Abstract
Reduced nitric oxide (NO) bioavailability contributes to endothelial dysfunction and hypertension. The endothelial isoform of NO synthase (eNOS) is responsible for the production of NO within endothelium. Loss of eNOS cofactor tetrahydrobiopterin to initial increase in oxidative stress leads to uncoupling of eNOS, in which the enzyme produces superoxide anion rather than NO, further substantiating oxidative stress to induce vascular pathogenesis. The current review focuses on recent advances on the molecular mechanisms and consequences of eNOS dysfunction in hypertension, and potential novel therapeutic strategies restoring eNOS function to treat hypertension.
Keywords: nitric oxide, endothelial nitric oxide synthase (eNOS), tetrahydrobiopterin, eNOS uncoupling, endothelial dysfunction, hypertension, oxidative stress, NADPH oxidase (NOX)
Introduction
There are at least 970 million people worldwide suffering from hypertension [1]. Patients with high blood pressure develop more cardiovascular complications [2]. Globally cardiovascular disease accounts for approximately 17 million deaths a year, nearly one third of the total mortality [3]. Among these, complications of hypertension account for 9.4 million deaths worldwide every year [3, 4]. It has become clear that nitric oxide (NO), produced by the endothelial isoform of NO synthase (eNOS) in the vascular endothelium, plays an important role in regulating blood pressure. Reduced NO bioavailability, which is considered a hallmark of endothelial dysfunction [5], plays an important role in mediating blood pressure elevation. Endothelial dysfunction also predicts atherosclerotic coronary and cerebral artery disease in hypertension. A better understanding of the molecular mechanisms regulating NO signaling under pathophysiological conditions is critically important in designing new therapeutic options. Therefore, in the present review we will discuss the following aspects of NO signaling that are relevant to hypertension: (1) nitric oxide and blood pressure regulation; (2) mechanisms of eNOS dysfunction; (3) consequences of eNOS uncoupling in hypertension; (4) potential novel therapies targeting uncoupled eNOS in hypertension.
1. Nitric oxide and blood pressure regulation
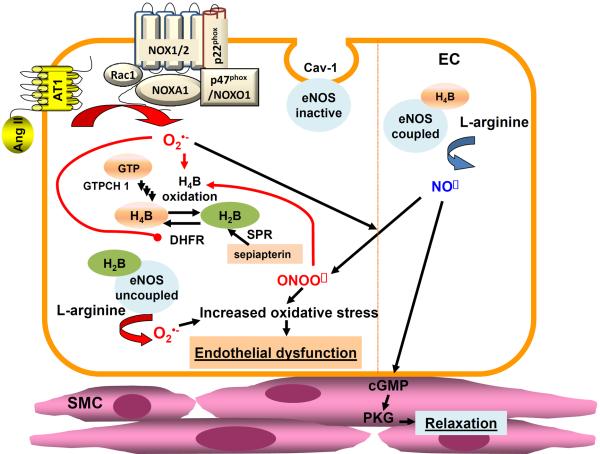
Accumulating evidence demonstrates a critical role of NO in blood pressure regulation. Released from endothelial cells, NO increases 3',5'-cyclic-guanosine monophosphate (cGMP) production and subsequent cGMP-dependent protein kinase (PKG) activation in the underneath vascular smooth muscle cells (VSMCs), resulting in vasodilatation [6, 7]. Pathophysiological regulation of NO signaling in endothelial cells and its relevance to VSMC regulation are summarized in Fig. 1. Previous studies have confirmed an essential role of NO in vasorelaxation of large human arteries [8]. In addition, impairment in NO mediated vasodilatation in brachial, coronary and renal arteries was also observed in patients with essential hypertension [9–12]. Moreover, the relaxation mediated by NO was depressed in mesenteric arteries of hypertensive rats with reduced renal mass, as the result of an arterial endothelial abnormality [13, 14]. In high-salt treated Dahl hypertensive rats, eNOS mRNA expression was down-regulated in mesenteric arterioles [15]. In deoxycorticosterone acetate-salt hypertensive rats, reduced eNOS phosphorylation resulted in reduced NO/cGMP signaling in mesenteric arteries [16]. Taken together, NO signaling plays an important role in both conduit and resistant arteries in the environment of hypertension. Of note, the mean blood pressure is 20 mm Hg higher in the eNOS knockout mice compared to its wild type littermates [17]. Therefore, understanding molecular mechanisms underlying impaired NO bioavailability and eNOS dysfunction in hypertension may prove beneficial in ultimately promoting development of novel therapeutics to treat hypertension.
Figure 1.
Endothelial dysfunction by eNOS uncoupling. Endothelial nitric oxide synthase (eNOS) produces nitric oxide (NO) to mediate vasorelaxation and preservation of vascular function. Tetrahydrobiopterin (H4B) is the key cofactor responsible for normal electron transfer from the reductase domain of one eNOS monomer to the oxygenase domain of the other monomer to produce NO, and in its deficiency, eNOS produces superoxide rather than NO, a process now referred to as eNOS uncoupling. H4B can be supplied by both de novo biosynthesis and salvage pathways. It is newly synthesized from GTP by activations of sequential enzymes of GTP hydrocyclolase 1 (GTPCH1), PTP synthase (PTPS), and sepiapterin reductase (SPR), or restored from its oxidized form H2B by the salvage enzyme dihydrofolate reductase (DHFR). SPR can also catalyze conversion of H4B precursor sepiapterin to H2B, prior to its conversion to H4B by DHFR. Pathological stimuli such as Ang II activates NADPH oxidase isoform 1 (NOX1) to produce superoxide in endothelial cells, which in turn cause peroxynitrite dependent oxidation of H4B and hydrogen peroxide dependent DHFR deficiency, leading to persistent reduction in H4B bioavailability. Superoxide production by uncoupled eNOS further sustains oxidative stress in the vasculature, resulting in endothelial dysfunction, impaired endothelium-dependent vasorelaxation, and elevated blood pressure.
Several mechanisms have been found responsible for NO deficiency in hypertension [9, 18–24]. Destruction of NO by superoxide anion leads to NO deficiency, endothelial dysfunction and high blood pressure. Among many enzymatic systems producing reactive oxygen species (ROS), NADPH oxidase (NOX), xanthine oxidase (XO), uncoupled eNOS, mitochondria, and cyclooxygenase (COX) have been extensively studied [18, 25]. Here we take COX for example, and the other ROS systems will be described in the later sections below. During the generation of prostanoids by COX, ROS are formed as by-products [26]. In most tissues, COX-1 is constitutively expressed, while COX-2 is often induced by a number of inflammation or growth factors [27]. In small resistance arteries of essential hypertensive patients, COX-2 is overexpressed and reduces nitric oxide availability, and COX-2 represents a major source of oxidative stress generation [28]. Indeed, endothelium-dependent contractions were triggered by acetylcholine (ACh) were abolished by COX-2 but not COX-1 inhibitors [29]. In addition, COX-2-derived prostaglandin F(2α) plays an important role in mediating endothelial dysfunction in renovascular hypertension [30]. Recently, Liu et al identified that uncoupling protein 2 inhibited oxidative stress and downregulated COX-2 expression to prevent endothelial dysfunction [31]. ROS from one source are able to trigger ROS production by activating other enzyme systems. For example, ROS produced by NOX can upregulate the expression of COX-2 by p38 MAPK-dependent mechanism, and also can induce eNOS uncoupling [32–34]. Oxidation of the eNOS cofactor tetrahydrobiopterin by peroxynitrite, a product of NO/superoxide interaction, induces eNOS uncoupling to produce superoxide rather than NO, further sustaining oxidative stress (see section 2.2).
Moreover, a defective L-arginine/NO pathway has been linked to NO deficiency in hypertension. Recent studies have confirmed that L-arginine transport is impaired in hypertensive and normotensive subjects with a genetic background of essential hypertension [20], and the offspring of essential hypertensive patients are characterized by a reduced response to acetylcholine linked to a defect in the nitric oxide pathway [19]. These data represent the link between L-arginine and the onset of essential hypertension. Furthermore, it has been shown that L-arginine supplementation improved endothelial dysfunction in hypertension [35]. The Km of eNOS for L-arginine is about 3 μM, but the L-arginine plasma concentration rarely falls below 60 μM in pathological conditions [36]. An elevation in asymmetric dimethylarginine (ADMA) levels may explain this “L-arginine paradox,” since ADMA is an endogenous competitive inhibitor of NO synthase [37]. Oxidative stress dependent increase in circulating ADMA could lead to eNOS uncoupling [38], vasoconstriction [39], and therefore a marked increase in blood pressure [40, 41]. Of note, elevated ADMA levels have been observed in hypertension, hypercholesterolemia, diabetes, and chronic kidney failure (CKD) [42]. Because ADMA is normally cleared by kidney, patients with CKD have more than 3–10 fold higher plasma ADMA levels, which would lead to eNOS dysfunction and hypertension [43, 44]. Regulation of eNOS function by H4B deficiency will be discussed in part 3.
2. Mechanisms of eNOS dysfunction
2.1. Synthesis of nitric oxide by NOS
Three NOS isoforms, neuronal (nNOS), inducible (iNOS), and endothelial (eNOS), catalyze the reaction of molecular oxygen with the amino acid substrate L-arginine to produce L-citrulline and NO [40, 45, 46]. nNOS is expressed in the central and peripheral nervous system, and produces NO that functions as a neurotransmitter [47]. It has been shown that nNOS is compensatorily up-regulated in hypertension [48]. Different from constitutively expressed nNOS and eNOS, the expression of iNOS is markedly increased by inflammatory cytokines [49]. Large quantities of NO produced by iNOS induce apoptosis to serve as a host defense mechanism, but also contribute to pathogenesis of chronic diseases [50]. eNOS, or NOS3, is constitutively expressed in vascular endothelial cells, and primarily located in the peri-nucleus, Golgi apparatus and caveolae [51, 52]. The NOS enzymes require several cofactors for enzymatic production of NO, including H4B, nicotinamide adenine dinucleotide phosphate (NADPH), flavin adenine dinucleotide (FAD), and flavin mononucleotide (FMN) [53]. During the process of NO synthesis, the electrons donated by NADPH at the C terminal reductase domain are transferred to the heme catalytic center of the N terminal oxidase domain, where activation of molecular oxygen is “coupled” to NO synthesis by two successive monooxygenations of L-arginine [46, 54, 55].
2.2. Reactive oxygen species and eNOS uncouping
Under some disease conditions, NOX activation increases local production of ROS, which oxidize H4B to induce H4B deficiency [56]. H4B promotes assembly of eNOS monomers into an active dimer, while lack of H4B results in inability of electron transfer to the N terminal oxygenase domain of the other eNOS monomer [57, 58]. Treatment of endothelial cells with peroxynitrite led to reduced eNOS activity and disruption of eNOS dimers [59]. Peroxynitrite is believed to be primarily responsible for oxidation of H4B to H2B in vivo, although H4B is sensitive to oxidation from many other ROS species [60, 61]. The oxidized form H2B can compete with H4B for eNOS interaction, and they share the same binding sites in the N terminal oxygenase domain of eNOS [62]. However, H2B cannot support eNOS cofactor activity, so this displacement results in eNOS uncoupling. Therefore, under oxidative stress conditions, the salvage pathway of H4B synthesis will become particularly important. Expanded discussions of mechanisms and contributions of eNOS uncoupling in hypertension are included in sections 3.1 and 3.2 below.
2.3. Phosphorylation Regulation of eNOS function
Regulation of eNOS function through phosphorylation has been well established. The activity is regulated through phosphorylation or dephosphorylation [63–66]. While phosphorylation of Ser615, 633, and 1177 results in the activation of eNOS, the phosphorylation of Thr495 reduces eNOS function [67]. Among these phosphorylated sites, Ser1177 and Thr495 are mostly investigated. The kinases taking part in eNOS phosphorylation at Ser1177, include AMPK, Akt, ERK1/2, and CaMK-II [68–71]. Reduced phosphorylation level at this site has been found in various of cardiovascular dieases, such as portal hypertension, diabetes, atherosclerosis, and myocardial infarction [67]. Of note, there seems to be a dynamic regulation of Ser1177 by transient hydrogen peroxide, acutely with ERK and chronically with Akt [33, 69, 71]. And Akt appears to be downstream of AMPK in this pathway [69]. In addition, a deficiency in Akt/eNOS signaling indicates impaired insulin signaling in type 2 diabetes. In aortas isolated from diabetic animals and in type 2 diabetic patients, Akt and eNOS phosphorylation was decreased [72, 73]. Reduced eNOSs1177 phosphorylation, vascular dysfunction, and elevated blood pressure have been observed in high-fat fed animals [74]. On the other hand, AMPK and PKC can phosphorylate eNOS at Thr495, and its increased phosphorylation may contribute to hypoxia, diabetes and cerebral ischaemia [67]. Modulation of eNOS activity by dynamic changes in its phosphorylation level at different phosphorylation sites appears to be an important mechanism of eNOS regulation under different pathophysiological conditions.
2.4. Other factors and eNOS dysfunction
In addition to L-arginine deficiency as discussed earlier, several other mechanisms have been implicated in causing eNOS dysfunction/uncoupling. These include acetylation of eNOS, S-glutathionylation of eNOS, and protein-protein interactions other than phosphorylations discussed above. Cigarette smoking (CS) induced oxidative stress downregulates silent information regulator protein 1 (SIRT1), leading to acetylation of eNOS and reduced NO production [75]. In addition, eNOS can also be acetylated by histone deacetylase 3 (HDAC3), which decreases NO production by reduced calmodulin association [76]. More recently, S-glutathionylation of eNOS has been proposed as alternative mechanism provoking eNOS uncoupling [77]. Its interrelationship with H4B deficiency, however, remains to be further investigated. Two highly conserved systeine residues, Cys 689 and Cys 908, in the C terminal reductase domain have been identified as sites of S-glutathionylation, which are important for eNOS function. Of note, eNOS S-glutathionylation in endothelial cells, accompanied by loss of NO and gain of superoxide, is associated with impaired endothelium-dependent vasodilation [77, 78]. Finally, the activity of eNOS can also be regulated by binding to its regulatory binding partners such as CaM, Caveolin-1 (Cav-1), Hsp90, and nitric oxide synthase interacting protein (NOSIP). For example, the association with Cav-1 inhibits eNOS activity and reduces NO production [79]. In Cav-1 knockout mice, both eNOS activity and vasorelaxation are enhanced in blood vessels [80]. In addition, Cav-1 deficiency was associated with attenuated Ang II-induced hypertension by inhibiting AT1a receptor-mediated uptake of Ang II in the renal proximal tubule [81].
3. Consequences/contributions of eNOS uncoupling in hypertension
3.1. Role of eNOS uncoupling in Angiotensin II dependent hypertension
Angiotensin II (Ang II) levels are often elevated in the kidney and plasma of patients with hypertension [82, 83]. AT1 receptor antagonists effectively reduce blood pressure in Ang II dependent hypertension [84, 85]. Our previous study has confirmed that eNOS uncoupling contributes to high blood pressure in Ang II infused mice, where aortic NO production was markedly decreased [86]. Dihydrofolate reductase (DHFR) overexpression or folic acid (FA) restoration of DHFR function effectively recoupled eNOS to reduce blood pressure [86, 87]. Moreover, in Ang II-infused hyperphenylalaninemia (hph)-1 mice, where 79% of the animals developed severe abdominal aortic aneurysm (AAA), oral FA administration completely prevented AAA from occurring [87]. eNOS uncoupling-mediated aneurysm formation was also prevented by FA in Ang II infused apoE null mice, and again this was attributed to targeted restoration of endothelial DHFR expression and activity [88]. The molecular mechanisms of Ang II induced eNOS uncoupling have been characterized by our group, which involves a rapid and transient activation of endothelial NOX, subsequent H2O2-dependent down-regulation of DHFR, and persistent H4B deficiency (Fig. 1) [33, 56, 89]. More specifically, the NOX isoform 1 (NOX1), has been identified as the mediator of Ang II-dependent uncoupling of eNOS in streptozotocin (STZ) induced diabetic mice [89] [56].
DHFR is the rate limiting salvage enzyme of H4B, and is responsible for maintaining normal H4B bioavailability by regeneration of H4B from its oxidized form H2B [90]. Therefore, impaired DHFR function is anticipated to lead to eNOS dysfunction [33]. Indeed, several studies have confirmed the crucial role of DHFR in maintaining H4B and NO bioavailability and hence the coupling state of eNOS [33, 86, 91]. RNAi inhibition of DHFR expression increased eNOS-dependent superoxide production, which was accompanied by reduced NO bioavailability, implying uncoupling of eNOS [33, 91]. Moreover, in angiotensin converting enzyme (ACE) knockout mice, where angiotensin II production was diminished, aortic DHFR protein abundance was significantly up-regulated [33]. All of these findings suggest that DHFR is critically involved in preserving eNOS coupling and blood pressure in the model of Ang II-induced hypertension. Of note, additional studies have also confirmed an important role of DHFR in regulating eNOS coupling/uncoupling activity and vascular function. Crabtree and his colleagues showed that DHFR takes part in controlling H4B/H2B ratio, which is different from the function of GTPCH1 that regulates total biopterin levels [91]. Although either DHFR or GTPCH1 knockdown reduced VEGF-dependent NO production, only DHFR RNAi led to formation of ROS [92], implying its role in preserving eNOS coupling. Moreover, DHFR expression was found down-regulated in an Ang II-dependent fashion during renal ischemia [93], which is similar to our findings in Ang II-infused hypertensive mice [86]. Furthermore, DHFR expression was decreased in 6 and 12 months old LDLR−/− animals, corresponding to impaired endothelial function [94].
3.2. Role of eNOS uncoupling in low renin, DOCA-salt hypertension
The association between a high salt intake and hypertension has been investigated for a long time [95]. An increase in dietary salt will lead to increased arterial blood pressure in individuals with salt-sensitive hypertension [96]. One commonly used model, namely deoxycorticosterone acetate (DOCA)-salt hypertension, was firstly established by Selye in 1943. Young rats were co-treated by DOCA and 1% NaCl solution for 7 weeks, and the MBP increased to 187/130 mmHg, compared to 110/80 mmHg in the sham controls [97]. Previous studies have confirmed that NOX is the initial source of ROS leading to H4B oxidation. H4B treatment prevented eNOS uncoupling, and blunted the blood pressure increase in DOCA-salt induced hypertension [98]. In addition, mitochondria may also contribute to the ET-1-dependent oxidative stress in DOCA-salt rats [99], while it is believed that Ang II levels are low in these animals. Recent research found an endothelial sepiapterin reductase (SPR) deficiency in aortic endothelial cells from DOCA-salt hypertensive mice [100]. SPR takes part in modulating H4B biosynthesis in both de novo synthetic pathway and salvage pathway, implicating its indispensable role in regulating NO bioavailability [101]. Interestingly, SPR overexpression increased H4B content, NO production, and NO-dependent vasorelaxation in both cultured cells and mouse models. RNAi of SPR had opposite effects [102]. Because SPR was lost in the endothelium of DOCA-salt induced hypertensive mice, supplementation of sepiapterin, which could be not metabolized to H2B before its conversion to H4B, had no effect in recoupling of eNOS. Nonetheless, combined administration of H4B and a NOX inhibitor apocynin fully restored NO bioavailability [100]. On a separate note, overexpression of the H4B synthetic enzyme GTP hydrocyclolase 1 (GTPCH1) was partially effective in improving endothelial function in DOCA-salt hypertensive rats [103]. This partial effect may be explainable by the SPR deficiency that prevents maximal biosynthesis of H4B in the presence of overexpressed GTPCH1.
4. Potential new therapies targeting uncoupled eNOS in hypertension
Given that eNOS uncoupling is one of the central pathogenic mechanisms of hypertension, restoration of adequate NO signaling via restoration of eNOS coupling activity in the blood vessels may serve as an important therapeutic strategy for hypertension. Restoration of cofactor bioavailability and inhibition of upstream pathways could represent promising strategies to recouple eNOS from its uncoupled state.
4.1. Restoration of cofactor bioavailability
H4B supplementation has a great therapeutic potential of improving endothelial dysfunction in hypertension [46]. It augments endothelium dependent vasodilation in both normotensive and hypertensive patients [22]. Basic experimental data from cultured cells and animal models support its efficacy in recoupling eNOS [92]. In addition, ascorbate (vitamin C) is important in maintaining H4B levels in the setting of vascular oxidative stress [104], and treatment of BAECs with both H4B and ascorbate prevented uncoupling of eNOS by ONOO− [105]. There are some evidences demonstrating that ascorbate improved endothelial function through regulation of eNOS in genetic model of hypertension [106], which is mediated by increasing H4B stability and its intracellular amount [107, 108]. Moreover, H4B has been used in various experimental models. In spontaneously hypertensive rats (SHR), H4B supplementation diminished eNOS dependent generation of ROS, while increasing NO production [109]. Oral administration of H4B reduced vascular ROS production, increased NO production detected by electron spin resonance (ESR), and blunted the increase in blood pressure in DOCA-salt hypertension [98]. However, there is a limitation in scope for the potential clinical use of H4B as a pharmaceutical drug, largely due to its chemical instability. H4B can be easily oxidized to 7, 8-H2B. Nevertheless, sapropterin dihydrochloride (6R-H4B) is a novel thermo and photostable H4B derivate that is commercially available for use as a phenylketonuria drug [110].
In addition, sepiapterin administration may be considered as another option to supply H4B. Sepiapterin is firstly metabolized to H2B by SPR, and further to H4B by DHFR [32]. Sepiapterin supplementation has been employed to recouple eNOS in cell culture and animal models. Treatment of BAECs with sepiapterin improved H4B and NO bioavailabilities [102]. Furthermore, administration of sepiapterin markedly improved endothelium dependent vasodilatation to different agonists [111]. All these data demonstrate that sepiapterin administration has the potential to be developed as an alternative treatment for hypertension.
4.2. Inhibition of upstream pathways
Since NOX has been identified as an initial activator to uncouple eNOS in both Ang II-dependent [86] and DOCA-salt induced hypertension [98, 100], it can be considered as a logical target for drug development for hypertension. Two isoforms, NOX1 and NOX2, have been shown to play crucial roles in hypertension, by promoting uncoupling of eNOS. The Ang II induced increase in blood pressure was found reduced in mice deficient in NOX1 [112], NOX2 [113] or their catalytic subunit p47phox [114]. Of note, p47phox has been shown to interact with NOX1 as well, in addition to well received notion of interacting with NOX2 [56, 115]. The impaired endothelial function in DOCA-salt hypertension was abolished in p47phox knockout mice [98]. Therefore, targeting the subunit or isoforms selectively and specifically might be beneficial to prevent or treat hypertension. Widely used NOX inhibitors include apocynin, an inhibitor of translocation of p47phox; statins, indirect inhibitors of cytosolic activator of NOX Rac1 [116], and Nox2ds-tat [117]. Recently, several inhibitors have been developed and found to be NOX specific [118]. VAS2870 and its derivative VAS3947 function as pan-NOX inhibitors [119, 120]. VAS2870 was found to inhibit NOX activity in smooth muscle cells [121] and human umbilical vein endothelial cells (HUVECs) [122]. Impaired acetylcholine-induced relaxation in SHR aortas was significantly attenuated by VAS2870 [120]. VAS3947 displays an improved solubility compared to VAS2870. It attenuated NOX activity completely in SHR aortas, without affecting either NOS activity or XO activity in PMA stimulated HL-60 cell [119].
Recent studies have identified GKT137831 as a specific inhibitor for NOX1 and NOX4 [123]. In human aortic endothelial cells (HAEC), high glucose induced NOX1 activation was attenuated by GKT137831 and by transfection of NOX1 siRNA. In addition, administration with GKT137831 into diabetic apolipoprotein E-deficient mice resulted in a significant attenuation of lesion formation, which was comparable to that seen in NOX1/apoE double knockout mice [123]. ML171 is a compound that was identified by cell-based high-throughput screening employing ROS detecting chemiluminescence in NOX1 overexpressing cells [124]. This inhibitor blocked the formation of functional invadopodia in human colon cancer cells, which is well established to be a NOX1 specific response [124]. Fulvene-5 efficiently inhibited NOX activity measured by hydrogen peroxide using a homovanillic acid (HVA) assay in 293 cells stably transfected with constitutively active NOX4 and COSphox cells harboring inducible Nox2/p47phox/p67phox complex, showing that Fluvene-5 can act as a NOX2 and NOX4 inhibitor [125]. Indeed NOX4-induced ROS production and arrhythmic phenotype in zebrafish was abolished by Fluvene 5 and dimethylamino Fluvene [126]. However, none of these NOX inhibitors has been tested in patients with hypertension, although they are highly promising drug candidates for hypertension via preservation of eNOS coupling activity. Given that Ang II is a potent NOX activator for subsequent induction of eNOS uncoupling, the attenuation of Ang II signaling is clearly another feasible strategy to inhibit eNOS uncoupling in hypertension.
Summary
In summary, this review gives an overview of a dynamic research field of how eNOS uncoupling contributes to hypertension, and how eNOS recoupling may serve as a novel and effective therapeutic strategy for hypertension. The major regulatory mechanisms of eNOS are listed in Table 1. Supplementation of H4B, its stable alternatives or precursor sepiapterin, or inhibition of NOX, may represent promising new therapeutics to preserve eNOS coupling activity for the treatment of hypertension.
Table 1.
Mechanisms regulatory of eNOS function
| Post translational regulation of eNOS | References |
|---|---|
| Uncoupling of eNOS | |
| H4B deficiency | |
| Oxidation by ONOO | [60] |
| Deficiency of GTPCH1 | [127, 128] |
| Deficiency of SPR | [100, 102] |
| Deficiency of DHFR | [86, 91, 129] |
| Deficiency of L-arginine | [130, 131] |
| Disruption of dimerization | [59] |
| Protein Modification | |
| Phosphorylation | |
| Phosphorylation at T495 | [65, 66, 132–134] |
| Dephosphorylation at S1177 | [65, 133–135] |
| Acetylation | |
| Downregulation of SIRT1 | [75, 136] |
| Inhibition of HDAC3 | [76] |
| S-glutathionylation* | [77] |
| Protein/protein interaction | |
| Dissociation with HSP90 | [137–139] |
| Binding to Caveolin-1 | [80, 138, 140] |
S-glutathionylation uncouples eNOS
Acknowledgements
This study was supported by National Institute of Health National Heart, Lung and Blood Institute (NHLBI) Grants HL077440 (HC), HL088975 (HC), HL108701 (HC, DGH), HL119968 (HC), an American Heart Association Established Investigator Award (EIA) 12EIA8990025 (HC), and an AHA Postdoctoral Fellowship Award 14POST20380966 (QL).
Footnotes
Competing Interests The authors have no conflicts of interest to report.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasan RS, Massaro JM, Wilson PW, Seshadri S, Wolf PA, Levy D, et al. Antecedent blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2002;105(1):48–53. doi: 10.1161/hc0102.101774. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2013. A global brief on Hypertension: Silent killer, global public health crisis. [Google Scholar]
- 4.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 6.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994;91(16):7583–7. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka Y, Tang G, Takizawa K, Otsuka K, Eghbali M, Song M, et al. Kv channels contribute to nitric oxide- and atrial natriuretic peptide-induced relaxation of a rat conduit artery. J Pharmacol Exp Ther. 2006;317(1):341–54. doi: 10.1124/jpet.105.096115. [DOI] [PubMed] [Google Scholar]
- 8.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91(5):1314–9. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 9.Panza JA, Garcia CE, Kilcoyne CM, Quyyumi AA, Cannon RO., 3rd Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91(6):1732–8. doi: 10.1161/01.cir.91.6.1732. [DOI] [PubMed] [Google Scholar]
- 10.Treasure CB, Klein JL, Vita JA, Manoukian SV, Renwick GH, Selwyn AP, et al. Hypertension and left ventricular hypertrophy are associated with impaired endothelium-mediated relaxation in human coronary resistance vessels. Circulation. 1993;87(1):86–93. doi: 10.1161/01.cir.87.1.86. [DOI] [PubMed] [Google Scholar]
- 11.Higashi Y, Oshima T, Ozono R, Watanabe M, Matsuura H, Kajiyama G. Effects of L-arginine infusion on renal hemodynamics in patients with mild essential hypertension. Hypertension. 1995;25(4 Pt 2):898–902. doi: 10.1161/01.hyp.25.4.898. [DOI] [PubMed] [Google Scholar]
- 12.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26(1–2):33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Kimura K, Nishio I. Impaired endothelium-dependent relaxation in mesenteric arteries of reduced renal mass hypertensive rats. Scand J Clin Lab Invest. 1999;59(3):199–204. doi: 10.1080/00365519950185724. [DOI] [PubMed] [Google Scholar]
- 14.Stankevicius E, Martinez AC, Mulvany MJ, Simonsen U. Blunted acetylcholine relaxation and nitric oxide release in arteries from renal hypertensive rats. J Hypertens. 2002;20(8):1571–9. doi: 10.1097/00004872-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Gadkari TV, Cortes N, Madrasi K, Tsoukias NM, Joshi MS. Agmatine induced NO dependent rat mesenteric artery relaxation and its impairment in salt-sensitive hypertension. Nitric Oxide. 2013;35:65–71. doi: 10.1016/j.niox.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasser JM, Sullivan JC, Elmarakby AA, Kemp BE, Pollock DM, Pollock JS. Reduced NOS3 phosphorylation mediates reduced NO/cGMP signaling in mesenteric arteries of deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 2004;43(5):1080–5. doi: 10.1161/01.HYP.0000122804.32680.c9. [DOI] [PubMed] [Google Scholar]
- 17.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377(6546):239–42. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 18.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–4. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 19.Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective L-arginine-nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;94(6):1298–303. doi: 10.1161/01.cir.94.6.1298. [DOI] [PubMed] [Google Scholar]
- 20.Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang WZ, et al. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation. 2004;110(24):3680–6. doi: 10.1161/01.CIR.0000149748.79945.52. [DOI] [PubMed] [Google Scholar]
- 21.Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Panza JA. Impairment of the nitric oxide-mediated vasodilator response to mental stress in hypertensive but not in hypercholesterolemic patients. J Am Coll Cardiol. 1998;32(5):1207–13. doi: 10.1016/s0735-1097(98)00391-x. [DOI] [PubMed] [Google Scholar]
- 22.Higashi Y, Sasaki S, Nakagawa K, Fukuda Y, Matsuura H, Oshima T, et al. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens. 2002;15(4 Pt 1):326–32. doi: 10.1016/s0895-7061(01)02317-2. [DOI] [PubMed] [Google Scholar]
- 23.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, et al. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23(8):1455–9. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 24.Hermann M, Flammer A, Luscher TF. Nitric oxide in hypertension. J Clin Hypertens (Greenwich) 2006;8(12 Suppl 4):17–29. doi: 10.1111/j.1524-6175.2006.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Youn J, Siu K, Li Q, Harrison DG, Cai H. Oxidase interactions in cardiovascular diseases. Systems Biology of Free Radicals and Antioxidants. 2014;Chapter 37:849–76. [Google Scholar]
- 26.Wong MS, Vanhoutte PM. COX-mediated endothelium-dependent contractions: from the past to recent discoveries. Acta Pharmacol Sin. 2010;31(9):1095–102. doi: 10.1038/aps.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feletou M, Huang Y, Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol. 2011;164(3):894–912. doi: 10.1111/j.1476-5381.2011.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virdis A, Bacca A, Colucci R, Duranti E, Fornai M, Materazzi G, et al. Endothelial dysfunction in small arteries of essential hypertensive patients: role of cyclooxygenase-2 in oxidative stress generation. Hypertension. 2013;62(2):337–44. doi: 10.1161/HYPERTENSIONAHA.111.00995. [DOI] [PubMed] [Google Scholar]
- 29.Wong SL, Leung FP, Lau CW, Au CL, Yung LM, Yao X, et al. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ Res. 2009;104(2):228–35. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]
- 30.Tian XY, Wong WT, Leung FP, Zhang Y, Wang YX, Lee HK, et al. Oxidative stress-dependent cyclooxygenase-2-derived prostaglandin f(2alpha) impairs endothelial function in renovascular hypertensive rats. Antioxid Redox Signal. 2012;16(4):363–73. doi: 10.1089/ars.2010.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, Liu J, Tian XY, Wong WT, Lau CW, Xu A, et al. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid Redox Signal. 2014;21(11):1571–81. doi: 10.1089/ars.2013.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong WT, Tian XY, Huang Y. Endothelial dysfunction in diabetes and hypertension: cross talk in RAS, BMP4, and ROS-dependent COX-2-derived prostanoids. J Cardiovasc Pharmacol. 2013;61(3):204–14. doi: 10.1097/FJC.0b013e31827fe46e. [DOI] [PubMed] [Google Scholar]
- 33.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102(25):9056–61. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong WT, Tian XY, Chen Y, Leung FP, Liu L, Lee HK, et al. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: implications on hypertension. Circ Res. 2010;107(8):984–91. doi: 10.1161/CIRCRESAHA.110.222794. [DOI] [PubMed] [Google Scholar]
- 35.Hishikawa K, Nakaki T, Suzuki H, Kato R, Saruta T. Role of L-arginine-nitric oxide pathway in hypertension. J Hypertens. 1993;11(6):639–45. doi: 10.1097/00004872-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–37. 37a–37d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr. 2004;134(10 Suppl):2842S–7S. doi: 10.1093/jn/134.10.2842S. discussion 53S. [DOI] [PubMed] [Google Scholar]
- 38.Antoniades C, Shirodaria C, Leeson P, Antonopoulos A, Warrick N, Van-Assche T, et al. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur Heart J. 2009;30(9):1142–50. doi: 10.1093/eurheartj/ehp061. [DOI] [PubMed] [Google Scholar]
- 39.Calver A, Collier J, Leone A, Moncada S, Vallance P. Effect of local intra-arterial asymmetric dimethylarginine (ADMA) on the forearm arteriolar bed of healthy volunteers. J Hum Hypertens. 1993;7(2):193–4. [PubMed] [Google Scholar]
- 40.Thomas GD, Zhang W, Victor RG. Nitric oxide deficiency as a cause of clinical hypertension: promising new drug targets for refractory hypertension. JAMA. 2001;285(16):2055–7. doi: 10.1001/jama.285.16.2055. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez J, Valls N, Brito R, Rodrigo R. Essential hypertension and oxidative stress: New insights. World J Cardiol. 2014;6(6):353–66. doi: 10.4330/wjc.v6.i6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu TM, Chung MY, Lin CC, Hsu CP, Lin SJ. Asymmetric dimethylarginine and clinical outcomes in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(7):1566–72. doi: 10.2215/CJN.08490910. [DOI] [PubMed] [Google Scholar]
- 43.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339(8793):572–5. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 44.Matsuguma K, Ueda S, Yamagishi S, Matsumoto Y, Kaneyuki U, Shibata R, et al. Molecular mechanism for elevation of asymmetric dimethylarginine and its role for hypertension in chronic kidney disease. J Am Soc Nephrol. 2006;17(8):2176–83. doi: 10.1681/ASN.2005121379. [DOI] [PubMed] [Google Scholar]
- 45.Daff S. NO synthase: structures and mechanisms. Nitric Oxide. 2010;23(1):1–11. doi: 10.1016/j.niox.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Alkaitis MS, Crabtree MJ. Recoupling the cardiac nitric oxide synthases: tetrahydrobiopterin synthesis and recycling. Curr Heart Fail Rep. 2012;9(3):200–10. doi: 10.1007/s11897-012-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27(11):2783–802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jurzik L, Froh M, Straub RH, Scholmerich J, Wiest R. Up-regulation of nNOS and associated increase in nitrergic vasodilation in superior mesenteric arteries in pre-hepatic portal hypertension. J Hepatol. 2005;43(2):258–65. doi: 10.1016/j.jhep.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 49.Seddon M, Shah AM, Casadei B. Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc Res. 2007;75(2):315–26. doi: 10.1016/j.cardiores.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 50.Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000;6(5):347–73. [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandez-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, et al. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol. 2006;174(3):369–77. doi: 10.1083/jcb.200601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villanueva C, Giulivi C. Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Radic Biol Med. 2010;49(3):307–16. doi: 10.1016/j.freeradbiomed.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li H, Poulos TL. Structure-function studies on nitric oxide synthases. J Inorg Biochem. 2005;99(1):293–305. doi: 10.1016/j.jinorgbio.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Wei CC, Wang ZQ, Tejero J, Yang YP, Hemann C, Hille R, et al. Catalytic reduction of a tetrahydrobiopterin radical within nitric-oxide synthase. J Biol Chem. 2008;283(17):11734–42. doi: 10.1074/jbc.M709250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stuehr DJ, Kwon NS, Nathan CF, Griffith OW, Feldman PL, Wiseman J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J Biol Chem. 1991;266(10):6259–63. [PubMed] [Google Scholar]
- 56.Youn JY, Gao L, Cai H. The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia. 2012;55(7):2069–79. doi: 10.1007/s00125-012-2557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bauersachs J, Schafer A. Tetrahydrobiopterin and eNOS dimer/monomer ratio--a clue to eNOS uncoupling in diabetes? Cardiovasc Res. 2005;65(4):768–9. doi: 10.1016/j.cardiores.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Cai S, Khoo J, Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res. 2005;65(4):823–31. doi: 10.1016/j.cardiores.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 59.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109(6):817–26. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103(9):1282–8. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 61.Crabtree MJ, Channon KM. Dihydrofolate reductase and biopterin recycling in cardiovascular disease. J Mol Cell Cardiol. 2009;47(6):749–51. doi: 10.1016/j.yjmcc.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol. 2008;294(4):H1530–40. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michel T, Li GK, Busconi L. Phosphorylation and subcellular translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1993;90(13):6252–6. doi: 10.1073/pnas.90.13.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia-Cardena G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem. 1996;271(44):27237–40. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 65.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276(21):17625–8. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 66.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88(11):E68–75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 67.Kolluru GK, Siamwala JH, Chatterjee S. eNOS phosphorylation in health and disease. Biochimie. 2010;92(9):1186–98. doi: 10.1016/j.biochi.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Youn JY, Wang T, Cai H. An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production. Circ Res. 2009;104(1):50–9. doi: 10.1161/CIRCRESAHA.108.178467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai H, Liu D, Garcia JG. CaM Kinase II-dependent pathophysiological signalling in endothelial cells. Cardiovasc Res. 2008;77(1):30–4. doi: 10.1093/cvr/cvm010. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci U S A. 2006;103(17):6530–5. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108(9):1341–8. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okon EB, Chung AW, Rauniyar P, Padilla E, Tejerina T, McManus BM, et al. Compromised arterial function in human type 2 diabetic patients. Diabetes. 2005;54(8):2415–23. doi: 10.2337/diabetes.54.8.2415. [DOI] [PubMed] [Google Scholar]
- 74.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104(9):1085–94. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem Biophys Res Commun. 2010;393(1):66–72. doi: 10.1016/j.bbrc.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jung SB, Kim CS, Naqvi A, Yamamori T, Mattagajasingh I, Hoffman TA, et al. Histone deacetylase 3 antagonizes aspirin-stimulated endothelial nitric oxide production by reversing aspirin-induced lysine acetylation of endothelial nitric oxide synthase. Circ Res. 2010;107(7):877–87. doi: 10.1161/CIRCRESAHA.110.222968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468(7327):1115–8. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zweier JL, Chen CA, Druhan LJ. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid Redox Signal. 2011;14(10):1769–75. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19(11):7289–304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293(5539):2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 81.Li XC, Gu V, Miguel-Qin E, Zhuo JL. Role of caveolin 1 in AT1a receptor-mediated uptake of angiotensin II in the proximal tubule of the kidney. Am J Physiol Renal Physiol. 2014 doi: 10.1152/ajprenal.00199.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39(2 Pt 2):316–22. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferrario CM. Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J Renin Angiotensin Aldosterone Syst. 2006;7(1):3–14. doi: 10.3317/jraas.2006.003. [DOI] [PubMed] [Google Scholar]
- 84.Crowley SD, Tharaux PL, Audoly LP, Coffman TM. Exploring type I angiotensin (AT1) receptor functions through gene targeting. Acta Physiol Scand. 2004;181(4):561–70. doi: 10.1111/j.1365-201X.2004.01331.x. [DOI] [PubMed] [Google Scholar]
- 85.Romero JC, Reckelhoff JF. State-of-the-Art lecture. Role of angiotensin and oxidative stress in essential hypertension. Hypertension. 1999;34(4 Pt 2):943–9. doi: 10.1161/01.hyp.34.4.943. [DOI] [PubMed] [Google Scholar]
- 86.Gao L, Chalupsky K, Stefani E, Cai H. Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity. J Mol Cell Cardiol. 2009;47(6):752–60. doi: 10.1016/j.yjmcc.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao L, Siu KL, Chalupsky K, Nguyen A, Chen P, Weintraub NL, et al. Role of uncoupled endothelial nitric oxide synthase in abdominal aortic aneurysm formation: treatment with folic acid. Hypertension. 2012;59(1):158–66. doi: 10.1161/HYPERTENSIONAHA.111.181644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siu KL, Miao XN, Cai H. Recoupling of eNOS with folic acid prevents abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E null mice. PLoS One. 2014;9(2):e88899. doi: 10.1371/journal.pone.0088899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes. 2007;56(1):118–26. doi: 10.2337/db06-0288. [DOI] [PubMed] [Google Scholar]
- 90.Nichol CA, Lee CL, Edelstein MP, Chao JY, Duch DS. Biosynthesis of tetrahydrobiopterin by de novo and salvage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vivo. Proc Natl Acad Sci U S A. 1983;80(6):1546–50. doi: 10.1073/pnas.80.6.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem. 2009;284(41):28128–36. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sugiyama T, Levy BD, Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J Biol Chem. 2009;284(19):12691–700. doi: 10.1074/jbc.M809295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seujange Y, Eiam-Ong S, Tirawatnapong T. Role of angiotensin II on dihydrofolate reductase, GTP-cyclohydrolase 1 and nitric oxide synthase expressions in renal ischemia-reperfusion. Am J Nephrol. 2008;28(4):692–700. doi: 10.1159/000126927. [DOI] [PubMed] [Google Scholar]
- 94.Miller JD, Chu Y, Castaneda LE, Serrano KM, Brooks RM, Heistad DD. Vascular function during prolonged progression and regression of atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2013;33(3):459–65. doi: 10.1161/ATVBAHA.112.252700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodriguez-Iturbe B, Vaziri ND. Salt-sensitive hypertension--update on novel findings. Nephrol Dial Transplant. 2007;22(4):992–5. doi: 10.1093/ndt/gfl757. [DOI] [PubMed] [Google Scholar]
- 96.O'Donaughy TL, Brooks VL. Deoxycorticosterone acetate-salt rats: hypertension and sympathoexcitation driven by increased NaCl levels. Hypertension. 2006;47(4):680–5. doi: 10.1161/01.HYP.0000214362.18612.6e. [DOI] [PubMed] [Google Scholar]
- 97.Selye H, Hall CE, Rowley EM. Malignant Hypertension Produced by Treatment with Desoxycorticosterone Acetate and Sodium Chloride. Can Med Assoc J. 1943;49(2):88–92. [PMC free article] [PubMed] [Google Scholar]
- 98.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111(8):1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Callera GE, Tostes RC, Yogi A, Montezano AC, Touyz RM. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin Sci (Lond) 2006;110(2):243–53. doi: 10.1042/CS20050307. [DOI] [PubMed] [Google Scholar]
- 100.Youn JY, Wang T, Blair J, Laude KM, Oak JH, McCann LA, et al. Endothelium-specific sepiapterin reductase deficiency in DOCA-salt hypertension. Am J Physiol Heart Circ Physiol. 2012;302(11):H2243–9. doi: 10.1152/ajpheart.00835.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Werner-Felmayer G, Golderer G, Werner ER. Tetrahydrobiopterin biosynthesis, utilization and pharmacological effects. Curr Drug Metab. 2002;3(2):159–73. doi: 10.2174/1389200024605073. [DOI] [PubMed] [Google Scholar]
- 102.Gao L, Pung YF, Zhang J, Chen P, Wang T, Li M, et al. Sepiapterin reductase regulation of endothelial tetrahydrobiopterin and nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2009;297(1):H331–9. doi: 10.1152/ajpheart.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Du YH, Guan YY, Alp NJ, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation. 2008;117(8):1045–54. doi: 10.1161/CIRCULATIONAHA.107.748236. [DOI] [PubMed] [Google Scholar]
- 104.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24(3):413–20. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 105.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278(25):22546–54. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 106.Ulker S, McKeown PP, Bayraktutan U. Vitamins reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension. 2003;41(3):534–9. doi: 10.1161/01.HYP.0000057421.28533.37. [DOI] [PubMed] [Google Scholar]
- 107.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276(1):40–7. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 108.Huang A, Vita JA, Venema RC, Keaney JF., Jr Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000;275(23):17399–406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 109.Cosentino F, Patton S, d'Uscio LV, Werner ER, Werner-Felmayer G, Moreau P, et al. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest. 1998;101(7):1530–7. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y, Janssens SP, Wingler K, Schmidt HH, Moens AL. Modulating endothelial nitric oxide synthase: a new cardiovascular therapeutic strategy. Am J Physiol Heart Circ Physiol. 2011;301(3):H634–46. doi: 10.1152/ajpheart.01315.2010. [DOI] [PubMed] [Google Scholar]
- 111.Tiefenbacher CP, Bleeke T, Vahl C, Amann K, Vogt A, Kubler W. Endothelial dysfunction of coronary resistance arteries is improved by tetrahydrobiopterin in atherosclerosis. Circulation. 2000;102(18):2172–9. doi: 10.1161/01.cir.102.18.2172. [DOI] [PubMed] [Google Scholar]
- 112.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, et al. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112(17):2677–85. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 113.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109(14):1795–801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 114.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, et al. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40(4):511–5. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free Radic Biol Med. 2006;41(2):193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 116.Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol. 2007;50(1):9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- 117.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24(9):471–8. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 118.Wingler K, Hermans JJ, Schiffers P, Moens A, Paul M, Schmidt HH. NOX1, 2, 4, 5: counting out oxidative stress. Br J Pharmacol. 2011;164(3):866–83. doi: 10.1111/j.1476-5381.2011.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, et al. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol. 2010;161(4):885–98. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, et al. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56(3):490–7. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 121.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, et al. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 2006;71(2):331–41. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 122.Stielow C, Catar RA, Muller G, Wingler K, Scheurer P, Schmidt HH, et al. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem Biophys Res Commun. 2006;344(1):200–5. doi: 10.1016/j.bbrc.2006.03.114. [DOI] [PubMed] [Google Scholar]
- 123.Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127(18):1888–902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 124.Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, et al. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol. 2010;5(10):981–93. doi: 10.1021/cb100219n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, et al. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. 2009;119(8):2359–65. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y, Shimizu H, Siu KL, Mahajan A, Chen JN, Cai H. NADPH oxidase 4 induces cardiac arrhythmic phenotype in zebrafish. J Biol Chem. 2014;289(33):23200–8. doi: 10.1074/jbc.M114.587196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Belik J, McIntyre BA, Enomoto M, Pan J, Grasemann H, Vasquez-Vivar J. Pulmonary hypertension in the newborn GTP cyclohydrolase I-deficient mouse. Free Radic Biol Med. 2011;51(12):2227–33. doi: 10.1016/j.freeradbiomed.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang S, Xu J, Song P, Wu Y, Zhang J, Chul Choi H, et al. Acute inhibition of guanosine triphosphate cyclohydrolase 1 uncouples endothelial nitric oxide synthase and elevates blood pressure. Hypertension. 2008;52(3):484–90. doi: 10.1161/HYPERTENSIONAHA.108.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cai S, Khoo J, Mussa S, Alp NJ, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia. 2005;48(9):1933–40. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- 130.Hallemeesch MM, Lamers WH, Deutz NE. Reduced arginine availability and nitric oxide production. Clin Nutr. 2002;21(4):273–9. doi: 10.1054/clnu.2002.0571. [DOI] [PubMed] [Google Scholar]
- 131.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108(16):2000–6. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 132.Chen F, Kumar S, Yu Y, Aggarwal S, Gross C, Wang Y, et al. PKC-dependent phosphorylation of eNOS at T495 regulates eNOS coupling and endothelial barrier function in response to G+ -toxins. PLoS One. 2014;9(7):e99823. doi: 10.1371/journal.pone.0099823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 134.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, et al. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276(19):16587–91. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 135.Sud N, Wedgwood S, Black SM. Protein kinase Cdelta regulates endothelial nitric oxide synthase expression via Akt activation and nitric oxide generation. Am J Physiol Lung Cell Mol Physiol. 2008;294(3):L582–91. doi: 10.1152/ajplung.00353.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104(37):14855–60. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Takahashi S, Mendelsohn ME. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J Biol Chem. 2003;278(33):30821–7. doi: 10.1074/jbc.M304471200. [DOI] [PubMed] [Google Scholar]
- 138.Gratton JP, Fontana J, O'Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem. 2000;275(29):22268–72. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 139.Shi Y, Baker JE, Zhang C, Tweddell JS, Su J, Pritchard KA., Jr Chronic hypoxia increases endothelial nitric oxide synthase generation of nitric oxide by increasing heat shock protein 90 association and serine phosphorylation. Circ Res. 2002;91(4):300–6. doi: 10.1161/01.res.0000031799.12850.1e. [DOI] [PubMed] [Google Scholar]
- 140.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, et al. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119(7):2009–18. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]