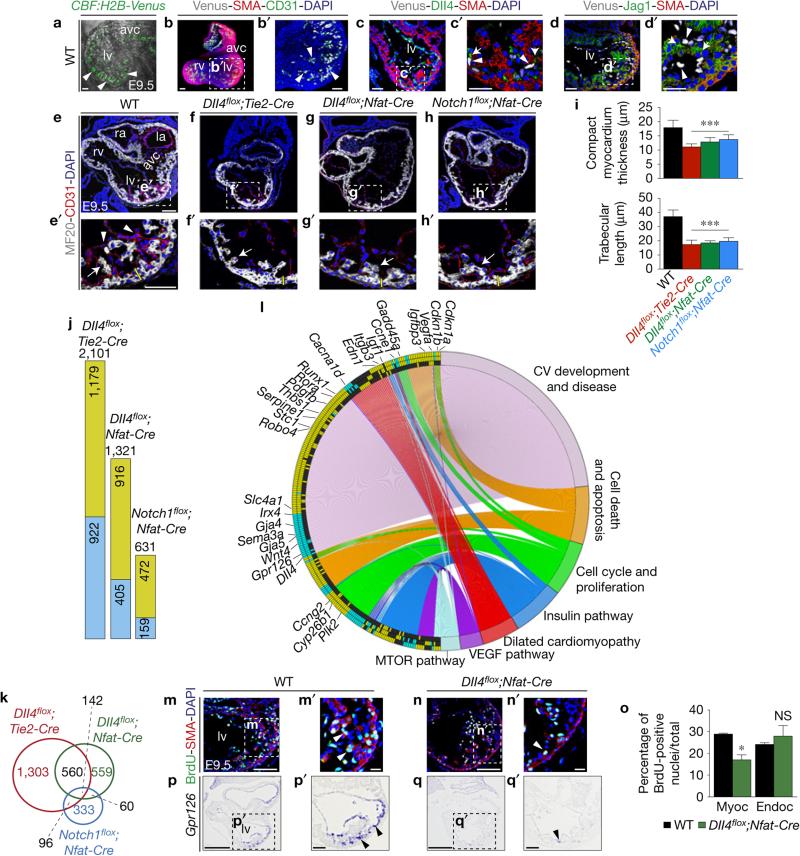
Using the single-cell-resolution Notch reporter line CBF:H2B-Venus (ref. 16), we mapped Notch activation in the developing ventricles in relation to the ligands Dll4 and Jag1. CBF:H2B-Venus was widely expressed in chamber endocardium of embryonic day 9.5 (E9.5) wild-type (WT) CBF:H2B-Venus embryos, and expression was abrogated in RBPJk- and Notch1-targeted mutants (Fig. 1a and Supplementary Fig. 1a). Whole-mount staining of E9.5 CBF:H2B-Venus hearts for α-smooth-muscle actin (SMA) and CD31, also known as Pecam1, confirmed endocardial Notch activity (Fig. 1b,b′ and Supplementary Video 1). At E9.5, Dll4 was preferentially expressed in the endocardium at the base of developing trabeculae, while Jag1 was expressed in trabecular myocardium, both coinciding with sites of CBF:H2B-Venus activity (Fig. 1c–d′). Amira-3D reconstructions of the early expression patterns of Dll4 (Supplementary Fig. 1b and Supplementary Data File 1), Jag1 (Supplementary Fig. 1b and Supplementary Data File 2) and N1ICD (Supplementary Fig. 1b and Supplementary Data File 3 and refs 12,15) supported these observations. Endocardial Dll4 expression weakened from E12.5 to E15.5 (Supplementary Fig. 1c,c′) and Dll4 transcription disappeared by E15.5 but persisted in coronary vessel endothelium (Supplementary Fig. 1e,e′). In contrast, Jag1 expression expanded to the compact myocardium but was weaker than in trabeculae and coronary vessels (Supplementary Fig. 1d,d′,f,f′,h,h′) whereas Notch activity was detected throughout endocardium and coronary vessel endothelium (Supplementary Fig. 1g,h′).
Figure 1.
Dll4–Notch1 signalling abrogation disrupts trabeculation and chamber-gene expression. (a) E9.5 WT CBF:H2B-Venus embryo, two-photon whole-mount image, left ventricle. Arrowheads indicate Venus expression. (b,b′) E9.5 WT CBF:H2B-Venus heart, whole-mount view of immunohistochemistry for Venus (grey), smooth-muscle actin (SMA, red) and CD31 (Pecam1) (green). (c,c′) E9.5 WT CBF:H2B-Venus heart, Dll4 (green), SMA (red) and GFP (grey) immunohistochemistry. (d,d′) E9.5 WT CBF:H2B-Venus heart, Jag1 (green), SMA (red) and GFP (grey) immunohistochemistry. Scale bars, 50 μm. (e–h′) Sarcomeric myosin (MF20) and CD31 immunohistochemistry in E9.5 WT (e,e′), Dll4flox/flox;Tie2-Cre/+(Dll4flox;Tie2-Cre) (f,f′), Dll4flox/flox;Nfatc1pan-Cre/+ (Dll4flox;Nfat-Cre) (g,g′) and Notch1flox/flox;Nfatc1pan-Cre/+ (Notch1flox;Nfat-Cre) sections (h,h′). Arrowheads, endocardium; arrows, trabecular myocardium. Yellow bars, compact myocardium thickness. In b–d′,e–h′ nuclei are DAPI-counterstained. Scale bars, 100 μm. (i) Quantification of compact myocardium thickness and trabecular length at E9.5. Data are mean ± s.d. (n = 12 sections from 3 WT and n = 12 sections from 3 mutant embryos of each genotype, ***P < 0.001, by Student's t-test). (j) Chart showing the total number of differentially expressed genes identified by RNA-seq (P < 0.05) in the indicated genotypes. Numbers in the green and blue sections indicate upregulated and downregulated genes, respectively. (k) Venn diagram representation of the comparative analysis of deregulated genes in the three genotypes. Numbers in black indicate the total number of genes common to at least two genotypes. (l) Circular plot of 257 differentially expressed genes, simultaneously presenting a detailed view of the relationships between expression changes (left semicircle perimeter) and processes (right semicircle perimeter). In the left semicircle perimeter, the inner ring represents Notch1flox;Nfat-Cre data, the middle ring Dll4flox;Nfat-Cre data and the outer ring Dll4flox;Tie2-Cre data. 28 of the 257 genes are named. Green, upregulated; blue, downregulated; black, unchanged. Details in Supplementary Table 1. (m–n′) E9.5 WT (m,m′) and Dll4flox;Nfat-Cre embryos (n,n′), BrdU immunostaining. The myocardium is SMA-counterstained (red). Arrowheads, BrdU-positive nuclei. Scale bars, 50 μm. (o) BrdU-positive nuclei as a percentage of total nuclei in myocardium and endocardium. Data are mean ± s.d. (n=3 WT and 3 n=mutant embryos, *P < 0.05 by Student's t-test; NS, not significant). (p–q′) Gpr126 ISH in E9.5 WT (p,p′) and Dll4flox;Nfat-Cre hearts (q,q′). Scale bars, 100 μm. Source data are available in Supplementary Table 4. avc, atrio-ventricular canal; la, left atrium; lv, left ventricle; ra, right atrium; rv, right ventricle; myoc, myocardium; endoc, endocardium.
Dll4–Notch1 signalling inactivation disrupts trabeculation and coronary vessel formation
We next crossed mice bearing a conditional Dll4flox allele17 with the pan-endothelial and pan-endocardial driver lines Tie2-Cre (ref. 18) and Nfatc1pan-Cre (ref. 4). Dll4flox inactivation with either driver severely disrupted trabeculation (Fig. 1e–g′) producing a phenotype similar to that of Notch1 inactivation (Fig. 1h,h′). At E9.5, myosin (MF20) and CD31/Pecam1 staining of mutant hearts revealed reductions in compact myocardium thickness (28%) and trabecular length (50%; Fig. 1i). N1ICD expression was below normal in the ventricular endocardium of E9.5 Dll4flox;Nfatc1pan-Cre and Dll4flox;Tie2-Cre mutants (Supplementary Fig. 2a,b). In Dll4flox;Tie2-Cre mutants, Hey2 transcription marked a thin compact myocardium and was reduced in the endocardium (Supplementary Fig. 2c). Mutant embryos also showed reduced EfnB2, Nrg1 and Bmp10 transcription (Supplementary Fig. 2c), known to depend on Notch activity12.
To determine the role of cardiac Dll4 at later stages, we bred Dll4flox mice with the driver Cdh5-CreERT, active in endocardium and vascular and coronary vessel endothelium19. At E15.5, Dll4flox;Cdh5-CreERT embryos had neck oedema, suggesting circulatory problems (Supplementary Fig. 3a,c). Ventricular wall thickness was reduced in Dll4flox;Cdh5-CreERT mutants (Supplementary Fig. 3b,d). Dll4, strongly expressed in WT coronary vasculature (Supplementary Fig. 3e,e′), was markedly reduced in mutants (Supplementary Fig. 3f,f′). N1ICD was still expressed in Dll4flox;Cdh5-CreERT chamber endocardium but was almost absent in intramyocardial coronary vessels (Supplementary Fig. 3g,h′). Dll4flox;Cdh5-CreERT mutant hearts showed normal Hey2 expression in compact myocardium (Supplementary Fig. 3i,j′) but the coronary artery endothelial markers HeyL, EfnB2 and Cx40, also known as Gja5, (Supplementary Fig. 3k–p′) were downregulated.
Cardiac Dll4–Notch1 gene profiling
To identify Notch targets, we profiled E9.5 WT, Dll4flox;Tie2-Cre, Dll4flox;Nfatc1pan-Cre and Notch1flox;Nfatc1pan-Cre hearts by RNA sequencing (RNA-seq). Dll4flox;Tie2-Cre mutants showed 2,101 affected genes (1,179 upregulated, 922 downregulated), Dll4flox;Nfatc1pan-Cre mutants 1,321 (916 upregulated, 405 downregulated) and Notch1flox;Nfatc1pan-Cre mutants 631 (472 upregulated, 159 downregulated; P <0.05; Fig. 1j and Supplementary Table 1). The three mutants shared 142 differentially expressed genes (Supplementary Table 1). Dll4flox;Tie2-Cre and Dll4flox;Nfatc1pan-Cre mutants shared 702 genes, Dll4flox;Tie2-Cre and Notch1flox;Nfatc1pan-Cre mutants 238, and Notch1flox;Nfatc1pan-Cre and Dll4flox;Nfatc1pan-Cre mutants 202 (Fig. 1k). Gene ontology (GO) classification revealed deregulation of genes involved in cardiovascular development and disease, cell cycle and proliferation, cell death, dilated cardiomyopathy and intercellular pathways (Fig. 1l and Supplementary Fig. 4a,b). Dll4flox;Tie2-Cre and Dll4flox;Nfatc1pan-Cre mutants showed upregulation of the negative regulators of cellular proliferation Cdkn1a (also known as p21), Cdkn1b, Gadd45a, Foxo3, Cbx7, Ccng2, Ndrg1, Myct1 and Runx1 (Fig. 1l). Consistently, cardiomyocyte proliferation was 42% lower in Dll4flox;Nfatc1pan-Cre mutants than in WT counterparts (Fig. 1m–o).
The three genotypes showed pronounced downregulation of Gpr126 (Fig. 1l and Supplementary Table 1), encoding an orphan adhesion G protein-coupled receptor20 whose inactivation in mice causes hypotrabeculation and ventricular wall thinning21,22. Gpr126 was transcribed in E9.5 WT endocardium and downregulated in Dll4flox;Nfatc1pan-Cre mutants (Fig. 1p–q′). Cardiac expression of zebrafish gpr126 was abrogated in Notch-deficient mib1ta52b mutant larvae (Supplementary Fig. 2d,e′) and in WT larvae treated with the Notch inhibitor RO4929097 (ref. 23; Supplementary Fig. 2d,d′,f,f′). Endocardial Gpr126 expression, the trabecular phenotype of Gpr126 mutant mice21,22, and Gpr126 downregulation in mouse and zebrafish Notch mutants suggest that it as a Notch target during trabeculation. To substantiate the relationship between Notch and Gpr126, we conducted real-time quantitative PCR with reverse transcription (qRT–PCR) on Dll4-stimulated HUVEC. Dll4 increased Gpr126 expression, an effect abolished by RO4929097 (Supplementary Fig. 2g). In silico analysis revealed two conserved putative RBPJK-binding sites in Gpr126 intron four. Luciferase reporter assays in bovine aortic endothelial cells (BAEC) showed that increasing amounts of N1ICD were able to transiently activate the Gpr126 reporter fivefold (Supplementary Fig. 2h).
Adult heart function requires signalling from myocardial Jag1 to endocardial Notch1
Jag1 inactivation with the early myocardial driver cTnT-Cre (ref. 24) did not affect trabeculation (Supplementary Fig. 5a); moreover, despite efficient deletion of Jag1flox in the ventricles, N1ICD expression was unaffected in E10.5 Jag1flox;cTnT-Cre mice (Supplementary Fig. 5a,b). Similarly, expression of Hey2, EfnB2, Nrg1 and Bmp10 seemed normal (Supplementary Fig. 5c). Jag1 thus seems not to be required for trabeculation and Notch1 activation in the early ventricle. In E13.5 Jag1flox;cTnT-Cre embryos, myocardial Jag1 expression is abrogated and endocardial N1ICD expression is markedly reduced (Supplementary Fig. 5d), indicating that at this stage Notch1 activity in the ventricles depends on Jag1 signalling.
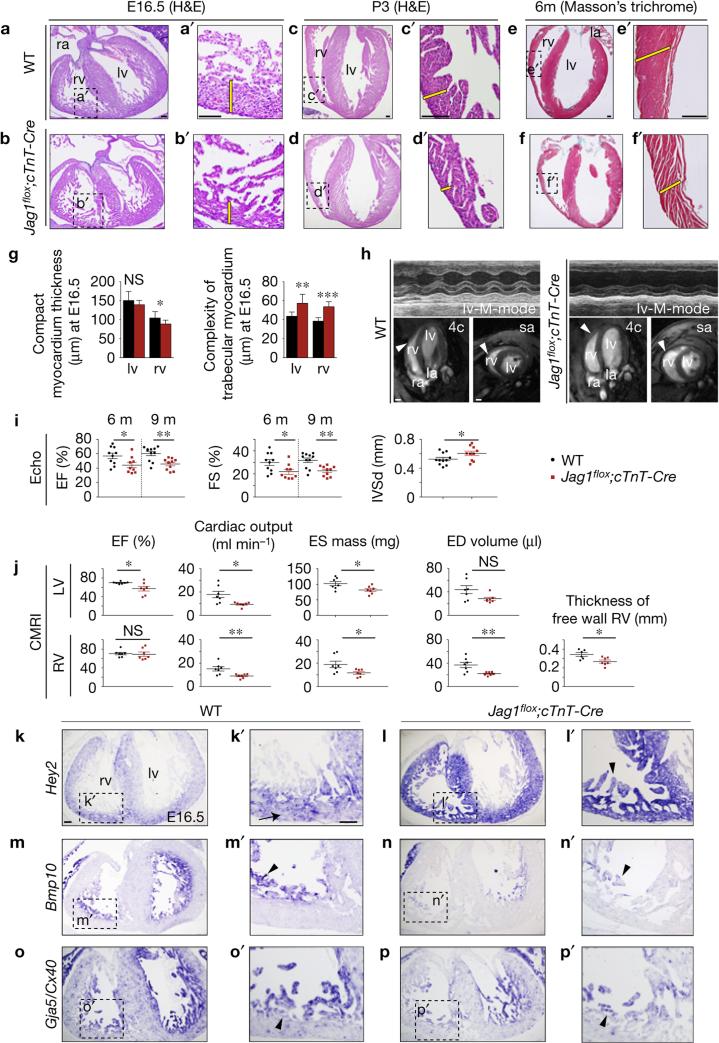
E16.5 WT embryos had a thick compact myocardium and compacting trabeculae, whereas Jag1flox;cTnT-Cre embryos revealed a 21% thinner compact myocardium in the right ventricle (Fig. 2a–b′,g). Mutant embryos also showed 30% and 42% higher trabecular complexity in the left and right ventricles (Fig. 2g). Increased trabecular complexity is a hallmark of LVNC and hypertrophic cardiomyopathies25. WT neonates had thick muscular ventricular walls (Fig. 2c,c′), but Jag1flox;cTnT-Cre littermates had dilated ventricles and a thin compact myocardium (Fig. 2d,d′). These defects were also apparent in mutant adult heart (Fig. 2e–f′). Ultrasonography revealed impaired left ventricular systolic function in adult Jag1flox;cTnT-Cre mutants, with reductions in ejection fraction, fractional shortening and diastolic interventricular septal wall thickness (Fig. 2h,i and Supplementary Video 2). High-resolution cardiac magnetic resonance imaging (CMRI) confirmed ventricular wall thinning in Jag1flox;cTnT-Cre animals (Fig. 2h and Supplementary Video 3). CMRI also revealed reductions in left ventricular ejection fraction, cardiac output, end-systolic mass, end-diastolic volume and the thickness of right ventricular free wall (Fig. 2j), which also showed segmental dyskinesia (Supplementary Video 4).
Figure 2.
Myocardial Jag1 inactivation disrupts chamber maturation and leads to cardiomyopathy and systolic dysfunction. (a–d′) Haematoxylin–eosin (H&E) staining of heart sections from WT and Jag1flox/flox;cTnT-Cre/+ (Jag1flox;cTnT-Cre) E16.5 embryos (a–b′) and postnatal day 3 (P3) neonates (c–d′). (e–f′) Masson's trichome staining in 6-month-old hearts. The yellow bars in a′–f′ indicate compact myocardium thickness. Abbreviations as in Fig. 1. Scale bars, 100 μm. (g) Quantification of compact myocardium thickness and trabecular complexity in E16.5 WT, Jag1flox;cTnT-Cre embryos. Data are mean ± s.d. (n = 16 sections from 4 WT and n = 16 sections from 4 mutant embryos, *P < 0.05, **P < 0.01, ***P < 0.001, by Student's t-test; NS, not significant). (h) Echocardiography and CMRI analysis of adult WT and Jag1flox;cTnT-Cre mice. Left: top, M-mode views of the left ventricle (lv) of a 6-month-old WT mouse. Bottom, CMRI analysis of a 6-month-old WT mouse. Images show four-chamber (4c) and short-axis (sa) views. Arrowheads mark the ventricular wall. Right: top, M-mode views of the left ventricle of a 6-month-old Jag1flox;cTnT-Cre mouse. Bottom, CMRI analysis of a 6-month-old mutant mouse. Arrowheads mark the thin ventricular wall. Scale bars, 2 mm. (i) Echocardiography analysis of left ventricular ejection fraction (EF) and fractional shortening (FS) measured at 6 and 9 months and diastolic interventricular septal wall thickness (IVSd) in WT and Jag1flox;cTnT-Cre mice. Data are mean ± s.d. (n = 10 WT and n = 9 mutants at 6 months and n = 11 WT and n = 10 mutants at 9 months, *P < 0.05 and **P < 0.01 by Student's t-test). (j) CMRI analysis of physiological and morphometric parameters in the left (LV) and right ventricles (RV) of 6-month-old WT and Jag1flox;cTnT-Cre mice. EF, ejection fraction; ES mass, end-systolic mass; ED volume, end-diastolic volume. Data are mean ± s.e.m. (n = 7 WT and n = 7 mutants, *P < 0.05 and **P <0.01 by Student's t-test; NS,=not significant). (k–p′) ISH in E16.5 WT and Jag1flox;cTnT-Cre heart sections. (k–l′) Hey2 expressed in compact myocardium (k′, arrow) is expanded to trabeculae in mutants (l′, arrowhead). (m–n′) Bmp10. (o–p′) Cx40. Arrowheads in m′,n′,o′,p′ indicate trabecular myocardium. Scale bars, 100 μm. Source data are available in Supplementary Table 4.
Jag1 is required for ventricular maturation
Chamber marker analysis in E16.5 Jag1flox;cTnT-Cre embryos revealed expansion of the compact myocardium marker Hey2 to the trabeculae (Fig. 2k–l′) and low expression of the trabecular markers Bmp10, Gja5/Cx40 (Fig. 2m–p′) and Anf (Supplementary Fig. 5e,f′), especially in the right ventricle.
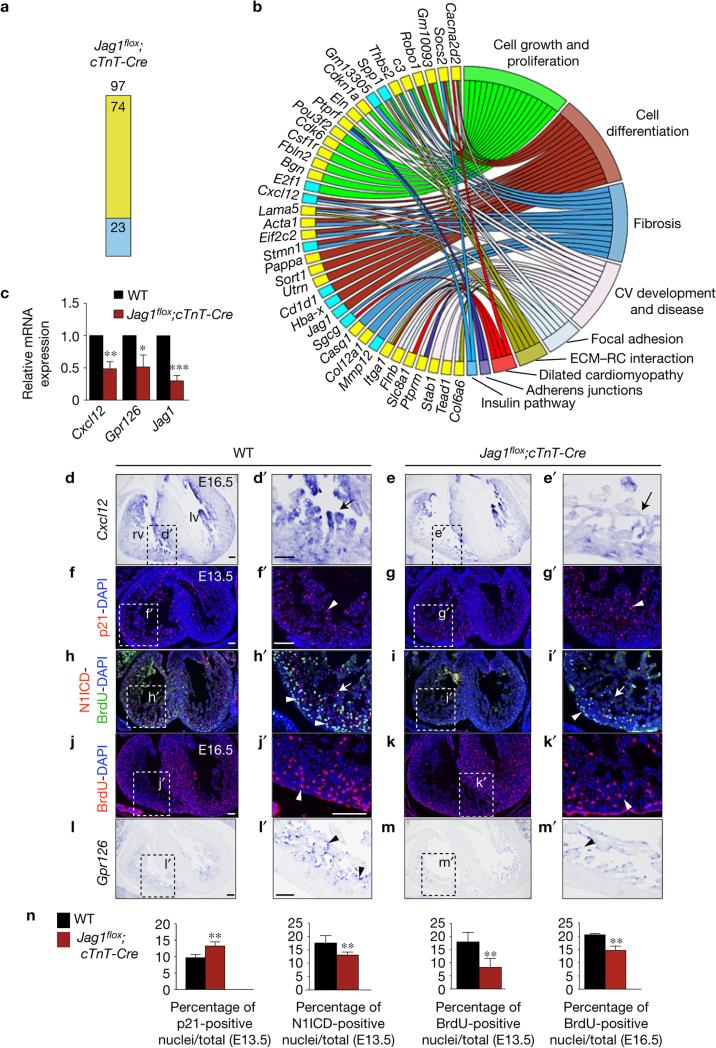
RNA-seq of E15.5 Jag1flox;cTnT-Cre ventricles identified 97 differentially expressed genes (P < 0.05): 74 upregulated, 23 downregulated (Fig. 3a and Supplementary Table 1). GO classification revealed involvement in essential cellular functions, cardiovascular development and disease, and dilated cardiomyopathy (Fig. 3b). E16.5 Jag1flox;cTnT-Cre mutants showed strong downregulation of the chemokine ligand gene Cxcl12 (Fig. 3b,c and Supplementary Table 1), with attenuated chamber myocardium expression at E16.5 (Fig. 3d–e′). Interestingly, Cxcl12/Cxcr4 signalling is crucial for coronary artery development26,27, a process simultaneous with and essential for compaction28. One upregulated gene was the cell-cycle inhibitor Cdkn1a/p21 (Fig. 3b and Supplementary Table 1). Consistent with RNA-seq, p21 expression was 30% above normal in E13.5 Jag1flox;cTnT-Cre mutant ventricles (Fig. 3f–g′,n) concomitantly with reductions in compact myocardium proliferation (56%) and endocardial N1ICD expression (28%; Fig. 3h–i′,n). This defect persisted at E16.5 when Jag1flox;cTnT-Cre embryos had a 25% below-normal proliferation of compact myocardium cardiomyocytes (Fig. 3j–k′,n). In situ hybridization (ISH) and qRT–PCR in E16.5 Jag1flox;cTnT-Cre mutants revealed reduced Gpr126 transcription (Fig. 3c,l–m′), similar to the situation in Dll4 mutants (Fig. 1p–q′).
Figure 3.
Jag1 gene profiling and validation. (a) Chart showing the number of deregulated genes identified by RNA-seq (P < 0.05) in Jag1flox;cTnT-Cre hearts. Yellow, upregulated; blue, downregulated. (b) Circular plot showing 39 representative differentially expressed genes from a total of 97 genes belonging to selected functional categories. Code colour as in a. The total number of deregulated genes and the genes represented in the circular plot can be found in Supplementary Table 1. (c) qRT–PCR analysis of Cxcl12, Gpr126 and Jag1 (as control) in E16.5 WT and mutant ventricles. Data are means ± s.d. (n = 3 pools of 3 WT ventricles per pool and n = 3 pools of 3 mutant ventricles per pool; *P < 0.05, **P < 0.01, ***P < 0.001 by Student's t-test). (d–e′) ISH of Cxcl12 in E16.5 WT and Jag1flox;cTnT-Cre hearts. Arrows point to myocardial expression. (f–i′) Immunostaining for p21 (red nuclei; arrowheads in f′,g′), N1ICD (red nuclei; arrowheads in h′,i′) and incorporated BrdU (green nuclei; arrows in h′,i′) in E13.5 WT and Jag1flox;cTnT hearts. (j–k′) Immunostaining of incorporated BrdU in E16.5 WT (j,j′) and Jag1flox;cTnT-Cre heart sections (k,k′). Arrowheads indicate BrdU-positive nuclei (red). (l–m′) ISH of Gpr126 in E16.5 WT and Jag1flox;cTnT-Cre hearts. Arrowheads indicate endocardial expression. (n) Quantification of p21-, N1ICD- and BrdU-positive nuclei in E13.5 and E16.5 Jag1flox;cTnT ventricles. Data are mean ± s.d. (n = 6 WT and 6 mutant embryos, except for E16.5 BrdU quantification, where n = 3 WT and n = 3 mutant embryos; *P < 0.01, by Student's t-test). Abbreviations as in Fig. 2. Scale bars, 100 μm. Source data are available in Supplementary Table 4.
Jag2 is required for chamber maturation and compaction together with Jag1
Targeted Mib1 inactivation in mouse myocardium causes LVNC cardiomyopathy15. We expected similar cardiac phenotypes in Mib1flox;cTnT-Cre and Jag1flox;cTnT-Cre mutants, assuming Jag1 as the only myocardial ligand and therefore the only potential Mib1 substrate. This was not the case: E16.5 Jag1flox;cTnT-Cre mutants showed a thin compact myocardium and altered ventricular patterning, and adult mutants showed structural and functional signs of cardiomyopathy and systolic dysfunction, but not LVNC (Fig. 2). The phenotypic differences between Mib1 and Jag1 mutants suggested that another Notch ligand might be acting in the myocardium.
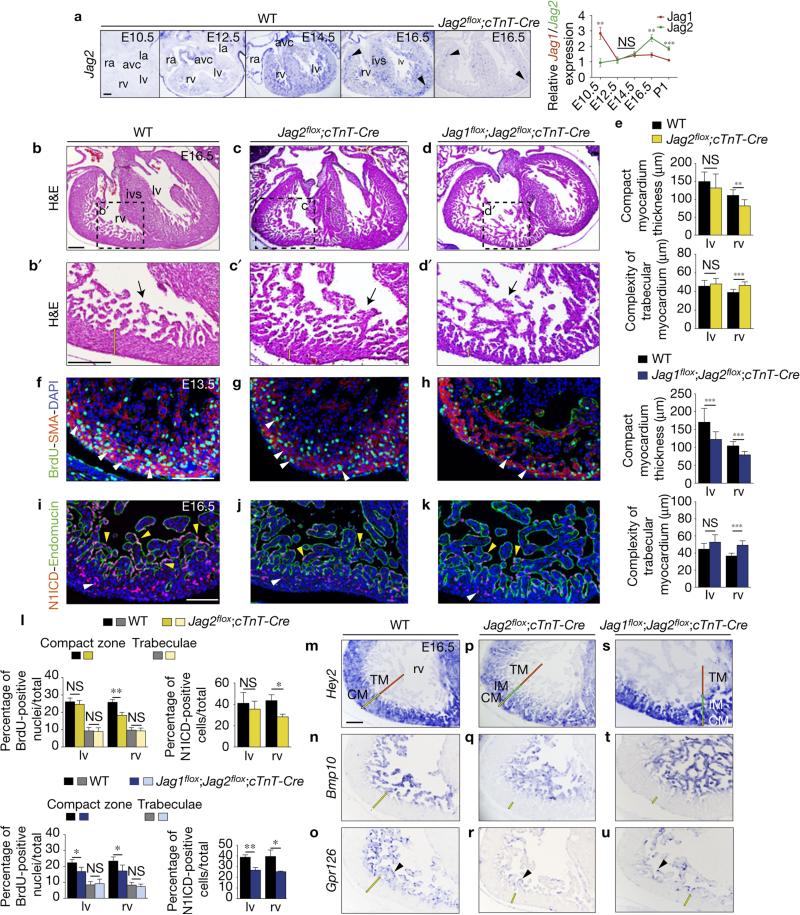
Myocardial expression of Jag2, a mammalian Jag1 paralogue, was upregulated from E10.5, (Fig. 4a). Jag2 upregulation paralleled Jag1 downregulation so that at E12.5–14.5 Jag1 and Jag2 expression levels were similar, with Jag2 levels higher thereafter (Fig. 4a). E16.5 Jag2flox;cTnT-Cre mice showed strongly diminished Jag2 expression in myocardium (Fig. 4a), and had a poorly structured ventricular septum and a 38% thinner compact myocardium, paralleled by a 20% increase in trabecular complexity (Fig. 4b–c′,e). Compact myocardium thinning was most likely due to the 30% reduced cardiomyocyte proliferation at E13.5, especially in the right ventricle (Fig. 4f,g,l). Consistent with action of Jag2 as a cardiac Notch ligand, N1ICD expression was 35% reduced in the right ventricle of E16.5 Jag2flox;cTnT-Cre embryos (Fig. 4i,j,l), indicating that myocardial Jag2 deletion disrupted endocardial Notch activation. Furthermore, Hey2, Bmp10 and Gpr126 were downregulated in Jag2flox;cTnT-Cre mutants (Fig. 4m–r).
Figure 4.
Myocardial Jag2, together with Jag1, is required for ventricular maturation and compaction. (a) ISH of Jag2 in heart sections from E10.5, E12.5, E14.5, E16.5 WT and E16.5 Jag2flox/flox;cTnT-Cre/+ (Jag2flox;cTnT-Cre) embryos. Abbreviations as in Fig. 1. cTnT-Cre-mediated deletion abrogates Jag2 expression in the myocardium but does not affect expression in coronary vessels (arrowheads). Chart shows relative ratios of Jag1 and Jag2 gene expression measured by qRT–PCR. Data are means ± s.d. (n=3 pools of 3 WT ventricles per pool for each stage analysed; **P < 0.01, ***P < 0.001, by Student's t-test; NS, not significant). (b–d′) H&E staining of E16.5 WT, Jag2flox;cTnT-Cre and Jag1flox;Jag2flox;cTnT-Cre hearts. The yellow bar in b′–d′ indicates the thickness of compact myocardium and the asterisk in c,d the defective ventricular septum. ivs, interventricular septum. (e) Chart showing quantification of compact myocardium thickness and complexity of trabecular myocardium in Jag2flox;cTnT-Cre and Jag1flox;Jag2flox;cTnT-Cre hearts. Data are mean ± s.d. (n = 9 sections from 3 WT and n = 9 sections from 3 Jag2flox;cTnT-Cre; n = 12 sections from 4 WT and n = 12 sections from 4 Jag1flox;Jag2flox;cTnT-Cre embryos; **P < 0.01, *** P < 0.001, determined by Student's t-test; NS, not significant). (f–h) BrdU (green) and SMA (red) immunostaining in E13.5 WT (f), Jag2flox;cTnT-Cre (g) and Jag1flox;Jag2flox;cTnT-Cre hearts (h). Nuclei are counterstained with DAPI (blue). (i–k) N1ICD immunostaining. In the E16.5 WT heart (i), N1ICD (red) labels endocardial nuclei (yellow arrowheads) delineated by endomucin staining (green), and coronary vessels (white arrowheads). Jag2flox;cTnT-Cre (j) and Jag1flox;Jag2flox;cTnT-Cre hearts (k) show below-normal N1ICD expression. (l) Quantification of BrdU- and N1ICD-positive cells in both genotypes. Data are mean ± s.d. (n = 3 WT and n = 3 Jag2flox;cTnT-Cre for BrdU and N1ICD stainings; n = 3 WT and n=4Jag1flox;Jag2flox;cTnT-Cre embryos for BrdU and N1ICD analyses; *P < 0.05, **P < 0.01, by Student's t-test; NS, not significant). (m–u) ISH analysis. Hey2 marks compact myocardium (CM, yellow bar) in m,p,s. The red and green bars in p,s mark the trabecular (TM) and intermediate myocardium (IM) respectively. Bmp10 labels TM in WT (n), Jag2flox;cTnT-Cre (q) and Jag1flox;Jag2flox;cTnT-Cre ventricles (t). (o,r,u) Gpr126 transcription. rv, right ventricle. Scale bars, 100 μm. Source data are available in Supplementary Table 4.
We next generated mice doubly deficient for myocardial Jag1 and Jag2. E16.5 Jag1flox;Jag2flox;cTnT-Cre embryos have a very thin compact myocardium, especially the ventricular apex region (Fig. 4d,d′), with a 27% thinner compact myocardium overall (Fig. 4e and Supplementary Fig. 6a). In contrast, trabecular complexity and trabecular area were respectively 26% and 72% above normal in the right ventricle of Jag1flox;Jag2flox;cTnT-Cre mice (Fig. 4e and Supplementary Fig. 6a), indicating defective compaction. Immunostaining of E16.5 Jag1flox;Jag2flox;cTnT-Cre heart sections for cTnT and endomucin, to label the myocardium and endocardium, showed a severe chamber phenotype (Supplementary Fig. 7a–b″), reminiscent of Mib1flox;cTnT-Cre mice15. Cardiomyocyte proliferation in E13.5 Jag1flox;Jag2flox;cTnT-Cre compact myocardium was 40% below normal in both ventricles (Fig. 4f,h,l). Endocardial Notch1 activity was also significantly reduced (32%, Fig. 4i,k,l). Hey2 delineated a thin compact myocardium with deep recesses (see below), Bmp10 was expressed in the trabeculae and endocardial Gpr126 expression was attenuated (Fig. 4m–o,s–u). Jag1 and Jag2 thus seem to play non-redundant roles in chamber development.
Manic Fringe modulates Notch ligand selectivity in ventricular endocardial cells
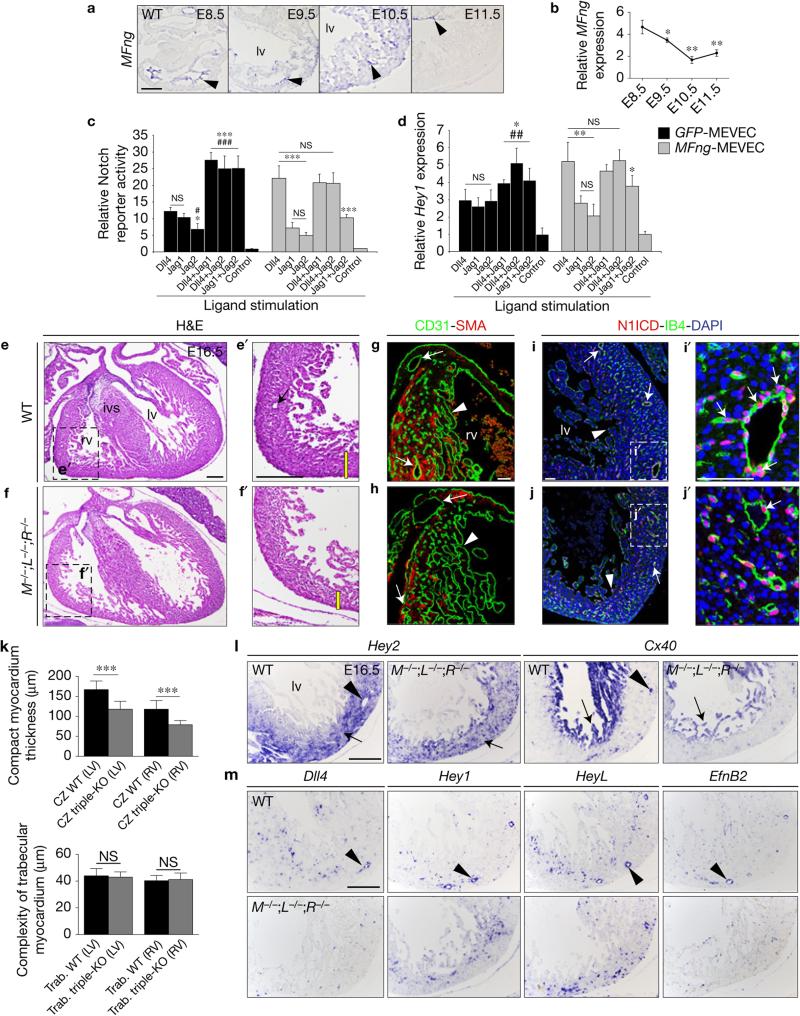
The Fringe family of glycosyltransferases elongate carbohydrates attached to the Notch extracellular EGF-like repeats, contributing to ligand selectivity29. On Notch glycosylation, Delta-Notch signalling is enhanced, and Jag/Ser–Notch signalling is diminished29,30. We first examined embryonic cardiac expression of the three mammalian Fringe paralogues by ISH and qRT–PCR. Manic fringe (MFng) was transcribed in chamber endocardium from E8.5, subsequently declining to a minimum at E11.5 (Fig. 5a,b). In contrast, lunatic fringe (LFng) was transcribed in epicardium and radical fringe (RFng) was not detected in the heart (Supplementary Fig. 8a,b). ISH data for MFng suggested that MFng activity in E8.5–10.5 chamber endocardium would favour endocardial Dll4 signalling to the Fringe-modified Notch receptor.
Figure 5.
MFng modulates Notch selectivity towards its ligands and systemic Fng abrogation disrupts coronary vessel development. (a) ISH analysis of MFng. Arrowheads point to expression in chamber endocardium (E8.5–10.5) and avc (E11.5). (b) Relative MFng expression by qRT–PCR from E8.5–11.5 ventricles. Data are means ± s.d. (n = 3 pools of 5 WT hearts at E8.5, and n = 3 pools of 3 ventricles per pool at E9.5–11.5, *P < 0.05, **P < 0.01, by Student's t-test). (c) Notch signalling activity measured by 10xCBF1–Luc reporter assay in MEVEC infected with control GFP lentivirus (GFP-MEVEC, black bars) or with a MFng lentivirus (MFng-MEVEC, grey bars) and stimulated with immobilized Notch ligands. Control represents the activity of the Notch reporter in non-stimulated MEVEC. (d) qRT–PCR analysis of Hey1 expression in GFP-MEVEC (black bars) or MFng-MEVEC (grey bars). Symbols: *, significant difference compared with Dll4; #, compared with Jag1. Data are mean ± s.d. (n = 4 independent biological replicates for each condition in c, and n = 3 for each condition in d; *, #P < 0.05, ## P < 0.01, ***, ### P < 0.001 by Student's t-test; NS, not significant). These data are representative of 3 independent experiments. (e–f′) H&E staining of E16.5 WT (e,e′) and M;L;RFng-deficient hearts (M−/−;L−/−;R−/− f,f′). The yellow bar in e′,f′ indicates the thickness of compact myocardium and the arrow in e′ points to a coronary vessel. (g,h) WT (g) and M−/−;L−/−;R−/− (h) ventricular sections stained with SMA (myocardium, red) and CD31 (Pecam1) (green), which labels the endocardium (arrowheads) and the endothelium of coronary vessels (arrows). (i–j′) N1ICD (red) stains chamber endocardium (i,j, arrowheads) and coronary vessels (i′–j′, arrows), co-stained with isolectin B4 (IB4, green). Nuclei are counterstained with DAPI (blue). (k) Quantification of compact myocardium thickness (CZ, myocardial compact zone) and trabecular myocardium complexity in E16.5 M−/−;L−/−;R−/− mice. Data are mean ± s.d. (n = 3 WT and n = 3 triple mutant embryos, ***P < 0.001, by Student's t-test; NS, not significant). (l,m) ISH analysis of Hey2 and Cx40 (l) and Dll4, Hey1, HeyL and EfnB2 (m) expression in WT and M−/−;L−/−;R−/− mice. Scale bars, 100 μm, except in g–j′ where scale bars are 50 μm. Source data are available in Supplementary Table 4.
We investigated the effect of MFng on ligand stimulation of Notch1, using isolated and immortalized mouse embryonic ventricular endocardial cells (MEVEC) transduced with lentiviral vector encoding MFng (MFng-MEVEC). We first examined the response of control GFP–MEVEC to stimulation with immobilized recombinant Dll4, Jag1 or Jag2 ligands31. As readouts we measured the activity of the Notch reporter CBF1–Luc (ref. 32; Fig. 5c) and transcript expression of endocardial Notch target Hey1 (ref. 33; Fig. 5d). Dll4 and Jag1 activated the Notch reporter to a similar extent, whereas Jag2 elicited a weaker response (Fig. 5c), but all three ligands induced a similar increase in Hey1 expression (Fig. 5d). Pairwise ligand combinations produced additive effects (Fig. 5c). In MFng-MEVEC Notch reporter activity and Hey1 expression were potentiated by Dll4, whereas the response to Jag1 and Jag2 remained weak (Fig. 5c,d). Combinations of Dll4+Jag1 and Dll4+Jag2 produced a similar response to stimulation with Dll4 alone (Fig. 5c,d), but the Jag1+Jag2 combination stimulated Notch signalling weakly (Fig. 5c,d).
Systemic Fng inactivation affects coronary vessel development
Our data suggest that MFng may regulate the spatio-temporal specificity of Notch ligand–receptor interaction in the developing chamber. In E8.5–10.5 chamber endocardium, MFng would favour endocardial Dll4 signalling to the Fringe-modified Notch receptor. MFng downregulation after E11.5 would allow Jag1 and Jag2 myocardial signalling to activate endocardial Notch1. We examined the effect of systemic Fng inactivation by generating mice triply deficient for M, L and RFng. Triple mutants develop to term and, although not found at Mendelian frequency at weaning, there are no reported embryonic lethal phenotypes34. Examination of M;L;RFng-deficient embryos at E10.5 revealed no obvious trabeculation defects, suggesting that Dll4 (and Jag1) is able to activate Notch signalling sufficiently. At E16.5, WT chamber development is well advanced (Fig. 5e,e′) and M−/−;L−/−;R−/− embryos had a 33% thinner compact myocardium (Fig. 5f,f′,k); moreover, CD31 staining revealed coronary vessel loss or defects (Fig. 5g,h). Interestingly, N1ICD expression seemed normal in ventricular endocardium but was attenuated in coronary vessels (Fig. 5i–j′). Hey2 expression marked the thin compact myocardium and Cx40 was expressed in trabeculae but did not label coronary vessels in M−/−;L−/−;R−/− mice (Fig. 5l). Coronary arteries of E16.5 Fng-deficient embryos also showed abnormal expression patterns of Dll4, Hey1, HeyL and EfnB2 (Fig. 5m), similar to E15.5 Dll4flox;Cdh5-CreERT mutant hearts (Supplementary Fig. 3k–p′). Systemic Fng abrogation disrupts coronary vessel development, crucial for myocardium nourishment, thus indirectly affecting compaction. During compaction MFng is expressed in coronary vessels (Fig. 6a), similarly to Dll4 (Supplementary Figs 1e,e′ and 3e,e′) suggesting that MFng may reinforce Dll4 signalling to Notch in the developing coronaries.
Figure 6.
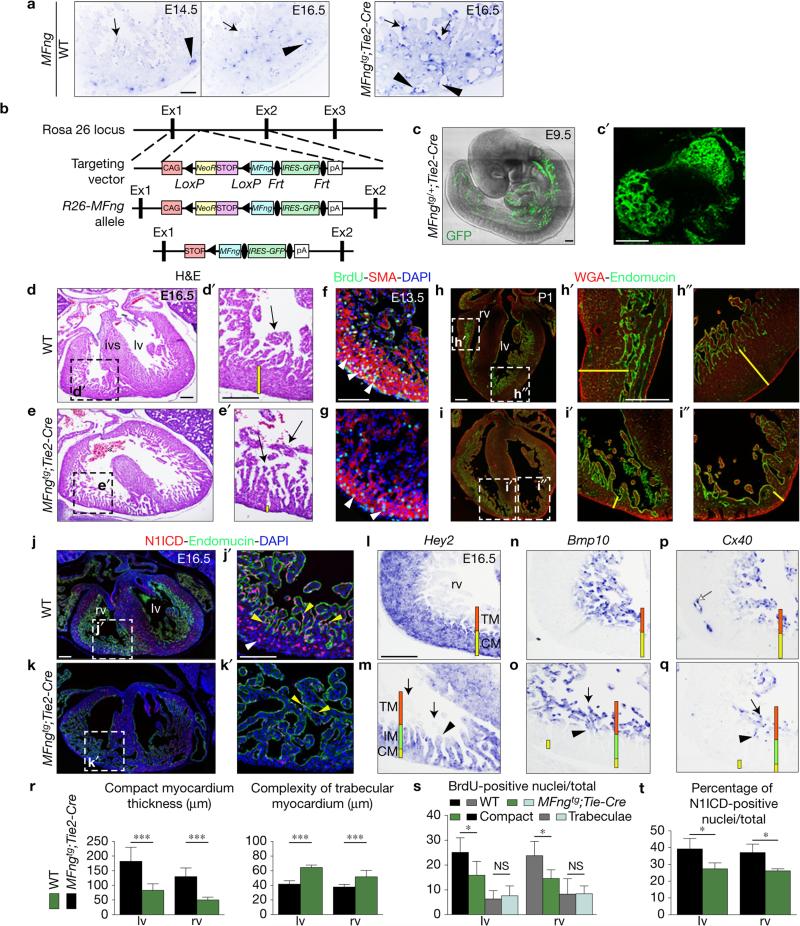
Forced MFng expression in the endocardium disrupts chamber development. (a) ISH showing MFng expression in coronary vessel endothelium (arrowheads) but not in endocardium (arrows) of E14.5–16.5 WT embryos, and ectopic MFng expression in endocardium (arrows) and in coronary vessels endothelium (arrowheads) of MFngtg;Tie2-Cre embryos. (b) Gene targeting strategy used to generate the conditional MFngtg line. (c) Whole-mount two-photon microscopy image of an E9.5 MFngtg/+;Tie2-Cre/+ embryo. (c′) Heart detail showing transgenic expression. (d–e′) H&E staining of E16.5 WT and MFngtg/tg;Tie2-Cre/+ (MFngtg;Tie2-Cre) ventricles. MFngtg;Tie2-Cre hearts show defective ventricular septum (e, asterisk). The yellow bar in d′,e′ marks the thickness of compact myocardium. (f,g) BrdU (green) and SMA (red) staining in E13.5 WT (f) and MFngtg;Tie2-Cre hearts (g). Nuclei are counterstained with DAPI (blue). (h–i″) Postnatal day 1 (P1) heart stained with wheat-germ agglutinin (WGA) and endomucin. The yellow bar in h′-i″ marks the thickness of compact myocardium. (j–k′) General view of the E16.5 WT (j) and MFngtg;Tie2-Cre hearts (k) stained for N1ICD and endomucin. N1ICD is expressed in WT endocardium (j′, yellow arrowheads) and coronaries (j′, white arrowhead) and very attenuated in the transgenic heart (k′). (l–q) ISH. Hey2 expression reveals a thick compact myocardium in the E16.5 WT heart (l) and a very thin and disorganized counterpart in the MFngtg;Tie2-Cre heart (m). Bmp10 and Cx40 label ventricular trabeculae in WT embryos (n,p) and both are expressed at the distal tip of trabeculae in MFngtg;Tie2-Cre embryos (o,q, arrows). Cx40 is also expressed in WT coronaries (p, white arrow) but not in transgenic embryos (q). The arrowhead (m,o,q) marks the transition between Bmp10−,Cx40− and Bmp10+,Cx40+ trabeculae. The red and yellow bars (l–q) mark the trabecular (TM) and compact myocardium (CM) and the green bar (m,o,q) marks the intermediate (IM) myocardium. Scale bars, 100 μm except in b,b′, where bars, 50 μm. (r) Quantification of ventricular morphological parameters of E16.5 WT (n = 3) and MFngtg;Tie2-Cre (n = 4) embryos. (s) BrdU-positive nuclei/total in E13.5 WT and MFngtg;Tie2-Cre hearts (n = 3 WT and n = 3 transgenic). (t) Quantification of N1ICD-positive nuclei in E16.5 WT (n = 3) and transgenic (n = 3) hearts. Data are mean ± s.d. *P < 0.05, ***P < 0.001, by Student's t-test; NS, not significant. Source data are available in Supplementary Table 4.
Forced MFng expression disrupts chamber development and compaction
Our in vitro data predicted that forced endocardial MFng expression would hamper Jag1- and Jag2-mediated Notch1 activation, producing a chamber phenotype similar to that caused by inactivation of both ligands (Fig. 4d,d′ and Supplementary Fig. 7b–b″) or Mib1 inactivation (Supplementary Fig. 7d–d″ and ref. 15). We generated a transgenic line (MFngtg) bearing a Rosa26-CAG-floxNeoSTOPflox-MFng-EGFP expression cassette (Fig. 6b). Tie2-Cre-mediated18 removal of the NeoSTOP sequences resulted in Rosa26-CAG-driven MFng-EGFP expression in vascular endothelium and endocardium (Fig. 6b,c,c′). At E16.5, transgenic mice with two MFng–EGFP copies had a dilated heart, with compact myocardium 50–60% thinner than WT (Fig. 6d,e,r and Supplementary Fig. 7c–c″), whereas trabecular complexity and trabecular area were 30% and >300% above normal (Supplementary Fig. 6a), indicating defective compaction. Cardiomyocyte proliferation was 38% impaired in compact myocardium of E13.5 transgenic embryos (Fig. 6f,g,s). Unlike the thick compact myocardium with some compacting trabeculae in WT neonate mice (Fig. 6h–h″), MFngtg;Tie2-Cre neonates had thin compact myocardium and non-compacted trabeculae in both ventricles, with no effect on cardiomyocyte size (Fig. 6i–i″). N1ICD expression was impaired in the ventricles of E16.5 MFngtg ;Tie2-Cre mice (Fig. 6j–k′,t), indicating that forced MFng endocardial expression disrupts Notch activation.
Hey2 expression in E16.5 MFngtg;Tie2-Cre mice delineated the thin compact myocardium and extended towards but did not reach the trabeculae tip (compare Fig. 6l,m), whereas Bmp10 and Cx40 were restricted to the distal trabeculae tip (compare Fig. 6n–q). Indeed, Hey2 and Bmp10 (or Cx40) expression in the trabeculae were complementary (compare Fig. 6m,o,q). In the MFngtg;Tie2-Cre ventricle, compact myocardium cardiomyocytes (Hey2+, Fig. 6m) seemed to enter the trabeculae and form an ‘intermediate myocardium’ (Hey2+, Bmp10−, Cx40−, Fig. 6m) defining a boundary between compact and trabecular myocardium (Hey2−, Bmp10+, Cx40+, Fig. 6o,q). A similar but more subtle phenotype was observed in Jag2flox;cTnT-Cre and Jag1flox;Jag2flox;cTnT-Cre hearts (Fig. 4m,n,p,q,s,t) but not Mib1flox;cTnT-Cre hearts15.
Upregulated MFng expression in coronary endothelium (Fig. 6a) and loss of Cx40 expression in coronaries of E16.5 MFngtg;Tie2-Cre mice (Fig. 6q) suggests that ectopic MFng might also affect compaction through alteration of the development of coronaries.
Comparative gene profiling
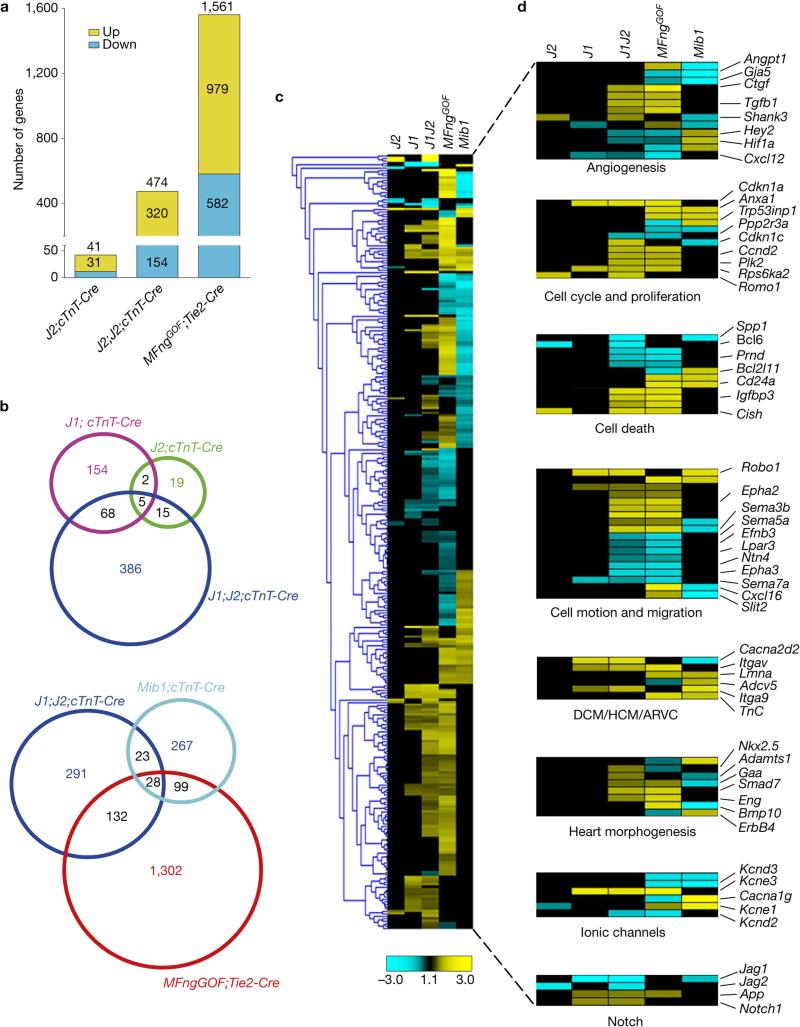
RNA-seq of E15.5 WT, Jag2flox;cTnT-Cre, Jag1flox;Jag2flox;cTnT-Cre and MFngtg;Tie2-Cre hearts was performed and compared with those from Jag1flox;cTnT-Cre (Fig. 3 and Supplementary Table 1) and Mib1flox;cTnT-Cre mice15. Jag2flox;cTnT-Cre hearts showed differential expression of 41 genes (31 upregulated, 10 downregulated), Jag1flox;Jag2flox;cTnT-Cre hearts showed 474 genes (320 upregulated, 154 downregulated) and MFngtg;Tie2-Cre hearts showed 1,561 (979 upregulated, 582 downregulated; Fig. 7a and Supplementary Table 1). Jag1flox;cTnT-Cre and Jag2flox;cTnT-Cre mutants shared 7 differentially expressed genes, Jag1flox;cTnT-Cre and Jag1flox;Jag2flox;cTnT-Cre mutants 73 genes and Jag2flox;cTnT-Cre and Jag1flox;Jag2flox;cTnT-Cre mutants 20 (Fig. 7b). Jag1flox;Jag2flox;cTnT-Cre and Mib1flox;cTnT-Cre mutants shared 51 genes, Jag1flox;Jag2flox;cTnT-Cre and MFngtg;Tie2-Cre 160 and MFngtg;Tie2-Cre and Mib1flox;cTnT-Cre 127 (Fig. 7b).
Figure 7.
Comparative expression profiling of Jag2flox;cTnT-Cre, Jag1flox;cTnT-Cre, Jag1flox;Jag2flox;cTnT-Cre, MFngGOF;Tie2-Cre (MFngtg;Tie2-Cre) and Mib1flox;cTnT-Cre mutants. GOF, gain of function. (a) Chart indicating the total number of deregulated genes identified by RNA-seq (P < 0.05) in E15.5 Jag2flox;cTnT-Cre mutants (J2;cTnT-Cre), Jag1flox;Jag2flox;cTnT-Cre mutants (J1;J2;cTnT-Cre) and MFngGOF;Tie2-Cre transgenic embryos (MFngGOF;Tie2-Cre). The total number of differentially expressed genes per genotype is indicated at the top of every column. Numbers in the yellow section indicate upregulated genes; numbers in the blue section indicate downregulated genes. (b) Venn diagram representation of the comparative analysis of the deregulated genes in the five genotypes analysed (Jag1flox;cTnT-Cre, Jag2flox;cTnT-Cre, Jag1flox;Jag2flox;cTnT-Cre, MFngGOF;Tie2-Cre and Mib1flox;cTnT-Cre). Top, comparison of J1 and J2 single and J1;J2;cTnT-Cre double mutants. Bottom, comparison of J1;J2;cTnT-Cre, Mib1;cTnT-Cre and MFngGOF;Tie2-Cre embryos. Numbers in black indicate the total number of genes common to at least two genotypes. (c) Heat map representation of the 372 genes differentially expressed in at least two of the five mutant genotypes. Yellow, upregulated; blue, downregulated; black, not significant change. (d) Subsets of genes clustered into functional classes (right panels). The total number of deregulated genes for each genotype and the genes represented in the heat map can be found in Supplementary Table 1.
The heat map in Fig. 7c shows the 319 genes differentially expressed in at least two of the five mutant genotypes. Jag2 inactivation affected fewer genes (41) than Jag1 (97), but many more genes were affected in the double Jag1flox;Jag2flox;cTnT-Cre mutant (474), indicating a synergistic effect of simultaneous inactivation (Fig. 7c). MFngtg;Tie2-Cre mice presented the largest number of deregulated genes, and genes shared with Jag1flox;Jag2flox;cTnT-Cre mutants generally changed in the same direction (Fig. 7c). The profile of Mib1flox;cTnT-Cre mutants was different, with expression of several deregulated genes showing the opposite changes to Jag1flox;Jag2flox;cTnT-Cre or MFngtg;Tie2-Cre mice (Fig. 7c).
GO analysis (Fig. 7d and Supplementary Table 1) identified alterations in genes involved in angiogenesis and vascular development, including the endocardial/endothelial Notch target Hey2 (refs 35,36) in at least two genotypes. Hey2 expression was down-regulated in Jag1flox;Jag2flox;cTnT-Cre and MFngtg;Tie2-Cre mice and upregulated in Mib1flox;cTnT-Cre mice (Fig. 7d and Supplementary Table 1 and ref. 15), reflecting its expanded expression from the compact myocardium to the large, non-compacted trabeculae15. Some of the deregulated genes in this category are involved in coronary artery development (Cxcl12; ref. 26, Hey2; ref. 37) and their down-regulated expression suggested defective coronary development in Jag1flox;Jag2flox;cTnT-Cre or MFngtg;Tie2-Cre mice. Genes involved in cardiac fibrosis (Ctgf (ref. 38), Tgfβ1 (ref. 39)) were upregulated in Jag1flox;Jag2flox;cTnT-Cre and MFngtg;Tie2-Cre mice. A second GO category included cell-cycle and proliferation genes, in which negative cell-cycle regulators (Cdkn1a/p21, Plk2, Rps6ka2 and Romo1) were upregulated in all genotypes except Mib1flox;cTnT-Cre mice (Fig. 7d). These results fit with the reduced cardiomyocyte proliferation in Jag1 and Jag2 mutants (Figs 3n and 4l) but not Mib1flox;cTnT-Cre mice15 (Supplementary Fig. 6b–d′). Cell-death-related genes were deregulated in Jag2flox;cTnT-Cre, Jag1flox;Jag2flox;cTnT-Cre and MFngtg;Tie2-Cre hearts, including the apoptosis inhibitors Spp1, Bcl6, Prnd and Bcl2l11 (downregulated) and the pro-apoptotic gene Cish (upregulated), suggesting altered cardiac cell homeostasis. Similar to what was found in Dll4flox and Notch1flox mutants (Fig. 1l), Semaphorin and Ephrin–Eph family members involved in cellular motion, axonal guidance and migration were deregulated. Semaphorins play key functions in cardiac morphogenesis and vascular patterning40,41. One down-regulated gene, Efnb3, is associated with the cardiac hypertrophic response in adult heart42. Genes related to dilated, hypertrophic and arrhythmogenic right ventricular cardiomyopathy (DCM, HCM and ARVC) were deregulated in Jag1flox;cTnT-Cre, Jag1flox;Jag2flox;cTnT-Cre, MFngtg;Tie2-Cre and Mib1flox;cTnT-Cre mice. Genes required for heart morphogenesis (Nkx2.5, Adamts1, Smad7, Gaa and Eng) were upregulated in Jag1flox;Jag2flox;cTnT-Cre mutants, whereas Bmp10 and ErbB4 expression changed in opposite directions in MFngtg;Tie2-Cre and Mib1flox;cTnT-Cre mice. Last, a set of genes encoding voltage-dependent potassium and calcium channels (Kcnd3, Kcne1, Kcne3, Kcnd2 and Cacna1g) was deregulated in Jag1flox;cTnT-Cre, MFngtg;Tie2-Cre and Mib1flox;cTnT-Cre mice.
DISCUSSION
Our studies demonstrate that heart chamber development is coordinated through sequential Notch1 receptor activation in the endocardium, first by endocardially expressed Dll4 and later by Jag1 and Jag2 signalling from the myocardium. This temporally patterned Notch1 activation is determined by MFng and elicits different morphogenetic processes in the developing ventricles: the early, MFng-favoured endocardial Dll4–Notch1 signalling promotes cardiomyocyte proliferation and differentiation leading to trabeculation. The late, myocardial Jag1/Jag2–endocardial Notch1 signalling, occurring after MFng (and Dll4) downregulation in the endocardium, supports myocardial patterning, maturation and compaction. Simultaneously, coronary endothelial Dll4 signalling to the Fng-modified-Notch1 receptor stimulates coronary vessel development.
RNA-seq of endocardial-specific Dll4 or Notch1 mutant hearts shows deregulation of a set of genes involved in crucial cellular processes, signalling, cardiovascular development and disease. Gpr126 is of particular interest because its expression is dependent on Notch signalling in endothelial cells, zebrafish and mouse. Moreover, targeted Gpr126 inactivation in mice disrupts trabeculation, revealing its crucial function in chamber development22. Work in zebrafish showed that an amino-terminal Gpr126 fragment rescues the trabeculation phenotype of gpr126 morphants22, suggesting that this fragment can function as a paracrine signal instructing adjacent trabecular cardiomyocytes. In response to Notch, Gpr126 might influence the proliferation and differentiation of neighbouring cardiomyocytes within the developing chamber. Accordingly, cardiomyocyte proliferation, essential for ventricular growth, is affected in endocardial Dll4 and Notch1 mutants. Indeed, we reported defective cardiomyocyte proliferation in RBPJk/CBF1 mutants, accompanied by reduced Bmp10 expression12. RNA-seq revealed increased expression of various negative cell-cycle regulators, suggesting that Notch signalling plays a general role in regulating cardiomyocyte proliferation. Proliferation and differentiation of embryonic cardiomyocytes are intimately connected43 and our data suggest that Notch regulates both processes. Gene profiling revealed no significant changes in EfnB2 or Nrg1, despite their downregulation after endothelial Dll4 deletion in the ISH analysis and their roles downstream of Notch during trabeculation12. This discrepancy might reflect the low expression of EfnB2 and Nrg1 in WT heart and the strict criteria for considering a gene-expression change as significant.
Jag1 is non-essential for trabeculation but is required for ventricular maturation. Jag1 inactivation in embryonic myocardium generated mice with a dilated heart, a thin compact myocardium, reduced ejection fraction and cardiomyopathy with systolic dysfunction. Myocardium-specific Jag1 mutants showed impaired proliferation and elevated p21 expression in compact myocardium cardiomyocytes, findings supported by the RNA-seq. Expression of various chamber-patterning markers was also impaired, indicating that Jag1 is required for ventricular patterning and maturation. Myocardial Jag1 mutants, like endocardial Dll4 mutants, showed reduced Gpr126 expression, indicating Gpr126 as an important Notch effector throughout chamber development. Myocardial expression of Jag2 increases from E12.5, mirroring Jag1 expression. Jag2 deletion also disrupts chamber maturation, and combined Jag1 and Jag2 deletion triggers a severe chamber phenotype reminiscent of LVNC.
MFng is a crucial temporal modulator of Notch ligand–receptor interaction during ventricular development. In vitro experiments show that in the absence of MFng, Dll4, Jag1 and Jag2 activate Notch to similar levels and that pairwise combinations of these ligands have an additive effect. In the presence of MFng, Dll4 becomes a Notch1 superactivator, but Jag1 and Jag2 activate Notch1 quite poorly, and do not affect the response to Dll4. The function of MFng as a Notch modulator has been described in embryonic vasculature, where Jag1 is co-expressed with Dll4 in endothelial cells and inhibits Notch activation31. In the developing ventricle the situation is different because Dll4 and Jag1 are expressed in different cell types. This MFng–Dll4–Notch1 relationship also exists in the coronaries, as indicated by our loss-of-function data showing that MFng and Dll4 are required for coronary vessel development and thus indirectly for compaction. Ectopic MFng overexpression in vascular endothelium and endocardium of MFngtg;Tie2-Cre mice causes a severe, LVNC-like chamber phenotype. RNA-seq identified a large set of differentially expressed genes overlapping those affected in Jag1flox;Jag2flox;cTnT-Cre mice. Thus, the data indicate that MFng overexpression throughout the endocardium effectively blocks Jag1 and Jag2 myocardial signalling to Notch1.
The chamber-patterning defects in Jag2flox;cTnT-Cre, Jag1flox;Jag2flox;cTnT-Cre and MFngtg;Tie2-Cre mice are emphasized by the detection of an ‘intermediate myocardium’ (Hey2+) in the trabeculae, proximal to the thin compact myocardium (Hey2+), in addition to the distal trabecular myocardium (Hey2−,Bmp10+, Cx40+). Supplementary Fig. 7 illustrates a feature common to all Notch mutants affecting compaction analysed here or previously15: the structural chamber defects are more severe in the right ventricle, perhaps reflecting the different developmental origins of the ventricular chambers44.
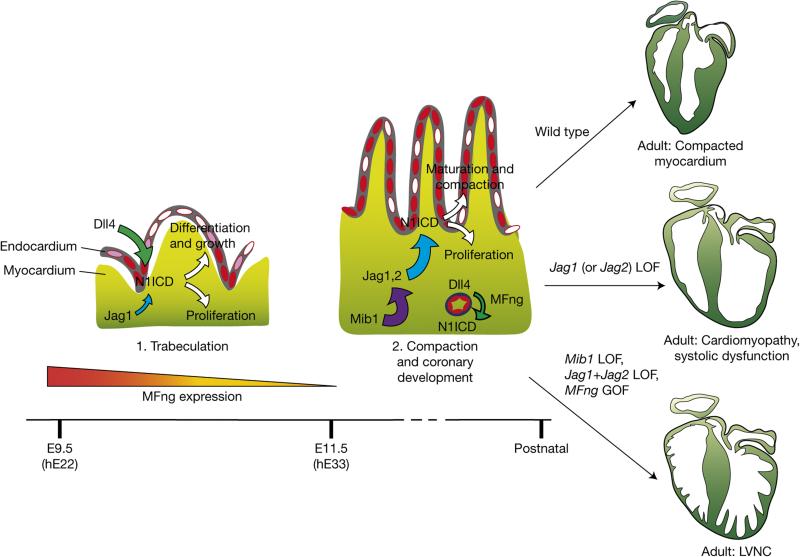
Our results are summarized in Fig. 8. Inactivation of the ubiquitin ligase MIB1 causes LVNC in mice and humans15. The current findings show that combined myocardial inactivation of Jag1 and Jag2 also causes LVNC, suggesting that both ligands are Mib1 substrates in the myocardium. This is supported by the LVNC-like phenotype after forced endocardial MFng expression (Fig. 8). Our findings shed light on the molecular mechanisms orchestrating ventricular chamber development, and the coordination between compaction and the formation of the coronary vessels that nourish the mature ventricles. Understanding these developmental processes has important implications for the management of heart disease.
Figure 8.
Sequential Notch ligand–receptor activation during ventricular chamber development. In the early ventricle (1) endocardial Dll4 and myocardial Jag1 can activate Notch1 in the endocardium. Expression of MFng in the endocardium favours Dll4 signalling to Notch1 (large green arrow) and Jag1 signalling is low (small blue arrow). Notch1 activation (red) promotes cardiomyocyte proliferation, trabecular growth and patterning. As chamber development proceeds (2), endocardial MFng expression reduces progressively (similarly to Dll4) allowing myocardial Jag1 to activate Notch1 in the myocardium. Jag2 expression is progressively upregulated in chamber myocardium, where it acts together with Jag1 to promote chamber proliferation, patterning and trabecular compaction to give rise to the functional ventricular wall (large blue arrow). At this time, MFng and Dll4 are required for Notch1 activation in coronary vessels (small green arrow). Inactivation of Jag1 (or Jag2) in the myocardium disrupts chamber maturation and leads to cardiomyopathy. Mib1 acts upstream of Jag1 and Jag2 in this process: Mib1 inactivation in the myocardium causes LVNC. A similar phenotype is produced when both Jag1 and Jag2 are deleted in the myocardium and when MFng is constitutively expressed in the endocardium (presumably causing persistent inhibition of Jag1 and Jag2 signalling). LOF, loss of function.
METHODS
Mouse strains and genotyping
Animal studies were approved by the CNIC Animal Experimentation Ethics Committee and by the Community of Madrid (Ref. PROEX 118/15). All animal procedures conformed to EU Directive 2010/63EU and Recommendation 2007/526/EC regarding the protection of animals used for experimental and other scientific purposes, enforced in Spanish law under Real Decreto 1201/2005. Mouse strains were CBF:H2B-Venus (ref. 16), Notch1 (ref. 45), RBPJk (ref. 46), Tie2-Cre (ref. 18), Nfatc1-Cre (ref. 4), cTnT-Cre (ref. 24), Cdh5(PAC)-CreERT2 (ref. 19), Dll4flox (ref. 17), Jag1flox (ref. 47), Jag2flox (ref. 48), Notch1flox (ref. 49), Mib1flox (ref. 50). Fringe mutant strains were bred and maintained as described previously34. For the generation of Rosa26-MFng Tg mice (Acc. No. CDB0917K: http://www.cdb.riken.jp/arg/mutant%20mice%20list.html), a cDNA encoding a native form of murine MFng was cloned into a modified version of the pROSA26-1 vector, preceded by the ubiquitous CAG promoter and a loxP-flanked ‘NeoR-STOP’ cassette and followed by a frt-flanked IRES–EGFP cassette and a bovine polyadenylation sequence. Gene targeting was done with TT2 embryonic stem cells and confirmed by Southern blotting. Mice were generated by injection of targeted embryonic stem cells into ICR embryos, and were backcrossed with the C57BL/6 strain. Details of genotyping will be provided on request.
Zebrafish experiments
Zebrafish were maintained and raised under standard conditions at 28 °C. We used WT AB and mibta52b fish51. WT embryos were treated for 15 h with RO4929097 (S1575, selleckchem.com, 10 μM) or with dimethylsulphoxide23 (DMSO). All embryos were fixed at 48 hpf in 4% PFA overnight and processed for in situ hybridization (ISH) as described previously52.
4-hydroxy-tamoxifen (4-OHT) induction
Dll4flox/+;Cdh5-CreERT/+ male mice were crossed with Dll4flox/flox females and pregnant females were administered with 4-OHT (H6278 Sigma) once by oral gavage at 12.5 days of gestation. Embryos were dissected at E15.5.
Histology and in situ hybridization
Haematoxylin–eosin (H&E) staining, in situ hybridization (ISH) on sections, and whole-mount ISH were performed as described previously52,53. Details of probes will be provided on request.
Whole-mount immunofluorescence
E9.5 CBF:H2B-Venus embryos were dissected and fixed for 2 h in 4% PFA at 4 °C. Embryos were permeabilized for 1 h with 0.5% Triton X-100/PBS and subsequently blocked for 1 h in Histoblock solution (5% goat serum, 3% BSA, 0.3% Tween-20 in PBS). Primary and secondary antibodies used are listed in Supplementary Table 2. After several washes in PBS-T (PBS containing 0.1% Tween-20) embryos were mounted in a 60 mm Petri dish with 1% agar. To detect endogenous GFP, fixed E9.5 CBF:H2B-Venus; CBF:H2B-Venus;N1KO and CBF:H2B-Venus;RBPJkKO embryos were imaged with a Zeiss 780 confocal microscope fitted with a ×20 objective with a dipping lens. Z-stacks were taken every 5 μm. 3D images were reconstructed with IMARIS software (Bitplane Scienific Software).
Immunohistochemistry
Paraffin sections (7 μm) were incubated overnight with primary antibodies, followed by 1 h incubation with a fluorescent-dye-conjugated secondary antibody. N1ICD, Dll4, Jag1 and p21 staining was performed using tyramide signal amplification54,55 (TSA). All antibodies used are listed in Supplementary Table 2.
Quantification of incorporated BrdU, p21 and N1ICD
Cell proliferation was analysed by detection of BrdU incorporation55. For p21 and N1ICD quantification, positive nuclei were divided by the total number of nuclei counted on sections (≥4) in six embryos of each genotype. Images were processed with ImageJ software. Statistical significance was assessed by Student's t-test.
Quantification of compact myocardium thickness and trabecular complexity
The method used was a modification of that described in refs 56,57. Briefly, 7 μm paraffin sections from E9.5 WT and mutant hearts were stained with anti-CD31 and anti-MF20 to facilitate visualization of ventricular structures. In E16.5 WT and mutant hearts endocardial cells were stained with anti-endomucin and myocardium with anti-cTnT. Confocal images were obtained with a NIKON A1R confocal microscope. ImageJ software was used for the measurements. In E16.5 heart sections, left and right ventricles were analysed separately. For each measurement, settings were kept constant for all images using the scale bar recorded in each image as the reference distance. The thickness of the compact myocardium was measured by dividing the ventricle into two main regions: the apex (at the bottom of a transverse section) and the basal region (at the top; see Supplementary Fig. 7a). Several measurements were taken in each region and the mean was expressed in micrometres. The complexity of trabecular myocardium indicates the size of the trabecular mesh of myocardial fibres and is considered a landmark of cardiomyopathy25. It was represented as the mean ratio, expressed in micrometres, obtained by dividing the length of each trabecula by its thickness. The ratio of trabecular area and compact myocardial thickness was measured by dividing the surface occupied by trabeculae in the ventricle (μm2) by the length of compact myocardium expressed in micrometres.
Imaging, photography and 3D reconstruction
E9.5 whole-embryos or hearts were imaged with a Zeiss 780 confocal microscope fitted with a ×20 objective with a dipping lens. Z-stacks were taken every 5 μm. 3D images were reconstructed with IMARIS software (Bitplane Scientific Software). WT E9.5 embryos were sectioned at 10 μm and stained for Dll4, Jag1 and N1ICD. Images were acquired with a confocal microscope (Leica TCS SPE) taking 10 optical sections per histological section. After acquisition, 3D reconstructions were performed as described previously58 and the resulting surfaces were exported to Adobe Acrobat Professional 9 Extended version to generate 3D interactive pdf files59.
Lentiviral production and infection
Full-length murine Manic Fringe (MFng) (ref. 31) was subcloned into the lentiviral vector pRLL-IRES-eGFP (Addgene). Concentrated lentiviruses expressing pRLL-IRES-eGFP or pRLL-MFng-IRES-eGFP were obtained as described previously60. Viruses were titrated in Jurkat cells, and infection efficiency (GFP-expressing cells) and cell death (propidium iodide staining) were monitored by flow cytometry.
Isolation and immortalization of MEVEC
Mouse embryonic ventricular endocardial cells (MEVEC) were obtained from ventricles of E10.5 embryos. Embryos were dissected in PBS and hearts were collected. Atria and the atrioventricular canal were removed and the remaining ventricles were mechanically dissociated. Isolated cells were collected in DMEM containing 20% FBS and centrifuged at 400g for 5 min. The cells were finally resuspended in 500 μl culture medium (DMEM, 20% FBS supplemented with penicillin/streptomycin and 100 μg ml−1 endothelial cell growth supplement (ECGS) (Sigma-Aldrich)), and seeded into one well of a MW24 plate pre-treated with collagen type I (4.08 mg ml−1; BD Bioscience). After 1 week, a confluent cobblestone morphology culture was observed and cells were transferred to one gelatinized p60 flask. MEVEC in passage 1 were infected with lentivirus expressing SV40-large T antigen in the presence of Polybrene (8 μg ml−1). Overexpression of SV40-large T antigen was determined by qRT–PCR. MEVEC cultures expressed the chamber endocardial markers Notch1, Dll4, Nfatc1, Nrg1 and Gpr126 (Supplementary Fig. 8c) and maintained their endocardial phenotype and cobblestone morphology over 20–25 passages.
Cell culture
Human umbilical vein endothelial cells (HUVEC) were purchased from Lonza, and cultured on gelatin-coated dishes until passage 9 in EGM-2 complete medium (Lonza) supplemented with penicillin/streptomycin. For expression analysis by qPCR, HUVEC were pre-treated with 10 μM γ-secretase inhibitor (RO4929097, Selleckchem) or vehicle (DMSO) and stimulated with Notch ligands as described above for MEVEC. Bovine aortic endothelial cells (BAEC) were purchased from Lonza and cultured in DMEM supplemented with penicillin/streptomycin plus 10%FBS.
Luciferase assays with Gpr126 genomic DNA fragments
The Evolutionary Conserved Region web platform (ECR; http://ecrbrowser.dcode.org) was used to identify RBPJ motifs in the Gpr126 gene. Two conserved RBPJ-binding sites were found in the intronic region, flanking the fifth and sixth exon of human, monkey, rat and mouse Gpr126. In human the conserved RBPJ sites are separated by 445 base pairs (bp). Genomic DNA from HUVEC was used as a template to amplify 726 bp of GPR126 containing the two conserved sites and the product was cloned into the KpnI and XhoI sites of the pGL3 promoter luciferase plasmid (Promega). The primer sequences were: 5′-ATGCGGTACCATTTATACTTGTTACTGTGC-3′ (forward, KpnI site underlined) and 5′-ATCGCTCGAGGGCCAAGAATTATAAAGAATGAG-3′ (reverse, XhoI site underlined). For luciferase assays we transiently co-transfected BAEC with the hGPR126 reporter plasmid (pGL3-hGPR126-Luciferase), Renilla plasmid, and increasing amounts of vector expressing the intracellular domain of Notch1 (0–200 ng) (pCS2-N1ICD). Twenty-four hours after transfection the cells were lysed, and luciferase activity was measured with the Dual Luciferase Assay System (Promega).
Notch stimulation with recombinant ligands and luciferase assays
For Notch stimulation with recombinant ligands, MW24 plates were incubated with anti-human-IgG-Fc, anti-mouse-IgG2a-Fc (Jackson), anti-His (Zymed), or a 1:1 mixture of antibodies (6.48 mg ml−1) for 30 min at 37 °C. After washing with PBS, plates were blocked for 1 h at 37 °C with 10% FBS in DMEM. Recombinant Dll4 (mouse Dll4–His R&D Systems no. 1389-D4), Jagged1 (rat Jag1-Fc, R&D Systems no. 599–JG) or Jagged2 (mouse Jag2-Fc, R&D Systems no. 4748–JG) was diluted in PBS and added to the dishes individually or in a 1:1 mixture at a final concentration of 18 nM. After 2 h at 37 °C, wells were washed and GFP-MEVEC and MFng-MEVEC were incubated on the coated plates at subconfluency overnight. For luciferase assays, stimulated cells were transiently co-transfected with the Notch reporter plasmid pGL3-10xCBF1-Luciferase and pGL3-Renilla luciferase at a 5:1 ratio using Lipofectamine and Plus reagents (Life Technologies). Twenty hours after transfection, luciferase activity was measured with the Dual Luciferase Assay System (Promega). Data are expressed as Firefly/Renilla luciferase ratios. Experiments were performed at least three times, with quadruplicate samples for each experiment. Data are presented as mean ± s.d. Differences were considered statistically significant at P < 0.05 (Student's t-test).
Quantitative RT–PCR
WT and mutant embryos at different stages were dissected in ice-cold PBS. Whole E8.5 hearts and ventricles of E9.5–14.5 embryos were separated from the rest of the body and RNA was extracted. For RNA extraction from cells stimulated in vitro with recombinant Notch ligands, the stimulation protocol was conducted as above in MW6 dishes and RNA was extracted after overnight incubation. Total RNA was purified using Trizol (Invitrogen). cDNA was synthesized with SuperScript III First Strand (Invitrogen), with 1 μg total RNA per reaction. Quantitative PCR was performed with Power SYBR Green Master Mix (Applied Biosystems, 4367659). Oligonucleotide sequences for real-time PCR analysis performed in this study are listed in Supplementary Table 3. Data are presented as mean ± s.d. Differences were considered statistically significant at P < 0.05 (Student's t-test).
RNA-Seq
RNA was isolated at E9.5 from whole hearts of WT and Dll4flox;Nfatc1-Cre embryos (32 per genotype), WT and Notch1flox;Nfatc1-Cre embryos (24 per genotype), and WT and Dll4flox;Tie2-Cre embryos (27 per genotype). RNA was pooled into four replicates for the first genotype pairs and three replicates for the last two genotype pairs. For Jag1flox;cTnT-Cre hearts, RNA was isolated from the ventricles of 12 WT and 12 mutant hearts at E15.5 and then pooled into three replicates. RNA was prepared using the standard Illumina TrueSeq RNA-Seq library preparation kit. Libraries were sequenced in a GAIIx Illumina sequencer using a 75 bp single end elongation protocol. For Jag2flox;cTnT-Cre and Jag1flox;Jag2flox;cTnT-Cre hearts, RNA was isolated from the ventricles of 8 WT and 12 mutant hearts at E15.5 and then pooled into two and three replicates respectively. For MFngGOF;Tie2-Cre hearts, RNA was isolated from the ventricles of 16 WT and 16 GOF hearts at E15.5 and then pooled into four replicates each. RNA was prepared using the NEBNext Ultra RNA Library Prep Kit for Illumina. Libraries were sequenced in a HiSeq2500 Illumina sequencer using a 61 bp single end elongation protocol. Sequenced reads were QC and pre-processed using cutadapt v1.6 (ref. 61) to remove adaptor contaminants. Resulting reads were aligned and gene expression quantified using RSEM v1.2.3 (ref. 62) over mouse reference GRCm38 and Ensembl genebuild 70. Gene differential expression was analysed using the EdgeR R package63. Data from Dll4flox;Tie2-Cre and Dll4flox;Nfatc1-Cre experiments were analysed together, as were data from Dll4flox;Nfatc1-Cre and Notch1flox;Nfatc1-Cre experiments, by applying batch correction using ComBat (ref. 64) to account for experimental differences. For Jag1flox and Jag2flox single and double Jag1flox;Jag2flox;cTnT-Cre experiments, data were analysed together applying batch correction to account for technical differences and adding the genotypes of both alleles as covariates. Mibflox;cTnT-Cre (E14.5) RNA-Seq from ref. 15 was reanalysed using the GRCm38 genebuild 70 reference. Genes showing altered expression with adjusted P < 0.05 were considered differentially expressed. For the set of differentially expressed genes a functional analysis was performed using Ingenuity Pathway Analysis Software and DAVID (ref. 65), and some of the enriched processes were selected according to relevant criteria related to the biological process studied. Unsupervised hierarchical clustering with Genesis Software66 was used to group genes according to the similarity of their expression profiles across the different Dll4 and Notch1 deficiency experiments. Using a new R visualization package called GOPlot, a chord plot67,68 was generated to better visualize the relationships between genes and the selected enriched processes.
Accession number
Data are deposited in the NCBI GEO database under accession number GSE67889.
Ultrasound
Left ventricle (LV) function and wall thickness were analysed by transthoracic echocardiography in male mice at 6 and 9 months of age. Mice were mildly anaesthetized by inhalation of isoflurane/oxygen (1–2%/98.75%) adjusted to obtain a target heart rate of 450 ± 50 beats per minute and examined with a 30 MHz transthoracic echocardiography probe. Images were obtained with Vevo 2100 (VisualSonics) from Jag1flox;cTnT-Cre, Jag2flox;cTnT-Cre and WT littermates. LV long-axis M-Mode views were obtained as described previously69. From these images, interventricular septum end-diastolic thickness was measured, and LV systolic function was assessed by estimating LV shortening fraction and LV ejection fraction69.
Cardiac MRI
Six-month-old male mice were anaesthetized by inhalation of isoflurane/oxygen (2%) and monitored by ECG and breathing rhythm (Model 1025, S.A. Instruments). Images were acquired with an Agilent/Varian 7 T system equipped with a DD1 console and an active shielded 205/120 gradient insert coil with 130 mT m−1 maximum strength and a combination of volume (transmission, TX) and two-channel phased array (reception, RX) coils (Rapid Biomedical GmbH) for transmit/receipt. Data were collected in a triggered cine gradient echo sequence with TE = 1.45 ms and TR 7 ms, FA 25°, 20 cardiac phases, and NA (number of averages) = 3. A total of 13 contiguous slices in the short-axis view were acquired, with a slice thickness of 0.8 mm. The field of view was 3 × 3 cm, with a 256 × 256 matrix.
Left ventricle (LV) and right ventricle (RV) function, end-diastolic volume, mass and wall thickness of the right ventricle were estimated by the Simpson method, which sums the RV end-diastolic areas from the 13 slices in the short-axis view and multiplies this figure by the 0.8 mm slice thickness. Images were processed using OsiriX (Pixmeo) and Medis MASS software (The Netherlands) to calculate every RV area by segmentation of end-diastolic epicardial and endocardial edge70.
Statistical analysis
Statistical analysis was carried out using Prism 5 (GraphPad). All statistical tests were performed using two-sided, unpaired Student's t-tests except for Fig. 2j where numerical data are presented as mean ± s.e.m; *P < 0.05, **P < 0.01 and ***P < 0.001. Sample size was chosen empirically according to previous experience in the calculation of experimental variability. No statistical method was used to predetermine sample size. All experiments were carried out with at least three biological replicates. The numbers of animals used are described in the corresponding figure legends. For adult image analysis by echo and CMRI we ensured that experimental groups were balanced in terms of animal age, sex and weight unless otherwise specified. Animals were genotyped before the experiment and were caged together and treated in the same way. Variance was comparable between groups throughout the manuscript. We chose the appropriate tests according to the data distributions. The experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment except for adult image analysis. Panels in Figs 1a–h′,m–n′,p–q′; 2a–f′,k–p′; 3d–m′; 4a,b–b′,f–k′,m–s; 5a,e–j′,i–m; 6a,c,d–i′,j–o and Supplementary Figs 1a–h; 2a,c,d–f′; 3a–p′; 5a,5c–f′; 6c–d′; 7a–d″ and 8a show a representative image of at least three independent experiments.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Y. Fukushima (Osaka U., Japan) for help with the generation of the R26-MFng targeting vector, RIKEN CDB (Japan) for producing the chimaeric mice, the CNIC Genomics Unit for RNA-seq, the CNIC Advance Imaging Unit for CMRI analysis, B. Zhou (Albert Einstein College, NYC, USA) for the NFATc1-Cre driver line, A. Martín-Pendas (CSIC, Salamanca, Spain), P. Muñoz-Canoves (UPF, Barcelona, Spain) and J. M. Pérez-Pomares (Málaga U., Spain) for critical reading of the manuscript and S. Bartlett (CNIC) and K. McCreath for English editing. Funds were from grants SAF2013-45543-R, RD12/0042/0005 (RIC) and RD12/0019/0003 (TERCEL) from the Spanish Ministry of Economy and Competitiveness (MINECO), FP7-ITN 215761 (NotchIT) and 28600 (CardioNeT) from the EU and a grant from the BBVA Foundation for Research in Biomedicine (2014), all to J.L.d.l.P. G.D'A. holds a PhD fellowship linked to grant FP7-ITN 215761 (NotchIT). The MINECO and the Pro-CNIC Foundation support the CNIC.
Footnotes
METHODS
Methods and any associated references are available in the online version of the paper.
Note: Supplementary Information is available in the online version of the paper
AUTHOR CONTRIBUTIONS
G.D'A., G.L., G.d.M.-N., B.M.-P. and M.S.B. performed experiments. C.T., W.W. and F.M. analysed the RNA-seq data, A.-K.H., A.U. and S.C. provided the CBF:H2B-Venus reporter, the MFng transgenic line and the M−/−;L−/−;R−/− embryos. R.B. advised on the Fng experiments and L.J.J.-B. evaluated ultrasonography and CMRI. J.L.d.l.P. designed experiments, reviewed the data and wrote the manuscript. All authors reviewed the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat. Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 2.Moorman AF, Christoffels VM. Cardiac chamber formation: development, genes, and evolution. Physiol. Rev. 2003;83:1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- 3.Tevosian SG, et al. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- 4.Wu B, et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151:1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wessels A, Sedmera D. Developmental anatomy of the heart: a tale of mice and man. Physiol. Genomics. 2003;15:165–176. doi: 10.1152/physiolgenomics.00033.2003. [DOI] [PubMed] [Google Scholar]
- 6.Ritter M, et al. Isolated noncompaction of the myocardium in adults. Mayo Clin. Proc. 1997;72:26–31. doi: 10.4065/72.1.26. [DOI] [PubMed] [Google Scholar]
- 7.Towbin JA. Left ventricular noncompaction: a new form of heart failure. Heart Fail. Clin. 2010;6:453–469. doi: 10.1016/j.hfc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 9.Captur G, Nihoyannopoulos P. Left ventricular non-compaction: genetic heterogeneity, diagnosis and clinical course. Int. J. Cardiol. 2010;140:145–153. doi: 10.1016/j.ijcard.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Sarma RJ, Chana A, Elkayam U. Left ventricular noncompaction. Prog. Cardiovasc. Dis. 2010;52:264–273. doi: 10.1016/j.pcad.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Arbustini E, Weidemann F, Hall JL. Left ventricular noncompaction: a distinct cardiomyopathy or a trait shared by different cardiac diseases? J. Am. Coll. Cardiol. 2014;64:1840–1850. doi: 10.1016/j.jacc.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grego-Bessa J, et al. Notch signaling is essential for ventricular chamber development. Dev. Cell. 2007;12:415–429. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh M, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 14.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luxan G, et al. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat. Med. 2013;19:193–201. doi: 10.1038/nm.3046. [DOI] [PubMed] [Google Scholar]
- 16.Nowotschin S, Xenopoulos P, Schrode N, Hadjantonakis AK. A bright single-cell resolution live imaging reporter of Notch signaling in the mouse. BMC Dev. Biol. 2013;13 doi: 10.1186/1471-213X-13-15. http://dx.doi.org/10.1186/1471-213X-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch U, et al. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J. Exp. Med. 2008;205:2515–2523. doi: 10.1084/jem.20080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisanuki YY, et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 20.Bjarnadottir TK, Fredriksson R, Schioth HB. The adhesion GPCRs: a unique family of G protein-coupled receptors with important roles in both central and peripheral tissues. Cell Mol. Life Sci. 2007;64:2104–2119. doi: 10.1007/s00018-007-7067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waller-Evans H, et al. The orphan adhesion-GPCR GPR126 is required for embryonic development in the mouse. PLoS ONE. 2010;5:e14047. doi: 10.1371/journal.pone.0014047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patra C, et al. Organ-specific function of adhesion G protein-coupled receptor GPR126 is domain-dependent. Proc. Natl Acad. Sci. USA. 2013;110:16898–16903. doi: 10.1073/pnas.1304837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munch J, Gonzalez-Rajal A, de la Pompa JL. Notch regulates blastema proliferation and prevents differentiation during adult zebrafish fin regeneration. Development. 2013;140:1402–1411. doi: 10.1242/dev.087346. [DOI] [PubMed] [Google Scholar]
- 24.Jiao K, et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Captur G, et al. Abnormal cardiac formation in hypertrophic cardiomyopathy: fractal analysis of trabeculae and preclinical gene expression. Circ. Cardiovasc. Genet. 2014;7:241–248. doi: 10.1161/CIRCGENETICS.113.000362. [DOI] [PubMed] [Google Scholar]
- 26.Ivins S, et al. The CXCL12/CXCR4 axis plays a critical role in coronary artery development. Dev. Cell. 2015;33:455–468. doi: 10.1016/j.devcel.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavallero S, et al. CXCL12 signaling is essential for maturation of the ventricular coronary endothelial plexus and establishment of functional coronary circulation. Dev. Cell. 2015;33:469–477. doi: 10.1016/j.devcel.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian X, Pu WT, Zhou B. Cellular origin and developmental program of coronary angiogenesis. Circ. Res. 2015;116:515–530. doi: 10.1161/CIRCRESAHA.116.305097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panin VM, Papayannopoulos V, Wilson R, Irvine KD. Fringe modulates Notch-ligand interactions. Nature. 1997;387:908–912. doi: 10.1038/43191. [DOI] [PubMed] [Google Scholar]
- 30.Yang LT, et al. Fringe glycosyltransferases differentially modulate Notch1 proteolysis induced by Delta1 and Jagged1. Mol. Biol. Cell. 2005;16:927–942. doi: 10.1091/mbc.E04-07-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 32.McKenzie GJ, et al. Nuclear Ca2+ and CaM kinase IV specify hormonal- and Notch-responsiveness. J. Neurochem. 2005;93:171–185. doi: 10.1111/j.1471-4159.2005.03010.x. [DOI] [PubMed] [Google Scholar]
- 33.Timmerman LA, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran JL, et al. Manic fringe is not required for embryonic development, and fringe family members do not exhibit redundant functions in the axial skeleton, limb, or hindbrain. Dev. Dynam. 2009;238:1803–1812. doi: 10.1002/dvdy.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luna-Zurita L, et al. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J. Clin. Invest. 2010;120:3493–3507. doi: 10.1172/JCI42666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe T, Koibuchi N, Chin MT. Transcription factor CHF1/Hey2 regulates coronary vascular maturation. Mech. Dev. 2010;127:418–427. doi: 10.1016/j.mod.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ. Res. 2015;116:1269–1276. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 39.Hinz B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Epstein JA, Aghajanian H, Singh MK. Semaphorin signaling in cardiovascular development. Cell Metab. 2015;21:163–173. doi: 10.1016/j.cmet.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toyofuku T, et al. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat. Cell Biol. 2004;6:1204–1211. doi: 10.1038/ncb1193. [DOI] [PubMed] [Google Scholar]
- 42.Dirkx E, et al. Nfat and miR-25 cooperate to reactivate the transcription factor Hand2 in heart failure. Nat. Cell Biol. 2013;15:1282–1293. doi: 10.1038/ncb2866. [DOI] [PubMed] [Google Scholar]
- 43.Hertig CM, Kubalak SW, Wang Y, Chien KR. Synergistic roles of neuregulin-1 and insulin-like growth factor-I in activation of the phosphatidylinositol 3-kinase pathway and cardiac chamber morphogenesis. J. Biol. Chem. 1999;274:37362–37369. doi: 10.1074/jbc.274.52.37362. [DOI] [PubMed] [Google Scholar]
- 44.Kelly RG, Buckingham ME, Moorman AF. Heart fields and cardiac morphogenesis. Cold Spring Harb. Perspect. Med. 2014;4:a015750. doi: 10.1101/cshperspect.a015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- 46.Oka C, et al. Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development. 1995;121:3291–3301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- 47.Mancini SJ, et al. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105:2340–2342. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- 48.Xu J, Krebs LT, Gridley T. Generation of mice with a conditional null allele of the Jagged2 gene. Genesis. 2010;48:390–393. doi: 10.1002/dvg.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radtke F, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 50.Koo BK, et al. An obligatory role of mind bomb-1 in notch signaling of mammalian development. PLoS ONE. 2007;2:e1221. doi: 10.1371/journal.pone.0001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C, Li Q, Lim CH, Qiu X, Jiang YJ. The characterization of zebrafish antimorphic mib alleles reveals that Mib and Mind bomb-2 (Mib2) function redundantly. Dev. Biol. 2007;305:14–27. doi: 10.1016/j.ydbio.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 52.de la Pompa JL, et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- 53.Kanzler B, Kuschert SJ, Liu YH, Mallo M. Hoxa-2 restricts the chondrogenic domain and inhibits bone formation during development of the branchial area. Development. 1998;125:2587–2597. doi: 10.1242/dev.125.14.2587. [DOI] [PubMed] [Google Scholar]
- 54.Del Monte G, Grego-Bessa J, Gonzalez-Rajal A, Bolos V, de la Pompa JL. Monitoring Notch1 activity in development: evidence for a feedback regulatory loop. Dev. Dynam. 2007;236:2594–2614. doi: 10.1002/dvdy.21246. [DOI] [PubMed] [Google Scholar]
- 55.Del Monte G, et al. Differential notch signaling in the epicardium is required for cardiac inflow development and coronary vessel morphogenesis. Circ. Res. 2011;108:824–836. doi: 10.1161/CIRCRESAHA.110.229062. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, et al. Inhibition of Notch2 by Numb/Numblike controls myocardial compaction in the heart. Cardiovasc. Res. 2012;96:276–285. doi: 10.1093/cvr/cvs250. [DOI] [PubMed] [Google Scholar]
- 57.Chen H, et al. Analysis of ventricular hypertrabeculation and noncompaction using genetically engineered mouse models. Pediatr. Cardiol. 2009;30:626–634. doi: 10.1007/s00246-009-9406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soufan AT, et al. Regionalized sequence of myocardial cell growth and proliferation characterizes early chamber formation. Circ. Res. 2006;99:545–552. doi: 10.1161/01.RES.0000239407.45137.97. [DOI] [PubMed] [Google Scholar]
- 59.de Boer BA, et al. The interactive presentation of 3D information obtained from reconstructed datasets and 3D placement of single histological sections with the 3D portable document format. Development. 2011;138:159–167. doi: 10.1242/dev.051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esteban V, et al. Regulator of calcineurin 1 mediates pathological vascular wall remodeling. J. Exp. Med. 2011;208:2125–2139. doi: 10.1084/jem.20110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMB J. 2011;17:10–12. [Google Scholar]
- 62.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12 doi: 10.1186/1471-2105-12-323. http://dx.doi.org/10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 65.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 66.Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 67.Walter W, Sanchez-Cabo F, Ricote M. GOplot: an R package for visually combining expression data with functional analysis. Bioinformatics. 2015;31:2912–2914. doi: 10.1093/bioinformatics/btv300. [DOI] [PubMed] [Google Scholar]
- 68.Krzywinski M, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cruz-Adalia A, et al. CD69 limits the severity of cardiomyopathy after autoimmune myocarditis. Circulation. 2010;122:1396–1404. doi: 10.1161/CIRCULATIONAHA.110.952820. [DOI] [PubMed] [Google Scholar]
- 70.van de Weijer T, et al. Geometrical models for cardiac MRI in rodents: comparison of quantification of left ventricular volumes and function by various geometrical models with a full-volume MRI data set in rodents. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H709–H715. doi: 10.1152/ajpheart.00710.2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.