Abstract
Objectives
To establish the prevalence and determinants of the 10-year risk of a cardiovascular disease (CVD) event in 25 – 74-year-old black Africans in Cape Town, South Africa, using Framingham laboratory- and non-laboratory-based and National Health and Nutrition Examination Survey (NHANES) I non-laboratory-based equations.
Methods
CVD risk factors were determined by questionnaires, clinical measurements and biochemical analyses. Survey logistic regression analyses assessed the sociodemographic determinants of CVD risk ≥20%.
Results
There were 1 025 participants, 369 men and 656 women. Mean 10-year risk for a CVD event by Framingham laboratory- and non-laboratory-based and NHANES I non-laboratory-based equations for men was 9.0% (95% confidence interval 7.7 – 10.3), 11.1% (9.6 – 12.6) and 9.0% (7.6 – 10.3), and for women 5.4% (4.7 – 6.1), 6.8% (5.9 – 7.7) and 8.7% (7.6 – 9.8). Correlations between laboratory- and non-laboratory-based scores were high (0.915 – 0.963). The prevalence of laboratory-based CVD risk ≥20% was 13.0% in men and 6.1% in women. In the logistic model for men, ≤7 years of education (odds ratio 3.09; 95% CI 1.67 – 5.71) and being unemployed (3.44; 1.21 – 9.81) compared with employed were associated with laboratory-based high risk. In women, high risk was associated with ≤7 years of education (4.20; 1.96 – 9.01), living in formal v. informal housing (2.74; 1.24 – 6.06) and being poor (middle v. lowest tertile 0.29; 0.13 – 0.66). In the Framingham non-laboratory-based logistic models there were no changes in the direction or significance of the variables except for housing, which was no longer significant in women.
Conclusions
Comparability of laboratory- and non-laboratory-based CVD risk estimates illustrates the utility of the latter in resource-constrained settings.
Treatment of cardiovascular disease (CVD) risk factors has traditionally been based on the presence or absence of a single CVD risk factor, such as hypertension, hyperlipidaemia or diabetes, without considering the continuous relationship between blood pressure (BP), blood glucose, blood cholesterol and cardiovascular risk.[1] While this approach appears straightforward, it may result in committing some individuals with only a small cardiovascular risk to years of unnecessary treatment or, conversely, neglecting to treat individuals with an overall higher risk.[1,2] This is because a combination of several slightly elevated risk factors may result in a much higher total risk than a single, more strikingly raised factor.[3,4]
Moreover, single risk factor approaches are neither cost-effective nor affordable for poorer individuals and in developing regions with limited resources.[2] Given the enormous burden of CVD and the high costs of management, it is therefore essential to prioritise cost-effective approaches that target high-risk individuals. Adoption of the multifactorial CVD risk assessment approach to identify high-risk individuals who need interventions is widely advocated, with the initiation of therapy based on the predicted absolute cardiovascular risk of the individual.[1] The total CVD risk assessment approach is particularly recommended as a cost-effective strategy in developing regions with scarce resources. Effective CVD prevention therefore warrants a paradigm shift from the treatment of single risk factors in isolation, to the management of total CVD risk with an improvement in the profile of all risk factors that will lead to the development of CVD.
Several computerised methods for estimating total cardiovascular risk have been developed. The first, best known and most frequently used risk estimation system was developed by the Framingham Heart Study researchers in the USA.[3,4] This score has been validated across different populations, modified for use in several countries and recommended by numerous international guideline committees for CVD prevention.
In South Africa (SA), the South African Heart Association and the Lipid and Atherosclerosis Society of Southern Africa,[5] as well as the Southern African Hypertension Society in conjunction with the National Department of Health (DoH),[6] have adopted a cardiovascular risk stratification approach for the management of CVD risk factors. Given the lack of prospective data in the African setting and the absence of locally validated total CVD risk assessment tools, and in view of the fact that the Framingham risk scores have been validated in white and black populations and are transportable to other culturally diverse populations, this approach has been considered appropriate for local use.[5]
Most scoring systems require expensive laboratory tests; however, recent advances include the development of models that use simple office-based predictors easily obtained in primary care and do not require laboratory testing. The body mass index (BMI) has replaced total cholesterol (TC) and high-density lipoprotein (HDL) cholesterol in these models. Considering the increasing burden of CVD and the need for cost-effective use of limited resources in the developing world, the introduction of non-laboratory-based tools in this milieu is highly relevant. The use of non-laboratory-based total CVD risk assessment has the potential to improve worldwide utility of the risk scores and enhance targeted CVD prevention efforts,[3,4] particularly in resource-constrained settings such as SA, where widespread laboratory availability is problematic and not economically feasible.
This study aimed to determine the 10-year risk of developing a CVD event in the black population of Cape Town using the Framingham laboratory- and non-laboratory-based equations. Additionally, the non-laboratory-based score developed using the National Health and Nutrition Examination Survey (NHANES) I population data in the USA was used to calculate total CVD risk. This equation was found to be highly correlated with commonly used laboratory scores in SA populations.[7] The sociodemographic determinants associated with high-risk scores (≥20%) were also ascertained.
Methods
Study population and sampling procedure
A sample of 25 – 74-year-old men and women in the predominantly black residential areas of Langa, Guguletu, Crossroads, Nyanga and Khayelitsha in Cape Town participated in this cross-sectional study in 2008/09. The sampling procedure for the current study included a three-stage cluster sampling and has been described in detail elsewhere.[8] The prespecified age and gender quotas included disproportionate sampling across age groups to ensure at least 50 men and women in each gender category. Among other criteria, individuals on tuberculosis or antiretroviral therapy or who had received cancer treatment within the past year were excluded. Additionally, participants with a self-reported history of ischaemic heart disease (IHD) or stroke were excluded for this analysis.
Data collection
Data collected by administered questionnaires included sociodemographic characteristics, medical history and self-reported tobacco use (World Health Organization (WHO) STEP-wise surveillance questionnaire).[9] Assets defining wealth were recorded and included ownership of consumer items such as a radio, television, telephone, refrigerator, personal computer, washing machine, motor vehicle, bicycle and electricity, and the source of drinking water and toilet facilities.
Height and weight were measured using standardised techniques. Three BP measurements were taken at 2-minute intervals and the average of the second and third measurements was used in the analyses.
Blood samples for lipid and glucose estimations were drawn following an overnight fast of 10 hours. A standard oral glucose tolerance test was then administered and blood samples taken 120 minutes later.[10] Blood samples were kept on ice and transported to the laboratory within 6 hours to be centrifuged, aliquoted and stored at −80° until the assays were performed.
Definitions
Diabetes was diagnosed according to the 1998 WHO criteria,[10] the use of hypoglycaemic agents or the subject having being told they were diabetic by a doctor/nurse. Hypertension was defined as BP ≥140/90 mmHg or using antihypertensive agents. BMI was computed as kg/m2. Smoking status was defined as currently smoking daily or occasionally.
The absolute risk of having a CVD event, defined as IHD, stroke, transient ischaemic attack or heart failure, within 10 years was calculated using the Framingham[11] and NHANES I equations.[12] A score ≥20% was considered to indicate high risk and 10 – 19.99% to indicate moderate risk.
Statistical analysis
Data analyses were done using STATA 12. Descriptive statistics, including crude prevalence, were calculated using the weights based on the sample design and adjusted for the realised sample. A principal component analysis of the pooled data, based on the assets that defined wealth, was used to develop an asset index[13] and categories of relative wealth were created using tertiles. The first component of the principal component analysis placed the highest loading on having a toilet and a tap inside the house and explained 31.0% of the variation. The second component seemed to measure the ownership of luxury items such as a car and a personal computer and explained 12.2% of the variation. All variables, except bicycle and telephone, had approximately equal loadings.
The equations for calculating the 10-year risk of developing a CVD event are gender-specific and include the variables of age, diabetes status, smoking status, treated and untreated systolic BP, TC and HDL cholesterol levels for the laboratory-based equations. BMI replaced lipids in the non-laboratory-based equations.[11,12] Concordance correlation coefficients determined the correlations of the mean scores calculated by the laboratory-based equations with the two non-laboratory based equations. The McNemar test compared the frequencies of estimated high risk calculated using the laboratory-based equations with the two non-laboratory based equations.
The univariate analyses are presented as mean or percentage values with 95% confidence intervals (CIs). The unadjusted survey-based odds ratio (OR) and 95% CI for the associations of the sociodemographic variables with total CVD risk ≥20% were calculated. On account of the high level of correlation between the risk scores, only the associations for the Framingham laboratory-based CVD risk scores are presented in men and women. Survey multiple logistic regression analyses determined the independent associations of the sociodemographic variables with estimated total CVD risk ≥20%. The same sociodemographic variables were included in the unadjusted and adjusted analyses. p-values are presented for the adjusted analyses.
The University of Cape Town’s Research and Ethics Committee approved the study. All participants signed informed consent.
Results
The realised study sample comprised 1 099 participants, 1 025 of whom (369 men and 656 women) were included in this analysis. Seventy-four participants with a self-reported history of IHD or stroke were excluded. The overall response rate was 86%, the non-responders, i.e. the selected people who the study team were unsuccessful in contacting, totalling 187 (79 men).
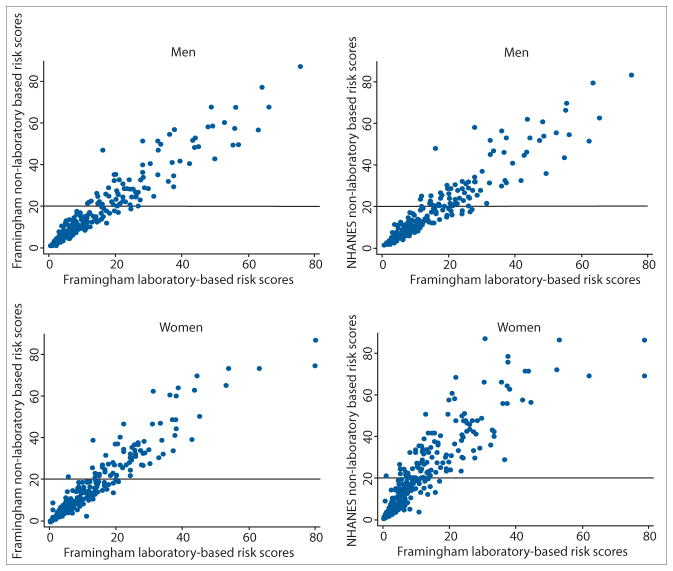
The overall mean 10-year risk for a CVD event estimated using the laboratory-based scores was low at 7.1% and significantly higher in men than in women (9.0% v. 5.4%; p<0.001) (Table 1). The non-laboratory-based mean scores were comparable to these laboratory-based estimates (Table 1 and Fig. 1). The concordance correlations between the Framingham laboratory and non-laboratory risk scores were very high at 0.936 for men and 0.924 for women. The concordance correlations between the Framingham laboratory-based and NHANES I risk scores were 0.950 and 0.786 in men and women, respectively.
Table 1.
Total CVD risk estimates using laboratory- and non-laboratory-based equations in men and women
| Men (N =369)
|
Women (N=656)
|
Total (N =1 025)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | |
| Framingham CVD risk using lipid profiles | |||||||||
| Mean | 9.0 | 7.7 – 10.3 | 5.4 | 4.7 – 6.1 | 7.1 | 6.4 – 7.9 | |||
| Moderate risk (10 – 19.99%) | 57 | 12.2 | 9.2 – 16.0 | 71 | 8.3 | 6.3 – 10.9 | 128 | 10.2 | 8.3 – 12.4 |
| High risk (≥20%)* | 67 | 13.0 | 9.9 – 17.0 | 55 | 6.1 | 4.6 – 8.0 | 122 | 9.4 | 7.6 – 11.6 |
| Framingham CVD risk using BMI | |||||||||
| Mean | 11.1 | 9.6 – 12.6 | 6.8 | 5.9 – 7.7 | 8.8 | 7.9 – 9.7 | |||
| Moderate risk (10 – 19.99%) | 71 | 16.2 | 12.6 – 20.7 | 83 | 10.3 | 8.2 – 12.8 | 154 | 13.1 | 11.0 – 15.5 |
| High risk (≥20%)* | 87 | 17.4 | 13.8 – 21.8 | 74 | 8.4 | 6.4 – 10.9 | 161 | 12.7 | 10.6 – 15.2 |
| CVD risk using BMI (NHANES I) | |||||||||
| Mean | 9.0 | 7.6 – 10.3 | 8.7 | 7.6 – 9.8 | 8.8 | 7.9 – 9.8 | |||
| Moderate risk (10 – 19.99%) | 64 | 14.5 | 10.9 – 19.0 | 84 | 10.1 | 8.0 – 12.7 | 148 | 12.2 | 10.1 – 14.6 |
| High risk (≥20%)† | 65 | 12.2 | 9.3 – 15.7 | 121 | 14.0 | 11.4 – 17.0 | 186 | 13.1 | 11.1 – 15.5 |
CVD = cardiovascular disease; CI = confidence interval; BMI = body mass index; NHANES = National Health and Nutrition Examination Survey.
Non-laboratory CVD risk scores used BMI instead of lipids. Men compared with women:
p<0.001;
p=0.390.
Fig. 1.
Scatterplots of the Framingham laboratory-based total CVD risk scores with the Framingham and NHANES non-laboratory-based CVD risk scores (CVD = cardiovascular disease; NHANES = National Health and Nutrition Examination Survey).
The prevalence of estimated high total CVD risk (≥20%) according to the laboratory-based scores was 13.0% in men and 6.1% in women (p<0.001) (Table 1). In men, the frequencies of estimated high risk were significantly different between the laboratory-based and the Framingham non-laboratory-based scores (p<0.001) but were similar between the former and the NHANES I non-laboratory scores (p=0.695). Taking the laboratory-based scores as the gold standard, the sensitivity was 97.0% and 79.1%, respectively, and the specificity 92.7% and 96.0%, respectively. In women, the frequencies of estimated high risk were significantly different for both comparisons (p<0.001). The sensitivity of 98.2% and 100%, respectively, was very high. The specificity for the comparison of estimated high risk between the two Framingham equations was 96.7% and that between the laboratory-based and the NHANES I non-laboratory scores 89.0%.
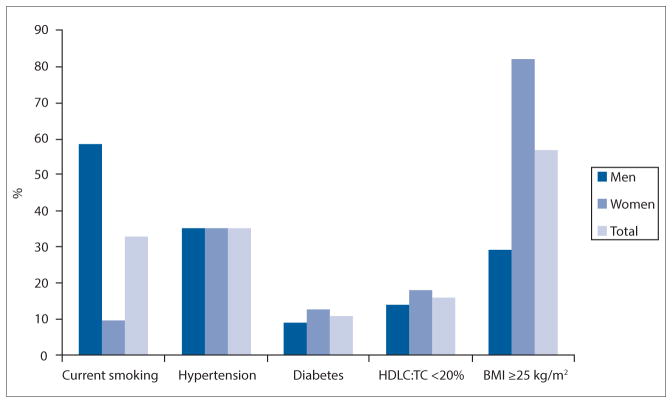
The prevalences of the individual CVD risk factors were similar in men and women for hypertension (p=0.985), diabetes (p=0.071) and HDL cholesterol:TC <20% (p=0.170) (Fig. 2). Men and women differed in smoking prevalence, which was significantly higher in men than in women (58.4% v. 9.8%; p<0.001). Overweight and obesity was significantly higher in women (82.0%) than in men (29.2%) (p<0.001).
Fig. 2.
Prevalence of cardiovascular disease risk score components presented according to gender. (Hypertension = blood pressure ≥140/90 mmHg or on hypertension treatment; diabetes = raised fasting glucose ≥7.0 mmol/l, 2-hour glucose ≥11.1 mmol/l or known diabetes; HDLC = high-density lipoprotein cholesterol; TC = total cholesterol; BMI = body mass index.)
As shown in Table 2, the adjusted odds for estimated high CVD risk by the Framingham laboratory-based scores were significantly associated with ≤7 years of education (p<0.001) and unemployment (p=0.021) in men. The significant adjusted odds for estimated high CVD risk by the laboratory-based scores in women were ≤7 years of education (p<0.001), better-quality housing (p=0.044) and being in the poorest wealth tertile (p=0.013). The work category with pensioners was associated with high CVD risk for both men and women because of the link with age, which is a function of the risk equation.
Table 2.
Prevalence and unadjusted and adjusted associations of sociodemographic factors with high total CVD risk (≥20% on laboratory-based estimates) in men and women
| Men
|
Women
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted
|
Adjusted
|
Unadjusted
|
Adjusted
|
|||||||||||
| N | % | OR | 95% CI | OR | 95% CI | p-value | N | % | OR | 95% CI | OR | 95% CI | p-value | |
| CVD risk ≥20% | 369 | 13.0 | 656 | 6.1 | ||||||||||
| Education (years) | ||||||||||||||
| >7 | 227 | 7.5 | 1.00 | 1.00 | 416 | 2.4 | 1.00 | 1.00 | ||||||
| ≤7 | 142 | 23.0 | 3.69 | 2.07 – 6.55 | 3.09 | 1.67 – 5.71 | <0.001 | 240 | 14.3 | 6.7 | 3.65 – 12.36 | 4.20 | 1.96 – 9.01 | <0.001 |
| % of life spent in the city | ||||||||||||||
| <50 | 127 | 9.6 | 1.00 | 1.00 | 301 | 4.6 | 1.00 | 1.00 | ||||||
| ≥50 | 242 | 14.9 | 1.64 | 0.83 – 3.24 | 1.29 | 0.58 – 2.86 | 0.529 | 355 | 7.5 | 1.69 | 0.90 – 3.17 | 1.64 | 0.74 – 3.62 | 0.220 |
| Work | <0.001 | <0.001 | ||||||||||||
| Employed | 88 | 3.2 | 1.00 | 1.00 | 133 | 2.5 | 1.00 | 1.00 | ||||||
| Unemployed | 227 | 9.7 | 3.22 | 1.21 – 8.56 | 3.44 | 1.21 – 9.81 | 0.021 | 397 | 2.9 | 1.18 | 0.38 – 3.68 | 1.12 | 0.36 – 3.54 | 0.844 |
| Other* | 54 | 47.7 | 27.4 | 9.33 – 80.14 | 22.10 | 7.22 – 67.66 | <0.001 | 126 | 25.1 | 13.20 | 4.74 – 36.73 | 9.66 | 3.22 – 29.00 | <0.001 |
| House | 0.136 | 0.044 | ||||||||||||
| Informal shack/other | 182 | 9.1 | 1.00 | 1.00 | 275 | 4.0 | 1.00 | 1.00 | ||||||
| Council/core house/hostel | 108 | 18.9 | 2.32 | 1.21 – 4.42 | 2.01 | 0.98 – 4.09 | 0.056 | 250 | 6.5 | 1.66 | 0.83 – 3.32 | 1.59 | 0.78 – 3.24 | 0.201 |
| Built formal unit (private) | 79 | 15.9 | 1.88 | 0.92 – 3.84 | 1.21 | 0.47 – 3.06 | 0.693 | 131 | 10.7 | 2.87 | 1.44 – 5.71 | 2.74 | 1.24 – 6.06 | 0.013 |
| Asset index | 0.270 | 0.013 | ||||||||||||
| 1st (poorest) | 137 | 9.3 | 1.00 | 1.00 | 210 | 7.8 | 1.00 | 1.00 | ||||||
| 2nd | 108 | 14.8 | 1.70 | 0.87 – 3.33 | 1.49 | 0.69 – 3.25 | 0.310 | 241 | 3.8 | 0.47 | 0.24 – 0.95 | 0.29 | 0.13 – 0.66 | 0.003 |
| 3rd (richest) | 124 | 15.4 | 1.78 | 0.89 – 3.59 | 2.10 | 0.85 – 5.14 | 0.105 | 205 | 7.1 | 0.91 | 0.47 – 1.74 | 0.44 | 0.18 – 1.05 | 0.065 |
CVD = cardiovascular disease; OR = odds ratio; CI – confidence interval.
Pensioners, homemakers, students and those receiving disability grants.
Bold font denotes significant findings with p<0.050
In the Framingham non-laboratory-based logistic models, compared with the laboratory- based analyses, there were no changes in the direction or significance of the variables except for housing, which was no longer significant in women (p=0.231). In the NHANES I logistic models, unlike the Framingham laboratory-based analyses, better housing (p=0.044) was associated with an estimated high CVD risk in men, while in women housing (p=0.133) and wealth (p=0.094) were no longer significant.
Discussion
The CRIBSA study, which is among the first to determine the 10-year risk of developing a CVD event in an urban African population in SA, found a modest prevalence of high risk (≥20%) in men and a low to moderate prevalence in women. This suggests that for cost-effective management, a relatively smaller proportion of participants require treatment compared with those with prevalent individual risk factors. For example, while only 13.0% of men and 6.1% of women would require interventions according to the Framingham laboratory-based high-risk estimates, hypertension prevalence was high at 35%. This is especially relevant in view of the potential burden that may be imposed on healthcare services if all individuals with only high BP were treated with medication. Basing treatment decisions on a total CVD risk assessment approach enables individuals who would benefit the most from treatment to be identified and also results in optimal and cost-effective management, as shown by Gaziano et al.[14] in an analysis of the SA hypertension guidelines.
The data confirm that the use of non-laboratory scores in clinical practice may be a feasible alternative in our setting. There was a high correlation between the mean laboratory-based and non-laboratory-based scores, and a high proportion of participants identified as at high risk on the laboratory-based estimates were also identified by the non-laboratory estimates. Gaziano et al.[7] also reported high correlations between laboratory- and non-laboratory-based scores in SA populations. Eliminating the need for costly laboratory measurements will enhance accessibility and utility of the total CVD risk assessment tool[4] and is particularly relevant in low-resource settings such as SA that face a high burden of multiple diseases.
Estimated high CVD risk by Framingham laboratory- and non-laboratory-based scores was found to be significantly related to unemployment in men, poverty in women and lower education levels in both. These are vulnerable groups that are likely to be less aware of the risks of CVD and may have difficulty in accessing healthcare because of financial constraints. There is therefore a pressing need to increase awareness of CVD among these individuals. Additionally, they should be targeted for screening and prevention of CVD and its risk factors, and their access to healthcare, if compromised, should be facilitated.
In view of the association of high CVD risk with poverty or lower socioeconomic status as defined by education level and asset index or wealth tertiles, the relation between better-quality housing and estimated high risk by the Framingham laboratory-based scores in women and the NHANES I scores in men was unexpected. That wealth, education and housing quality may not be well correlated possibly accounts for these findings, underscoring the fact that socioeconomic dynamics are complex and further research in this area is needed.
Despite the high prevalence of CVD risk factors in both men and women, the two-fold greater predicted high risk of Framingham-based CVD in men compared with women is a function of the equations, which allocate greater weight to men. In women, while high scores identify those at high risk, lower scores do not sufficiently ensure that individual women are at low risk. It is difficult for women aged <75 years, even with several markedly elevated risk factors, to be classified as at high risk.[15] Factors beyond the risk scores therefore need to be considered when determining therapy in women. These models do not incorporate all CVD risk factors, particularly physical activity and stress. They also do not consider the duration of exposure to a risk factor, or include relevant family history.[15] In addition, for CVD risk management in women, medical and lifestyle history, markers of preclinical disease and other conditions need to be considered when determining the intensity of preventive therapy. Recommendations also include a lower cut-point for defining high risk in women as ≥10% 10-year risk for all CVD compared with the traditionally ≥20% 10-year risk estimate utilised.[15] The prevalence of high risk in women would then be 14.4%, 18.7% and 24.1% by the laboratory-based and non-laboratory-based Framingham and NHANES 1 scores, respectively.
Nevertheless, while there are some recognised limitations, 10-year risk estimations represent an improvement over clinical judgement alone for appropriate risk stratification. Considering that a systematic approach to total CVD risk estimation seems to result in better risk factor control,[4] the weaknesses of the CVD risk prediction models should not defer the control of the major risk factors through this cost-effective approach.[2] Total CVD risk assessment is likely to remain an important therapeutic component for individuals and populations.
Study limitations
Limitations of the study included the low sample realisation in men (64%), which necessitated higher sampling weights and a loss of precision. The application in this study of risk estimation tools developed primarily in populations of European origin in wealthy regions with different incidences of CVD events to our population is another limitation.
Conclusion
Despite the high prevalence of many individual CVD risk factors, the estimates of a ≥20% 10-year risk for a CVD event in the black population of Cape Town were moderate in men and low to moderate in women, depending on the equation used. This implies that for optimal cost-effective management of CVD in this population, fewer individuals require medical intervention compared with those with raised single risk factors. The comparability of the Framingham laboratory- and non-laboratory-based CVD risk estimates illustrates the utility of the latter in this resource-limited setting and could further optimise cost-effective strategies. In view of the fact that in this study high CVD risk was associated with unemployed men, the poorest women and less-educated adults, CVD management needs to target these vulnerable groups. Future research will be required to determine whether the total CVD risk approach results in improved health outcomes and benefits for the healthcare system in SA.
Acknowledgments
We thank the participants, fieldworkers, Medical Research Council research nurse fieldworkers Debbie Jonathan and Theresa Gogela, fieldwork co-ordinator Erica April, study manager Serena van Haght, statisticians Nomonde Gwebushe, Rebecca Shanmugam and Ria Laubscher, and Dr Kirsty Bobrow. We thank the City of Cape Town for provision of the aerial maps.
Funding. This work was supported by an unrestricted grant from Servier Laboratories (South Africa), the Medical Research Council of South Africa, the Initiative for Cardiovascular Health Research in Developing Countries (IC Health) Foundation Council, and Brigham and Women’s Hospital, Harvard University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest. NL has received honoraria from Novartis for serving on the steering committee for the Navigator Trial and travel support from Novo Nordisk, Eli Lilly Laboratories and Sanofi Aventis. All other authors report no potential conflicts of interest, including specific financial interests, relevant to the subject of this manuscript.
References
- 1.Ker JA, Oosthuizen H, Rheeder P. Decision-making using absolute cardiovascular risk reduction and incremental cost-effectiveness ratios: A case study. Cardiovasc J Afr. 2008;19(2):97–101. [PMC free article] [PubMed] [Google Scholar]
- 2.Mendis S. The contribution of the Framingham Heart Study to the prevention of cardiovascular disease: A global perspective. Prog Cardiovasc Dis. 2010;53(1):10–14. doi: 10.1016/j.pcad.2010.01.001. http://dx.doi.org/10.1016/j.pcad.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Bitton A, Gaziano TA. The Framingham Heart Study’s impact on global risk assessment. Prog Cardiovasc Dis. 2010;53(1):68–78. doi: 10.1016/j.pcad.2010.04.001. http://dx.doi.org/10.1016/j.pcad.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooney MT, Dudina A, D’Agostino R, Graham IM. Cardiovascular risk-estimation systems in primary prevention: Do they differ? Do they make a difference? Can we see the future? Circulation. 2010;122(3):300–310. doi: 10.1161/CIRCULATIONAHA.109.852756. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.852756. [DOI] [PubMed] [Google Scholar]
- 5.Klug E. South African dyslipidaemia guideline consensus statement. S Afr Med J. 2012;102(3):178–187. doi: 10.7196/samj.5502. [DOI] [PubMed] [Google Scholar]
- 6.Seedat YK, Croasdale MA, Milne FJ, et al. South African hypertension guideline 2006. S Afr Med J. 2006;96(4):337–362. [PubMed] [Google Scholar]
- 7.Gaziano TA, Pandya A, Steyn K, et al. Comparative assessment of absolute cardiovascular disease risk characterization from non-laboratory-based risk assessment in South African populations. BMC Med. 2013;11(1):1–11. doi: 10.1186/1741-7015-11-170. http://dx.doi.org/10.1186/1741-7015-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peer N, Steyn K, Lombard C, Lambert EV, Vythilingum B, Levitt NS. Rising diabetes prevalence among urban-dwelling black South Africans. PloS One. 2012;7(9):e43336. doi: 10.1371/journal.pone.0043336. http://dx.doi.org/10.1371/journal.pone.0043336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonita R, deCourten M, Dwyer T, Jamrozik K, Winkelmann R. Surveillance of Risk Factors for Non-communicable Diseases: The WHO STEPwise approach. Geneva: World Health Organization; 2001. [Google Scholar]
- 10.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Geneva: World Health Organization; 1999. [Google Scholar]
- 11.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 12.Gaziano TA, Young CR, Fitzmaurice G, Atwood S, Gaziano JM. Laboratory-based versus non-laboratory-based method for assessment of cardiovascular disease risk: The NHANES I Follow-up Study cohort. Lancet. 2008;371(9616):923–931. doi: 10.1016/S0140-6736(08)60418-3. http://dx.doi.org/10.1016/S0140-6736(08)60418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data – or tears: An application to educational enrollments in states of India. Demography. 2001;38(1):115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 14.Gaziano TA, Steyn K, Cohen DJ, Weinstein MC, Opie LH. Cost-effectiveness analysis of hypertension guidelines in South Africa: Absolute risk versus blood pressure level. Circulation. 2005;112(23):3569–3576. doi: 10.1161/CIRCULATIONAHA.105.535922. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.535922. [DOI] [PubMed] [Google Scholar]
- 15.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women – 2011 update: A guideline from the American Heart Association. Circulation. 2011;123(11):1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. http://dx.doi.org/10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]