Abstract
Purpose
To study corneal reinnervation and sensation recovery in Herpes zoster Ophthalmicus (HZO).
Methods
Two patients with HZO were studied over time with serial corneal esthesiometry and laser in vivo confocal microscopy (IVCM). A Boston keratoprosthesis (B-KPro) type 1 was implanted and the explanted corneal tissues were examined by immunofluorescence histochemistry for βIII-tubulin to stain for corneal nerves.
Results
The initial central corneal IVCM performed in each patient, showed a complete lack of the subbasal nerve plexus, which was in accordance with severe loss of sensation (0 of 6 cm) measured by esthesiometry. When IVCM was repeated 2 years later prior to undergoing surgery, Case 1 showed a persistent lack of central subbasal nerves and sensation (0 of 6). In contrast, Case 2 showed regeneration of the central subbasal nerves (4,786 µm/mm2) with partial recovery of corneal sensation (2.5 of 6 cm). Immunostaining of the explanted corneal button in Case 1 showed no corneal nerves while Case 2, showed central and peripheral corneal nerves. Eight months after surgery, IVCM was again repeated in the donor tissue around the B-KPro in both patients, to study innervation of the corneal transplant. Case 1 showed no nerves, while Case 2 showed new nerves growing from the periphery into the corneal graft.
Conclusions
We demonstrate that regaining corneal innervation and function is possible in patients with HZO as shown by corneal sensation, IVCM, and ex-vivo immunostaining, indicating zoster neural damage is not always permanent and it may recover over an extended period of time.
Keywords: Herpes Zoster Ophthalmicus, Corneal nerves, Corneal sensation, Corneal reinnervation, In vivo confocal microscopy
INTRODUCTION
Herpes zoster Ophthalmicus (HZO) is a painful and debilitating condition caused by reactivation of the varicella-zoster virus (VZV) from latent chicken pox infection in the first division of the trigeminal sensory ganglia (V-1). Antibodies against VZV are found in nearly 100% of the population over the age of 50. Approximately 30% will develop herpes zoster in their lifetimes, resulting in one million new cases annually in the United States alone.1 The risk of Herpes zoster increases significantly, from a low of between 1.1 and 2.9 per 1000 person-years in people younger than 50 years to 4.6 and 6.9 per 1000 person-years, respectively, in the age groups 50 to 59 and 60 to 69. The age groups 70 to 79 and 80 years or older have the highest incidence, with 9.5 and 10.9 per 1000 persons-years respectively, due to age-related decrease in VZV-specific cell-mediated immunity.2 HZO accounts for 10% to 20% of all cases of herpes zoster.3
The relationship between zoster infection and destruction of neurons and nerves has been well established,4 with neurologic damage beginning even before the characteristic zoster appears.5,6 After exiting the Vth ganglion, the trigeminal ophthalmic branch divides into the nasociliary, frontal, and lacrimal branches. The nasociliary nerve, in turn, innervates the anterior and posterior ethmoidal sinuses, the skin of eyelids, conjunctiva, sclera, iris, choroid, tip of the nose, and, most importantly for the present study, the cornea.7 Herpes zoster keratitis may involve one or all of the complications of viral invasion, including infectious pseudodendrites, disciform immune keratitis, necrotic interstitial keratitis, endotheliitis and, most difficult of all, neurotrophic keratopathy with or without sterile trophic ulcers and possible melting and perforation.3,8 Because of the high rate of post-operative inflammation, as well as poor epithelial and stromal healing, corneal surgery for HZO is relatively rare. Further, there is no longitudinal data on the potential for nerve regeneration.
Corneal in vivo confocal microscopy (IVCM) is an emerging technique for the study of the cornea at the cellular level, providing images comparable to in vitro histochemical methods. IVCM has an enormous potential, being a non-invasive procedure that images the living cornea, to study both its physiological and pathological states, including local infectious, immune, and systemic disease alterations. IVCM enables the examination of corneal nerves, allowing the study of nerve alterations in different ocular diseases, after corneal surgery, and in systemic diseases.9 In a previous cross-sectional study in patients with HZO, we performed clinical slit-lamp examination, measured corneal sensation in the central cornea of both eyes, and correlated these findings with corneal nerve morphology and density using IVCM.10 Two of these patients, the subjects of this study, were followed with serial pre-operative IVCM and later underwent surgery, each receiving a Type 1 Boston keratoprosthesis. The corneal buttons, removed at the time of surgery, underwent specialized neuronal immunohistochemistry to evaluate the anatomic status of the corneal nerves. We observed nerve regeneration as shown by corneal sensation, IVCM, and ex-vivo immunostaining. Our data demonstrates that some patients with HZO neurokeratopathy can regain corneal innervation and function.
MATERIALS AND METHODS
This is a prospective, longitudinal study. The subjects were patients from the Cornea & Refractive Surgery Service of the Massachusetts Eye & Ear Infirmary, Boston, Massachusetts. The study was Health Insurance Portability and Accountability Act compliant, conducted in accordance with the requirements of the Declaration of Helsinki and was approved by the Institutional Review Board/Ethics Committee from the institution. Both participants signed a written informed consent. Detailed history was taken from the patients.
Corneal Sensation
We examined both patients by slit-lamp bio-microscopy and measured bilateral central and peripheral corneal sensation with a Cochet-Bonnet esthesiometer (Luneau Ophthalmologie, Chartres, France). This test mechanically stimulates corneal nerves, by touching the tip of a retractable 6 cm length monofilament nylon thread of 0.12 mm diameter against the anterior corneal surface, decreasing in steps of 1.0 cm if a positive response was not obtained. If a positive response was obtained, the thread was advanced by 0.5 cm. Measurements were repeated twice and averaged and the longest filament length resulting in a positive response was considered the corneal sensitivity threshold.
In Vivo Confocal Microscopy (IVCM)
Laser scanning in vivo confocal microscopy (Heidelberg Retina Tomograph 3 with the Rostock Cornea Module, Heidelberg Engineering GmbH, Heidelberg, Germany) of the central cornea was performed in the two cases during the course of their follow-ups using the technique previously described.11 Briefly, this microscope uses a 670-nm red wavelength diode laser source and it is equipped with a 63× objective immersion lens with a numerical aperture of 0.9 (Olympus, Tokyo, Japan). The laser confocal microscope provides images that represent a coronal section of the cornea of 400 × 400 µm, which is 160,000 µm2, at a selectable corneal depth and is separated from adjacent images by approximately 1 to 4 µm and lateral resolution of 1 µm/pixel. A disposable sterile polymethylmethacrylate cap filled with a layer of hydroxypropyl methylcellulose 2.5% (Gen teal gel, Novartis Ophthalmics), was mounted in front of the Rostock Cornea Module optics for each examination. One drop of topical anesthesia of 0.5% proparacaine hydrochloride (Alcaine, Alcon) was instilled in both eyes, followed by a drop of hydroxypropyl methylcellulose 2.5% (GenTeal gel, Novartis Ophthalmics) in both eyes. A total of 6 to 8 scans in sequence and volume scan mode were obtained in the center, mid-periphery and periphery of each cornea with special emphasis on the subbasal nerve plexus. An experienced masked observer (AC) selected 3 representative images for corneal subbasal nerve analysis from the central and peripheral cornea of each eye from at least 50 good quality images from each cornea that satisfied the criteria for selection. The best-focused and complete images of the basal epithelial layer and anterior to the Bowman layer, and having the whole image in the same layer and good contrast, were selected and analyzed using the NeuronJ plugin for ImageJ (NIH free software).
Immunohistochemistry
Both patients underwent keratoprosthesis type I surgery. The patient’s host corneas were excised during surgery. One half cornea was sent for routine pathology. The other half was immediately fixed in 4% paraformaldehyde post-excision in the operating room and stored for 24 hours at 4°C in preparation for immunohistochemistry for corneal nerves. At 24 hours the speciman underwent three washes with phosphate buffered saline (PBS), 15 minutes each. To block nonspecific staining and to enhance penetration, corneas were incubated in 2% bovine serum albumin (BSA) and 0.3% Triton X, diluted in PBS (PBS-BSA) for 3 hours at room temperature before addition of conjugated primary antibody. Corneas were then incubated with mouse monoclonal neuron-specific Northern Lights™ (NL) 557-conjugated anti-βIII-tubulin antibody (Clone Tuj, 1:150, R&D Systems, Minneapolis, MN) or isotype control for 72 hours at 4°C and gently shaken. After 3 thorough washes with PBS for 15 minutes each, corneas were whole-mounted and covered with mounting medium (Vector, Burlingame, CA) and analyzed using confocal laser scanning microscope (Olympus Fluoview BX50WI inverted microscope and 10×/0.4 numerical aperture (NA), 20×/0.5 NA or 60×/1.42 NA objectives). The corneas were analyzed both in the center and periphery.
Case 1
An 88-year-old Caucasian woman was referred to the Cornea Service at the Massachusetts Eye & Ear Infirmary with a 3-year history of recurring manifestations of HZO in her left eye (OS). The initial presentation was with marked vesicular dermatitis over the trigeminal ophthalmic nerve area and multiple small corneal epithelial non-dendritic defects. A one-week course of Valtrex (1 gram per mouth TID) resolved the acute disease. A few weeks later she developed ocular pain, iritis, and an increase of her intraocular pressure from 18mmHg to 40mmHg, presumably due to trabeculitis. This reactivation resolved completely within two weeks on treatment with 1% prednisolone acetate and Cosopt™. By year 2 after initial presentation, her vision OS had dropped from 20/70 to counting fingers (CF) due to corneal opacification.
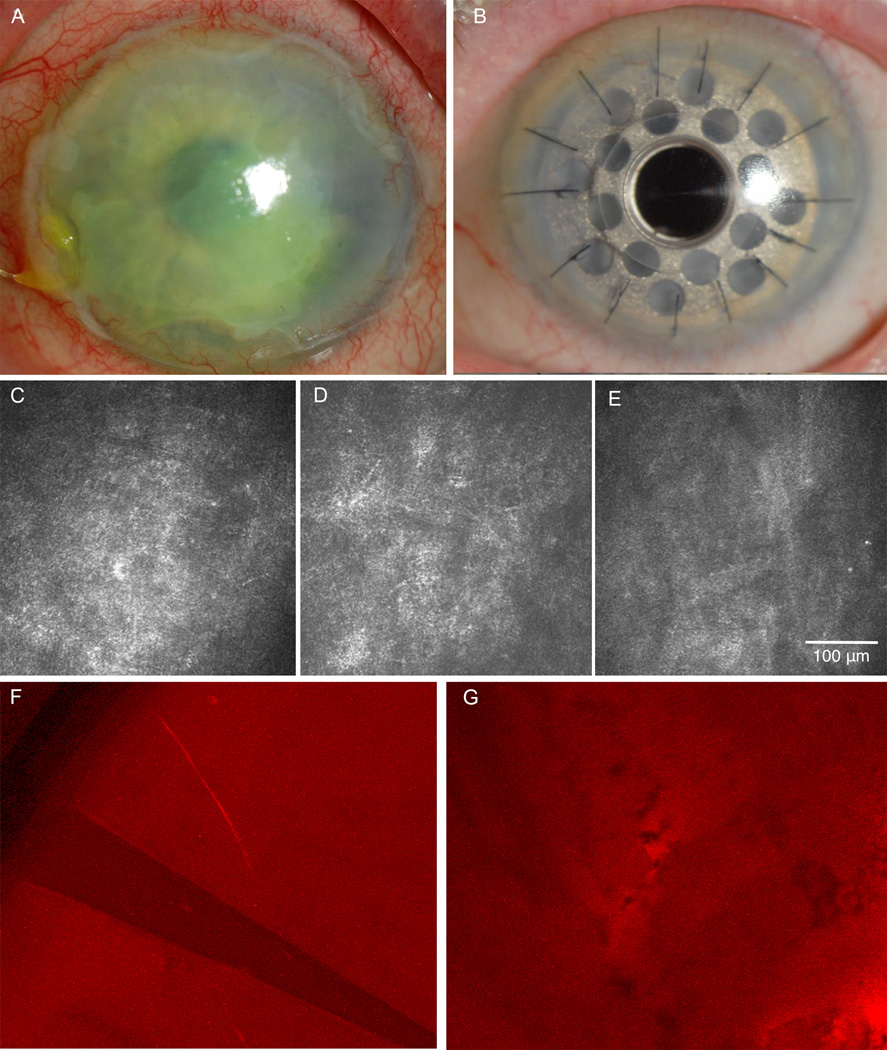
On our first exam in year 4 of her illness, visual acuity was 20/30 in her right eye (OD) and CF at 9 feet OS. IOP was 16mmHg OU. Corneal sensation was 5.5 of 6cm OD and 0 of 6cm OS with a large neurotrophic ulcer and about 10% corneal thinning. There was no iritis. A Kontur therapeutic lens was placed, doxycycline 100 mg/day initiated, and topical fluoroquinolone, mild steroid and glaucoma medications continued. The ulcer healed within a month over an irregular stroma (Fig. 1). She remained stable over the next 6 months, at which time laser IVCM was performed. The laser IVCM performed demonstrated a complete lack of the central subbasal corneal nerve plexus (Fig. 1), which was in accordance with the absolute loss of sensation.
Figure 1. Herpes Zoster Ophthalmicus Case 1.
A) Slit-lamp picture of neurotrophic keratopathy 2 years before surgery. B) Slit-lamp picture post implantation of Boston type 1 keratoprosthesis. C) In vivo confocal microscopy (IVCM) at the beginning of the follow up period. Lack of subbasal nerve plexus resulted in corneal sensation of zero. D) IVCM shortly before surgery showing the consistent absence of subbasal corneal nerve plexus. E) IVCM of the corneal tissue surrounding the Boston keratoprosthesis 8 months after surgery, showing continued absence of nerves. F) Immunohistochemistry of the peripheral corneal button shows absence of the subbasal nerve plexus and just one stromal nerve. G) Immunohistochemistry of the central corneal button reveals absence of the subbasal nerve plexus.
During her follow-up period, she suffered at various times a number of recurring forms of HZO. These included trigeminal pain of 4 on a scale of 10, dendritic keratitis (5 episodes), neurotrophic ulcers with 20% of central corneal thinning (3 episodes), disciform edema (3 episodes), iritis (3 episodes) and trabeculitis (2 episodes). These complications were resolved on various combinations of valacyclovir, topical steroids, fluoroquinolones, glaucoma medications and eventually a lateral tarsorrhaphy. At two years a large non-healing neurotrophic ulcer developed, that eventually resulted in a scarred irregular cornea. A mature cataract was present as well. She was thought to be a poor candidate for traditional corneal transplantation due to her anesthetic cornea and a type 1 Boston keratoprosthesis (Boston KPro) was recommended. The patient underwent another laser IVCM evaluation 1 week prior to surgery, revealing again a total lack of subbasal central corneal nerves and complete lack of central corneal sensation (0 of 6).
An uneventful KPro triple procedure (aphakic Boston KPro in a fresh corneal carrier graft, open-sky extracapsular cataract extraction, and placement of a plano posterior chamber intraocular lens) was performed.12 Immunohistochemistry analysis for corneal nerves with anti beta-III tubulin staining of the explanted corneal button demonstrated a lack of central corneal nerves, confirming the lack of innervation as shown by IVCM and the total absence of central corneal sensation. However, in the peripheral cornea, stromal nerves were seen in several independent areas (Fig. 1). Vision improved to 20/40 at 3 months after surgery with a stable KPro and normal IOP. At the five-month visit, she noted worsened vision to 20/70. A macular OCT showed mild cystoid macular edema (CME). Prednisolone acetate 1% was increased to 4 times daily and her vision improved to 20/60 at month 8. IVCM of the patient and donor corneal tissue surrounding the Boston keratoprosthesis (Fig. 1) at 8 months after surgery showed absence of corneal nerves (Fig. 1). Ten months after surgery, she again presented with worsened vision to 20/400 with a stable KPro and normal IOP. Repeat OCT showed worsened CME. Topical 0.1% nepafenac drops were added and a retina consult was obtained. The retinal specialist added a topical carbonic anhydrase inhibitor and oral acetazolamide. The patient was unable to tolerate the oral medication due to dizziness and it was discontinued. At the last follow-up 16 months after the KPro, the macular edema had improved by OCT but vision remained 20/400 as sequelae of the macular edema. The KPro remained stable and the intraocular pressure was well controlled.
Case 2
A 70-year-old Caucasian woman was referred with a 3-months history of HZO OS. She initially presented with a trigeminal vesicular rash and significant pain (7/10) despite treatment with Valtrex and Percocet. Treatment with gabapentin 1200 mg twice a day and 75 mg of nortriptyline once daily were initiated. Best-corrected visual acuity was 20/20 OD and 20/80 OS, intraocular pressures were 20mmHg OD and 35mmHg OS due to acute trabeculitis. The cornea OS demonstrated microcystic edema, stromal edema, and keratic precipitates (endotheliitis), but the anterior chamber was clear. Corneal sensation was 6 of 6 cm OD, and 0 of 6 cm OS. Treatment with 1% prednisolone acetate 4 times a day, glaucoma drops (timolol/dorzolamide), amitriptyline 50 mg daily improved her visual acuity to 20/30 within 2 days with resolving corneal edema. Her intraocular pressure improved to 15mmHg.
Over the ensuing 5 years she had several recurring forms of HZO including constant major trigeminal pain (7/10), which became bearable to “just mild itch” with continued gabapentin at 600 mg 4 times a day, nortriptyline 75 mg daily, and addition of lidocaine 5% skin gel 4–6/day. Over the 5 years gabapentin was slowly tapered to 600 mg twice a day with pain controlled. The total of other complications included dendritic keratitis (1 episode), neurotrophic ulcers with mild central corneal thinning (1 episode), disciform edema/endotheliitis (4 episodes), and trabeculitis (2 episodes). These complications were resolved on various combinations of topical steroids, fluoroquinolones, glaucoma medications, famciclovir, and lateral tarsorrhaphy. By year 2 vision OS had dropped from 20/30 to fluctuation between 20/100 and count fingers due to corneal scarring and irregular astigmatism. A dense cataract developed. Cataract surgery was performed, resulting in best post-operative vision of 20/60.
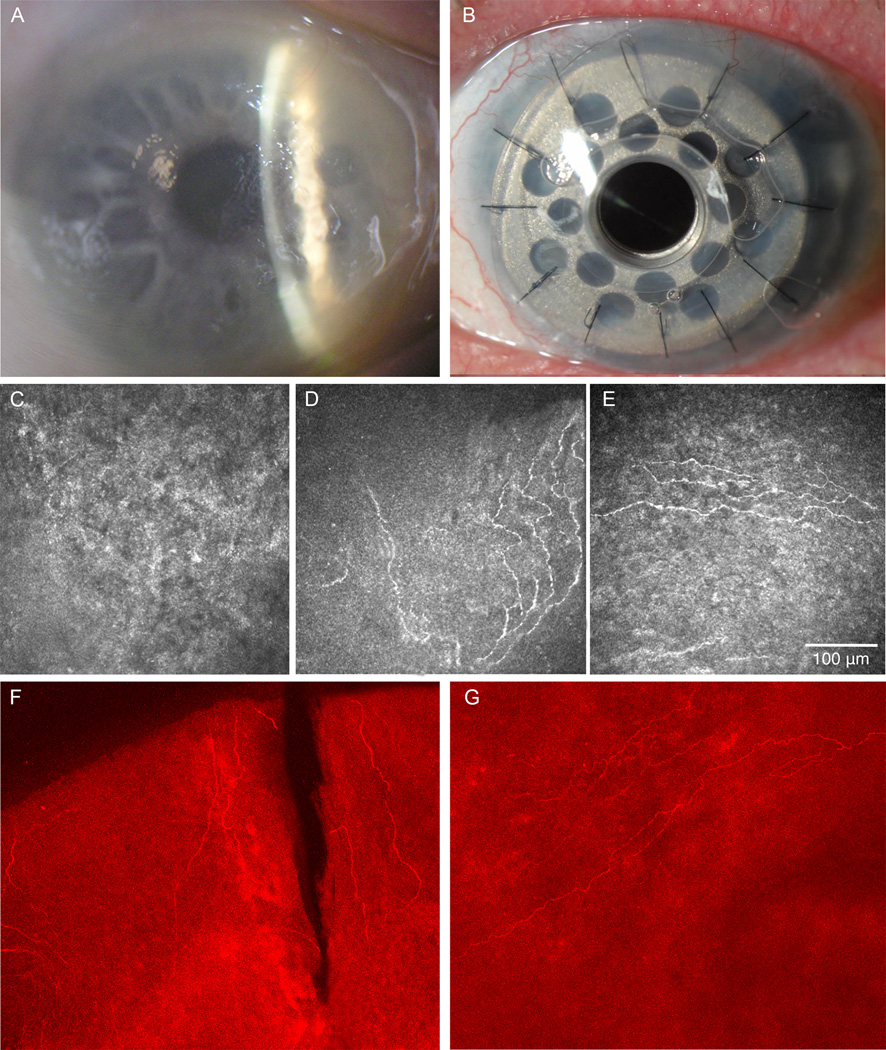
Laser IVCM was performed three times over the initial 4 years demonstrating complete absence of the central corneal subbasal nerve plexus, which correlated with total loss of central corneal sensation as measured (0/6 cm) (Fig. 2). However, at her 5th year of follow-up, esthesiometry showed an increase in central corneal sensation to 2.5/6 cm OS. At that time, laser IVCM was performed and demonstrated reappearance of central corneal subbasal nerve plexus, showing a density of 4,786 µm/mm2 (Fig. 2).
Figure 2. Herpes Zoster Ophthalmicus Case 2.
A) Slit-lamp picture of neurotrophic keratopathy 3 years before surgery. B) Slit-lamp picture post-implantation of Boston type 1 keratoprosthesis. C) In vivo confocal microscopy (IVCM) at the beginning of the follow up period showing absence of subbasal nerve plexus; corneal sensation was zero. D) IVCM before surgery after 3 years of follow-up showing the presence of some subbasal corneal nerves and some hyper-reflective dendritiform cells. Corneal sensation was 2.5 out of 6. E) IVCM of the corneal tissue surrounding the Boston keratoprosthesis 8 months after surgery, showing the presence of fine new nerves in the subbasal layer in the transplanted corneal button. F) Immunohistochemistry of the peripheral corneal button reveals presence of corneal nerves in the subbasal layer. G) Immunohistochemistry of the central corneal button with presence of corneal subbasal nerves.
Her vision ultimately declined to 20/200 due to worsening neurotrophic keratopathy (Fig. 2) with corneal melting, irregular surface and mild neovascularization and a Boston KPro was recommended. A type 1 Boston KPro was placed (Fig. 2). The immunohistochemistry study of the explanted corneal button for neuron-specific beta III tubulin demonstrated the presence of peripheral corneal nerves throughout the corneal periphery. In addition, in contrast to case 1, case 2 demonstrated central corneal nerves in two quadrants (Fig. 2). These findings confirmed the pre-operative IVCM findings that showed the presence of central corneal nerves and the partial recovery of corneal sensation (Fig. 2).
Postoperatively, at day one the uncorrected visual acuity was 20/50. Over the subsequent 16 months, vision has varied from 20/25 to 20/60, with a stable KPro. IVCM of the recipient and donor corneal tissue surrounding the Boston keratoprosthesis 8 months after surgery clearly showed the regeneration of fine subbasal corneal nerves in the periphery of the donor corneal button (Fig 2).
DISCUSSION
The cornea is the most densely innervated tissue in the human body, with approximately 19,000 to 44,000 axons in the subbasal plexus supplied by the terminal branches of the ophthalmic division of the trigeminal nerve as ciliary nerves.13 Once the nerves enter the anterior stroma from the periphery, they give off branches that penetrate the Bowman layer throughout the central and peripheral cornea. These branches then divide and run parallel to the superficial corneal surface, between the Bowman layer and the basal epithelium, forming the subbasal nerve plexus that supplies the overlying corneal epithelium.13,14 Corneal nerves not only protect the ocular surface through an elaborate mechanism of sensation and induction of the blink reflex, but they also release numerous neurotrophic factors, which regulate the epithelial integrity, proliferation and wound healing.13–15
A two-dimensional reconstruction map of the normal human subbasal corneal nerve plexus was produced by Patel et al16 using IVCM on one eye in a normal subject once a week for a duration of 6 weeks. The subbasal nerve pattern migrated centripetally from the corneal periphery toward an inferocentral whorl. Rate measurements from this study provided evidence that the living human subbasal corneal nerve plexus undergoes continuous centripetal movement of identifiable branch points of up to 26 micron per week. This created marked pattern changes in the plexus over a 6-week period and indicated that the corneal subbasal nerve plexus is a highly dynamic structure with good probability of repairing itself after damage.17
Corneal nerves of the adult human eye have been studied extensively both ex vivo and in vivo.7,14,18 However, ex vivo studies of human corneal nerves by light and electron microscopy may generate unreliable results, as human corneal nerves are known to degenerate within the first hours of death.14 It is to be noted that our specimen were fixed in 4% paraformaldehyde in the operating room immediately post-excision resulting in high potential for obviating the vast majority, if not all, neuronal degeneration. With a growing interest in non-invasive techniques to study live tissue physiology in different pathologic conditions, the development and application of in vivo confocal microscopy has enabled ophthalmologists to study the cornea at a quasi-histopathological level in patients real-time. This methodology has proven useful in the diagnosis and management of a variety of diseases via a rapid, noninvasive, precise technique with low interobserver variability.9
This study of severe cases of Herpes zoster ophthalmicus reveals the impact of this infection on corneal nerves through in vivo corneal esthesiometry, in-vivo confocal imaging, and ex-vivo immunohistological techniques. While our first case had persistent central corneal anesthesia throughout the 6-year observation period and no evidence of corneal nerve regrowth on sequential central IVCMs, the case did demonstrate the presence of isolated stromal peripheral corneal nerves. Our second case showed a surprising regeneration of corneal nerves and recovery of some corneal sensation (2.5/6), after 5 years of anesthetic cornea, as well as within months after corneal transplantation. Together, these findings demonstrate that corneal regeneration is possible in some cases of HZO, despite a prolonged period of corneal anesthesia, and that in vivo confocal microscopy is a powerful tool to monitor the corneal status. Further, the Boston keratoprosthesis post-operative anterior segment course was excellent in both cases.
Post-mortem studies reveal that nerve fiber bundles in the subbasal plexus of the human cornea form a regular dense meshwork with equal density over a large central and central-peripheral area. Because of their size, the majority of the fibers can be classified as C-fibers which are non-myelinated fibers in the cornea but fast conductors of sensory neuronal signals.14 Because of the relatively high incidence of surgical complications, particularly poor healing after penetrating keratoplasty due to neurokeratopathy, there has been a paucity of reports on the histopathology of zoster keratitis. Nine eyes and 4 corneal buttons surgically obtained from 13 patients with HZO were examined at different time points after clinical onset of HZO (range, 1 day-19 years; median, 36 months). Histopathologic changes associated with HZO included corneal stromal vascularization, granulomatous reaction to Descemet's membrane, perineuritis and perivasculitis of the long posterior ciliary nerves and arteries.19–21
In acute cases where eyes were taken at autopsy, Hedges and Albert reported nongranulomatous inflammation of the iris, ciliary body, choroid, and trabecular meshwork but normal corneal stroma.22 Studies on chronic HZO described a keratitis characterized by various combinations of epidermalization of epithelium, lipid keratopathy, intense stromal vascular scarring, and giant cell granulomatous reaction to Descemet's membrane.23,24 In none of these studies was there any report of neuronal status despite the fact that neurotrophic keratitis incidence may vary from 8.6 to 31.5% depending on age of HZO patients.25 It has been postulated that neurotrophic keratopathy was the result of corneal anesthesia secondary to VZV trigeminal ganglionitis and mesencephalic/pons nuclear damage, as well as aqueous tear deficiency due to loss of the nasolacrimal reflex, and decreased blink reflex.26–30
Corneal anesthesia may be evident at the time of onset of HZO or may develop over weeks to years. IVCM studies showing damage to or complete loss of the corneal subbasal nerve plexus in both HSV and HZO demonstrate physically in the living patient why the corneas become anesthetic and unable to heal.10,13,31 About 25 percent of all HZO patients will develop clinical signs of neurotrophic keratitis due to permanent corneal anesthesia.32 The only previous study describing pathological corneal nerve alterations in HZO by IVCM is a study performed by our group in 27 patients with unilateral HZO demonstrating a profound and significant bilateral loss of the corneal nerve plexus as compared to controls. Loss of corneal sensation strongly correlated with subbasal nerve plexus alterations as shown by IVCM.10 In addition, there is single case report by Patel et al.33 who found a marked decrease in the subbasal nerve plexus in a patient with HZO. This demonstrates that patients with HZO may have several levels of nerve damage both on a morphological level as well as on a functional level. We also found that there is an increased immune response correlated to decreased innervation (Cavalcanti B, et al. Inves Ophthalmol Vis Sci 2013;277:ARVO E-Abstract 2159) This, together with the fact that nerves can regenerate, suggest that improved control of immune responses in HZO may benefit patients and potentially prevent loss of corneal nerves.
To our knowledge, this is the first report, demonstrating spontaneous corneal nerve regeneration in a case with severe neurotrophic zoster keratopathy. Previously, Petersen et al.34 assessed cutaneous innervation following herpes zoster and found no recovery at 6 months. The authors concluded that a much longer period of observation is needed to determine if zoster-affected skin is ever re-innervated. In the present study one of our two patients did recover partial corneal innervation and sensation preoperatively after 5.3 years of follow-up. This nerve regrowth was confirmed by IVCM, corneal esthesiometry and ex-vivo immunohistochemistry. Furthermore, we observed postoperative re-innervation of the corneal graft carrier of the Boston KPro by IVCM. Future studies in animal models may be able to study whether the re-innervation after HZO corresponds to recovery of damaged nerves or new nerves that grow into the cornea. In the case of postoperative re-innervation of the corneal graft, we believe these are new nerves growing from the host into the periphery of the graft.
A limitation of in vivo confocal microscopy studies is that the area and volume of the cornea sampled is small. Therefore, the findings cannot necessarily be extrapolated to the entire cornea. To try to overcome this limitation, we image the cornea in both sequence and volume scan mode throughout the center and periphery of the cornea. In addition, considering the “in vivo” nature of this imaging technique, histological markers cannot be used to identify the structures of interest. Hence, we performed ex-vivo immunohistochemistry of the extracted corneal buttons to study the presence of nerves and confirm the in vivo confocal findings.
In addition to the extended observation time, a variety of therapies are coming to the fore as enhancers of neuronal regeneration and recovery of sensation. Studies on the effect of omega-3 fatty acids or other lipid mediators on increased corneal nerve density and epithelial proliferation in dry eye strongly suggests that increased dietary intake of fish oils would have a beneficial effect on neurokeratopathy.35 Stimulating the injured cornea with pigment epithelial derived factor in the presence of docosahexaenoic acid results in synthesis of neuroprotectin D1, a derivative of lipooxygenase activity leading to regeneration of corneal nerves.36 Autologous serum tears also harbors neurotrophic growth factors, such as substance P and Nerve growth factor (NGF).37 Recent reports have shown improvement of visual acuity and increase in corneal sensation in a variety of patients with neurotrophic keratopathy.37,38 Particularly, Rao et al.38 using IVCM and Cochet-Bonnet sensation testing revealed statistically significant improvement in corneal sensation and innervation, visual acuity, and staining. NGF eye drops are also of great interest and potential efficacy. Bonini et al.,39 in an uncontrolled study reported the use of murine NGF to heal the neurotrophic keratopathy in 45 corneas of 47 patients with return of corneal sensation and/or healing of all ulcers. There were only 3 recurrences requiring one retreatment period (trigeminal nerve resection). Recently, the American Food and Drug Administration (FDA) has granted orphan drug status to a recombinant human NGF- based treatment for neurotrophic keratitis (Dompe Center for Research & Development of l’Aquila, Italy).
The study presented herein shows that corneal re-innervation is possible in certain cases of zoster neurotrophic keratopathy. Further studies are needed to explain the numerous factors essential to the regrowth of corneal nerves and the recovery of corneal sensation not only in herpetic disease, but also in the wide variety of infectious, immune, and systemic diseases that affect the integrity of normal corneal function. Once we identify and understand these factors we may move forward in their synthesis and clinical application to heal both corneal and numerous other forms of neurotrophic disease.
Acknowledgments
Financial Support: Johnstone Fund (DP-L), NIH K08-EY020575 (PH), NIH K12-EY016335 (PH), Stevens Fund (DP-L), Falk Medical Research Foundation (PH)
Footnotes
Conflict of Interest: The authors have no financial/conflicting interests to disclose
REFERENCES
- 1.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 2.Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–753. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(Suppl):3–12. doi: 10.1016/j.ophtha.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Oaklander AL, Romans K, Horasek S, et al. Unilateral postherpetic neuralgia is associated with bilateral sensory neuron damage. Ann Neurol. 1998;44:789–795. doi: 10.1002/ana.410440513. [DOI] [PubMed] [Google Scholar]
- 5.Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, et al. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635–645. doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- 6.Arvin A. Aging, immunity, and the varicella-zoster virus. N Engl J Med. 2005;352:2266–2267. doi: 10.1056/NEJMp058091. [DOI] [PubMed] [Google Scholar]
- 7.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90:478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Pavan-Langston D, Yamamoto S, Dunkel EC. Delayed herpes zoster pseudodendrites. Polymerase chain reaction detection of viral DNA and a role for antiviral therapy. Arch Ophthalmol. 1995;113:1381–1385. doi: 10.1001/archopht.1995.01100110041023. [DOI] [PubMed] [Google Scholar]
- 9.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–177. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamrah P, Cruzat A, Dastjerdi MH, et al. Unilateral Herpes Zoster Ophthalmicus Results in Bilateral Corneal Nerve Alteration: An In Vivo Confocal Microscopy Study. Ophthalmology. 2013;120:40–47. doi: 10.1016/j.ophtha.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52:5136–5143. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todani A, Gupta P, Colby K. Type I Boston keratoprosthesis with cataract extraction and intraocular lens placement for visual rehabilitation of herpes zoster ophthalmicus: the "KPro Triple". Br J Ophthalmol. 2009;93:119. doi: 10.1136/bjo.2008.146415. [DOI] [PubMed] [Google Scholar]
- 13.Müller LJ, Marfurt CF, Kruse F, Tervo TMT. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 14.Müller LJ, Vrensen GF, Pels L, et al. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997;38:985–994. [PubMed] [Google Scholar]
- 15.Guthoff RF, Wienss H, Hahnel C, Wree A. Epithelial innervation of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2005;24:608–613. doi: 10.1097/01.ico.0000154384.05614.8f. [DOI] [PubMed] [Google Scholar]
- 16.Patel DV, McGhee CNJ. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005;46:4485–4488. doi: 10.1167/iovs.05-0794. [DOI] [PubMed] [Google Scholar]
- 17.Patel DV, McGhee CNJ. In vivo laser scanning confocal microscopy confirms that the human corneal sub-basal nerve plexus is a highly dynamic structure. Invest Ophthalmol Vis Sci. 2008;49:3409–3412. doi: 10.1167/iovs.08-1951. [DOI] [PubMed] [Google Scholar]
- 18.Al-Aqaba MA, Alomar T, Miri A, et al. Ex vivo confocal microscopy of human corneal nerves. Br J Ophthalmol. 2010;94:1251–1257. doi: 10.1136/bjo.2009.178244. [DOI] [PubMed] [Google Scholar]
- 19.Wenkel H, Rummelt C, Rummelt V, et al. Detection of varicella zoster virus DNA and viral antigen in human cornea after herpes zoster ophthalmicus. Cornea. 1993;12:131–137. doi: 10.1097/00003226-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Wenkel H, Rummelt V, Fleckenstein B, Naumann GO. Detection of varicella zoster virus DNA and viral antigen in human eyes after herpes zoster ophthalmicus. Ophthalmology. 1998;105:1323–1330. doi: 10.1016/S0161-6420(98)97042-7. [DOI] [PubMed] [Google Scholar]
- 21.Maudgal PC, Missotten L, De Clercq E, Descamps J. Varicella-zoster virus in the human corneal endothelium: a case report. Bulletin de la Societe Belge d Ophtalmologie. 1980;190:71–86. [PubMed] [Google Scholar]
- 22.Hedges TR, Albert DM. The progression of the ocular abnormalities of herpes zoster. Histopathologic observations of nine cases. Ophthalmology. 1982;89:165–177. doi: 10.1016/s0161-6420(82)34842-3. [DOI] [PubMed] [Google Scholar]
- 23.Naumann G, Gass JD, Font RL. Histopathology of herpes zoster ophthalmicus. Am J Ophthalmol. 1968;65:533–541. doi: 10.1016/0002-9394(68)93869-5. [DOI] [PubMed] [Google Scholar]
- 24.Zaal MJ, Maudgal PC, Rietveld E, Suir EP. Chronic ocular zoster. Curr Eye Res. 1991;10(Suppl):125–130. doi: 10.3109/02713689109020368. [DOI] [PubMed] [Google Scholar]
- 25.Ghaznawi N, Virdi A, Dayan A, et al. Herpes zoster ophthalmicus: comparison of disease in patients 60 years and older versus younger than 60 years. Ophthalmology. 2011;118:2242–2250. doi: 10.1016/j.ophtha.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Reske-Nielsen E, Oster S, Pedersen B. Herpes zoster ophthalmicus and the mesencephalic nucleus. A neuropathological study. Acta Pathol Microbiol Immunol Scand A. 1986;94:263–269. doi: 10.1111/j.1699-0463.1986.tb02993.x. [DOI] [PubMed] [Google Scholar]
- 27.Mahalingam R, Wellish M, Wolf W, et al. Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N Engl J Med. 1990;323:627–631. doi: 10.1056/NEJM199009063231002. [DOI] [PubMed] [Google Scholar]
- 28.Watson CP, Deck JH, Morshead C, et al. Post-herpetic neuralgia: further post-mortem studies of cases with and without pain. Pain. 1991;44:105–117. doi: 10.1016/0304-3959(91)90124-G. [DOI] [PubMed] [Google Scholar]
- 29.Heigle TJ, Pflugfelder SC. Aqueous tear production in patients with neurotrophic keratitis. Cornea. 1996;15:135–138. doi: 10.1097/00003226-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Head H, Campbell AW, Kennedy PG. The pathology of Herpes Zoster and its bearing on sensory localisation. Rev Med Virol. 1997;7:131–143. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liesegang TJ. Corneal complications from herpes zoster ophthalmicus. Ophthalmology. 1985;92:316–324. doi: 10.1016/s0161-6420(85)34034-4. [DOI] [PubMed] [Google Scholar]
- 33.Patel DV, McGhee CN. Laser scanning in vivo confocal microscopy demonstrating significant alteration of human corneal nerves following herpes zoster ophthalmicus. Arch Neurol. 2010;67:640–641. doi: 10.1001/archneurol.2010.62. [DOI] [PubMed] [Google Scholar]
- 34.Petersen KL, Rice FL, Farhadi M, et al. Natural history of cutaneous innervation following herpes zoster. Pain. 2010;150:75–82. doi: 10.1016/j.pain.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 35.He J, Bazan HE. Omega-3 fatty acids in dry eye and corneal nerve regeneration after refractive surgery. Prostaglandins Leukot Essent Fatty Acids. 2010;82:319–325. doi: 10.1016/j.plefa.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenchegowda S, Bazan HEP. Significance of lipid mediators in corneal injury and repair. J Lipid Res. 2010;51:879–891. doi: 10.1194/jlr.R001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. 2004;111:1115–1120. doi: 10.1016/j.ophtha.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;94:584–591. doi: 10.1136/bjo.2009.164780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonini S, Lambiase A, Rama P, et al. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107:1347–1351. doi: 10.1016/s0161-6420(00)00163-9. – discussion 1351–2. [DOI] [PubMed] [Google Scholar]