Introduction
Whether the menopause-related reduction in estradiol (E2) levels1 contributes to the higher risk of cardiovascular disease (CVD) in women after menopause 2,3 has been a matter of prolonged debate. Observational studies have reported that hormone therapy (HT) is associated with a reduction in coronary heart disease (CHD) risk and total mortality,4-8 which supports a cardio-protective role for E2. On the other hand, clinical trials of HT demonstrated no such protective effect among postmenopausal women.9,10
Previous studies evaluating the role of endogenous E2 or HT on CVD risk have assumed that the pattern of endogenous E2 decline over the menopause transition is identical for all women.4-8,11-18 However, recent studies suggest that the pattern of E2 decline around the final menstrual period (FMP) varies among women, and women can be classified into distinct groups based upon these patterns.19,20 Findings from the Study of Women's Health Across the Nation (SWAN) demonstrated that not all women experience a linear decline in E2 around their FMP. 20 E2 may rise 4-to-5 years before the FMP and then decline either immediately before or later after the FMP. Similarly, E2 levels post-FMP may not be the same among all postmenopausal women, and may increase slightly in some but decrease in others. In SWAN, 29% of postmenopausal women have circulating E2 levels that are consistently 10-15 pg/mL higher than other postmenopausal women.20
Similar to the E2 decline, an increase in follicle stimulating hormone (FSH) accompanies the menopause transition1 and may also contribute to the higher risk of CVD after menopause.11,12,17 The magnitude of FSH increase over the transition may not be the same in all postmenopausal women.20 In SWAN, almost 41% women have a postmenopausal FSH level that is 30-80 mIU/mL higher than that of other women.20
The primary purpose of this paper is to evaluate whether different patterns of E2 and FSH around the time of the FMP are related to levels of subclinical measures of atherosclerosis 21-23 after menopause, independent of CVD risk factors. Given the known higher risk of CVD after menopause, 2,3 a time period of significant decline in E2 and rise in FSH, 1 we hypothesize that women with higher E2 levels before FMP but lower thereafter as well as those with a lower rise of FSH over the menopause transition will have the most favorable profile for subclinical measures of atherosclerosis.
Methods
Study population
SWAN is a large longitudinal multi-ethnic cohort study of women transitioning through the menopause. SWAN was conducted at seven sites and recruited White women and one additional racial/ethnic group at each site (Black in Pittsburgh, Boston, the Detroit area, and Chicago; Japanese in Los Angeles; Hispanic in New Jersey; Chinese in the Oakland area of California).24 Baseline (1996–1997) eligibility criteria included: aged 42-52 years, having a uterus and ≥ one ovary, not using oral-contraceptives or HT, and having ≥ one menstrual cycle in the last 3 months before baseline. Study protocols were approved by the institutional review board at each site and participants provided written informed consent.
Of the 3302 women enrolled in SWAN, 1552 completed the carotid ultrasound assessments (all sites except Los Angeles). Women were excluded from the trajectory analyses due to missing FMP (due to surgery or HT use) or having less than 3 visits with hormone data (n=633). For the analyses linking hormone trajectories with subclinical atherosclerosis, women with a history of stroke or myocardial infarction were excluded (n=63). The final analyses included 856 women. Women excluded were more likely to be Black or White (p<0.001) and to have a worse CVD risk profile, but similar subclinical measures of atherosclerosis.
Subclinical measures of atherosclerosis
Carotid images were obtained at study sites using a Terason t3000 Ultrasound System (Teratech Corp., Burlington, MA) equipped with 5-12 MHz linear array transducer, and were read centrally using the semi-automated AMS reading software at the Ultrasound Research Laboratory (University of Pittsburgh). 25 IMT measures were obtained by tracing electronically the lumen–intima interface and the media–adventitia interface across a 1-cm segment of the near and far walls of the right and left distal common carotid arteries (CCAs), 1 cm proximal to the carotid bulb. One measurement was generated for each pixel over the area, for a total of approximately 140 measures for each segment. The average and maximal values for these measures were recorded for all 4 segments, with the mean of the average and maximal readings of all 4 segments used in analyses.
Adventitial Diameter (AD) was measured directly as the distance from the adventitial–medial interface on the near wall to the medial–adventitial interface on the far wall at end-diastole across the same CCA segments used for IMT measurement. Reproducibility of IMT and AD was excellent with an intraclass correlation coefficient between sonographers ≥ 0.77, and between readers ≥ 0.89.
The presence of any carotid plaque (cPlaque) was evaluated in the left and right carotid artery in each of 5 segments (distal and proximal CCA, carotid bulb, and proximal internal and external carotid arteries). 26 cPlaque was defined as a distinct area protruding into the vessel lumen that was at least 50% thicker than the adjacent IMT (intraclass correlation coefficient ≥ 0.86). 27
E2 and FSH Trajectories
Circulating hormones were measured at baseline and at 12 follow-up visits. Women provided fasting blood samples during the early follicular phase (days 2-5 of the menstrual cycle). Fasting samples were obtained within 90 days of the recruitment anniversary date if a timed sample could not be obtained. Accordingly, cycle day of blood draw was reported as either days 2-5 or outside that period. E2 and FSH assays were measured by a central laboratory duplicate and singlicate, respectively. E2 levels were measured with a modified, off-line ACS-180 (E2-6) chemiluminometric immunoassay (Bayer Diagnostics Corporation, Norwood, MA).28 The lower limit of detection (LLD) was between 1 and 7 pg/mL. The inter- and intra-assay coefficients of variation averaged were 10.6% and 6.4%, respectively. FSH levels were measured with a two-site chemiluminometric immunoassay. The LLD was between 0.4 and 1.0 mIU/mL. The inter- and intra-assay coefficients of variation were 11.4% and 3.8%, respectively. The previously described trajectories of E2 and FSH over 8.3 years before/after FMP 20 were re-identified among the current SWAN subsample over 9.6 years before/after FMP (see data analyses section).
FMP determination
FMP date was determined from bleeding data that were collected every annual follow-up visit. At each clinical visit, participants were asked about months of amenorrhea and date of the last menstrual period. FMP date indicated 12 months of amenorrhea since the last menstrual period for no other cause (e.g., hysterectomy, bilateral oophorectomy).
CVD risk factors and other potential covariates
Concurrent with subclinical measures of atherosclerosis, CVD risk factors were drawn from the 12th clinic visit and included height and weight to calculate BMI (kg/m2), systolic blood pressure, and self-reported physical activity level assessed via a modified Baecke scores of habitual physical activity. 29 Fasting lipids, glucose, and insulin assays were measured on a Siemens ADVIA 2400 automated chemistry analyzer utilizing Siemens ADVIA chemistry system reagents at the University of Michigan Pathology Laboratory. Lipids were determined from EDTA-treated plasma,30,31 except for low density lipoprotein cholesterol (LDL-C), which was calculated using Friedewald equation.32 Serum insulin was measured using radioimmunoassay. Glucose was measured using a two-step enzymatic reaction that utilizes hexokinase and glucose-6 phosphate dehydrogenase enzymes. The HOMA index was calculated as (fasting insulin (mU/L) * fasting glucose (mmol/L) / 22.5).33 Women were classified as diabetic by visit 12 based on longitudinal fasting glucose data and use of insulin or anti-diabetic agents. Ever smoking was defined as yes if participants self-reported smoking at any study visit.
Education level was assessed at baseline. Ever use of medications (Yes: antihypertensive, antidiabetics, lipid-lowering, or anticoagulants) and of HT over the study period were considered as two separate covariates.
Data Analyses
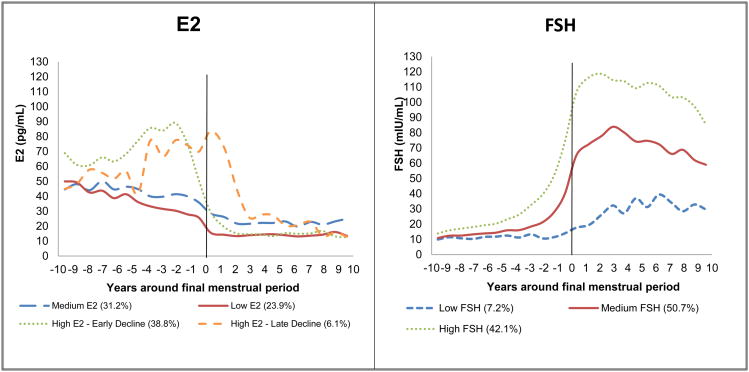
Trajectories of hormones were first created, and then linked to subclinical measures of atherosclerosis available at the 12th annual SWAN visit. Group based growth trajectory modeling 34 was utilized to re-identify the previously described trajectories of E2 and FSH, 20 among the current subset of SWAN participants, Figure 1. Visits at which women were on HT were dropped (less than 1% of the eligible visits for trajectory analyses) from trajectory analyses. Similar to our previous publication,20 the time scale was anchored to the FMP, however this analysis includes a longer maximum time before and after the FMP of 9.6 years. Hormone values were log transformed before estimating the trajectories since their distributions were not normal. Each participant was assigned to one of the identified E2 or FSH trajectories based on highest posterior (predicted) probability.
Figure 1. Trajectories of E2 and FSH over the menopausal transition*.
*Trajectories were adjusted for study site, time-varying age and time-varying cycle date of blood draw
Associations between E2 and FSH trajectory groups and each subclinical measure of atherosclerosis were estimated in linear or logistic regression models as appropriate. Models were first adjusted for age, race/ethnicity, education, and site, with additional adjustment for covariates associated with subclinical measures of atherosclerosis at p<0.05. Analyses were performed with SAS v9.2 (SAS Institute, Cary, NC).
All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Results
At visit 12, women were, on average, 59 years old and approximately 8 years post-FMP. Participants were 46.7% White, 30.7% Black, 16.5% Chinese and 6.1% Hispanic.
The four distinct trajectories of E2 in this analytic sample were: 1) low E2 before and after FMP (Low E2: 23.9%); 2) medium E2 before FMP but stable and high E2 after FMP (Medium E2: 31.2%); 3) high rise of E2 before FMP followed by early decline at FMP (High E2- early decline: 38.8%); 4) high rise of E2 before FMP followed by late decline after FMP (High E2-late decline: 6.1%), Figure 1. The three distinct trajectories of FSH rise were: 1) low rise before/after FMP (Low FSH: 7.2%), medium rise before/after FMP (Medium FSH: 50.7%); 3) high rise before/after FMP (High FSH: 42.1%), Figure 1.
CVD risk factors and subclinical atherosclerosis measures by hormone trajectory groups (Tables 1 and 2)
Table 1. Characteristics of study population at visit 12 by E2 groups.
| Characteristics | Low E2 N=207 | Medium E2 N=256 | High E2 - Early Decline N=350 | High E2 - Late Decline N=43 | P value |
|---|---|---|---|---|---|
| Age, years, Mean(SD) | 59.98 (2.68) | 59.14 (2.65) | 59.59 (2.64) | 58.35 (2.38) | 0.0002 |
| Race, n (%) | <0.001 | ||||
| Black | 26 (12.56) | 133 (51.95) | 94 (26.86) | 10 (23.26) | |
| White | 76 (36.71) | 116 (45.31) | 187 (53.43) | 21 (48.84) | |
| Chinese | 81 (39.13) | 3 (1.17) | 52 (14.86) | 5 (11.63) | |
| Hispanic | 24 (11.59) | 4 (1.56) | 17 (4.86) | 7 (16.28) | |
| Education, n (%) | 0.009 | ||||
| ≤ High school/some college | 99 (48.29) | 159 (62.6) | 176 (50.87) | 23 (54.76) | |
| College degree/post college | 106 (51.71) | 95 (37.4) | 170 (49.13) | 19 (45.24) | |
| BMI, kg/m2, Mean (SD) | 27.39 (6.33) | 33.67 (7.70) | 27.95 (6.47) | 31.04 (8.26) | <0.001 |
| SBP, mmHg, Mean (SD) | 118.87 (18.22) | 125.00 (16.83) | 120.57 (17.07) | 123.74 (21.93) | 0.001 |
| DBP, mmHg, Mean (SD) | 71.72 (10.85) | 75.69 (9.47) | 74.04 (10.02) | 74.06 (12.26) | 0.001 |
| HDL-C, mg/dL, Mean (SD) | 62.72 (16.29) | 58.50 (15.33) | 64.74 (15.49) | 62.35 (18.22) | <0.001 |
| LDL-C, mg/dL, Mean (SD) | 118.11 (29.89) | 119.67 (32.58) | 122.69 (31.83) | 118.05 (33.01) | 0.37 |
| Triglycerides, mg/dL, Median (Q1, Q3) | 105.50 (78.00, 155.00) | 92.00 (74.00, 128.00) | 91.00 (66.00, 126.00) | 108.00 (86.00, 148.00) | 0.0004 |
| HOMA index, Median (Q1, Q3) | 1.74 (1.11, 3.45) | 2.81 (1.62, 4.86) | 1.74 (1.10, 3.08) | 2.48 (1.45, 3.93) | <0.001 |
| Physical activity score,* Mean (SD) | 7.53 (1.83) | 7.49 (1.80) | 7.71 (1.93) | 7.19 (1.87) | 0.22 |
| Diabetes, n (%) | 17 (8.21) | 43 (16.8) | 24 (6.86) | 9 (20.93) | <0.001 |
| Ever Smoker, n (%) | 65 (41.4) | 104 (48.6) | 127 (45.52) | 16 (50.00) | 0.55 |
| Ever use of medications,† n (%) | 102 (58.96) | 168 (71.79) | 169 (54.52) | 26 (66.67) | 0.001 |
| Mean-IMT, mm, Mean (SD) | 0.77 (0.11) | 0.82 (0.12) | 0.78 (0.11) | 0.79 (0.13) | <0.001 |
| Maximal-IMT, mm, Mean (SD) | 0.90 (0.13) | 0.96 (0.14) | 0.91 (0.13) | 0.92 (0.14) | <0.001 |
| AD, mm, Mean (SD) | 7.12 (0.60) | 7.31 (0.66) | 7.12 (0.67) | 7.03 (0.60) | 0.001 |
| Presence of plaque, n (%) | 103 (49.76) | 117 (45.88) | 139 (39.71) | 18 (41.86) | 0.12 |
| Time since FMP, year, Mean (SD) | 8.59 (3.21) | 7.34 (3.05) | 8.20 (3.22) | 6.65 (3.78) | <0.001 |
| Ever use hormone therapy, n (%) | 11 (5.31) | 12 (4.69) | 18 (5.14) | 2 (4.65) | 0.99 |
Higher scores indicating more physical activity
Antihypertensive, antidiabetic, lipid lowering, or anticoagulants medications
Table 2. Characteristics of study population at visit 12 by FSH groups.
| Characteristics | Low FSH N=53 | Medium FSH N=431 | High FSH N=372 | P value |
|---|---|---|---|---|
| Age, years, Mean(SD) | 58.66 (2.50) | 59.17 (2.54) | 59.97 (2.76) | <0.001 |
| Race, n (%) | <0.001 | |||
| Black | 27 (50.94) | 144 (33.41) | 92 (24.73) | |
| White | 16 (30.19) | 201 (46.64) | 183 (49.19) | |
| Chinese | 1 (1.89) | 62 (14.39) | 78 (20.97) | |
| Hispanic | 9 (16.98) | 24 (5.57) | 19 (5.11) | |
| Education, n (%) | 0.09 | |||
| ≤ High school/some college | 32 (61.54) | 242 (56.54) | 183 (49.86) | |
| College degree/post college | 20 (38.46) | 186 (43.46) | 184 (50.14) | |
| BMI, kg/m2, Mean (SD), | 37.63 (9.07) | 30.75 (7.34) | 27.30 (6.11) | <0.001 |
| SBP, mmHg, Mean (SD) | 129.77 (14.91) | 122.27 (17.95) | 119.75 (17.42) | 0.0003 |
| DBP, mmHg, Mean (SD) | 76.61 (10.33) | 74.41 (9.99) | 73.09 (10.53) | 0.03 |
| HDL-C, mg/dL, Mean (SD) | 52.98 (12.06) | 60.60 (15.50) | 65.51 (16.22) | <0.001 |
| LDL-C, mg/dL, Mean (SD) | 106.94 (28.32) | 120.06 (32.64) | 122.84 (30.54) | 0.003 |
| Triglycerides, mg/dL, Median (Q1, Q3) | 110.00 (89.00, 146.00) | 98.00 (74.00, 140.00) | 88.00 (68.00, 124.00) | 0.001 |
| HOMA index, Median (Q1, Q3) | 3.94 (2.22, 7.08) | 2.44 (1.33, 3.99) | 1.61 (1.06, 3.03) | <0.001 |
| Physical activity score,* Mean (SD) | 7.06 (1.78) | 7.56 (1.85) | 7.67 (1.88) | 0.08 |
| Diabetes, n (%) | 13 (24.53) | 57 (13.23) | 23 (6.18) | <0.001 |
| Ever Smoker, n (%) | 17 (50.00) | 167 (47.99) | 128 (42.67) | 0.35 |
| Ever use of medications,† n (%) | 39 (79.59) | 249 (64.18) | 177 (55.49) | 0.002 |
| Mean-IMT, mm, Mean (SD) | 0.79 (0.09) | 0.81 (0.13) | 0.77 (0.11) | 0.001 |
| Maximal-IMT, mm, Mean (SD) | 0.92 (0.09) | 0.94 (0.15) | 0.90 (0.13) | 0.0001 |
| AD, mm, Mean (SD) | 7.32 (0.71) | 7.21 (0.67) | 7.11 (0.62) | 0.02 |
| Presence of plaque, n (%) | 17 (32.08) | 197 (45.81) | 163 (43.82) | 0.16 |
| Time since FMP, year, Mean (SD) | 7.92 (3.89) | 7.41 (3.21) | 8.59 (3.06) | <0.001 |
| Ever use hormone therapy, n (%) | 1 (1.89) | 20 (4.64) | 22 (5.91) | 0.40 |
Higher scores indicating more physical activity
Antihypertensive, antidiabetic, lipid lowering, or anticoagulants medications
CVD risk factors at visit 12 (Table 1) or at baseline (Supplemental-Table 1) in women experiencing either the High E2-early decline or the Low E2 trajectory were similar, except for triglycerides, which were higher in the Low E2 group. The CVD risk profiles in the High E2-early decline and the Low E2 groups were more favorable than in the Medium E2 or the High E2-late decline groups. Women with the High E2- early decline pattern had the lowest prevalence of cPlaque, while women with the Medium E2 and High E2- late decline patterns had the thickest IMT measures, Table 1. The Low FSH group had a worse CVD risk factor profile at both visit 12 (Table 2) and baseline (Supplementary-Table 2), however their IMT measures and prevalence of cPlaque were more favorable than those in the Medium or High FSH groups, Table 2.
Multivariable analyses of subclinical atherosclerosis measures and hormone trajectory groups (Table 3 & 4)
Table 3. Multivariable associations between E2 groups and subclinical measures of atherosclerosis.
| Groups | Mean-IMT, mm | Maximal-IMT, mm | AD, mm | cPlaque (Y/N) | ||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | OR (95%CI) | P value | |
| Model 1 | ||||||||
| Medium E2 | 0.029 (0.013) | 0.03 | 0.038 (0.015) | 0.01 | 0.167 (0.073) | 0.02 | 0.949 (0.599, 1.504) | 0.82 |
| High E2 - Early decline | 0.0001 (0.011) | 0.99 | 0.002 (0.012) | 0.89 | 0.015 (0.061) | 0.81 | 0.651 (0.443, 0.957) | 0.03 |
| High E2 - Late decline | 0.011 (0.020) | 0.59 | 0.016 (0.023) | 0.49 | -0.030 (0.111) | 0.79 | 0.848 (0.421, 1.707) | 0.64 |
| Low E2 | reference | reference | reference | reference | ||||
| Model 2 | ||||||||
| Medium E2 | 0.034 (0.015) | 0.03 | 0.038 (0.018) | 0.03 | 0.139 (0.083) | 0.10 | 0.709 (0.398, 1.265) | 0.24 |
| High E2 - Early decline | 0.010 (0.013) | 0.44 | 0.008 (0.015) | 0.58 | 0.113 (0.069) | 0.10 | 0.547 (0.338, 0.885) | 0.01 |
| High E2 - Late decline | 0.014 (0.024) | 0.56 | 0.014 (0.027) | 0.60 | -0.075 (0.127) | 0.56 | 1.151 (0.483, 2.738) | 0.75 |
| Low E2 | reference | reference | reference | reference | ||||
| Model 3 | ||||||||
| Medium E2 | 0.023 (0.016) | 0.15 | 0.029 (0.019) | 0.11 | 0.054 (0.087) | 0.53 | 0.687 (0.373, 1.265) | 0.23 |
| High E2 - Early decline | 0.004 (0.014) | 0.74 | 0.007 (0.016) | 0.64 | 0.063 (0.072) | 0.39 | 0.568 (0.341, 0.945) | 0.03 |
| High E2 - Late decline | 0.014 (0.024) | 0.55 | 0.018 (0.028) | 0.51 | -0.042 (0.130) | 0.75 | 0.907 (0.372, 2.213) | 0.83 |
| Low E2 | reference | reference | reference | reference | ||||
E2 groups are modeled as posterior probabilities of group membership
Model 1: Adjusted for study site, age at visit 12, ethnicity, and education
Model 2: Model 1 plus ever smoking, ever use of medications, and visit 12 BMI, systolic blood pressure, LDL-C, HDL-C, triglycerides, HOMA –IR, and physical activity
Model 3: Model 1 plus ever smoking, ever use of medications, and baseline BMI, systolic blood pressure, LDL-C, HDL-C, triglycerides, HOMA –IR, and physical activity
Table 4. Multivariable associations between FSH groups and subclinical measures of atherosclerosis.
| Groups | Mean-IMT, mm | Maximal-IMT, mm | AD, mm | cPlaque (Y/N) | ||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | OR (95%CI) | P value | |
| Model 1 | ||||||||
| High FSH | 0.002 (0.018) | 0.93 | 0.002 (0.021) | 0.94 | -0.220 (0.101) | 0.03 | 1.271 (0.663, 2.437) | 0.47 |
| Medium FSH | 0.033 (0.017) | 0.06 | 0.039 (0.020) | 0.05 | -0.118 (0.099) | 0.23 | 1.524 (0.805, 2.884) | 0.20 |
| Low FSH | reference | reference | reference | reference | ||||
| Model 2 | ||||||||
| High FSH | 0.043 (0.023) | 0.06 | 0.061 (0.026) | 0.02 | 0.133 (0.123) | 0.28 | 1.395 (0.593, 3.282) | 0.45 |
| Medium FSH | 0.066 (0.022) | 0.002 | 0.085 (0.025) | 0.001 | 0.173 (0.118) | 0.14 | 1.628 (0.717, 3.699) | 0.24 |
| Low FSH | reference | reference | reference | reference | ||||
| Model 3 | ||||||||
| High FSH | 0.038 (0.024) | 0.11 | 0.054 (0.027) | 0.05 | 0.173 (0.129) | 0.18 | 1.642 (0.650, 4.151) | 0.29 |
| Medium FSH | 0.063 (0.023) | 0.01 | 0.081 (0.026) | 0.002 | 0.146 (0.124) | 0.24 | 2.107 (0.866, 5.127) | 0.10 |
| Low FSH | reference | reference | reference | reference | ||||
FSH groups are modeled as posterior probabilities of group membership
Model 1: Adjusted for study site, age at visit 12, ethnicity, and education
Model 2: Model 1 plus ever smoking, ever use of medications, and visit 12 BMI, systolic blood pressure, LDL-C, HDL-C, triglycerides, HOMA –IR, and physical activity
Model 3: Model 1 plus ever smoking, ever use of medications, and baseline BMI, systolic blood pressure, LDL-C, HDL-C, triglycerides, HOMA –IR, and physical activity
Independent of socio-demographics, and visit 12, or baseline CVD risk factors, the High E2-early decline group was less likely to have cPlaque than the Low E2 group. On the other hand, the Medium E2 group had significantly greater IMT than the Low E2 group in models adjusted for visit 12 CVD risk factors. Adjusting for baseline CVD risk factors attenuated the association between the Medium E2 group and IMT, Table 3. For FSH groups, adjusting for socio-demographics, and visit 12, or baseline CVD risk factors, both the Medium and High FSH trajectory groups had significantly thicker IMT, Table 4.
Additional adjustment for ever-use of HT and time since FMP did not change the above findings. Interactions with ethnicity were not significant.
Discussion
This study is the first to examine variations in the patterns of E2 decline and FSH rise around FMP in relation to postmenopausal subclinical atherosclerosis. We found for the first time, that E2 and FSH trajectories over the FMP are associated with subclinical atherosclerosis differently. Findings for E2 trajectories suggest that compared to women with low E2 before and after their FMP, women with higher E2 before their FMP but lower E2 thereafter (High E2-early decline group) may be more protected from developing atherosclerosis after menopause. For FSH, women experiencing a lower FSH rise after menopause (Low group) may be at lower risk of atherosclerosis development than those who experienced either a medium or a high rise FSH over the transition. These findings were not explained by differences in demographics and/or CVD risk factors between the trajectory groups, which suggest an independent role of the time course of hormone trajectories on risk of atherosclerosis development after menopause.
We found that women with higher E2 levels before their FMP but lower E2 thereafter (High E2-early decline) were ∼43% less likely to have cPlaque than women with low E2 before and after FMP (Low E2). Ovarian E2 production in a woman's fertile years' results in circulating E2 that is up to two log-orders higher than that of a postmenopausal woman. Consistently higher E2 production during these premenopausal years may explain the lower prevalence of cPlaque in the High E2-early decline group after menopause.35 Premenopausal women without CHD have normal mean plasma concentrations of E2, approximately 80 pg/ml.36 In the current study, E2 levels prior to the FMP among women in the High E2-early decline ranged from 60 to 89 pg/ml.
The pathways by which premenopausal-estrogen reduces the development of atherosclerosis are thought to be related in part to the well-established roles of endogenous estrogen in improving the lipid/lipoprotein profile, endothelial function, and arterial vasodilation.35 In the current study, the High E2-early decline group had a better CVD risk profile at both baseline and visit 12. Adjusting for baseline risk factors rather than those from visit 12 did not change our findings, suggesting that atherosclerosis present after menopause could potentially be influenced by the premenopausal-estrogen exposure independent of CVD risk factors.
Our findings of thicker IMT in the Medium E2 group (medium levels of E2 before FMP, but stable and high postmenopausal-E2 levels) compared with the Low E2 group in models adjusted for concurrent CVD risk factors may be surprising given that the premenopausal E2 level was moderately higher than that among the Low E2 group. The potential reason for that is not fully understood. It is possible that at this medium level of premenopausal-E2 (35-50 pg/mL) women may not be as protected as when premenopausal-E2 is higher as found among the High E2-Early decline group (60-89 pg/mL). As described above, premenopausal women without CHD have normal mean concentration of E2 of approximately 80 pg/ml, 36 a level that is higher than the premenopausal-E2 level found among the Medium E2 group.
Another potential explanation of the thicker IMT among the Medium E2 group compared with the Low E2 group could be due to the stable and high postmenopusal-E2 level found in the Medium E2 group, but not among the Low E2 group. Higher postmenopausal-estrogen levels have been found to be associated with greater insulin resistance, 37 inflammatory marker levels, 38 and a more pro-atherogenic lipid profile.39, 40 In agreement with this notion, women who experienced the Medium E2 trajectory also had a worse CVD risk profile than the Low E2 group. Alternatively, the high postmenopausal-E2 levels in women who had thicker mean and maximal IMT after menopause could represent an endogenous response to the process of inflammation/atherosclerosis, rather than a causative factor in the development of atherosclerosis.13,41 Estrogens can be synthesized locally in the artery wall.42 Women who experienced the Medium E2 trajectory in the current study had an adverse cardiovascular risk profile at baseline and visit 12 compared to the Low E2 trajectory group. This supports the possibility that the high-postmenopausal E2 level found to be associated with thicker IMT measures could be an endogenous response to an already worse risk profile. In agreement with this finding, when adjusting for baseline levels of CVD risk factors rather than levels at visit12, the associations between the Medium E2 group and thicker IMT measures were attenuated. Further studies should investigate the potential impact of levels of E2 before and after menopause on CVD risk.
Although women in the Medium and High FSH groups were more likely to be White or Chinese and to have a better CVD risk profile at both baseline and visit 12 compared to the Low FSH group, their IMT measures were significantly less favorable, independent of demographics and CVD risk factors measured at either baseline or visit 12. These findings suggest a potential independent role of lifetime exposure to high or increasing FSH, a cardinal marker of menopause,1 on the vasculature. Our findings are consistent with the few studies that have evaluated associations between FSH and subclinical atherosclerosis. 11,12,17 The mechanisms by which lifetime exposure to FSH might impact IMT after menopause are not known.
The present results should be interpreted in the context of several limitations. The analyses linking the trajectory groups and subclinical measures of atherosclerosis were cross-sectional since subclinical measures of atherosclerosis were only available at visit 12. Irrespective of that, the hormone trajectory groups were created based on extensive longitudinal data over 9.6 years across FMP. Women without an observed FMP were excluded, and therefore our results cannot be generalized to all women. The current analyses were limited by the small numbers of Hispanics. Additionally, due to limited sample sizes in certain trajectory groups (High E2-late decline and Low FSH), analysis in these groups could be under powered to detect significant associations with subclinical measures of atherosclerosis. Many dietary habits such as fat and phytoestrogens intake could have an effect on plasma level of estrogen. We did not have this information across FMP and therefore we were not able to account for this in the current analyses. Finally, the use of group based trajectory analyses to classify women to certain trajectory group using the posterior probabilities may introduce misclassification bias that may weaken the observed associations between the true hormone trajectories and subclinical atherosclerosis.
In this well-characterized cohort of midlife -women followed over the menopause transition, the current study highlights the importance of evaluating the time course of sex hormones when assessing their associations with subclinical atherosclerosis. It shows for the first time that E2 and FSH trajectories over the menopause transition are significantly associated with atherosclerosis risk after menopause. Our results suggest that relative to low levels of E2, higher E2 levels before menopause may contribute to lower atherosclerotic risk after menopause, while higher E2 levels after menopause may be associated with higher risk. The detected associations were independent of socio-demographics and CVD risk factors. These findings may reflect an endogenous hormone response to atherosclerosis developing over time, or direct detrimental actions of elevated E2 after menopause. Further studies are needed to determine the potential role of higher postmenopausal-E2 levels on atherosclerosis development. The current study suggests that women with low FSH rise over their menopause transition may be at lower risk of atherosclerosis than women with medium or high FSH rises. These findings were not driven by CVD risk factors.
The menopause transition is a critical time period of women's life. During this transition, women are subjected to hormonal alterations that could potentially increase their risk to develop CVD after menopause. To minimize the risk of developing CVD after menopause, CVD prevention strategies should be initiated early in the transition. These strategies should be focused on women experiencing low E2 levels accompanied by a deleterious CVD risk profile before their FMP.
Supplementary Material
Supplemental-Table 1. Characteristics of study population at baseline by E2 groups
Supplemental-Table 2. Characteristics of study population at baseline by FSH groups
Acknowledgments
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA – Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Funding: The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Disclosures: Dr. El Khoudary reports grants from NIH, during the conduct of the study; Dr. Santoro reports Bayer, Inc: investigator initiated grant support (money paid to my institution and none to me); Menogenix: stock options (have received no money to date), Mrs. Chen, and Drs. Tepper, Brooks, Thurston, Janssen, Harlow, Barinas-Mitchell, and Selzer, report grants from NIH, during the conduct of the study; Dr. Derby reports grants from NIH-NIA 2UO1AG012535, during the conduct of the study; Dr. Jackson reports the following American Journal of Medicine (editor) modest, McKesson (consultant) modest, NIH (study section) modest, Up-To-Date (author) modest, American College of Cardiology (consultant) modest, Spry Publishing (author) modest; Drs. McConnell and Matthews report grants from NIA, during the conduct of the study.
References
- 1.Randolph JF, Jr, Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, Vuga M. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96:746–754. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2015;27(131):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Gorodeski GI. Impact of the menopause on the epidemiology and risk factors of coronary artery heart disease in women. Exp Gerontol. 1994;29:357–375. doi: 10.1016/0531-5565(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 4.Psaty BM, Heckbert SR, Atkins D, Lemaitre R, Koepsell TD, Wahl PW, Siscovick DS, Wagner EH. The risk of myocardial infarction associated with the combined use of estrogens and progestins in postmenopausal women. Arch Intern Med. 1994;154:1333–1339. [PubMed] [Google Scholar]
- 5.Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453–461. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 6.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 7.Henderson BE, Paganini-Hill A, Ross RK. Decreased mortality in users of estrogen replacement therapy. Arch Intern Med. 1991;151:75–78. [PubMed] [Google Scholar]
- 8.Prentice RL, Langer RD, Stefanick ML, Howard BV, Pettinger M, Anderson GL, Barad D, Curb JD, Kotchen J, Kuller L, Limacher M, Wactawski-Wende J Women's Health Initiative Investigators. Combined analysis of Women's Health Initiative observational and clinical trial data on postmenopausal hormone treatment and cardiovascular disease. Am J Epidemiol. 2006;163:589–599. doi: 10.1093/aje/kwj079. [DOI] [PubMed] [Google Scholar]
- 9.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progesterone Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis. 2012;225:180–186. doi: 10.1016/j.atherosclerosis.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celestino Catão Da Silva D, Nogueira De Almeida Vasconcelos A, Cleto Maria Cerqueira J, De Oliveira Cipriano Torres D, Oliveira Dos Santos AC, De Lima Ferreira Fernandes Costa H, Bregieiro Fernandes Costa LO. Endogenous sex hormones are not associated with subclinical atherosclerosis in menopausal women. Minerva Ginecol. 2013;65:297–302. [PubMed] [Google Scholar]
- 13.Naessen T, Bergquist J, Lind L, Kushnir MM. Higher endogenous estrogen levels in 70-year-old women and men: an endogenous response to counteract developing atherosclerosis? Menopause. 2012;19:1322–1328. doi: 10.1097/gme.0b013e31825ea8c1. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang P, Vaidya D, Dobs A, Golden SH, Szklo M, Heckbert SR, Kopp P, Gapstur SM. Sex hormone levels and subclinical atherosclerosis in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2009;204:255–261. doi: 10.1016/j.atherosclerosis.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karim R, Hodis HN, Stanczyk FZ, Lobo RA, Mack WJ. Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. J Clin Endocrinol Metab. 2008;93:131–138. doi: 10.1210/jc.2007-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M. Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol. 2002;155:437–445. doi: 10.1093/aje/155.5.437. [DOI] [PubMed] [Google Scholar]
- 17.Munir JA, Wu H, Bauer K, Bindeman J, Byrd C, Feuerstein IM, Villines TC, Taylor AJ. The perimenopausal atherosclerosis transition: relationships between calcified and noncalcified coronary, aortic, and carotid atherosclerosis and risk factors and hormone levels. Menopause. 2012;19:10–15. doi: 10.1097/gme.0b013e318221bc8d. [DOI] [PubMed] [Google Scholar]
- 18.Jeon GH, Kim SH, Yun SC, Chae HD, Kim CH, Kang BM. Association between serum estradiol level and coronary artery calcification in postmenopausal women. Menopause. 2010;17:902–907. doi: 10.1097/gme.0b013e3181d76768. [DOI] [PubMed] [Google Scholar]
- 19.Hale GE, Hughes CL, Burger HG, Robertson DM, Fraser IS. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause. 2009;16:50–59. doi: 10.1097/GME.0b013e31817ee0c2. [DOI] [PubMed] [Google Scholar]
- 20.Tepper PG, Randolph JF, Jr, McConnell DS, Crawford SL, El Khoudary SR, Joffe H, Gold EB, Zheng H, Bromberger JT, Sutton-Tyrrell K. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women's Health across the Nation (SWAN) J Clin Endocrinol Metab. 2012;97:2872–2880. doi: 10.1210/jc.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Jensen-Urstad K, Jensen-Urstad M, Johansson J. Carotid artery diameter correlates with risk factors for cardiovascular disease in a population of 55-year-old subjects. Stroke. 1999;30:1572–1576. doi: 10.1161/01.str.30.8.1572. [DOI] [PubMed] [Google Scholar]
- 23.Eigenbrodt ML, Sukhija R, Rose KM, Tracy RE, Couper DJ, Evans GW, Bursac Z, Mehta JL. Common carotid artery wall thickness and external diameter as predictors of prevalent and incident cardiac events in a large population study. Cardiovasc Ultrasound. 2007;5:11. doi: 10.1186/1476-7120-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, Lobo AR, editors. Menopause: Biology and Pathology. New York: Academic Press; 2000. pp. 175–88. [Google Scholar]
- 25.Wendelhag I, Gustavsson T, Suurküla M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol. 1991;11:565–577. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 26.Thompson T, Sutton-Tyrrell K, Wildman RP, Kao A, Fitzgerald SG, Shook B, Tracy RP, Kuller LH, Brockwell S, Manzi S. Progression of carotid intima-media thickness and plaque in women with systemic lupus erythematosus. Arthritis Rheum. 2008;58:835–842. doi: 10.1002/art.23196. [DOI] [PubMed] [Google Scholar]
- 27.Sutton-Tyrrell K, Wolfson SK, Jr, Thompson T, Kelsey SF. Measurement variability in duplex scan assessment of carotid atherosclerosis. Stroke. 1992;23:215–220. doi: 10.1161/01.str.23.2.215. [DOI] [PubMed] [Google Scholar]
- 28.England BG, Parsons GH, Possley RM, McConnell DS, Midgley AR. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clinical Chemistry. 2002;48:1584–1586. [PubMed] [Google Scholar]
- 29.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 30.Steiner P, Freidel J, Bremner W, Stein E. Standardization of micro-methods for plasma cholesterol, triglyceride and hdl-cholesterol with the lipid research clinics' methodology. J Clin Chem Clin Biochem. 1981;19:850. [Google Scholar]
- 31.Izawa S, Okada M, Matsui H, Horita Y. A new direct method for measuring hdl cholesterol which does not produce any biased values. J Med Pharm Sci. 1997;37:1385–1388. [Google Scholar]
- 32.Friedewald WT, Levy RI, Frederickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparation ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 33.Matthews D, Hosker J, Rudenski A, Naylor B, Teacher D, Turner R. Homeostasis model assessment: insulin resistance and b cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 34.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 35.Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am J Cardiol. 2002;89:12E–17E. doi: 10.1016/s0002-9149(02)02405-0. discussion 17E-18E. [DOI] [PubMed] [Google Scholar]
- 36.Bairey Merz CN1, Johnson BD, Sharaf BL, Bittner V, Berga SL, Braunstein GD, Hodgson TK, Matthews KA, Pepine CJ, Reis SE, Reichek N, Rogers WJ, Pohost GM, Kelsey SF, Sopko G WISE Study Group. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol. 2003;41:413–419. doi: 10.1016/s0735-1097(02)02763-8. [DOI] [PubMed] [Google Scholar]
- 37.Golden SH, Dobs AS, Vaidya D, Szklo M, Gapstur S, Kopp P, Liu K, Ouyang P. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92:1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- 38.Folsom AR, Golden SH, Boland LL, Szklo M. Association of endogenous hormones with C-reactive protein, fibrinogen, and white blood count in post-menopausal women. Eur J Epidemiol. 2005;20:1015–1022. doi: 10.1007/s10654-005-3657-0. [DOI] [PubMed] [Google Scholar]
- 39.Lambrinoudaki I, Christodoulakos G, Rizos D, Economou E, Argeitis J, Vlachou S, Creatsa M, Kouskouni E, Botsis D. Endogenous sex hormones and risk factors for atherosclerosis in healthy Greek postmenopausal women. Eur J Endocrinol. 2006;154:907–916. doi: 10.1530/eje.1.02167. [DOI] [PubMed] [Google Scholar]
- 40.Vaidya D, Dobs A, Gapstur SM, Golden SH, Hankinson A, Liu K, Ouyang P. The association of endogenous sex hormones with lipoprotein subfraction profile in the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2008;57:782–790. doi: 10.1016/j.metabol.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naessen T, Sjogren U, Bergquist J, Larsson M, Lind L, Kushnir MM. Endogenous steroids measured by high-specificity liquid chromatography-tandem mass spectrometry and prevalent cardiovascular disease in 70-year-old men and women. J Clin Endocrinol Metab. 2010;95:1889–1897. doi: 10.1210/jc.2009-1722. [DOI] [PubMed] [Google Scholar]
- 42.Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–C118. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental-Table 1. Characteristics of study population at baseline by E2 groups
Supplemental-Table 2. Characteristics of study population at baseline by FSH groups