Abstract
Objective To assess the risks of amputation, blindness, severe kidney failure, hyperglycaemia, and hypoglycaemia in patients with type 2 diabetes associated with prescribed diabetes drugs, particularly newer agents including gliptins or glitazones (thiazolidinediones).
Design Open cohort study in primary care.
Setting 1243 practices contributing data to the QResearch database in England.
Participants 469 688 patients with type 2 diabetes aged 25-84 years between 1 April 2007 and 31 January 2015.
Exposures Hypoglycaemic agents (glitazones, gliptins, metformin, sulphonylureas, insulin, and other) alone and in combination.
Main outcome measures First recorded diagnoses of amputation, blindness, severe kidney failure, hyperglycaemia, and hypoglycaemia recorded on patients’ primary care, mortality, or hospital records. Cox models estimated hazard ratios for diabetes treatments adjusting for potential confounders.
Results 21 308 (4.5%) and 32 533 (6.9%) patients received prescriptions for glitazones and gliptins during follow-up, respectively. Compared with non-use, glitazones were associated with a decreased risk of blindness (adjusted hazard ratio 0.71, 95% confidence interval 0.57 to 0.89; rate 14.4 per 10 000 person years of exposure) and an increased risk of hypoglycaemia (1.22, 1.10 to 1.37; 65.1); gliptins were associated with a decreased risk of hypoglycaemia (0.86, 0.77 to 0.96; 45.8). Although the numbers of patients prescribed gliptin monotherapy or glitazones monotherapy were relatively low, there were significantly increased risks of severe kidney failure compared with metformin monotherapy (adjusted hazard ratio 2.55, 95% confidence interval 1.13 to 5.74). We found significantly lower risks of hyperglycaemia among patients prescribed dual therapy involving metformin with either gliptins (0.78, 0.62 to 0.97) or glitazones (0.60, 0.45 to 0.80) compared with metformin monotherapy. Patients prescribed triple therapy with metformin, sulphonylureas, and either gliptins (adjusted hazard ratio 5.07, 95% confidence interval 4.28 to 6.00) or glitazones (6.32, 5.35 to 7.45) had significantly higher risks of hypoglycaemia than those prescribed metformin monotherapy, but these risks were similar to those involving dual therapy with metformin and sulphonylureas (6.03, 5.47 to 6.63). Patients prescribed triple therapy with metformin, sulphonylureas, and glitazones had a significantly reduced risk of blindness compared with metformin monotherapy (0.67, 0.48 to 0.94).
Conclusions We have found lower risks of hyperglycaemia among patients prescribed dual therapy involving metformin with either gliptins or glitazones compared with metformin alone. Compared with metformin monotherapy, triple therapy with metformin, sulphonylureas, and either gliptins or glitazones was associated with an increased risk of hypoglycaemia, which was similar to the risk for dual therapy with metformin and sulphonylureas. Compared with metformin monotherapy, triple therapy with metformin, sulphonylureas, and glitazones was associated with a reduced risk of blindness. These results, while subject to residual confounding, could have implications for the prescribing of hypoglycaemic drugs.
Introduction
Type 2 diabetes is associated with increased risks of macrovascular complications (such as heart failure and cardiovascular disease), microvascular complications (including lower limb amputation, blindness, kidney failure), and hypoglycaemic and hyperglycaemic attacks. Higher levels of glycated haemoglobin (HbA1c) are associated with higher risks of both microvascular and microvascular complications.1 The UK Prospective Diabetes Study (UKPDS) showed a reduced risk of microvascular complications with intensive hypoglycaemic treatment, particularly with metformin, but no conclusive benefit for cardiovascular outcomes.2 3 Additional observational analyses of the UKPDS cohort suggested that any lowering of HbA1c is likely to reduce risk of microvascular complications as well as cardiovascular outcomes.1
Apart from the original UKPDS studies, clinical trial evidence for newer hypoglycaemic agents—such as glitazones (thiazolidinediones) and gliptins—has been largely based on surrogate endpoints such as reduction of HbA1c rather than hard clinical endpoints such as increased survival rates or reduced incidence of complications. While the assumption that lowering HbA1c will result in a net clinical benefit seems plausible, evidence suggests that it is not always reliable. Phenformin, a biguanide, was withdrawn from the market in 1978 because of concerns about increased risk of cardiovascular events and lactic acidosis.4 In the United Kingdom, troglitazone (an insulin sensitising glitazone) was withdrawn from the market in 1997 a few months after its launch because of hepatotoxicity.5 The drug was withdrawn from the United States three years later.
Other newer hypoglycaemic agents have subsequently shown unexpected increases in cardiovascular endpoints.6 7 8 9 For example, rosiglitazone, another glitazone, was associated with an increased incidence of heart failure.6 This resulted in the drug’s withdrawal from Europe, India, New Zealand, and South Africa in 2010-11, although it continues to be available in the USA, albeit with limitations. Uncertainty therefore remains over the longer term comparative clinical risks and benefits among patients prescribed diabetes drugs, particularly gliptins and glitazones alone and in combination with other treatments.10 11 Regulatory agencies have responded to this uncertainty, not by requiring evidence of direct clinical benefit but by requiring evidence that new hypoglycaemic drugs are not associated with harmful increases in cardiovascular events.12 13 This has led to industry funded, controversial non-inferiority trials such as the RECORD study, in which rosiglitazone was assumed to be non-inferior so long as the upper limit of the 95% confidence interval of the hazard ratio for cardiovascular disease was less than 1.20.14 15 While the RECORD study confirmed an increased risk of heart failure with rosiglitazone, it was unable to rule out an increased risk of myocardial infarction.14
The lifelong nature of diabetes, together with the marked increase in its prevalence and the inclusion of prescribing recommendations in guidelines,16 are likely to lead to increases in the numbers of patients prescribed different diabetes drugs. Given the impracticability and ethical difficulties of head to head trials, there is a need to quantify risks of clinical outcomes in large representative populations of patients with type 2 diabetes prescribed these drugs over longer periods. This information can complement information from meta-analyses of clinical trials where available, although these sources can have publication bias, lack sufficient reporting of outcomes, and have insufficient duration of follow-up or power to make relevant comparisons for effects on clinical endpoints.11 17 Although the focus of attention for new diabetes drugs has shifted from HbA1c alone to include cardiovascular outcomes, relatively little clinical trial evidence has accrued as to whether the newer diabetes drugs increase or decrease risk of other diabetes complications including lower limb amputation, blindness, kidney failure, serious hyperglycaemic or hypoglycaemic episodes, although these are the complications of particular importance to patients.18 There is also a lack of evidence on how these drugs are used, alone and in combination, in real world populations. This is important given that there are now a plethora of individual hypoglycaemic agents available from at least six different drug classes.
We therefore carried out a cohort study using a large UK primary care database with linked general practice, mortality, and hospital admissions data. This study aimed to investigate the associations between different classes of diabetes drugs and the risks of microvascular complications (severe kidney failure, blindness, and amputation), and serious hyperglycaemic or hypoglycaemic events. We were particularly interested in the risks associated with the newer agents, including glitazones and gliptins. In a separate analysis not included in this paper, we have compared the risks of heart failure, cardiovascular disease, and all cause mortality between different classes of diabetes drugs in patients with type 2 diabetes.
Methods
Setting and data source
We did a population based open cohort study of patients in England aged 25-84 years with a diagnosis of type 2 diabetes. We used a large population of primary care patients derived from version 40 of the QResearch database (www.qresearch.org). QResearch is a continually updated, patient level, pseudonymised database with event level data extending back to 1989. QResearch currently includes clinical and demographic data from 1243 general practices in England and two practices in Scotland covering a population of over 24 million patients, collected in the course of routine healthcare by general practitioners and associated staff.
The primary care data includes demographic information, diagnoses, prescriptions, referrals, laboratory results, and clinical values. Diagnoses are recorded using the Read code classification.19 QResearch has been used for a wide range of clinical research including the assessment of unintended effects of commonly prescribed medicines.20 21 22 23 24 25 The primary care data are linked at individual patient level to Hospital Episode Statistics, and mortality records from the Office for National Statistics (ONS). Hospital Episode Statistics provides details of all inpatient admissions in the UK’s health service since 1997, including primary and secondary causes coded by ICD-10 (international classification of diseases, 10th revision) and operations and interventions coded by OPCS-4 (OPCS classification of interventions and procedures version 4). ONS provides details of all deaths in England with primary and underlying causes, also coded using ICD-10. Patient records are linked using a project specific pseudonymised NHS number, which is valid and complete for 99.8% of primary care patients, 99.9% for ONS mortality records, and 98% for hospital admissions records.1
Inclusion and exclusion criteria
We included all QResearch practices in England who had been using the Egton Medical Information Systems (EMIS) computer system for at least a year. We initially identified an open cohort of patients aged 25-84 years registered with eligible practices between 1 April 2007 and 31 January 2015. We chose this study period because both pioglitazone and gliptins were available in the UK for the full study period. We then selected patients with diabetes if they had a Read code for diabetes or more than one prescription for a hypoglycaemic drug.
We excluded patients as having type 1 diabetes if they had been diagnosed under the age of 35 years and prescribed insulin.26 We also excluded patients without a postcode related deprivation score. We determined an entry date to the cohort for each patient, which was the latest of the following: date of diagnosis of diabetes, 25th birthday, date of registration with the practice plus one year, date on which the practice computer system was installed plus one year, and the beginning of the study period. We excluded patients with an existing diagnosis of an outcome of interest at the study entry date from the analysis of that outcome.
We used an incident user design for patients prescribed glitazones, gliptins (our main exposures of interest), or insulin to reduce bias.27 We defined incident users as patients without a prescription for these drugs in the 12 months before the study entry date as in other studies,28 and we excluded people who had received any of these drugs in the previous 12 months. We included prevalent users of metformin or sulphonylureas in the study cohort, because glitazones and gliptins are usually prescribed after monotherapy with metformin or sulphonylureas. Exclusion of prevalent users of metformin or sulphonylureas would have substantially reduced the numbers of new users of glitazones and gliptins, which were our main exposures of interest.
Outcomes
We had five primary outcomes based on diagnoses and procedures recorded either in the patient’s primary care record or in their linked hospital record or mortality record during follow-up:
Lower limb amputation—including hindquarter, above knee, or below knee amputation
Blindness—including blindness in one or both eyes, registered blindness, and severe visual impairment
Severe kidney failure—including kidney dialysis, kidney transplant, chronic kidney disease stage 5
Hyperglycaemia—including hyperosmolar hyperglycaemic coma, diabetic ketoacidosis
Hypoglycaemia—including spontaneous, reactive, drug induced hypoglycaemia events requiring paramedic or hospital admission or resulting in death
We used Read codes to identify recorded diagnoses from the primary care records. We used ICD-10 clinical codes and OPCS-4 procedure codes to identify incident cases from hospital and ONS mortality records. Web appendix 1 lists the clinical codes used to identify each outcome. We used the earliest recorded date from any of the three data sources as the index date for the diagnosis for each outcome. Patients were censored at the earliest date of the first recorded diagnosis of the outcome of interest, death, deregistration with the practice, last upload of computerised data, or the study end date (31 January 2015).
Exposure data
Our primary exposures of interest were new use of gliptins and new use of glitazones during the study period. We extracted details of all individual prescriptions for all types of hypoglycaemics for each patient including the prescription date and the type. We partitioned the follow-up time into different treatment periods, where each period corresponded to treatment with a particular type or combination of hypoglycaemic drugs, or could be a period of no treatment with any hypoglycaemic drugs. If the patient changed to a different type of treatment or to a different combination of treatments, we classified that as a separate treatment period. For example, consider a patient who was prescribed metformin alone on cohort entry for 12 months, then was prescribed both glitazones and metformin for another 24 months, and then had a treatment free period for six months until they were censored. This patient would have three treatment periods (metformin only for 12 months, metformin and glitazones for 24 months, and no treatment for six months).
We determined the duration of each treatment period by calculating the number of days between the earliest issue date and the latest issue date for the type of treatment prescribed. If another treatment was added before the initial treatment was stopped, the treatment period on the initial treatment alone was the number of days between the earliest issue date for the initial treatment and the earliest issue date for the next treatment. We added 90 days to the last prescription date as an estimate of the date on which the patient stopped treatment (the stop date). We made this assumption to allow for events that occur during a withdrawal period to be attributed to the medication rather than counting as unexposed time.
For the analysis, we used six binary exposure variables for each treatment period to indicate treatment with any of the diabetes drugs grouped into six drug classes: glitazones, gliptins, metformin, sulphonylureas, insulin, and other oral hypoglycaemic drugs (including α-glucosidase inhibitors, glinides, sodium-glucose cotransporter 2 inhibitors, guar). These drug classes were not split into individual drugs in the analyses. This exposure classification allowed for patients to be on different combinations of these drugs during a treatment period. To further assess associations for different specific treatment combinations (such as dual therapy with metformin and glitazones), we also categorised treatments during each treatment period into one categorical variable with 21 mutually exclusive categories. These mutually exclusive categories included a no current treatment group and 20 categories for mono, dual, and triple combinations of drugs.
Confounding variables
We considered confounding variables that were likely to be associated with the risk of the diabetes complications according to the established literature28 29 30 31 32 or with the likelihood of receiving treatment for different hypoglycaemic drugs. These included:
Age at study entry
Sex
Number of years since diagnosis of diabetes (categorised as <1, 1-3, 4-6, 7-10, ≥11)32
Calendar year
Smoking status (non-smoker, ex-smoker, light smoker (1-9 cigarettes/day), moderate smoker (10-19 cigarettes/day), heavy smoker (≥20 cigarettes/day), not recorded)
Ethnic group (categorised as white/not recorded, Indian, Pakistani, Bangladeshi, other Asian, black African, black Caribbean, Chinese, other including mixed)29
Townsend deprivation score
Complications (severe kidney failure,32 one or more episodes of hyperglycaemia, one or more episodes of hypoglycaemia, amputation, blindness—other than where the complication was the outcome of interest in which case patients with an existing diagnosis of the outcome of interest at the study entry date were excluded)
Comorbidities (cardiovascular disease,32 heart failure, peripheral vascular disease, valvular heart disease, chronic kidney disease, atrial fibrillation,29 hypertension,29 rheumatoid arthritis29)
Prescription drugs (statins, aspirin, anticoagulants, thiazides, angiotensin converting enzyme (ACE) inhibitors/angiotensin blockers, calcium channel blockers)
Clinical values (body mass index,32 cholesterol:high density lipoprotein (HDL) ratio,29 systolic blood pressure,29 serum creatinine, HbA1c1 32).
We evaluated confounders at the start of each treatment period for comorbidities, other complications, other prescribed medications, smoking status, and clinical values. For comorbidities and other complications, we identified whether patients had a diagnosis recorded before the relevant treatment period. For prescribed medications, we defined patients as treated at the start of the relevant period of diabetes drug treatment if they had at least two prescriptions for the other type of drug, including one in the 28 days before the treatment period and one after the start date. For smoking status and continuous variables (systolic blood pressure, body mass index, creatinine, cholesterol:HDL ratio, and HbA1c), we used the most recent recorded value immediately before the relevant treatment period. We categorised duration of diabetes as this had a highly skewed distribution, with a substantial proportion of newly diagnosed patients due to the open cohort design.
Analysis
We used Cox proportional hazards models to assess the associations between the six different classes of hypoglycaemic drugs and risk of each of our outcomes, adjusting for potential confounding variables. We used the Cox model for analysis rather than a competing risks analysis, because it is considered more appropriate for aetiological analyses such as this study whereas competing risks analyses tend to be most useful for prediction modelling or estimating absolute risks.33 34 35 To account for patients starting and stopping different treatments and changing between treatments, we treated hypoglycaemic exposures as time varying exposures.
In the analysis, we calculated unadjusted and adjusted hazard ratios for the six different diabetes drug classes (each as a binary variable indicating use or no use) with adjustment for the confounding variables and the other classes of hypoglycaemic drugs. We also calculated unadjusted and adjusted hazard ratios for the mutually exclusive treatment combinations comparing each treatment category with no current treatment and also with metformin alone. To determine whether significant differences existed between classes or individual drugs, we carried out Wald’s tests. We tested for interactions between the six different drug classes and age, sex, HbA1c, and body mass index.
We used multiple imputation with chained equations to replace missing values for continuous values and smoking status, and used these values in our main analyses.36 37 38 We did this for each study outcome and included the censoring indicator for the outcome, the log of survival time, all the confounding variables, and the diabetes drug treatment variables in the imputation model. We log-transformed body mass index, HbA1c, creatinine, cholesterol, and HDL cholesterol before imputation, because they had skewed distributions. We carried out five imputations and combined results using Rubin’s rules.
To evaluate the robustness of our results and assess the effect of confounding variables, we added the confounding variables to our model in blocks and compared the adjusted hazard ratios. We assessed four models:
Model A: diabetes drug classes adjusted for age, sex, ethnicity, deprivation, calendar year, duration of diabetes plus other diabetes drugs
Model B: model A plus comorbidities (hypertension, cardiovascular disease, atrial fibrillation, chronic renal disease, rheumatoid arthritis, valvular heart disease, peripheral vascular disease) plus existing complications (history of hypoglycaemia, hyperglycaemia, amputation, severe kidney failure, blindness) plus use of other drugs (statins, aspirin, anticoagulants, diuretics, ACE inhibitors/angiotensin blockers, β blockers, calcium channel blockers)
Model C (primary analysis model): model B plus clinical values (body mass index, cholesterol:HDL ratio, systolic blood pressure, serum creatinine, HbA1c)
Model D: model C plus interaction terms
We also carried out a sensitivity analysis (model E) by excluding prevalent users of sulphonylureas from the study cohort so that the hazard ratios for sulphonylureas are based on incident users, and we refitted the primary analysis model (model C).
We included all the eligible patients in the database to maximise the power and also generalisability of the results. We used a P value less than 0.01 (two tailed) to determine statistical significance. Hazard ratios calculated as at least 1.10 or calculated as 0.90 or lower were considered as clinically important. We used Stata (version 13.1) for all analyses.
Patient involvement
Patients were not involved in setting the research question, the outcome measures, the design, or implementation of the study. Patient representatives from the QResearch Advisory Board have written the information for patients on the QResearch website about the use of the database for research. They have also advised on dissemination including the use of lay summaries describing the research and its results.
Results
Overall study population
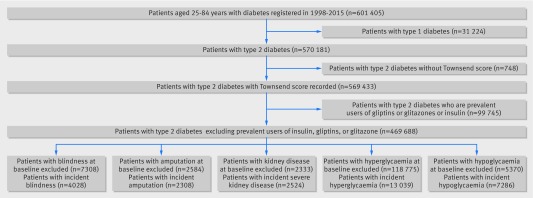
Overall, 1243 QResearch practices in England met the inclusion criteria. We identified a cohort of 601 405 patients aged 25-84 years with diabetes (fig 1). We sequentially excluded 31 224 (5.2%) patients with type 1 diabetes, 748 (0.1%) without a Townsend deprivation score, and 99 745 (16.6%) prescribed glitazones, gliptins, or insulin in the 12 months before the study entry date. These exclusions left 469 688 patients with type 2 diabetes in the study cohort. Figure 1 also shows the numbers of patients with each outcome at baseline who were excluded from the analysis of that outcome as well as the numbers of incident outcomes observed during follow-up.
Fig 1 Flow of patients through study
Baseline characteristics
In total, 274 324 (58.4%) of the patients in the study cohort received prescriptions for one or more diabetes drugs during follow-up. 21 308 (4.5%) were prescribed glitazones, 32 533 (6.9%) prescribed gliptins, 256 024 (54.5%) prescribed metformin, 134 570 (28.7%) prescribed sulphonylureas, 19 791 (4.2%) prescribed insulin, and 12 062 (2.6%) prescribed other oral hypoglycaemic agents. Of those patients receiving prescriptions for glitazones, 19 051 (89.4%) received pioglitazone with the remainder receiving rosiglitazone.
Table 1 shows the characteristics of patients prescribed each of the six classes of diabetes drugs during follow-up based on the last recorded value before the medication was first prescribed (or at study entry for patients already prescribed sulphonylureas, metformin, or other hypoglycaemics at baseline). The groups were similar for most characteristics except for increased levels of comorbidities other than hypertension in patients prescribed insulin, and reduced levels of prescriptions for statins and aspirin in patients prescribed metformin compared with the other drugs.
Table 1.
Characteristics of patients with type 2 diabetes
| Glitazones | Gliptins | Metformin | Sulphonylureas | Insulin | Other hypoglycaemic agents | |
|---|---|---|---|---|---|---|
| Total No of patients exposed | 21 308 | 32 533 | 256 024 | 134 570 | 19 791 | 12 062 |
| Mean age at study entry (SD) | 63.0 (11.9) | 63.3 (12.1) | 64.6 (13.1) | 66.2 (12.9) | 64.5 (12.7) | 60.0 (11.9) |
| Mean Townsend score (SD) | 0.4 (3.5) | 0.5 (3.5) | 0.6 (3.6) | 0.6 (3.6) | 0.5 (3.6) | 0.8 (3.6) |
| Male | 12 658 (59.4) | 18 871 (58.0) | 146 690 (57.3) | 79 284 (58.9) | 11 499 (58.1) | 6509 (54.0) |
| Time since diagnosis of diabetes | ||||||
| Newly diagnosed | 4412 (20.7) | 10 166 (31.2) | 100 690 (39.3) | 33 363 (24.8) | 6223 (31.4) | 2895 (24.0) |
| 1-3 years ago | 5996 (28.1) | 8590 (26.4) | 62 951 (24.6) | 32 604 (24.2) | 3999 (20.2) | 3115 (25.8) |
| 4-6 years ago | 5033 (23.6) | 6561 (20.2) | 43 477 (17.0) | 28 187 (20.9) | 3628 (18.3) | 2570 (21.3) |
| 7-10 years ago | 3389 (15.9) | 4288 (13.2) | 28 054 (11.0) | 21 768 (16.2) | 3128 (15.8) | 1874 (15.5) |
| >10 years ago | 2478 (11.6) | 2928 (9.0) | 20 852 (8.1) | 18 648 (13.9) | 2813 (14.2) | 1608 (13.3) |
| Ethnicity recorded | 19 130 (89.8) | 29 396 (90.4) | 228 962 (89.4) | 119 507 (88.8) | 17 264 (87.2) | 10 947 (90.8) |
| White or not recorded | 17 112 (80.3) | 26 104 (80.2) | 204 915 (80.0) | 107 537 (79.9) | 17 001 (85.9) | 10 135 (84.0) |
| Indian | 997 (4.7) | 1662 (5.1) | 11 732 (4.6) | 5978 (4.4) | 476 (2.4) | 420 (3.5) |
| Pakistani | 811 (3.8) | 1132 (3.5) | 7425 (2.9) | 3972 (3.0) | 389 (2.0) | 290 (2.4) |
| Bangladeshi | 586 (2.8) | 713 (2.2) | 7282 (2.8) | 3980 (3.0) | 370 (1.9) | 374 (3.1) |
| Other Asian | 476 (2.2) | 720 (2.2) | 5873 (2.3) | 2947 (2.2) | 234 (1.2) | 164 (1.4) |
| Caribbean | 473 (2.2) | 795 (2.4) | 6376 (2.5) | 3700 (2.7) | 549 (2.8) | 278 (2.3) |
| Black African | 392 (1.8) | 676 (2.1) | 5715 (2.2) | 2977 (2.2) | 350 (1.8) | 161 (1.3) |
| Chinese | 84 (0.4) | 95 (0.3) | 983 (0.4) | 513 (0.4) | 36 (0.2) | 34 (0.3) |
| Other | 377 (1.8) | 636 (2.0) | 5723 (2.2) | 2966 (2.2) | 386 (2.0) | 206 (1.7) |
| Smoking status recorded | 21 215 (99.6) | 32 399 (99.6) | 255 186 (99.7) | 134 080 (99.6) | 19 569 (98.9) | 12 003 (99.5) |
| Non-smoker | 11 374 (53.4) | 17 116 (52.6) | 132 634 (51.8) | 69 849 (51.9) | 9393 (47.5) | 6126 (50.8) |
| Ex-smoker | 6252 (29.3) | 9725 (29.9) | 78 935 (30.8) | 41 438 (30.8) | 6142 (31.0) | 3726 (30.9) |
| Light smoker | 2170 (10.2) | 3358 (10.3) | 25 678 (10.0) | 13 846 (10.3) | 2413 (12.2) | 1252 (10.4) |
| Moderate smoker | 730 (3.4) | 1121 (3.4) | 9395 (3.7) | 4661 (3.5) | 832 (4.2) | 441 (3.7) |
| Heavy smoker | 689 (3.2) | 1079 (3.3) | 8544 (3.3) | 4286 (3.2) | 789 (4.0) | 458 (3.8) |
| Comorbidities | ||||||
| Cardiovascular disease | 2962 (13.9) | 5325 (16.4) | 48 066 (18.8) | 28 895 (21.5) | 4596 (23.2) | 1992 (16.5) |
| Heart failure | 302 (1.4) | 737 (2.3) | 6943 (2.7) | 5069 (3.8) | 960 (4.9) | 374 (3.1) |
| Peripheral vascular disease | 1008 (4.7) | 1576 (4.8) | 12 458 (4.9) | 8467 (6.3) | 1519 (7.7) | 544 (4.5) |
| Valvular heart disease | 379 (1.8) | 914 (2.8) | 7378 (2.9) | 4606 (3.4) | 765 (3.9) | 292 (2.4) |
| Hypertension | 12 520 (58.8) | 19 293 (59.3) | 150 219 (58.7) | 80 776 (60.0) | 11 117 (56.2) | 7310 (60.6) |
| Atrial fibrillation | 929 (4.4) | 1980 (6.1) | 17 327 (6.8) | 10 574 (7.9) | 1890 (9.5) | 657 (5.4) |
| Chronic kidney disease | 388 (1.8) | 593 (1.8) | 3067 (1.2) | 4183 (3.1) | 1165 (5.9) | 224 (1.9) |
| Rheumatoid arthritis | 719 (3.4) | 1237 (3.8) | 9718 (3.8) | 5382 (4.0) | 842 (4.3) | 460 (3.8) |
| Prior complications | ||||||
| Existing severe kidney failure | 54 (0.3) | 74 (0.2) | 509 (0.2) | 825 (0.6) | 213 (1.1) | 36 (0.3) |
| Existing blindness | 260 (1.2) | 383 (1.2) | 3715 (1.5) | 2404 (1.8) | 360 (1.8) | 170 (1.4) |
| Existing amputation | 85 (0.4) | 125 (0.4) | 1239 (0.5) | 894 (0.7) | 161 (0.8) | 65 (0.5) |
| At least one previous episode of hypoglycaemia | 288 (1.4) | 286 (0.9) | 2247 (0.9) | 1946 (1.4) | 337 (1.7) | 215 (1.8) |
| At least one previous episode of hyperglycaemia | 7921 (37.2) | 10 054 (30.9) | 68 839 (26.9) | 46 341 (34.4) | 7279 (36.8) | 3914 (32.4) |
| Other medications | ||||||
| Anticoagulants | 642 (3.0) | 1419 (4.4) | 9409 (3.7) | 5989 (4.5) | 1344 (6.8) | 540 (4.5) |
| Thiazides | 3444 (16.2) | 4346 (13.4) | 31 291 (12.2) | 16 972 (12.6) | 2386 (12.1) | 1844 (15.3) |
| ACE inhibitors | 9318 (43.7) | 12 939 (39.8) | 83 847 (32.7) | 48 960 (36.4) | 7750 (39.2) | 5362 (44.5) |
| Angiotension 2 blockers | 3399 (16.0) | 4895 (15.0) | 28 629 (11.2) | 16 976 (12.6) | 2633 (13.3) | 2088 (17.3) |
| Calcium channel blockers | 5613 (26.3) | 8105 (24.9) | 55 674 (21.7) | 32 141 (23.9) | 5034 (25.4) | 3328 (27.6) |
| Statins | 15 512 (72.8) | 21 383 (65.7) | 137 574 (53.7) | 77 865 (57.9) | 12 640 (63.9) | 8451 (70.1) |
| Aspirin | 7890 (37.0) | 9684 (29.8) | 68 013 (26.6) | 41 647 (30.9) | 7057 (35.7) | 4096 (34.0) |
Data are no (%) of patients unless stated otherwise. Values represent those recorded before starting treatment or at study entry for prevalent users. Treatment groups not mutually exclusive. ACE=angiotension converting enzyme; SD=standard deviation.
Table 2 shows levels of recording and mean values for HbA1c, body mass index, cholesterol:HDL ratio, systolic blood pressure, and serum creatinine before the start of treatment (or at study entry for patients already prescribed sulphonylureas, metformin, or other hypoglycaemics at baseline). Highest levels of recording were for HbA1c, which were in excess of 97% for all six drug groups. Lowest levels of recording were for cholesterol:HDL ratios, which were above 84% for all drug groups. Overall, at least 82% of patients had complete data for all five clinical values across all drug groups. Apart from higher mean levels of HbA1c in patients before exposure to insulin and the other hypoglycaemic groups, and higher levels of creatinine among those prescribed sulphonylureas or insulin, mean values were similar across the six groups. Web table 1 shows mean values before starting the 20 different treatment combinations. The mean values for HbA1c tended to be higher for patients starting triple therapy (as high values of HbA1c will tend to trigger changes in therapy).
Table 2.
Recorded clinical values for patients before starting diabetes medication or at study entry for prevalent users
| Glitazones | Gliptins | Metformin | Sulphonylureas | Insulin | Other hypoglycaemic agents | |
|---|---|---|---|---|---|---|
| Total patients exposed | 21 308 | 32 533 | 256 024 | 134 570 | 19 791 | 12 062 |
| No (%) of patients with values recorded | ||||||
| HbA1c | 21 251 (99.7) | 32 474 (99.8) | 253 219 (98.9) | 133 170 (99.0) | 19 255 (97.3) | 12 022 (99.7) |
| Body mass index | 21 120 (99.1) | 32 224 (99.1) | 252 290 (98.5) | 132 477 (98.4) | 19 436 (98.2) | 11 749 (97.4) |
| Cholesterol:HDL ratio | 18 264 (85.7) | 29 307 (90.1) | 224 504 (87.7) | 115 991 (86.2) | 16 723 (84.5) | 10 619 (88.0) |
| Systolic blood pressure | 21 306 (100.0) | 32 529 (100.0) | 255 892 (99.9) | 134 487 (99.9) | 19 765 (99.9) | 12 057 (100.0) |
| Creatinine | 21 288 (99.9) | 32 509 (99.9) | 255 381 (99.7) | 134 244 (99.8) | 19 655 (99.3) | 12 044 (99.9) |
| All above values recorded | 18 097 (84.9) | 29 010 (89.2) | 220 119 (86.0) | 113 843 (84.6) | 16 270 (82.2) | 10 340 (85.7) |
| Mean values (SD) recorded | ||||||
| HbA1c (mmol/mol) | 66.8 (18.9) | 68.4 (18.4) | 61.4 (18.7) | 64.9 (19.9) | 75.4 (22.7) | 70.9 (19.6) |
| Body mass index | 31.7 (6.0) | 31.7 (5.9) | 30.6 (5.9) | 30.1 (5.8) | 30.2 (6.1) | 34.1 (6.6) |
| Cholesterol:HDL ratio | 3.8 (1.3) | 3.9 (1.3) | 3.8 (1.3) | 3.8 (1.3) | 4.0 (1.4) | 4.0 (1.3) |
| Systolic blood pressure (mm Hg) | 133.1 (14.8) | 132.3 (14.7) | 132.5 (15.3) | 132.8 (15.9) | 131.7 (16.9) | 132.5 (14.9) |
| Creatinine (μmol/L) | 87.1 (33.7) | 84.9 (33.3) | 84.8 (30.1) | 92.1 (47.7) | 99.3 (62.0) | 83.5 (34.6) |
Treatment groups not mutually exclusive. HDL=high density lipoprotein; SD=standard deviation.
Risks associated with use of each medication group
Table 3 shows the number of incident cases of each outcome for patients during periods of exposure to each of the six treatment classes during follow-up. The treatment classes in table 3 are not mutually exclusive—for example, the row for glitazones includes any use of glitazones, whether as monotherapy, dual therapy, or triple therapy. Similarly, the adjusted hazard ratios for model C shown in table 3 give an overall risk for the use of each drug group compared with non-use of that drug group, having adjusted for use of other diabetes drugs and the potential confounders listed in the footnote.
Table 3.
Number of incident events, person years, rates, and adjusted hazard ratios for each study outcome by use of diabetes drug
| Study outcome/diabetes drug group | No of events | No of person years | Rate per 10 000 person years of exposure | Adjusted hazard ratios (95% CI)* |
|---|---|---|---|---|
| Blindness | ||||
| Glitazones | 79 | 54 981 | 14.4 | 0.71 (0.57 to 0.89) |
| Gliptins | 117 | 70 197 | 16.7 | 0.83 (0.69 to 1.01) |
| Metformin | 2021 | 1 045 943 | 19.3 | 0.70 (0.66 to 0.75) |
| Sulphonylureas | 1220 | 494 418 | 24.7 | 0.96 (0.89 to 1.04) |
| Insulin | 202 | 56 153 | 36.0 | 1.48 (1.28 to 1.72) |
| Other hypoglycaemic agents | 52 | 27 715 | 18.8 | 0.86 (0.65 to 1.13) |
| Hyperglycaemia | ||||
| Glitazones | 180 | 32 966 | 54.6 | 0.88 (0.76 to 1.02) |
| Gliptins | 265 | 46 686 | 56.8 | 0.93 (0.82 to 1.05) |
| Metformin | 5368 | 712 777 | 75.3 | 0.65 (0.62 to 0.67) |
| Sulphonylureas | 2216 | 297 347 | 74.5 | 0.99 (0.94 to 1.04) |
| Insulin | 415 | 32 321 | 128.4 | 1.69 (1.53 to 1.87) |
| Other hypoglycaemic agents | 130 | 17 531 | 74.2 | 0.95 (0.80 to 1.14) |
| Hypoglycaemia | ||||
| Glitazones | 355 | 54 507 | 65.1 | 1.22 (1.10 to 1.37) |
| Gliptins | 319 | 69 719 | 45.8 | 0.86 (0.77 to 0.96) |
| Metformin | 3905 | 1 047 042 | 37.3 | 0.58 (0.55 to 0.61) |
| Sulphonylureas | 4064 | 491 712 | 82.7 | 2.93 (2.78 to 3.09) |
| Insulin | 1133 | 53 882 | 210.3 | 4.57 (4.27 to 4.89) |
| Other hypoglycaemic agents | 176 | 27 313 | 64.4 | 1.07 (0.92 to 1.25) |
| Amputation | ||||
| Glitazones | 62 | 55 551 | 11.2 | 0.77 (0.60 to 1.00) |
| Gliptins | 99 | 71 007 | 13.9 | 0.88 (0.71 to 1.08) |
| Metformin | 1195 | 1 059 266 | 11.3 | 0.70 (0.64 to 0.77) |
| Sulphonylureas | 819 | 502 028 | 16.3 | 1.05 (0.95 to 1.15) |
| Insulin | 199 | 56 798 | 35.0 | 1.64 (1.41 to 1.91) |
| Other hypoglycaemic agents | 45 | 28 028 | 16.1 | 0.93 (0.69 to 1.25) |
| Kidney failure | ||||
| Glitazones | 54 | 55 677 | 9.7 | 1.05 (0.80 to 1.38) |
| Gliptins | 64 | 71 251 | 9.0 | 0.99 (0.77 to 1.27) |
| Metformin | 562 | 1 064 018 | 5.3 | 0.41 (0.37 to 0.46) |
| Sulphonylureas | 881 | 502 691 | 17.5 | 1.21 (1.10 to 1.33) |
| Insulin | 235 | 56 573 | 41.5 | 1.45 (1.25 to 1.68) |
| Other hypoglycaemic agents | 35 | 28 166 | 12.4 | 0.80 (0.56 to 1.14) |
Hazard ratio for each diabetes drug group is compared with patients not prescribed that particular medication.
*Hazard ratios adjusted for: sex; age; calendar year; duration since diagnosis of diabetes (five levels); ethnicity (nine levels); Townsend deprivation score; smoking status (five levels); use of anticoagulants, thiazides, ACE inhibitors, angiotensin 2 blockers; calcium channel blockers; statins; aspirin; existing complications (blindness, hyperglycaemia, hypoglycaemia, amputation, severe kidney failure); hypertension; cardiovascular disease; atrial fibrillation; chronic renal disease; rheumatoid arthritis; valvular heart disease; peripheral vascular disease; body mass index; systolic blood pressure; HbA1c; serum creatinine; cholesterol:high density lipoprotein ratio. Hazard ratios also mutually adjusted for use of each of the other diabetes drug classes.
For our main exposures of interest, we found:
Compared with non-use, glitazones were significantly associated with a 29% decreased risk of blindness; and a 22% increased risk of hypoglycaemia.
Compared with non-use, gliptins were significantly associated with a 14% decreased risk of hypoglycaemia.
In addition for the other diabetes drug groups, we found:
Compared with non-use, metformin was associated with a significantly decreased risk of all five outcomes: reductions in risk of 30% for blindness, 35% for hyperglycaemia, 42% for hypoglycaemia, 30% for amputation, and 59% for severe kidney failure.
Compared with non-use, sulphonylureas were significantly associated with a 193% increased risk of hypoglycaemia and a 21% increased risk of severe kidney failure.
Compared with non-use, insulin was associated with significantly increased risks of all outcomes: increases of 48% for blindness, 69% for hyperglycaemia, 357% for hypoglycaemia, 64% for amputation, and 45% for severe kidney failure.
Compared with non-use, the other hypoglycaemic group was not associated with a significant increase or decrease in risk of any complication though the person years of exposure for this group were lower than the other groups in the analysis.
The only significant interaction involving glitazones or gliptins was between glitazones and age for hypoglycaemia (web table 2; model D), where the increased risk of hypoglycaemia associated with glitazone use became more marked with increasing age.
Web table 2 also shows the results from analyses adding confounders in separate blocks (models A and B). Generally, inclusion of comorbidities and existing complications in the model (model B) tended to slightly reduce hazard ratios (compared with model A), which was most marked for sulphonylureas and insulin. Further inclusion of clinical values (table 3; model C) only resulted in small changes to the hazard ratios with the exception of severe kidney failure which had some larger increases, for example, from 0.74 (95% confidence interval 0.56 to 0.97) to 1.05 (0.80 to 1.38) for glitazones.
The sensitivity analysis excluding the 73 445 prevalent users of sulphonylureas at study entry showed some increases in adjusted hazard ratios for sulphonylureas compared with model C (web table 2; model E), with the largest increase for hypoglycaemia (increase from 2.93 to 3.99).
Risks associated with different treatment combinations
Table 4 shows a more detailed breakdown of 21 mutually exclusive treatment categories, including a “no current treatment group” that included 0.7 million person years free of any hypoglycaemic medication. The table shows the number of events for each clinical outcome for each of the treatment categories.
Table 4.
Number of incident events for each outcome, person years of exposure, and rates per 10 000 person years of exposure for mutually exclusive diabetes treatment groups
| Blindness | Hyperglycaemia | Hypoglycaemia | Amputation | Kidney failure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of events; PY | Rate | No of events; PY | Rate | No of events; PY | Rate | No of events; PY | Rate* | No of events; PY | Rate | |||||
| Periods with no current treatment | 1541; 692 694 | 22.2 | 6905; 497 577 | 138.8 | 1850; 695 332 | 26.6 | 800; 702 512 | 11.4 | 1124; 701 350 | 16.0 | ||||
| Monotherapy | ||||||||||||||
| Metformin alone | 1053; 578 632 | 18.2 | 3407; 428 157 | 79.6 | 546; 583 302 | 9.4 | 504; 586 234 | 8.6 | 299; 588 181 | 5.1 | ||||
| Sulphonylureas alone | 334; 87 687 | 38.1 | 499; 54 551 | 91.5 | 817; 87 839 | 93.0 | 182; 89 826 | 20.3 | 546; 88 327 | 61.8 | ||||
| Insulin alone | 61; 16 247 | 37.5 | 156; 9572 | 163.0 | 383; 15 307 | 250.2 | 74; 16 463 | 44.9 | 147; 16 025 | 91.7 | ||||
| Glitazones alone | 3; 1651 | 18.2 | 9; 1149 | 78.3 | 3; 1668 | 18.0 | 3; 1690 | 17.8 | 6; 1675 | 35.8 | ||||
| Gliptins alone | 7; 2487 | 28.1 | 14; 1929 | 72.6 | 2; 2468 | 8.1 | 3; 2536 | 11.8 | 14; 2524 | 55.5 | ||||
| Other hypoglycaemic agents alone | 3; 1591 | 18.9 | 3; 1056 | 28.4 | 12; 1516 | 79.2 | 0; 1601 | 0.0 | 13; 1572 | 82.7 | ||||
| Dual therapy | ||||||||||||||
| Metformin and sulphonylureas | 676; 309 595 | 21.8 | 1315; 186 517 | 70.5 | 2251; 308 091 | 73.1 | 450; 313 843 | 14.3 | 184; 315 690 | 5.8 | ||||
| Metformin and insulin | 57; 18 124 | 31.5 | 123; 10 463 | 117.6 | 291; 17 393 | 167.3 | 54; 18 182 | 29.7 | 13; 18 422 | 7.1 | ||||
| Metformin and glitazones | 25; 17 805 | 14.0 | 45; 12 082 | 37.2 | 23; 17 727 | 13.0 | 10; 17 942 | 5.6 | 7; 17 990 | 3.9 | ||||
| Metformin and gliptins | 28; 23 879 | 11.7 | 81; 17 503 | 46.3 | 28; 23 831 | 11.7 | 22; 24 092 | 9.1 | 7; 24 172 | 2.9 | ||||
| Metformin and other hypoglycaemic agents | 15; 9187 | 16.3 | 46; 6314 | 72.9 | 30; 9099 | 33.0 | 11; 9306 | 11.8 | 3; 9355 | 3.2 | ||||
| Sulphonylureas and insulin | 30; 4364 | 68.7 | 37; 2510 | 147.4 | 156; 4159 | 375.1 | 23; 4497 | 51.1 | 55; 4354 | 126.3 | ||||
| Sulphonylureas and glitazones | 7; 3467 | 20.2 | 18; 1898 | 94.8 | 57; 3401 | 167.6 | 11; 3524 | 31.2 | 15; 3506 | 42.8 | ||||
| Sulphonylureas and gliptins | 14; 4293 | 32.6 | 13; 2688 | 48.4 | 31; 4276 | 72.5 | 10; 4399 | 22.7 | 23; 4375 | 52.6 | ||||
| Sulphonylureas and other hypoglycaemic agents | 2; 1076 | 18.6 | 3; 648 | 46.3 | 18; 1045 | 172.2 | 4; 1072 | 37.3 | 7; 1084 | 64.6 | ||||
| Triple therapy | ||||||||||||||
| Metformin, sulphonylureas, and insulin | 37; 11 313 | 32.7 | 56; 6072 | 92.2 | 194; 11 034 | 175.8 | 28; 11 449 | 24.5 | 8; 11 545 | 6.9 | ||||
| Metformin, sulphonylureas, and glitazones | 36; 24 733 | 14.6 | 74; 13 390 | 55.3 | 199; 24 487 | 81.3 | 26; 24 994 | 10.4 | 18; 25 098 | 7.2 | ||||
| Metformin, sulphonylureas, and gliptins | 53; 31 204 | 17.0 | 115; 19 326 | 59.5 | 188; 30 881 | 60.9 | 49; 31 511 | 15.6 | 13; 31 703 | 4.1 | ||||
| Metformin, sulphonylureas, and other hypoglycaemic agents | 20; 9871 | 20.3 | 44; 5805 | 75.8 | 56; 9744 | 57.5 | 14; 9986 | 14.0 | 5; 10 057 | 5.0 | ||||
| All other drug combinations | 26; 13 797 | 18.8 | 76; 8484 | 89.6 | 151; 13 623 | 110.8 | 30; 13 990 | 21.4 | 17; 14 026 | 12.1 | ||||
| Total | 4028; 1 863 697 | 21.6 | 13 039; 128 7691 | 101.3 | 7286; 1 866 223 | 39.0 | 2308; 1 889 649 | 12.2 | 2524; 189 1031 | 13.3 | ||||
Data are number of incident events, number of person years of exposure (PY), and rate per 10 000 person years of exposure.
Table 5 shows the corresponding adjusted hazard ratios for each treatment group category compared with monotherapy with metformin. For glitazones, we saw a significant increase in the risk of severe kidney failure for monotherapy with glitazones (155% increase), but no significant associations between glitazone monotherapy and risk of any other complications, compared with monotherapy with metformin. However, glitazone monotherapy was relatively uncommon (table 4). Dual therapy with glitazones and metformin was associated with a significantly decreased risk of hyperglycaemia (reduction of 40%). Dual therapy with glitazones and sulphonylureas was associated with a 93% increased risk of hyperglycaemia, a 106% increased risk of amputation, a 114% increased risk of severe kidney failure, and more than a 13-fold increased risk of hypoglycaemia compared with metformin monotherapy. Triple therapy with metformin, sulphonylureas, and glitazones was associated with a decreased risk of blindness (33% reduction) and more than a sixfold increased risk of hypoglycaemia.
Table 5.
Adjusted hazard ratios (95% CI) for each study outcome* compared with metformin monotherapy
| Study outcome | |||||
|---|---|---|---|---|---|
| Blindness | Hyperglycaemia | Hypoglycaemia | Amputation | Kidney failure | |
| Monotherapy | |||||
| Metformin alone | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Sulphonylureas alone | 1.42 (1.25 to 1.62) | 1.38 (1.25 to 1.52) | 7.32 (6.55 to 8.18) | 1.44 (1.20 to 1.71) | 2.63 (2.25 to 3.06) |
| Insulin alone | 1.96 (1.50 to 2.55) | 2.48 (2.11 to 2.92) | 21.17 (18.48 to 24.25) | 2.68 (2.08 to 3.46) | 2.88 (2.32 to 3.57) |
| Glitazones alone | 0.98 (0.31 to 3.03) | 1.50 (0.78 to 2.89) | 1.83 (0.59 to 5.70) | 1.88 (0.60 to 5.84) | 2.55 (1.13 to 5.74) |
| Gliptins alone | 1.39 (0.66 to 2.93) | 1.44 (0.85 to 2.43) | 0.83 (0.21 to 3.33) | 1.03 (0.33 to 3.20) | 3.52 (2.04 to 6.07) |
| Other hypoglycaemic agents alone | 0.82 (0.26 to 2.55) | 0.32 (0.08 to 1.29) | 6.44 (3.63 to 11.42) | — | 1.17 (0.63 to 2.18) |
| Dual therapy | |||||
| Metformin and sulphonylureas | 0.94 (0.85 to 1.04) | 1.02 (0.95 to 1.09) | 6.03 (5.47 to 6.63) | 1.08 (0.95 to 1.23) | 0.76 (0.62 to 0.92) |
| Metformin and insulin | 1.58 (1.21 to 2.07) | 1.95 (1.63 to 2.34) | 14.34 (12.40 to 16.59) | 1.78 (1.34 to 2.37) | 1.13 (0.65 to 1.98) |
| Metformin and glitazones | 0.87 (0.58 to 1.29) | 0.60 (0.45 to 0.80) | 1.35 (0.89 to 2.05) | 0.57 (0.30 to 1.06) | 0.71 (0.33 to 1.50) |
| Metformin and gliptins | 0.72 (0.49 to 1.04) | 0.78 (0.62 to 0.97) | 1.23 (0.84 to 1.80) | 0.84 (0.55 to 1.30) | 0.59 (0.28 to 1.25) |
| Metformin and other hypoglycaemic agents | 0.83 (0.50 to 1.38) | 1.06 (0.79 to 1.41) | 2.77 (1.92 to 4.01) | 0.93 (0.51 to 1.70) | 0.57 (0.18 to 1.79) |
| Sulphonylureas and insulin | 2.13 (1.47 to 3.09) | 2.62 (1.89 to 3.63) | 23.91 (19.89 to 28.75) | 2.15 (1.40 to 3.29) | 3.56 (2.64 to 4.82) |
| Sulphonylureas and glitazones | 0.80 (0.38 to 1.69) | 1.93 (1.21 to 3.07) | 13.22 (10.04 to 17.39) | 2.06 (1.13 to 3.76) | 2.14 (1.27 to 3.61) |
| Sulphonylureas and gliptins | 1.32 (0.78 to 2.25) | 0.98 (0.57 to 1.69) | 5.93 (4.12 to 8.53) | 1.30 (0.69 to 2.45) | 3.21 (2.08 to 4.93) |
| Sulphonylureas and other hypoglycaemic agents | 0.69 (0.17 to 2.76) | 0.73 (0.24 to 2.27) | 12.12 (7.57 to 19.41) | 2.02 (0.75 to 5.43) | 2.38 (1.11 to 5.09) |
| Triple therapy | |||||
| Metformin, sulphonylureas, and insulin | 1.36 (0.97 to 1.89) | 1.46 (1.12 to 1.91) | 13.17 (11.14 to 15.57) | 1.30 (0.88 to 1.91) | 1.06 (0.53 to 2.16) |
| Metformin, sulphonylureas, and glitazones | 0.67 (0.48 to 0.94) | 0.99 (0.79 to 1.25) | 6.32 (5.35 to 7.45) | 0.70 (0.47 to 1.05) | 1.21 (0.75 to 1.96) |
| Metformin, sulphonylureas and gliptins | 0.82 (0.62 to 1.08) | 1.09 (0.91 to 1.32) | 5.07 (4.28 to 6.00) | 0.99 (0.73 to 1.33) | 0.68 (0.39 to 1.20) |
| Metformin, sulphonylureas and other hypoglycaemic agents | 0.91 (0.59 to 1.43) | 1.03 (0.76 to 1.38) | 4.31 (3.26 to 5.68) | 0.85 (0.50 to 1.45) | 0.93 (0.38 to 2.26) |
| All other drug combinations | 0.94 (0.64 to 1.39) | 1.43 (1.14 to 1.80) | 9.02 (7.50 to 10.83) | 1.25 (0.86 to 1.82) | 1.60 (0.98 to 2.62) |
| No medication | 1.40 (1.29 to 1.52) | 1.58 (1.51 to 1.65) | 3.06 (2.77 to 3.37) | 1.44 (1.28 to 1.62) | 1.85 (1.61 to 2.12) |
Treatment categories are mutually exclusive.
*Hazard ratios adjusted for sex; age; duration since diagnosis of diabetes (five levels); ethnicity (nine levels); Townsend deprivation score; smoking status (five levels); use of anticoagulants, thiazides, ACE inhibitors, angiotensin 2 blockers, calcium channel blockers, statins, aspirin; existing complications (blindness, hyperglycaemic coma, hypoglycaemia, amputation, severe kidney failure); hypertension; cardiovascular disease; atrial fibrillation; chronic renal disease; rheumatoid arthritis; valvular heart disease; peripheral vascular disease; body mass index; systolic blood pressure; HbA1c; serum creatinine; and cholesterol:high density lipoprotein ratio.
Gliptin monotherapy was associated with more than a threefold increased risk of severe kidney failure compared with monotherapy with metformin (adjusted hazard ratio 3.52; 95% confidence interval 2.04 to 6.07), but there were no other significant associations although gliptin monotherapy was relatively uncommon (table 4). Dual therapy with gliptins and metformin was associated with a 22% decreased risk of hyperglycaemia. Dual therapy with gliptins and sulphonylureas was associated with nearly a sixfold increased risk of hypoglycaemia and more than a threefold increased risk of severe kidney failure. Triple therapy with gliptins, metformin and sulphonylureas was associated with a fivefold increased risk of hypoglycaemia (5.07; 4.28 to 6.00). The increased risks of hypoglycaemia in patients prescribed triple therapy with metformin, sulphonylureas, and either gliptins or glitazones were similar to those involving dual therapy with metformin and sulphonylureas (adjusted hazard ratio 6.03, 95% confidence interval 5.47 to 6.63).
Web table 3 shows the results for the different treatment combinations compared with periods of no hypoglycaemic treatment. Web table 4 shows the results for the different treatment combinations compared with no treatment having dropped prevalent users of sulphonylureas at study entry. Overall, the adjusted hazard ratios for glitazones and gliptins were similar to those in web table 3 except confidence intervals were wider due to reduced numbers and there were fewer significant associations.
Discussion
Key findings
This cohort study has found clinically important differences between different hypoglycaemic drugs (alone and in combination) and the risk of five key outcomes (blindness, amputation, severe kidney failure, hyperglycaemia, and hypoglycaemia) in patients with type 2 diabetes. Our study aimed to look at a range of complications in relation to drug treatment whereas many previous studies have either been clinical trials that have had limited sample sizes and durations; or observational studies that have focused on one particular drug or drug combination.
Overall, we found a reduced risk of blindness and an increased risk of hypoglycaemia associated with glitazones, and a reduced risk of hypoglycaemia associated with gliptins. A more detailed analysis according to combinations of treatments showed some differences according to whether glitazones and gliptins were prescribed as monotherapy or dual or triple therapy with other agents. Our results indicate that gliptins and glitazones are associated with an increased risk of kidney failure compared with metformin monotherapy only when used as monotherapy or in combination with sulphonylureas, despite adjustments for serum creatinine and other risk factors at baseline. However, the risks when used in combination with sulphonylureas are similar to the risk with sulphonylureas monotherapy. This suggests either a drug-drug interaction in the dual therapy, where the other drug used has a modifying effect on risk, or indication bias when these drugs are used as monotherapy since this combination is mainly likely to be in patients with contraindications to metformin, as recommended in guidelines. For example, the British National Formulary indicates that metformin should be avoided in patients with significant renal impairment owing to increased risk of lactic acidosis.
Although the numbers prescribed gliptin monotherapy or glitazones monotherapy were relatively low, the findings appear consistent with other reports of safety of the renally excreted gliptins.39 But other data in relation to this outcome are limited. Our finding for glitazones is consistent with increased risks of incident chronic kidney disease reported in a observational study of almost 4000 users of pioglitazone followed between 2003 and 2009 in Taiwan.40 Although pioglitazone is extensively metabolised in the liver,41 pioglitazone is also known to increase fluid and sodium retention particularly in patents with renal impairment.42 This, together with the concerns regarding possible increased risk of bladder cancer, has led to the withdrawal of pioglitazone use in France and Germany and caution regarding its use in Switzerland.43
Dual treatment combinations for glitazones or gliptins with metformin showed reduced risks of hyperglycaemia compared with metformin monotherapy and no significant increases in risk for the other outcomes. Triple therapy involving metformin, sulphonylureas, and glitazones was associated with a significantly decreased risk of blindness, and an increased risk of hypoglycaemia. Similarly, triple therapy of metformin, sulphonylureas, and gliptins was associated with a significantly increased risk of hypoglycaemia.
Overall, triple therapy involving gliptins or glitazones does not appear to have consistent measurable advantages compared with dual therapy or monotherapy with metformin for these five clinical outcomes. This triple combination also showed substantially increased risks of hypoglycaemia when compared against metformin monotherapy although risks were similar to those involving dual therapy with metformin and sulphonylureas.
Comparison with previous studies
Clinical trial evidence for newer hypoglycaemic agencies, such as glitazones and gliptins, has been largely based on surrogate endpoints such as reduction of HbA1c rather than hard clinical endpoints such as reduced incidence of complications as in our study. The UKPDS-34 study3 provided the original clinical trial evidence for the use of metformin as first line treatment of choice for patients with type 2 diabetes since it was associated with a greater reduction in diabetes related endpoints and fewer hypoglycaemic attacks than sulphonylureas. Designed in the 1970s and running over a 20 year period, part of the UKPDS cohort was randomised with 342 patients randomised to metformin and 411 patients to diet alone. The researchers reported a 32% reduction (95% confidence interval 13% to 47%) for any diabetes related endpoint.3 Although the numbers randomised to each arm were small and the numbers of clinical endpoints even smaller (there were only six events of amputation in the metformin group and nine in the diet alone group), the results are comparable with the reduction in risk reported in our study for metformin monotherapy compared with diet only.
Strengths and limitations
Ideally we would now have ongoing published meta-analyses of randomised trials comparing clinical outcomes among patients prescribed different diabetes drugs alone and in combination compared with no medication. These meta-analyses would form the basis of national guidelines and robust systems would be in place to routinely monitor the use of these drugs for unintended or adverse outcomes. However, nearly all clinical trials of diabetes drugs have been designed using intermediate or surrogate outcomes such as changes in HbA1c rather than relevant clinical outcomes. The criteria set by regulators regarding new diabetes agents12 13 and the impracticability of undertaking adequately powered head to head trials of clinical outcomes for existing drugs means that we need to use alternative methods for establishing the necessary evidence base for clinical outcomes. Therefore, the question becomes how best to assemble the evidence for clinical outcomes using observational linked data sources, how to minimise attendant biases, and how to interpret the findings with due caution.
Generalisability
To our knowledge, this is the largest study based on an ethnically diverse contemporaneous, representative population of patients with type 2 diabetes. We included all eligible patients to minimise selection bias. Therefore, we think the results are likely to be generalisable to similar populations of patients with type 2 diabetes.
Clinical outcomes
Strengths of our analysis were the inclusion of hard clinical endpoints based on clinical diagnoses or procedural codes recorded on at least one of three linked electronic data sources. Use of all three linked data sources was designed to minimise under-ascertainment of outcomes, which would otherwise lead to under-estimation of absolute risks. The clinical outcomes were based on clinical diagnoses made by the treating clinician or hospital procedural codes rather than formally adjudicated events as would occur in a clinical trial. Although some patients may have been incorrectly recorded as having a particular outcome, UK general practices have good levels of accuracy and completeness in recording clinical diagnoses.44 Also, the diagnostic validity of such diagnoses in general practice has been shown to be high.45 Possible ascertainment bias of outcomes is unlikely to vary according to the type of hypoglycaemic prescribed so would not explain the associations we have found.
Exposure to medication
We had detailed exposure information on hypoglycaemic agents prescribed throughout the follow-up period, enabling us to develop a detailed categorisation of drug exposure time with 20 different treatment groups including combinations of treatments. This categorisation enabled us to account for switching between different treatments or treatment combinations, and we were able to account for real world prescribing patterns over a long period, allowing multiple comparisons not only between drugs but also between different drug combinations. The recording of prescriptions issued in UK general practices has very high levels of completeness.46 Our study analysed prescribed medication rather than medication actually taken by the patients, although renewal of prescriptions is likely to indicate drug use because patients need to initiate repeat prescriptions. This could result in misclassification of exposure if patients were prescribed medication that they did not actually take and could underestimate associations between diabetes drug use and clinical outcomes. Unlike previous studies, we have included comparisons of risk against periods of no treatment6 as well as against metformin monotherapy. The first comparison is important because about 40% of patients with type 2 diabetes are managed without hypoglycaemic treatments throughout follow-up.
Our study included over 55 000 person years of exposure to glitazones. The predominant glitazone was pioglitazone, which was prescribed to 90% of the glitazone users. Our study included over 70 000 person years of exposure to gliptins, which represents one of the largest studies to date. The predominant gliptin prescribed in our dataset was sitagliptin, which was prescribed to 80% of those gliptin users. There are currently too few patients prescribed linagliptin, saxagliptin, and vildagliptin to support separate analyses by individual drug; this was a limitation of the study because there may be differences between individual gliptins and their effect on HbA1c.47 However, the numbers of patients on different types of gliptin is likely to increase over time and further analyses can be undertaken once more data has accrued. We did not split the other drug classes such as sulphonylureas into different drug subtypes because they were not the main focus of the study, but potential heterogeneity could exist in risk of the outcomes across different subtypes for each drug class in the analyses.
Assessment of other types of bias
Other types of bias that can affect observational studies include recall bias, indication bias, and channelling bias. Recall bias will not have occurred because data on prescriptions for hypoglycaemia and confounding variables were recorded before the clinical outcomes. We restricted the study population to patients with type 2 diabetes to limit indication bias (which occurs when patients are prescribed drugs for a condition associated with the risk of the adverse event under consideration). We used an incident user design to reduce confounding and biases that can otherwise arise from adjustment for intermediate characteristics in the causal path.27 We saw some differences at baseline between patients prescribed different treatment groups (tables 1 and 2), although these were predominantly increased levels of comorbidities for insulin and lower levels of concurrent use of medication (such as statins and aspirin) for metformin.
To reduce channelling bias (where the choice of a particular drug is influenced by patient characteristics), we adjusted for a wide range of potential confounding variables including demographic characteristics, different comorbidities, clinical values, and concurrent medication. We decided to use the most recent clinical values before changes in treatment since these are the values most likely to be used to inform those decisions. We did not use repeated values or changes in values over long periods because these data are not necessarily recorded consistently and would tend to increase proportions of missing data. However, we are unable to exclude the possibility of residual confounding since there may be other unmeasured patient characteristics that affected selection of hypoglycaemic agents.
As in other similar studies,28 we excluded prevalent users of insulin at baseline but left patients subsequently prescribed insulin in for the rest of the analysis because it is part of the treatment ladder and some of these patients will also have had other medications of interest during follow-up. Although insulin was not the primary exposure of interest, patients prescribed insulin had higher risks of all complications (tables 3 and 5) despite adjustment for higher levels of comorbidity. It is unlikely that this increased risk was a direct result of treatment with insulin. Instead, residual confounding and reverse causality could have occurred—that is, the insulin treated group was at much higher risk of complications than the groups treated with diet or oral medication, and it is this that results in their apparently worse outcomes and not their treatment with insulin. For example, in table 2, the insulin treated group had the highest HbA1c and creatinine values before treatment, although both factors were adjusted for in the analyses. An alternative explanation could be that patients have symptoms that lead to an amputation or sight problems before being diagnosed with these conditions and that the symptoms are associated with subsequent use of insulin rather than glitazones or gliptins.
Although randomised controlled trials of hypoglycaemic treatments are not influenced by residual confounding, they tend to be small, of short duration, and might not report on relevant clinical outcomes. An alternative design would an observational study of a cohort of patients specifically assembled for the purpose rather than routinely collected data as in our study. Studies using routinely collected data are susceptible to missing data, but in our study, over 99% of patients had smoking status recorded, 87% had ethnic group recorded, and over 82% had of patients had complete data for all five clinical values (table 2). We also used multiple imputation to impute missing data. Other problems with routine data include coding errors and variable timing between measurements of risk factors in patients because of differences in when patients present to their general practitioner. Advantages of using routinely collected data rather than a purposeful cohort include size, efficiency, better generalisability, and less susceptibility to selection bias or attrition bias.
We fitted several different models and carried out sensitivity analyses that showed some heterogeneity of results. The results are therefore sensitive to the assumptions made in the study design and modelling and have uncertainty; however, our findings were generally consistent across the different analyses for glitazones and gliptins.
Conclusions
We have found lower risks of hyperglycaemia among patients prescribed dual therapy involving metformin with either gliptins or glitazones compared with metformin alone. Compared with metformin monotherapy, triple therapy with metformin, sulphonylureas, and either gliptins or glitazones was associated with an increased risk of hypoglycaemia, which was similar in magnitude to the risk for dual therapy with metformin and sulphonylureas. Compared with metformin monotherapy, triple therapy with metformin, sulphonylureas, and glitazones was associated with a reduced risk of blindness. These results, while subject to residual confounding, could have implications for prescribing of hypoglycaemic drugs.
What is already known
Type 2 diabetes is associated with increased risks of microvascular complications (including lower limb amputation, blindness, kidney failure) and hypoglycaemic and hyperglycaemic attacks
Clinical trial evidence for glitazones and gliptins is largely based on surrogate endpoints (such as reduction of HbA1c) rather than hard clinical endpoints (such as reduced incidence of complications)
There is a need to quantify risks of clinical outcomes in large representative populations of patients with type 2 diabetes prescribed these drugs over longer periods of time
What this study adds
Although the numbers of patients prescribed gliptin monotherapy or glitazone monotherapy were relatively low, we found an increased risk of severe kidney failure compared with metformin monotherapy despite adjustments for serum creatinine and other risk factors at baseline
We found reduced risks of hyperglycaemia among patients prescribed dual therapy of metformin with either gliptins or glitazones, compared with metformin monotherapy
Compared with metformin monotherapy, triple therapy with metformin, sulphonylureas, and either gliptins or glitazones was associated with an increased risk of hypoglycaemia; compared with metformin monotherapy, triple therapy with metformin, sulphonylureas, and glitazones was associated with a reduced risk of blindness
Web Extra.
Extra material supplied by the author
Web appendix 1: Clinical codes used to identify severe kidney failure
Web appendix 2: Clinical codes used to identify blindness
Web appendix 3: Clinical codes used to identify amputation
Web appendix 4: Clinical codes used to identify hyperglycaemia
Web appendix 5: Clinical codes used to identify hypoglycaemia
Web appendix 6: Web tables
We acknowledge the contribution of EMIS practices who contribute to QResearch and acknowledge EMIS and the University of Nottingham for expertise in establishing, developing, and supporting the database. We acknowledge the Office of National Statistics for providing mortality data. Hospital Episode Statistics 2015 have been reused with permission of the Health and Social Care Information Centre (all rights reserved). This work and any views expressed within it are solely those of the co-authors and not of any affiliated bodies or organisations.
Contributorship: JHC initiated the study, undertook the literature review, data extraction, data manipulation and primary data analysis, and wrote the first draft of the paper. CC contributed to the design, analysis, data manipulation, interpretation, and drafting of the paper. Both authors had access to all the data for this project. JHC is the guarantor.
Funding: There was no external funding for this project.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: JHC is professor of clinical epidemiology at the University of Nottingham and codirector of QResearch—a not for profit organisation that is a joint partnership between the University of Nottingham and EMIS (leading commercial supplier of IT for 60% of general practices in the UK); JHC is a paid director of ClinRisk, which produces open and closed source software to ensure the reliable and updatable implementation of clinical risk algorithms within clinical computer systems to help improve patient care; CC is professor of medical statistics in primary care at the University of Nottingham and a paid consultant statistician for ClinRisk.
Ethical approval: The project was reviewed in accordance with the QResearch agreement with Trent multicentre ethics committee (reference 03/4/021).
Data sharing: The patient level data from the QResearch database are specifically licensed according to its governance framework. See www.qresearch.org for further details.
The lead author, Julia Hippisley-Cox, affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as have been explained.
References
- 1.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-12. 10.1136/bmj.321.7258.405 pmid:10938048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53. 10.1016/S0140-6736(98)07019-6 pmid:9742976. [DOI] [PubMed] [Google Scholar]
- 3. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) [correction in: Lancet 1998;352:1557]. Lancet 1998;352:854-65. 10.1016/S0140-6736(98)07037-8 pmid:9742977. [DOI] [PubMed] [Google Scholar]
- 4.Nattrass M, Alberti KG. Biguanides. Diabetologia 1978;14:71-4. 10.1007/BF01263443 pmid:631459. [DOI] [PubMed] [Google Scholar]
- 5.Wikipedia. Troglitzone 2015. https://en.wikipedia.org/wiki/Troglitazone.
- 6.Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 2009;339:b4731 10.1136/bmj.b4731 pmid:19959591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dormandy JA, Charbonnel B, Eckland DJ, et al. PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279-89. 10.1016/S0140-6736(05)67528-9 pmid:16214598. [DOI] [PubMed] [Google Scholar]
- 8.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007;298:1180-8. 10.1001/jama.298.10.1180 pmid:17848652. [DOI] [PubMed] [Google Scholar]
- 9.Scirica BM, Bhatt DL, Braunwald E, et al. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317-26. 10.1056/NEJMoa1307684 pmid:23992601. [DOI] [PubMed] [Google Scholar]
- 10.Diamond GA, Bax L, Kaul S. Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med 2007;147:578-81. 10.7326/0003-4819-147-8-200710160-00182 pmid:17679700. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez AV, Walker E, Ioannidis JP, Kattan MW. Challenges in meta-analysis of randomized clinical trials for rare harmful cardiovascular events: the case of rosiglitazone. Am Heart J 2008;156:23-30. 10.1016/j.ahj.2008.03.002 pmid:18585493. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health and Human Services FaDA, Center for Drug Evaluation and Research. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf.
- 13.European Medicine Agency CfMPfHU. Guideline on clinical investigation of medicinal products in the treatment of diabetes mellitus. 2010. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/10/WC500115945.pdf
- 14.Home PD, Pocock SJ, Beck-Nielsen H, et al. RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373:2125-35. 10.1016/S0140-6736(09)60953-3 pmid:19501900. [DOI] [PubMed] [Google Scholar]
- 15.Psaty BM, Furberg CD. The record on rosiglitazone and the risk of myocardial infarction. N Engl J Med 2007;357:67-9. 10.1056/NEJMe078116 pmid:17551162. [DOI] [PubMed] [Google Scholar]
- 16.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577-96. 10.1007/s00125-012-2534-0 pmid:22526604. [DOI] [PubMed] [Google Scholar]
- 17.Engel SS, Golm GT, Shapiro D, Davies MJ, Kaufman KD, Goldstein BJ. Cardiovascular safety of sitagliptin in patients with type 2 diabetes mellitus: a pooled analysis. Cardiovasc Diabetol 2013;12:3 10.1186/1475-2840-12-3 pmid:23286208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9. 10.1177/027298902400448902 pmid:12150599. [DOI] [PubMed] [Google Scholar]
- 19.Wikipedia. Readcodes 2015. https://en.wikipedia.org/wiki/Read_code.
- 20.Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005;330:1366-74. 10.1136/bmj.330.7504.1366 pmid:15947398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005;331:1310-6. 10.1136/bmj.331.7528.1310 pmid:16322018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010;340:c2197 10.1136/bmj.c2197 pmid:20488911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker C, Coupland C, Hippisley-Cox J. Antipsychotic drugs and risk of venous thromboembolism: nested case-control study. BMJ 2010;341:c4245 10.1136/bmj.c4245 pmid:20858909. [DOI] [PubMed] [Google Scholar]
- 24.Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to bisphosphonates and risk of gastrointestinal cancers: series of nested case-control studies with QResearch and CPRD data. BMJ 2013;346:f114 10.1136/bmj.f114 pmid:23325866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coupland C, Hill T, Morriss R, Arthur A, Moore M, Hippisley-Cox J. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database. BMJ 2015;350:h517 10.1136/bmj.h517 pmid:25693810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hippisley-Cox J, Pringle M. Prevalence, care, and outcomes for patients with diet-controlled diabetes in general practice: cross sectional survey. Lancet 2004;364:423-8. 10.1016/S0140-6736(04)16765-2 pmid:15288740. [DOI] [PubMed] [Google Scholar]
- 27.Johnson ES, Bartman BA, Briesacher BA, et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf 2013;22:1-6. 10.1002/pds.3334 pmid:23023988. [DOI] [PubMed] [Google Scholar]
- 28.Eurich DT, Simpson S, Senthilselvan A, Asche CV, Sandhu-Minhas JK, McAlister FA. Comparative safety and effectiveness of sitagliptin in patients with type 2 diabetes: retrospective population based cohort study. BMJ 2013;346:f2267 10.1136/bmj.f2267 pmid:23618722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475-82. 10.1136/bmj.39609.449676.25 pmid:18573856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke PM, Gray AM, Briggs A, et al. UK Prospective Diabetes Study (UKDPS) Group. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747-59. 10.1007/s00125-004-1527-z pmid:15517152. [DOI] [PubMed] [Google Scholar]
- 31.Davis TME, Coleman RL, Holman RR. UKPDS Group. Ethnicity and long-term vascular outcomes in Type 2 diabetes: a prospective observational study (UKPDS 83). Diabet Med 2014;31:200-7. 10.1111/dme.12353 pmid:24267048. [DOI] [PubMed] [Google Scholar]
- 32.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 2004;27:1879-84. 10.2337/diacare.27.8.1879 pmid:15277411. [DOI] [PubMed] [Google Scholar]
- 33.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41:861-70. 10.1093/ije/dyr213 pmid:22253319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170:244-56. 10.1093/aje/kwp107 pmid:19494242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pintilie M. Analysing and interpreting competing risk data. Stat Med 2007;26:1360-7. 10.1002/sim.2655 pmid:16900575. [DOI] [PubMed] [Google Scholar]
- 36.Steyerberg EW, van Veen M. Imputation is beneficial for handling missing data in predictive models. J Clin Epidemiol 2007;60:979 10.1016/j.jclinepi.2007.03.003 pmid:17689816. [DOI] [PubMed] [Google Scholar]
- 37.Moons KGM, Donders RART, Stijnen T, Harrell FE Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 2006;59:1092-101. 10.1016/j.jclinepi.2006.01.009 pmid:16980150. [DOI] [PubMed] [Google Scholar]
- 38.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods 2002;7:147-77. 10.1037/1082-989X.7.2.147 pmid:12090408. [DOI] [PubMed] [Google Scholar]
- 39.Eligar VS, Bain SC. A review of sitagliptin with special emphasis on its use in moderate to severe renal impairment. Drug Des Devel Ther 2013;7:893-903.pmid:24039399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee M-Y, Hsiao P-J, Yang Y-H, Lin K-D, Shin S-J. The association of pioglitazone and urinary tract disease in type 2 diabetic Taiwanese: bladder cancer and chronic kidney disease. PLoS One 2014;9:e85479 10.1371/journal.pone.0085479 pmid:24427312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budde K, Neumayer H-H, Fritsche L, Sulowicz W, Stompôr T, Eckland D. The pharmacokinetics of pioglitazone in patients with impaired renal function. Br J Clin Pharmacol 2003;55:368-74. 10.1046/j.1365-2125.2003.01785.x pmid:12680885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004;27:256-63. 10.2337/diacare.27.1.256 pmid:14693998. [DOI] [PubMed] [Google Scholar]
- 43.Zanchi A, Lehmann R, Philippe J. Antidiabetic drugs and kidney disease--recommendations of the Swiss Society for Endocrinology and Diabetology. Swiss Med Wkly 2012;142:w13629.pmid:22987488. [DOI] [PubMed] [Google Scholar]
- 44.Majeed A. Sources, uses, strengths and limitations of data collected in primary care in England. Health Stat Q 2004;21:5-14.pmid:15615148. [PubMed] [Google Scholar]
- 45.Meier CR, Derby LE, Jick SS, Vasilakis C, Jick H. Antibiotics and risk of subsequent first-time acute myocardial infarction. JAMA 1999;281:427-31. 10.1001/jama.281.5.427 pmid:9952202. [DOI] [PubMed] [Google Scholar]
- 46.Majeed A. Sources, uses, strengths and limitations of data collected in primary care in England.HSQ, 2004:21. [PubMed] [Google Scholar]
- 47.Esposito K, Chiodini P, Maiorino MI, et al. A nomogram to estimate the HbA1c response to different DPP-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of 98 trials with 24 163 patients. BMJ Open 2015;5:e005892 10.1136/bmjopen-2014-005892 pmid:25687897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix 1: Clinical codes used to identify severe kidney failure
Web appendix 2: Clinical codes used to identify blindness
Web appendix 3: Clinical codes used to identify amputation
Web appendix 4: Clinical codes used to identify hyperglycaemia
Web appendix 5: Clinical codes used to identify hypoglycaemia
Web appendix 6: Web tables