Abstract
Neuromyelitis optica (NMO) is an autoimmune disorder of the central nervous system that usually presents with acute myelitis and/or optic neuritis. Recently, some brain magnetic resonance imaging findings have been described in NMO that are important in the differential diagnosis. Pencil-thin, leptomeningeal, and cloud-like enhancement may be specific to NMO. These patterns are usually seen during relapses. Recognizing these lesions and enhancement patterns may expedite the diagnosis and allows early effective treatment. The purpose of this article is to review the latest knowledge and to share our experience with the contrast enhancement patterns of NMO brain lesions.
Keywords: Neuromyelitis optica, Magnetic resonance imaging, Brain, neuroimaging
1. Introduction
Neuromyelitis optica (NMO) is an autoimmune disorder of the central nervous system that typically presents with acute transverse myelitis (TM) and/or optic neuritis (ON) attacks. NMO is now known to be associated with antibodies to aquaporin 4 (AQP4), which is highly concentrated in astrocytic end-feet at the blood–brain barrier [1].
Brain, orbits, and spinal cord magnetic resonance imaging (MRI) has an increasing role in the early diagnosis of NMO [2,3]. Early diagnosis and appropriate treatment of NMO are clinically important. First of all, preventing future attacks and related severe disability is the major goal of treatment in NMO. Secondly, some multiple sclerosis (MS) treatments, especially natalizumab and interferon beta, may worsen the disease [4–6].
Enhancement patterns of the brain lesions in NMO have some unique features, and sometimes, in the presence of characteristic lesions, they might help to make a specific diagnosis. The purpose of this article is to review the latest knowledge and to share our experience with the contrast enhancement patterns of NMO brain lesions. With the data and images provided in this review, we hope to familiarize readers with the different types of lesion enhancement characteristics that might indicate NMO.
1.1. Brain lesions
Presence of a brain MRI that is not diagnostic of MS also remains part of the current diagnostic criteria [7] (Table 1). These criteria should be used to make a diagnosis of NMO. Excluding NMO based on these criteria is not advised, considering reported brain lesions that may meet MS criteria and spinal cord lesions that are not long enough to meet longitudinally extensive transverse myelitis (LETM) definition (contiguous T2 signal abnormality ≥3 vertebral segments) [2].
Table 1.
Diagnostic criteria for the diagnosis of NMO [7]
| Acute myelitis |
| Optic neuritis |
And at least 2 of the 3 following supportive criteria:
|
After discovery of the antibody to AQP4 in 2004, various brain lesions have been reported in NMO [8]. AQP4 protein is expressed mainly in astrocytes, in high densities in perivascular and subpial astrocytic end-feet. It is widely distributed in the central nervous system, and thus the manifestations of the disease would not be expected to be restricted to the spine and optic nerves. Lesions in the hypothalamus and thalamus (diencephalic lesions), and periependymal lesions around cerebral aqueduct, third and fourth ventricles, dorsal medulla, and area postrema are regarded as typical locations for NMO lesions as these regions also represent areas of high AQP4 distribution [9–12]. Periventricular lesions that have a parallel orientation (i.e., not Dawson’s fingers); diffuse heterogenous hyperintensities in the splenium of the corpus callosum; corticospinal tract lesions; large, extensive confluent cerebral hemispheric lesions; and nonspecific punctate T2 hyperintense lesions have also been described in NMO patients [12–14]. Brain lesions in NMO tend to be asymptomatic; however, intractable hiccups, vomiting, and nausea may signify lesions involving the area postrema and the medullary floor of the fourth ventricle; narcolepsy and hypothermia may point to hypothalamic lesions; and seizures and headaches are nongeneralizing presenting symptoms also seen in NMO [15–19].
1.2. Brain contrast enhancement patterns
Table 2 outlines the published literature about contrast enhancement patterns of brain lesions in NMO [20–35]. We did a PubMed search for articles published between 2000 and 2014 using the word “neuromyelitis optica.” All orginal articles, case reports, and reviews about NMO and neuromyelitis optica spectrum disease, including MRI findings, published in English and providing a digital object identifier number, were reviewed. The year and origin of the study, and the number, race, and sex of the patients were noted. The provided seropositivity and seronegativity for NMO immunoglobulin G (IgG) were included the table. The number and percentage of the patients that were demonstrated to have enhancement of the brain lesions and described enhancement pattern or patterns, if provided, were listed.
Table 2.
Published literaturea about brain MRI lesion enhancement patterns in NMO patients
| First author, year (ref) | Country | No of patients | F/M | NMO IgG/AQP4 Ab+ | Enhanced lesions % (n) | Described pattern, % (n) |
|---|---|---|---|---|---|---|
| Eichel, 2008 [20] | Israel | 2 | 1/1 | 50% | 1 of 2 patientsb | Not described |
| Ito, 2009 [21] | Japan | 18 | 18/0 | 100% | 56% (10) | Cloud-like, 90% (9) Isolated, 10% (1) |
| Matsushita, 2009 [22] | Japan | 5 | 4/1 | 100% | 1 of 5 patients | Not described |
| Magana, 2009 [23] | USA | 4 | 4/0 | 100% | 3 of 4 patients | Not described |
| Kim W., 2010 [24] | Korea | 78 | 71/7 | 100% | 13% (10) | Cloud-like (not classified) |
| Kim J. E., 2011 [25] | Korea | 17 | 14/3 | 79% (11 of 14 patients) | 35% (6) | Cloud-like, 29% (5) Isolated, 6% (1) |
| Kim W., 2011 [26] | Korea | 15 | 14/1 | 100% | 3 of 9 patientsc | Not described |
| Newey, 2011 [27] | USA | 1 | 1 | 100% | 1 patient | Multiple ring enhancement |
| Tahara, 2012 [28] | Japan | 3 | 3/0 | 100% | 3 patients | Leptomeningeal and cortical enhancement |
| McGraw, 2012 [29] | USA | 1 | 1 F | 100% | 1 patient | Periependymal ventricular enhancement |
| Banker, 2012 [30] | USA | 2 | 2/0 | 100% | 2 patients | Pencil-thin ependymal enhancement |
| Cheng, 2013 [31] | China | 16 | 14/2 | 100% | 1 of 16 patients | Open ring enhancement |
| Iorio, 2013 [32] | Italy | 37 | 23/14 | 43% (16) | 94% (15, IgG+) 71% (21, IgG−) |
Not described |
| Huh, 2014 [33] | Korea | 67 | 57/10 | 100% | 21% (12 of 57 patients) | Cloud-like, 12% (8) Leptomeningeal, 3% (2) Ring,1% (1) Open ring,1% (1) |
| Long, 2014 [34] | China | 47 | 45/2 | 100% | 25.5% | Pencil-thin, 75% (9 of 12) Cloud-like, 25% (3 of 12) Isolated, 8.3% (1 of 12) |
| Pekcevik, 2014 [35] | USA | 1 | 1 F | 100% | 1 patient | Perivascular enhancement |
AQP4 Ab: aquaporin-4 antibody, F: female, M: male.
English literature, Pubmed.
Enhancement was seen in the seronegative patient.
Brain MRI available only in nine patients.
According to the previous reports, nonenhancing brain lesions appear to be more common in NMO, but in most of those reports, brain MRIs were not acquired during an acute attack of the disease. One would expect more enhancing lesions when the studies are obtained during acute attack. Additionally, as shown in Table 2, the enhancement patterns were mostly described in NMO-IgG positive patients. Recent studies have also demonstrated contrast-enhancing brain lesions in NMO IgG-negative patients [20,32]. Furthermore given the lower sensitivity of the previous NMO IgG tests, there would probably be more NMO-seropositive patients with current highly sensitive tests than those included in previous studies.
Gadolinium enhancement is the result of the disruption of the blood–brain barrier (BBB) [36]. AQP4 is a water channel protein and responsible for the bidirectional water flow between blood and brain. It is highly expressed in astrocytic end-feet and plays a critical role in forming the BBB. Antibody to AQP4 may cause the degradation or down-regulation of the water channel protein, damage the astrocytes, and result in breakdown of the BBB. When the breakdown of the BBB occurs, this allows the secondary influx of humoral or cellular immune components that attack the adjacent brain regions [21,37,38]. The ongoing disruption and damage to the BBB might explain the enhancement of brain lesions in NMO. Nonetheless, some parenchymal lesions in NMO may not show enhancement. For example, large, extensive parenchymal lesions, which are similar in appearance to posterior reversible encephalopathy or acute disseminated encephalomyelitis (ADEM), may be reversible and may not enhance, suggesting preserved integrity of the BBB [22–24].
1.3. Patchy parenchymal enhancement (“cloud-like” enhancement)
Patchy, inhomogeneous, subtle parenchymal enhancement with ill-defined margins, so called “cloud-like” enhancement, was first described by Ito et al. [21] and was proposed to be specific to NMO (Fig. 1). It has been the most commonly reported enhancement pattern in NMO patients since then in the literature [21,24,25,33,34]. The association of this enhancement pattern with NMO is helpful in differential diagnosis because it is different from the nodular or ring enhancement, which is more prominent and well defined, usually seen in MS [21] (Fig. 2). Cloud-like enhancement is sometimes associated with linear periependymal enhancement, which may be a more characteristic enhancement pattern for NMO [34]. When cloud-like enhancement is seen together with periependymal enhancement, it may look like a “flame” (Fig. 1) or “smoke coming out of mouth” (Fig. 3).
Fig. 1.
Cloud-like enhancement. T1-weighted contrast-enhanced images of a 57-year-old female with seropositive NMO shows patchy, ill-defined parenchymal enhancement (arrows). Images were obtained during acute TM attack, and there is also upper spinal cord enhancement (arrowhead).
Fig. 2.
Typical (A) nodular and (B) ring enhancement in two different patients with multiple sclerosis (arrows).
Fig. 3.
Cloud-like enhancement associated with linear periependymal enhancement in a patient with seropositive NMO. T1-weighted contrast-enhanced image of a 62-year-old male during both acute TM and ON attack shows periependymal enhancement associated with slight, ill-defined parenchymal enhancement. It looks like “smoke coming out of mouth.”
1.4. Periependymal linear enhancement
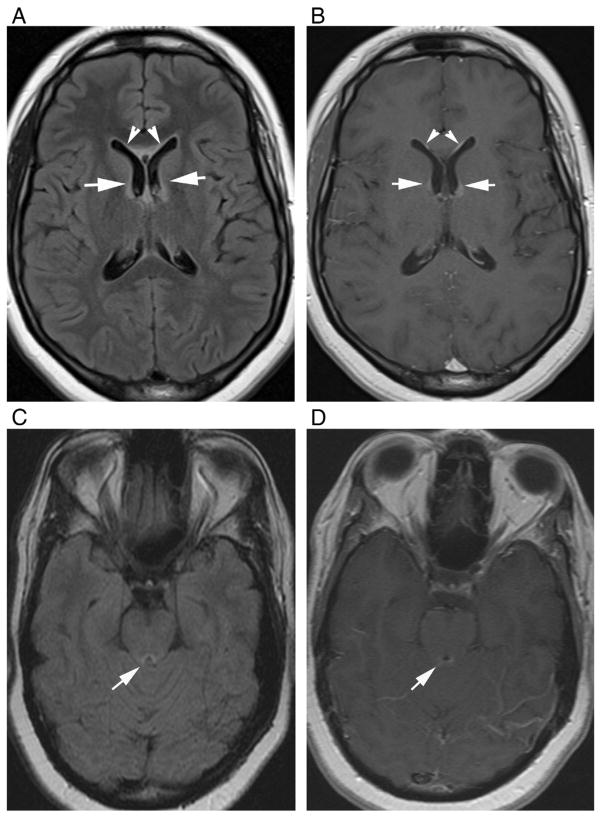
This is a thin, linear enhancement, surrounding the lateral, third, or fourth ventricles and/or cerebral aqueduct, which is recently described in NMO [29,30,34]. The ependymal cells and astrocytes are known to be rich in AQP4, and enhancement of these regions during relapses may make this pattern more characteristic to NMO than other described patterns [29,30]. It is usually associated with periventricular linear hyperintensities on fluid-attenuated inversion recovery (FLAIR) images and may be seen during acute TM and/or ON attacks [34] (Fig. 4). Periependymal linear enhancement and FLAIR periependymal linear hyperintensity could reflect an earlier phase of the disease. It follows the margins of the ventricles, may sometimes be interrupted or focal, and disappears after acute attack [29,30].
Fig. 4.
Periependymal linear enhancement in two different patients with seropositive NMO during first acute TM attack. (A) Axial FLAIR and (B) T1-weighted contrast-enhanced images of a 21-year-old female show periependymal linear hyperintensity and linear enhancement along the frontal horns of lateral ventricles. (C) Axial FLAIR and (D) T1-weighted contrast-enhanced images of a 35-year-old female demonstrate periependymal linear hyperintensity and linear enhancement around the cerebral aqueduct (arrows).
Periependymal enhancement can be associated with “cloud-like” enhancement, in periventricular and deep white matter, as described above (Fig. 3). It is not always seen as “pencil-thin” or linear and sometimes may be thick and irregular [30,34]. With progression of the disease, probably because of increased damage over time, it may appear thicker and extend into the brain parenchyma (Fig. 5).
Fig. 5.
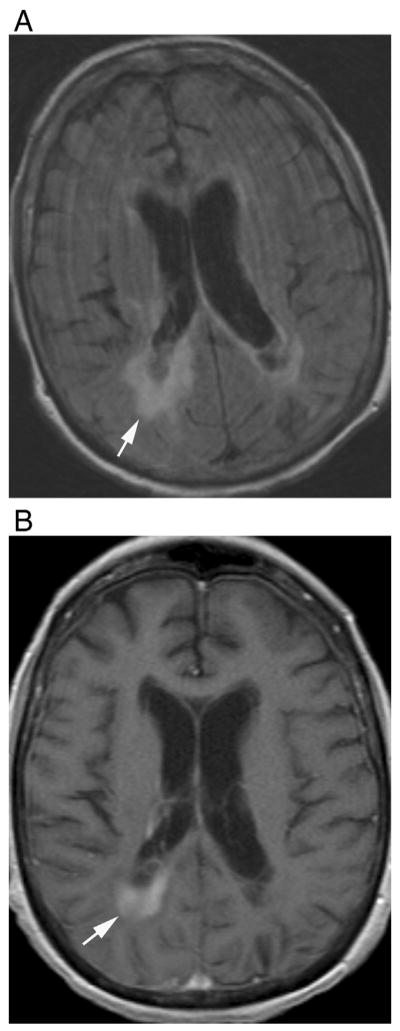
Periependymal linear enhancement. (A) Axial fluid-attenuated recovery and (B) T1-weighted contrast-enhanced images show thicker periependymal enhancement in a 73-year-old male during ON attack later in the course of the disease.
The differential diagnosis of periependymal enhancement includes infectious ventriculitis and neoplasms such as lymphoma and sarcoidosis.
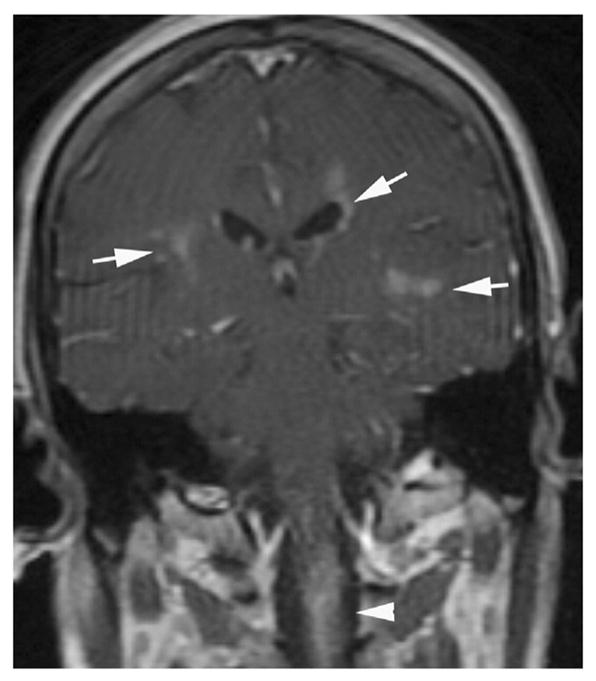
1.5. Leptomeningeal enhancement
Blood–brain barrier microvasculature consists of two capillary cell components (pericytes and endothelia), which are typically surrounded by astrocytic end-feet processes [39]. The dominant water channel protein, AQP4, is confined to the astrocytes end-feet processes. Antibody to AQP4 binds to surface of microvessels, pia, and Virchow–Robin sheaths and damages the astrocytes. Thus, leptomeningeal enhancement is probably a result of functional impairment of AQP4 water channels in the pial and subpial surfaces [34]. According to this mechanism, one might also expect perivascular enhancement in NMO patients [35].
Leptomeningeal enhancement can be linear or thick and extensive [28,34]. Recently, leptomeningeal enhancement around the brainstem was described in a study with NMO IgG-positive patients [34]. Enhancement of the leptomeninges might be mistaken for infectious disorders, granulomatous diseases such as sarcoidosis, or neoplastic diseases, especially when MRI is performed early during the disease course (Fig. 6). Moreover, these NMO patients can be symptomatic and present with encephalopathy-like symptoms, making differential diagnosis even more difficult [28].
Fig. 6.
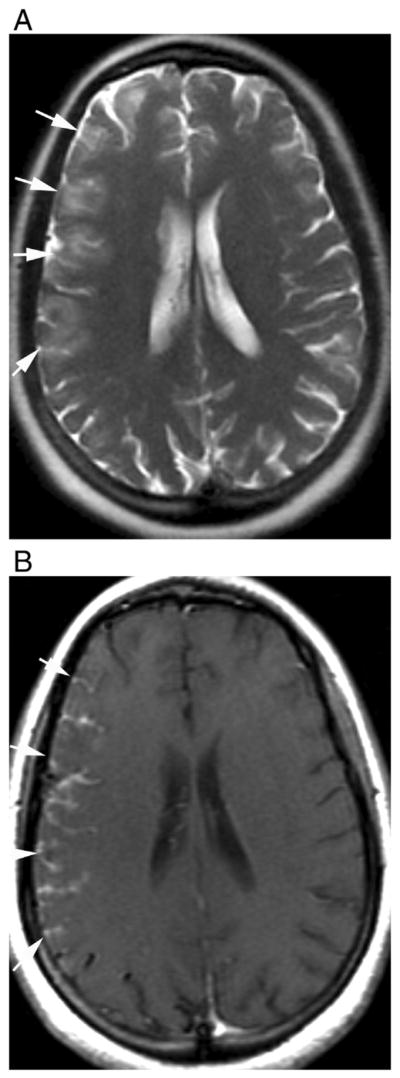
Leptomeningeal enhancement in a previously healthy 28-year-old female that was later diagnosed as seropositive NMO. (A) Axial T2-weighted fast spin-echo shows cortical hyperintensities and sulcal effacement in the right cerebral hemisphere without white matter signal abnormalities. (B) T1-weighted contrast-enhanced images demonstrate thick leptomeningial and cortical enhancement.
1.6. Enhancement of the brainstem, hypothalamus, and corticospinal tract lesions
Besides the periventricular areas, the hypothalamus, medullary floor of the fourth ventricle, and area postrema express AQP4 abundantly [6]. Lesions in these locations are known to be characteristic of NMO [40]. Hypothalamic involvement is found more commonly in patients with NMO compared to ADEM or MS. Bilateral involvement of the hypothalamus was helpful in differentiating NMO from MS in a recent study [41]. Lesions in those areas usually show faint enhancement with poorly defined margins (Figs. 7 and 8).
Fig. 7.
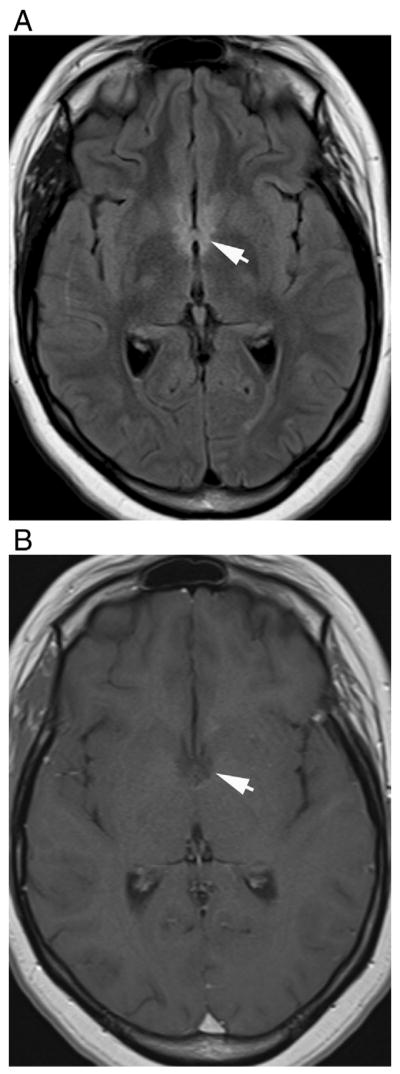
Bilateral involvement of the hypothalamus in a 21-year-old female with seropositive NMO. Axial (A) fluid-attenuated recovery and (B) T1-weighted contrast-enhanced images demonstrate hyperintense lesions in hypothalamus that show faint, poorly defined enhancement (arrows).
Fig. 8.
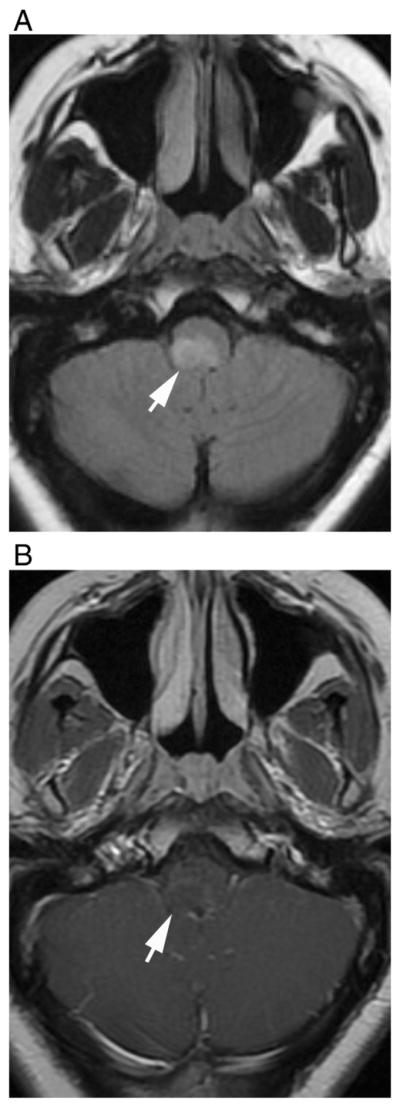
Dorsal medullary lesion in a 28-year-old female that was later diagnosed as seropositive NMO. Axial (A) fluid-attenuated recovery and (B) T1-weighted contrast-enhanced images show hyperintense lesion in dorsal medulla that show faint enhancement with poorly defined margins (arrows).
Corticospinal tract lesions, which may be either bilateral or unilateral and extend from internal capsule to pons, were previously described in NMO [12,24]. These lesions could be analogous with LETM in spinal cord and may cause contralateral hemiparesis [24]. The pyramidal tract is not one of the CNS areas that AQP4 is highly distributed, so the etiology of this involvement is still not clear [24]. There has been no previously described enhancement pattern in these lesions.
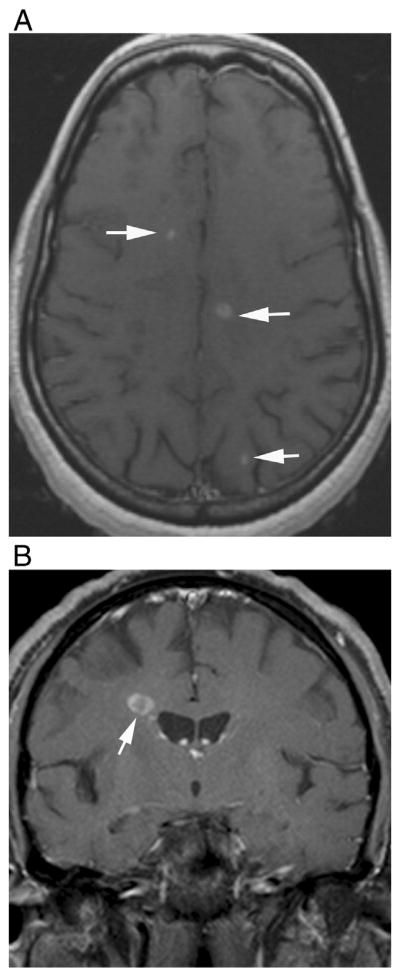
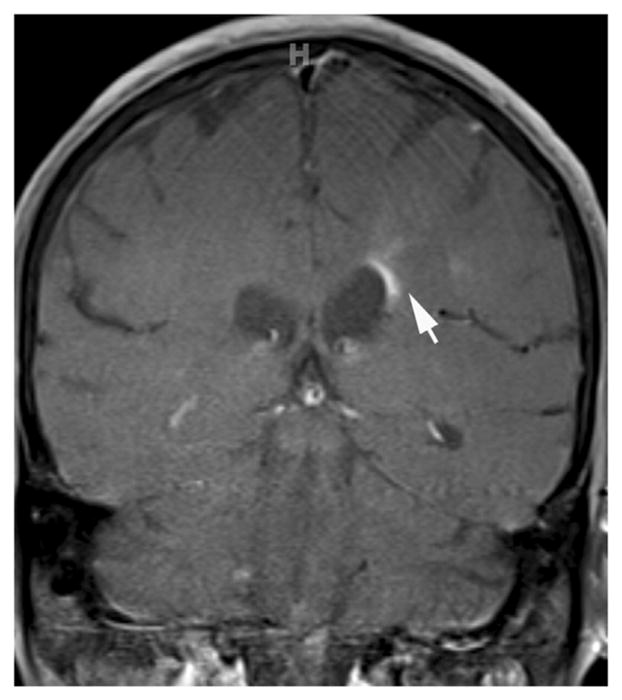
1.7. Isolated, ring, and open ring enhancement
Isolated enhancement, ring, and open ring enhancements are considered to be specific to MS, and they are rarely seen in NMO patients [21,25,27,31,33,34]. However, although rare, these intense, well-defined enhancement patterns were described before, especially in seronegative NMO patients [14] (Fig. 9).
Fig. 9.
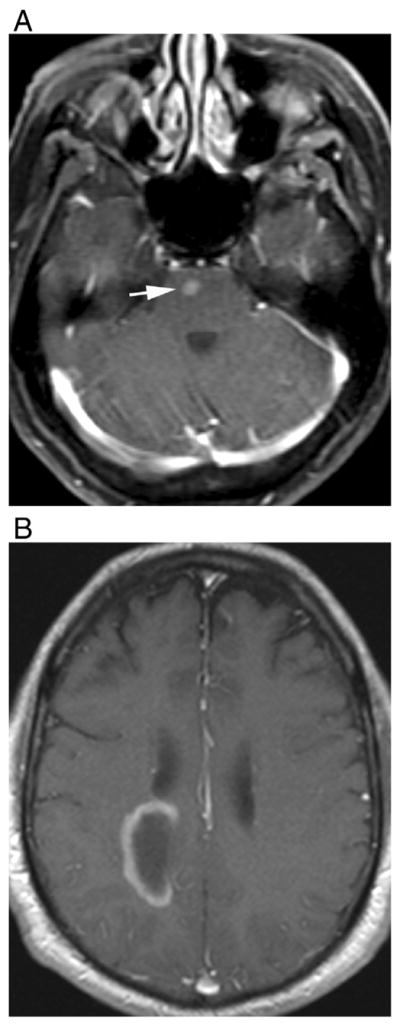
Multiple-sclerosis-like enhancement in two different patients. T1-weighted contrast-enhanced images demonstrate (A) isolated, well-defined nodular enhancement in the pons in a 57-year-old female with seropositive NMO and (B) open ring enhancement in a 65-year-old male with seronegative NMO.
1.8. Enhancement of optic nerves and optic chiasm
Optic neuritis may present with similar clinical and imaging features in both MS and NMO, but long segment enhancement that extends into chiasm and even optic tracts, and bilateral involvement are more suggestive of NMO than MS [6,42] (Fig. 10).
Fig. 10.
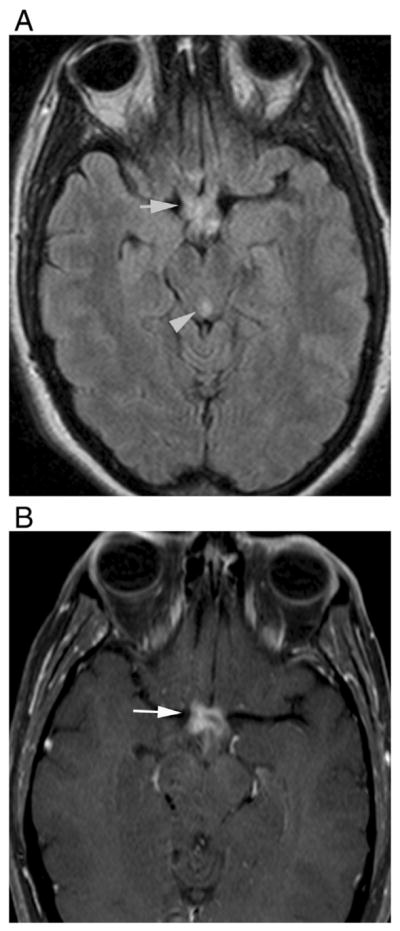
Enhancement of the prechiasmatic optic nerves and optic chiasm in a 44-year-old female during bilateral ON attack. (A) Axial fluid-attenuated recovery image shows hyperintensity in posterior parts of the optic nerves, chiasma, and optic tractus (arrow). There is hyperintensity around the cerebral aqueduct as well (arrowhead). (B) T1-weighted contrast-enhanced image demonstrates marked enhancement (arrow). Bilateral, long segment enhancement, usually including also the optic chiasm and sometimes optic tracts, is characteristic for NMO.
2. Conclusion
Magnetic resonance imaging findings are important in the differential diagnosis of NMO. Although nonenhancing brain lesions on brain MRI have been reported to be more common, there are some enhancement patterns (cloud-like enhancement, periependymal linear enhancement, leptomeningeal enhancement, enhancement in the hypothalamus, medullary floor of the fourth ventricle and area postrema, and bilateral long segment optic nerve enhancement that extends chiasma) that may be specific to NMO. These patterns are more common in seropositive patients and usually seen during relapses. Recognizing these lesions and enhancement patterns that are more typical of NMO may expedite the diagnosis and treatment.
Acknowledgments
The authors thank Professor David M. Yousem for critical review of the manuscript.
Footnotes
Conflict of interest statements: Dr. Yeliz Pekcevik reports no disclosure. Dr. Gunes Orman reports no disclosure. Dr. In Ho Lee reports no disclosure. Maureen A. Mealy received honoraria from the International Organization of Multiple Sclerosis Nurses and from EMD Serono. Dr. Michael Levy receives research support from the National Institutes of Health, Guthy Jackson Charitable Foundation, Viropharma, Acorda, Sanofi, NeuralStem, and Genentech and serves as a consultant for Chugai Pharmaceuticals, GlaxoSmithKline, and Medimmune. Dr. Izlem Izbudak received research support from Bayer and Siemens. There are no direct financial or other conflicts of interest in relation to the current publication.
Contributor Information
Yeliz Pekcevik, Email: yelizpekcevik@yahoo.com.
Gunes Orman, Email: gorman1@jhmi.edu.
In Ho Lee, Email: leeinho1974@hanmail.net.
Maureen A. Mealy, Email: mmealy1@jhmi.edu.
Michael Levy, Email: mlevy@jhmi.edu.
Izlem Izbudak, Email: iizbuda1@jhmi.edu.
References
- 1.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–12. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 2.Trebst C, Jarius S, Berthele A, Paul F, Schippling S, Wildemann B, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS) J Neurol. 2014;261:1–16. doi: 10.1007/s00415-013-7169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downer JJ, Leite MI, Carter R, Palace J, Küker W, Quaghebeur G. Diagnosis of neuromyelitis optica (NMO) spectrum disorders: is MRI obsolete? Neuroradiology. 2012;54:279–85. doi: 10.1007/s00234-011-0875-x. [DOI] [PubMed] [Google Scholar]
- 4.Palace J, Leite M, Nairne A, Vincent A. Interferon beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch Neurol. 2010;67:1016–7. doi: 10.1001/archneurol.2010.188. [DOI] [PubMed] [Google Scholar]
- 5.Juryńczyk M, Zaleski K, Selmaj K. Natalizumab and the development of extensive brain lesions in neuromyelitis optica. J Neurol. 2013;260:1919–21. doi: 10.1007/s00415-013-6965-4. [DOI] [PubMed] [Google Scholar]
- 6.Tackley G, Kuker W, Palace J. Magnetic resonance imaging in neuromyelitis optica. Mult Scler. 2014:14. doi: 10.1177/1352458514531087. pii: 1352458514531087. [DOI] [PubMed]
- 7.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–9. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 8.Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006;63:390–6. doi: 10.1001/archneur.63.3.390. [DOI] [PubMed] [Google Scholar]
- 9.Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome) Neurology. 1999;53:1107–14. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 10.Makhani N, Bigi S, Banwell B, Shroff M. Diagnosing neuromyelitis optica. Neuroimaging Clin N Am. 2013;23:279–91. doi: 10.1016/j.nic.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Liu Y, Duan Y, Li K. Brain MRI abnormalities in neuromyelitis optica. Eur J Radiol. 2011;80:445–9. doi: 10.1016/j.ejrad.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Cabrera-Gomez JA, Kister I. Conventional brain MRI in neuromyelitis optica. Eur J Neurol. 2012;19:812–9. doi: 10.1111/j.1468-1331.2011.03565.x. [DOI] [PubMed] [Google Scholar]
- 13.Matthews L, Marasco R, Jenkinson M, Küker W, Luppe S, Leite MI, et al. Distinction of seropositive NMO spectrum disorder and MS brain lesion distribution. Neurology. 2013;80:1330–7. doi: 10.1212/WNL.0b013e3182887957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Feng F, Yang Y, Li J, Ma L. MR imaging findings of the corpus callosum region in the differentiation between multiple sclerosis and neuromyelitis optica. Eur J Radiol. 2012;81:3491–5. doi: 10.1016/j.ejrad.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Popescu BF, Lennon VA, Parisi JE, Howe CL, Weigand SD, Cabrera-Gómez JA, et al. Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications. Neurology. 2011;76:1229–37. doi: 10.1212/WNL.0b013e318214332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apiwattanakul M, Popescu BF, Matiello M, Weinshenker BG, Lucchinetti CF, Lennon VA, et al. Intractable vomiting as the initial presentation of neuromyelitis optica. Ann Neurol. 2010;68:757–61. doi: 10.1002/ana.22121. [DOI] [PubMed] [Google Scholar]
- 17.Baba T, Nakashima I, Kanbayashi T, Konno M, Takahashi T, Fujihara K, et al. Narcolepsy as an initial manifestation of neuromyelitis optica with anti-aquaporin-4 antibody. J Neurol. 2009;256:287–8. doi: 10.1007/s00415-009-0139-4. [DOI] [PubMed] [Google Scholar]
- 18.McKeon A, Lennon VA, Lotze T, Tenenbaum S, Ness JM, Rensel M, et al. CNS aquaporin-4 autoimmunity in children. Neurology. 2008;71:93–100. doi: 10.1212/01.wnl.0000314832.24682.c6. [DOI] [PubMed] [Google Scholar]
- 19.Choi SI, Lee YJ, Kim do W, Yang JY. A case of neuromyelitis optica misdiagnosed as cervicogenic headache. Korean J Pain. 2014;27:77–80. doi: 10.3344/kjp.2014.27.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichel R, Meiner Z, Abramsky O, Gotkine M. Acute disseminating encephalomyelitis in neuromyelitis optica: closing the floodgates. Arch Neurol. 2008;65:267–71. doi: 10.1001/archneurol.2007.59. [DOI] [PubMed] [Google Scholar]
- 21.Ito S, Mori M, Makino T, Hayakawa S, Kuwabara S. “Cloud-like enhancement” is a magnetic resonance imaging abnormality specific to neuromyelitis optica. Ann Neurol. 2009;66:425–8. doi: 10.1002/ana.21753. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita T, Isobe N, Matsuoka T, Ishizu T, Kawano Y, Yoshiura T, et al. Extensive vasogenic edema of anti-aquaporin-4 antibody-related brain lesions. Mult Scler. 2009;15:1113–7. doi: 10.1177/1352458509106613. [DOI] [PubMed] [Google Scholar]
- 23.Magaña SM, Matiello M, Pittock SJ, McKeon A, Lennon VA, Rabinstein AA, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology. 2009;72:712–7. doi: 10.1212/01.wnl.0000343001.36493.ae. [DOI] [PubMed] [Google Scholar]
- 24.Kim W, Park MS, Lee SH, Kim SH, Jung IJ, Takahashi T, et al. Characteristic brain magnetic resonance imaging abnormalities in central nervous system aquaporin-4 auto-immunity. Mult Scler. 2010;16:1229–36. doi: 10.1177/1352458510376640. [DOI] [PubMed] [Google Scholar]
- 25.Kim JE, Kim SM, Ahn SW, Lim BC, Chae JH, Hong YH, et al. Brain abnormalities in neuromyelitis optica. J Neurol Sci. 2011;302:43–8. doi: 10.1016/j.jns.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Kim W, Kim SH, Lee SH, Li XF, Kim HJ. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler. 2011;17:1107–12. doi: 10.1177/1352458511404917. [DOI] [PubMed] [Google Scholar]
- 27.Newey CR, Bermel RA. Fulminant cerebral demyelination in neuromyelitis optica. Neurology. 2011;77:193. doi: 10.1212/WNL.0b013e3182242d6e. [DOI] [PubMed] [Google Scholar]
- 28.Tahara M, Ito R, Tanaka K, Tanaka M. Cortical and leptomeningeal involvement in three cases of neuromyelitis optica. Eur J Neurol. 2012;19:e47–8. doi: 10.1111/j.1468-1331.2012.03667.x. [DOI] [PubMed] [Google Scholar]
- 29.McGraw CA, Swerdlow ML, Robbins MS. Acute reversible periependymal ventricular enhancement in neuromyelitis optica. Eur J Neurol. 2012;19:e57–8. doi: 10.1111/j.1468-1331.2012.03696.x. [DOI] [PubMed] [Google Scholar]
- 30.Banker P, Sonni S, Kister I, Loh JP, Lui YW. Pencil-thin ependymal enhancement in neuromyelitis optica spectrum disorders. Mult Scler. 2012;18:1050–3. doi: 10.1177/1352458511431730. [DOI] [PubMed] [Google Scholar]
- 31.Cheng C, Jiang Y, Chen X, Dai Y, Kang Z, Lu Z, et al. Clinical, radiographic characteristics and immunomodulating changes in neuromyelitis optica with extensive brain lesions. BMC Neurol. 2013;13:72. doi: 10.1186/1471-2377-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iorio R, Damato V, Mirabella M, Evoli A, Marti A, Plantone D, et al. Distinctive clinical and neuroimaging characteristics of longitudinally extensive transverse myelitis associated with aquaporin-4 autoantibodies. J Neurol. 2013;260:2396–402. doi: 10.1007/s00415-013-6997-9. [DOI] [PubMed] [Google Scholar]
- 33.Huh SY, Min JH, Kim W, Kim SH, Kim HJ, Kim BJ, et al. The usefulness of brain MRI at onset in the differentiation of multiple sclerosis and seropositive neuromyelitis optica spectrum disorders. Mult Scler. 2014;20:695–704. doi: 10.1177/1352458513506953. [DOI] [PubMed] [Google Scholar]
- 34.Long Y, Chen M, Zhang B, Gao C, Zheng Y, Xie L, et al. Brain gadolinium enhancement along the ventricular and leptomeningeal regions in patients with aquaporin-4 antibodies in cerebral spinal fluid. J Neuroimmunol. 2014;269:62–7. doi: 10.1016/j.jneuroim.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Pekcevik Y, Izbudak I. Perivascular enhancement in a patient with neuromyelitis optica spectrum disease during an optic neuritis attack. J Neuroimaging. 2014 doi: 10.1111/jon.12198. http://dx.doi.org/10.1111/jon.12198. [DOI] [PubMed]
- 36.Levy LM. Exceeding the limits of the normal blood–brain barrier: quo vadis gadolinium? AJNR Am J Neuroradiol. 2007;28:1835–6. doi: 10.3174/ajnr.A0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain. 2007;130:1224–34. doi: 10.1093/brain/awm047. [DOI] [PubMed] [Google Scholar]
- 38.Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125:1450–61. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic–spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–7. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63:964–8. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Wu A, Zhang B, Chen S, Men X, Lin Y, et al. Comparison of deep gray matter lesions on magnetic resonance imaging among adults with acute disseminated encephalomyelitis, multiple sclerosis, and neuromyelitis optica. Mult Scler. 2014;20:418–23. doi: 10.1177/1352458513499420. [DOI] [PubMed] [Google Scholar]
- 42.Khanna S, Sharma A, Huecker J, Gordon M, Naismith RT, Van Stavern GP. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. J Neuroophthalmol. 2012;32:216–20. doi: 10.1097/WNO.0b013e318254c62d. [DOI] [PubMed] [Google Scholar]