Abstract
The “illusory truth” effect refers to the phenomenon whereby repetition of a statement increases its likelihood of being judged true. This phenomenon has important implications for how we come to believe oft-repeated information that may be misleading or unknown. Behavioral evidence indicates that fluency or the subjective ease experienced while processing a statement underlies this effect. This suggests that illusory truth should be mediated by brain regions previously linked to fluency, such as the perirhinal cortex (PRC). To investigate this possibility, we scanned participants with fMRI while they rated the truth of unknown statements, half of which were presented earlier (i.e., repeated). The only brain region that showed an interaction between repetition and ratings of perceived truth was PRC, where activity increased with truth ratings for repeated, but not for new, statements. This finding supports the hypothesis that illusory truth is mediated by a fluency mechanism and further strengthens the link between PRC and fluency.
INTRODUCTION
Every day we encounter unknown claims that we come to accept after repeated exposure, such as “Vikings wore horns on their helmets,” or “The Great Wall of China is visible from space.” Our belief in these statements is partly due to the “illusory truth” effect (Hasher, Goldstein, & Toppino, 1977), where repeated statements appear more truthful than new statements (for a review, see Dechene, Stahl, Hansen, & Wanke, 2010). This effect has clear ramifications for decisions we make in our daily lives as even repetition from untrustworthy (Henkel & Mattson, 2011; Begg, Anas, & Farinacci, 1992) or fictional (Marsh, Meade, & Roediger, 2003) sources makes statements more believable; “if everybody appears to be saying that climate science is corrupt, or that the MMR vaccine causes autism, it takes on the appearance of fact” (Giles, 2010, p. 43). Consistent with this, exposure to misconceptions about vaccines and autism increases mistrust of vaccines, even in a sample not predisposed to such a viewpoint (Betsch, Renkewitz, Betsch, & Ulshöfer, 2010). Thus, how we process repeated information has many real-world implications for how we learn (Herzog & Hertwig, 2013) and can even lead to increased false memories (Zaragoza & Mitchell, 1996).
Repeated information is easier to process at both sensory (i.e., perceptual) and semantic (i.e., conceptual) levels (Whittlesea, 1993). Recent work suggests that this processing fluency drives illusory truth, wherein ease of processing is interpreted as evidence of truth (Reber & Unkelbach, 2010; Reber & Schwarz, 1999; Kelley & Lindsay, 1993). Consistent with this, illusory truth can occur even without repetition. For example, people assign higher truth ratings to rhyming than nonrhyming aphorisms (McGlone & Tofighbakhsh, 2000), to statements in high-contrast rather than low-contrast fonts (Parks & Toth, 2006; Reber & Schwarz, 1999), and to statements embedded in a congruent relative to an incongruent context (Parks & Toth, 2006).
Beyond the illusory truth effect, fluency is thought to influence a variety of inferential decisions. As examples, fluent words appear more familiar (Lindsay & Kelley, 1996; Jacoby & Whitehouse, 1989), fluent names more famous (Jacoby, Woloshyn, & Kelley, 1989), fluent exemplars more frequent (Tversky & Kahneman, 1973), and fluent paintings more appreciated (Belke, Leder, Strobach, & Carbon, 2010). This consistency across cognitive tasks suggests that there is a common mechanism driving these fluency effects (e.g., Unkelbach & Greifeneder, 2013; Alter & Oppenheimer, 2009).
The hypothesis that fluency drives the illusory truth effect could be bolstered by neuroimaging evidence that regions associated with fluency are also associated with illusory truth. Surprisingly, the neural mechanisms underpinning the perceived truth of repeated claims remain largely unknown, and to our knowledge, only one fMRI study uses an illusory truth paradigm. Mitchell, Dodson, and Schacter (2005) exposed participants to ambiguous claims paired with either an explicit label (“true” or “false”) or no label. Participants later judged the truthfulness of these statements, as well as new ones. The fMRI analyses, however, focused on statements explicitly labeled as “true” or “false,” rather than the unlabeled statements that provided insight into illusory truth. In other words, their neuroimaging data address memory for sources of information (i.e., labels), rather than the biasing influence of repetition on evaluations of claims.
Although little is known about the neural correlates of illusory truth, several lines of functional neuroimaging and patient lesion research implicate the perirhinal cortex (PRC). First, this region subserves recognition memory and familiarity-based recognition, in particular (e.g., Bowles et al., 2007; for a review, see Eichenbaum, Yonelinas, & Ranganath, 2007). Not only does PRC differentiate between objectively old and new stimuli (Henson, Cansino, Herron, Robb, & Rugg, 2003), but it also tracks perceived oldness or memory confidence (Wang, Ranganath, & Yonelinas, 2014; Danckert, Gati, Menon, & Köhler, 2007; Daselaar, Fleck, & Cabeza, 2006; Montaldi, Spencer, Roberts, & Mayes, 2006; Gonsalves, Kahn, Curran, Norman, & Wagner, 2005). Second, it is involved in semantic judgments and semantic memory (Bruffaerts et al., 2013; Bowles et al., 2007; Taylor, Moss, Stamatakis, & Tyler, 2006; Davies, Graham, Xuereb, Williams, & Hodges, 2004). Finally, PRC is critical in differentiating between primed (i.e., fluently processed) and unprimed semantic information in tasks assessing conceptual implicit memory (Wang et al., 2014; Heusser, Awipi, & Davachi, 2013; Voss, Federmeier, & Paller, 2012; Wang, Lazzara, Ranganath, Knight, & Yonelinas, 2010; O’Kane, Insler, & Wagner, 2005).
Paralleling the cognitive literature, these patient and neuroimaging data suggest a common fluency-related mechanism in PRC (Wang & Yonelinas, 2012; Dew & Cabeza, 2011; Henke, 2010). Direct evidence for this comes from our recent recognition memory study that found higher rates of false alarms to unstudied words that were primed with a masked conceptual associate, which encouraged fluent processing (Dew & Cabeza, 2013). This effect was linked to changes in PRC activity, raising the possibility that PRC may support other fluency effects, such as illusory truth. Thus, the current study investigates whether PRC is sensitive to repetition and perceived truth during judgments of truth, thus mediating illusory truth. Evidence for a mediating role of PRC would offer valuable support for the idea that a common mechanism supports various types of fluency-based decision-making. We tested this hypothesis by exposing participants to unknown claims (e.g., Eagle syndrome is caused by a calcified ligament), after which they rated the truthfulness of these and new statements in the MRI scanner. We predicted that participants would perceive repeated statements to be truer than new statements, with the additional novel prediction that PRC activity would mediate this effect.
METHODS
Participants
Thirty-one native English speakers from Duke University and the surrounding community participated for monetary compensation. The Duke University Institutional Review Board approved all procedures. Seven participants were excluded, three due to technical malfunctions with the scanner or testing computer and four due to issues with performance (two fell asleep, one had chance performance, and the fourth failed to use the entire scale). The final sample included 24 participants (age M = 23.17, SEM = 0.68; education M = 15.33, SEM = 0.41; 10 women).
Materials
Materials consisted of 360 trivia statements collected from the Internet and referred to either unknown or known facts. For unknown facts, we created a true framing (e.g., The inhabitable part of the world is the toponym) and a matching false framing (e.g., The inhabitable part of the world is the ecumene) that referred to a plausible, but incorrect, alternative. Two thirds of the statements were unknown; of these items, half were true and the other half were false (counterbalanced across participants). Extensive piloting demonstrated minimal differences in truth ratings between unknown true and unknown false facts, regardless of their framing. As a result, we collapsed across framing in our analyses. The remaining one third of the items were known facts (e.g., The capital city of Spain is called Madrid). These statements all appeared in a true framing, and participants reliably and confidently rated them as true during piloting. This ceiling effect prevented their inclusion in our illusory truth analyses.
Design and Procedure
Following informed consent, participants completed the exposure phase. They rated 180 trivia statements for subjective interest on a 6-point scale from 1 (very interesting) to 6 (very uninteresting), with the scale being reversed in half the participants. Each statement was presented for 4 sec, followed by a 1-sec ISI fixation. To enhance fluency and maximize illusory truth, participants completed this task twice.
Participants then entered the MRI scanner to perform the truth rating phase (i.e., is this statement true or false) and an episodic recognition task (i.e., is this statement old or new) in four separate counterbalanced ABBA runs. The imaging data in the latter task is not relevant to the illusory truth effect and is not discussed further. In the task of interest, participants assigned truth ratings on a 6-point confidence scale from 1 (definitely true) to 6 (definitely false), with the scale being reversed in half the participants. In addition to the warning that they would encounter true and false statements, participants knew that some statements appeared earlier in the experiment (i.e., were repeated), whereas others were new. Overall, participants rated the truth of 60 repeated unknown, 60 new unknown, 30 repeated known, and 30 new known statements, divided equally across the two knowledge task runs. The different types of statements were intermixed, and each was presented for 5 sec with a 3-sec mean ISI fixation.
Image Acquisition
Images were collected on a 3-T General Electric scanner with an eight-channel head coil at the Duke University Brain Imaging and Analysis Center. Functional images were acquired using a SENSE spiral sequence (64 × 64 matrix, repetition time = 2000 msec, echo time = 27 msec, field of view = 24 cm, flip angle = 60°) and consisted of 34 axial slices acquired in an interleaved fashion. Slice thickness was 3.8 mm, resulting in 3.75 × 3.75 × 3.8 mm voxels. Additionally, high-resolution structural images were collected using a 3-D, T1-weighted FSPGR sequence (256 × 256 matrix, 166 slices, 1 mm isotropic voxels).
Data were preprocessed and analyzed with SPM8 (fil.ion.ucl.ac.uk/spm). After discarding the first three scans of each block, the functional data for each participant were slice time-corrected, realigned, and coregistered to their respective anatomical images. The anatomical images were then segmented into separate gray and white matter images that were used with the DARTEL toolbox to create average gray and white matter templates. These templates were used to normalize the functional and anatomical images into MNI (Montreal Neuroimaging Institute) space. Lastly, the spatially normalized functional data were spatially smoothed with an 8-mm isotropic FWHM Gaussian filter.
Image Analysis
To investigate illusory truth, we focused our analyses on 3 (maybe false), 4 (maybe true), and 5 (probably true) responses to unknown statements. Participants made too few 1 (definitely false), 2 (probably false), and 6 (definitely true) responses to include in the analyses. Additionally, as discussed above, known statements served as fillers and were not analyzed. Statistical analyses were performed in SPM8 using the general linear model. A high-pass filter of 128 sec and grand mean scaling were applied to the data, and serial correlations in the time series were accounted for using the autoregressive model (AR[1]). The functional data were modeled using a canonical hemodynamic response function with the stimulus onsets serving as event onsets. Repeated and new statements that received a 3, 4, or 5 truth rating were modeled as conditions of interest. All other trials were modeled separately according to trial type. Additional covariates of no interest included the six motion parameters estimated during realignment, baseline and session effects, and global mean and motion outliers obtained from the Artifact Detection Toolbox (nitrc.org/projects/artifact_detect).
Analyses were conducted using an uncorrected threshold of p < .001 and a cluster size (cs) of no less than 10 contiguous voxels (Lieberman & Cunningham, 2009). To assess illusory truth, we conducted a 2 (repetition: repeated, new) × 3 (perceived truth: 3, 4, 5) ANOVA in a second-level random-effects analysis. The primary contrast was an F-contrast of the interaction between repetition and perceived truth. We also highlight the main effects of repetition and perceived truth in the results, and these t contrasts were exclusively masked by the interaction contrast described above (p < .05 uncorrected). Lastly, to assess within-participant (i.e., trial-by-trial) correlations between neural activity and behavioral responses, each trial was modeled in a separate model, yielding different beta values for each trial and each participant using the LSS approach (Mumford, Turner, Ashby, & Poldrack, 2012). For each individual, beta values from the seed cluster of interest were z-scored and entered into ordered logistic regression analyses using the behavioral response on each trial as the ordinal dependent variable. Mean standardized coefficients for repeated and new statements were then submitted to t tests (p < .05) for analyses.
RESULTS
Behavioral Results
Consistent with the illusory truth effect, participants assigned higher truth ratings to repeated (M = 4.07, SEM = 0.08) than new (M = 3.80, SEM = 0.08) statements (t(23) = 3.30, p = .004, Cohen’s d = 0.73). In addition, participants responded significantly faster to repeated (M = 3567 msec, SEM = 99 msec) than new (M = 4242 msec,SEM = 111 msec) statements (t(23) = −12.66, p < .001, d = 1.28). These patterns remain consistent when we restricted our analyses to 3 (maybe false), 4 (maybe true), and 5 (probably true) responses (excluding 12% of repeated unknown and 11% of new unknown statements), the bins used in the fMRI analyses. Critically, participants assigned higher truth ratings to repeated (M = 4.03, SEM = 0.04) than new (M = 3.80, SEM = 0.08) statements (t(23) = 4.99, p < .001; d = 0.66); in addition, they responded more quickly to repeated (M = 3619 msec, SEM = 107 msec) than to new (M = 4234 msec, SEM = 110 msec) statements (F(1, 23) = 79.01, p < .001; ) in each response bin (ts(23) > −4.06, ps < .001; ds > 0.86).
Imaging Results
First we examined which brain regions were sensitive to repetition. Consistent with the fMRI literature on involuntary memory (Hall et al., 2014; Habib & Nyberg, 2008), several parietal and posterior midline components of the episodic retrieval network exhibited greater activity for repeated relative to new statements regardless of truth (see Table 1), even though participants were not instructed to discriminate between repeated and new statements. No suprathreshold clusters exhibited greater activity for new relative to repeated statements. Second, we examined which regions were sensitive to perceived truth. One cluster in the right occipital fusiform cortex (Z = 3.39; x = 38, y = −68, z = −15; cs = 12) exhibited greater activity as a function of decreasing perceived truth, possibly reflecting greater visual (reading) attention to statements that seemed ambiguous. No suprathreshold clusters exhibited increasing activity as a function of increasing perceived truth.
Table 1.
Greater Activity for Repeated than New Statements
| Region | Hem | x | y | z | Z | cs |
|---|---|---|---|---|---|---|
| Parieto-occipital | ||||||
| Precuneus | L | −11 | −68 | 34 | 6.99 | 72 |
| R | 8 | −68 | 34 | 5.56 | 108 | |
| Lateral occipital cortex | L | −45 | −60 | 38 | 6.14 | 200 |
| Angular gyrus | L | −38 | −56 | 38 | 6.09 | |
| R | 45 | −52 | 42 | 5.41 | 223 | |
| Superior parietal lobule | R | 45 | −41 | 57 | 3.29 | |
| Midline | ||||||
| Posterior cingulate | L | −4 | −30 | 27 | 4.83 | 64 |
| R | 4 | −19 | 27 | 4.55 | ||
| Paracingulate gyrus | L | −8 | 34 | 30 | 3.77 | 40 |
| R | 8 | 34 | 34 | 3.44 | ||
| Frontal | ||||||
| Frontal pole | L | −26 | 49 | 0 | 3.95 | 88 |
| R | 19 | 56 | −4 | 3.51 | 19 |
Hem = Hemisphere; cs = cluster size.
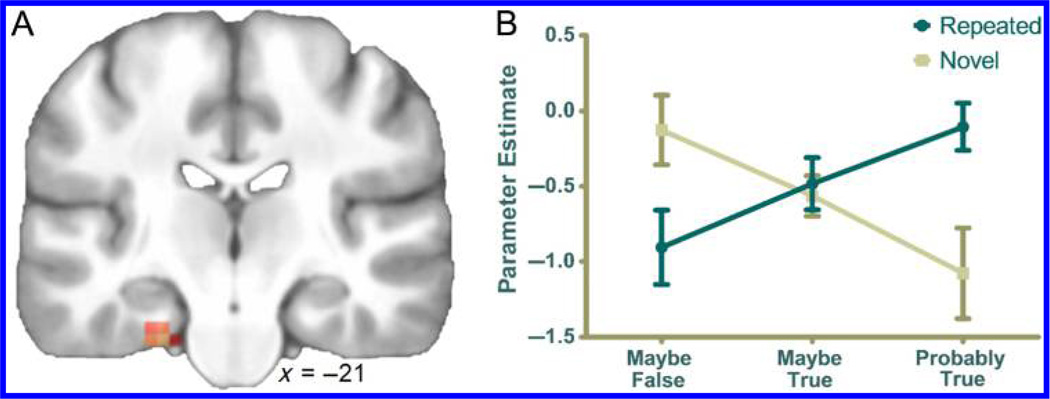
Most importantly, we targeted the neural correlates of illusory truth by examining the interaction between repetition and perceived truth. That is, we tested for activity changes across response bins (3, 4, 5) that differed between repeated and new statements. As illustrated in Figure 1A, this F-contrast yielded only one suprathreshold cluster in the whole brain. Consistent with our expectations, this cluster was in PRC (Z = 3.91; x = −22, y = −19, z = −30; cs = 10) and survived correction for multiple comparisons within a PRC mask.1 A post hoc analysis indicated that there was a significant positive linear trend (F(1, 23) = 5.81, p = .024; ) for repeated statements (i.e., increasing activity as a function of perceived truth) and a significant negative linear trend (F(1, 23) = 8.25, p = .009; ) for new statements (see Figure 1B). The crossover interaction between these two linear trends was significant (F(1, 23) = 9.19, p = .006; ). To confirm the linearity of these effects, we tested for quadratic trends and found that they were all nonsignificant (repeated statements, new statements, and interaction: all Fs < 1).
Figure 1.
Brain regions exhibiting an interaction between perceived truth and repetition. (A) The only region to exhibit a significant perceived truth by repetition interaction was the left perirhinal cortex. (B) Repeated statements exhibited in increasing activity as a function of perceived truth, whereas the opposite pattern occurred for new statements. Error bars reflect SEM.
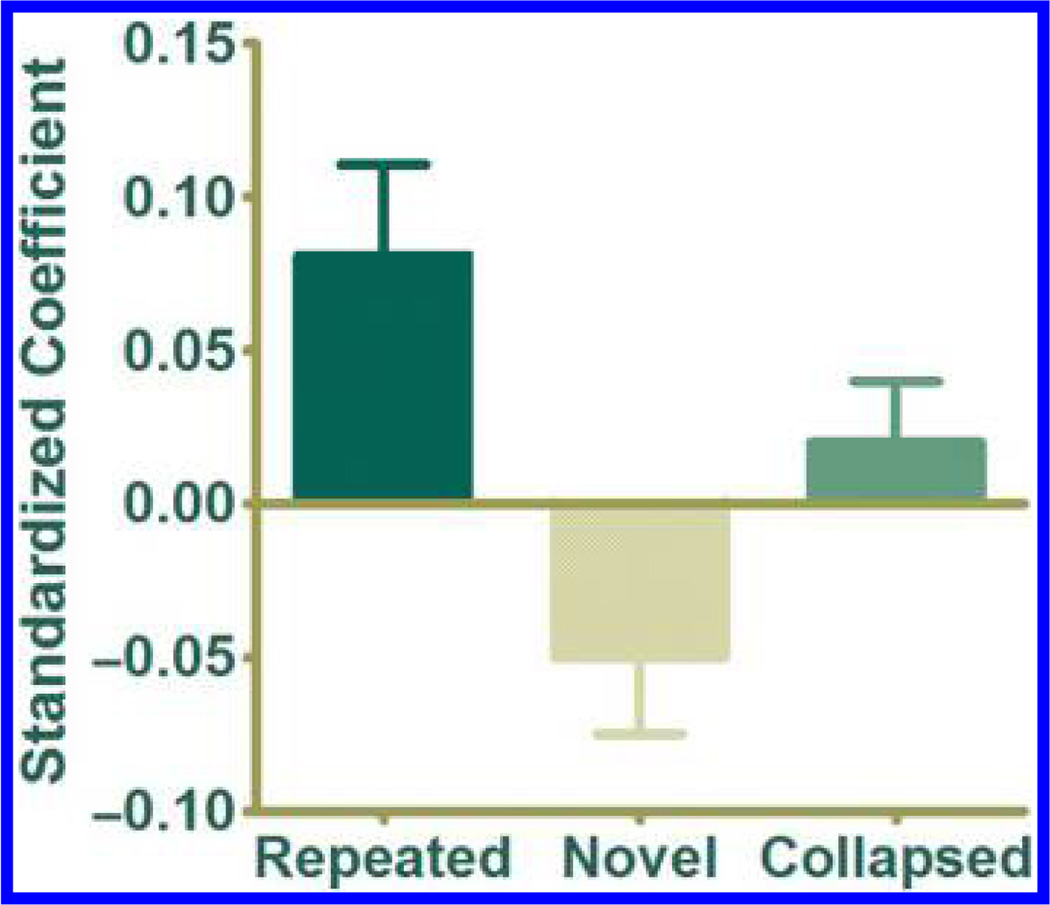
Our previous contrast identifying PRC showed activity changes that occurred with different truth ratings on average (i.e., collapsing across trials within a given bin). In addition, we were interested in whether trial-by-trial judgments of truth were associated with concurrent changes in PRC activity. We extracted mean parameter estimates from the PRC cluster described above for each individual trial in each individual participant. Separate regressions were then conducted for repeated and new statements for each participant, with these parameter estimates as the dependent variable. As illustrated in Figure 2, this analysis offered converging results; increases in trial-by-trial PRC activity predicted increases in the perceived truth of repeated statements (β = 0.08, SEM = 0.03; t(23) = 2.73, p = .012; d = 0.56), whereas decreases in PRC activity predicted increases in the perceived truth of new statements (β = −0.05, SEM = 0.02; t(23) = −1.95, p = .063; d = 0.40). Additionally, the difference between these two conditions was statistically significant (t(23) = 3.19, p = .004; d = 0.99).On the other hand, PRC activity neither positively nor negatively predicted truth when collapsing across repeated and new statements (β = 0.02, SEM = 0.02; t(23) = 1.17, p > .250).
Figure 2.
An analysis of trial-by-trial activity in this PRC cluster yielded converging results; activity for repeated statements positively predicted perceived truth, activity for new statements negatively predicted perceived truth, and activity for repeated and new statements collapsed neither positively nor negatively predicted perceived truth. Error bars reflect SEM.
DISCUSSION
The present work is one of the first to illuminate the neural correlates of illusory truth, a well-documented effect in the cognitive and social psychology literatures (Dechene et al., 2010). Our findings corroborate a fluency account of illusory truth and contribute to a larger literature implicating PRC in fluency-based inferences. The behavioral data replicate previous findings, with repeated statements receiving higher truth ratings than new statements. In addition, the neuroimaging analyses indicate that activity in PRC mediated illusory truth. Specifically, activity increased as a function of perceived truth of repeated (i.e., fluent) statements but decreased as a function of perceived truth of new statements. Moreover, an analysis of trial-by-trial activity revealed a similar effect: PRC activity positively predicted perceived truth of repeated statements and negatively predicted perceived truth of new statements.
Previous imaging work shows that PRC is related to both familiarity- and semantic-based memory retrieval. PRC not only exhibits activity changes in response to repetition during explicit recognition (Henson et al., 2003) and conceptual priming (Wang et al., 2014; Heusser et al., 2013; Voss et al., 2012; O’Kane et al., 2005) but also exhibits activity changes as a function of the memory strength or familiarity of items during item recognition, often irrespective of its actual old/new status (Wang et al., 2014; Danckert et al., 2007; Daselaar et al., 2006; Montaldi et al., 2006; Gonsalves et al., 2005). These data accord with the notion that a common neural mechanism support fluency-based mnemonic decisions (Wang & Yonelinas, 2012; Dew & Cabeza, 2011; Henke, 2010) and also complement cognitive proposals positing that a common mechanism can explain fluency effects across judgment tasks (Unkelbach & Greifeneder, 2013; Alter & Oppenheimer, 2009). Consistent with this notion, a recent study examining how masked conceptual priming influences recognition judgments and PRC activity demonstrated that the effects of repetition on PRC during mnemonic tasks may be mediated by fluency (Dew & Cabeza, 2013).
The current study extends previous work by illuminating the effect of fluency on PRC activity in a non-mnemonic task. Our results accord with Dew and Cabeza (2013) in suggesting that PRC activity changes may not only index episodic memory, but also fluency. That is, we did not observe a main effect of repetition in PRC, but rather an interaction between repetition and truth ratings: Activity increased as a function of perceived truth for repeated statements and decreased as a function of perceived truth for new statements. Although this interaction is not directly comparable to existing studies reporting a main effect of repetition or fluency, it accords with evidence of PRC activity increases for repeated stimuli during the implicit retrieval of novel semantic associations (Reber, Luechinger, Boesiger, & Henke, 2014; Henke et al., 2003) and during the perceptual discrimination of unfamiliar faces (Mundy, Downing, Dwyer, Honey, & Graham, 2013).
However, the direction of the effects in this study diverge from the more common finding of PRC activity reductions during fluent processing (e.g., familiarity or conceptual priming), including the pattern reported by Dew and Cabeza (2013). Rather, our result are consistent with evidence of repetition-related activity increases in ventral temporal regions for unfamiliar or novel stimuli (Gagnepain et al., 2008; Soldan, Zarahn, Hilton, & Stern, 2008; Fiebach, Gruber, & Supp, 2005; Henson, 2001; Henson, Shallice, & Dolan, 2000). Henson (2003) suggests that this repetition enhancement of repeated unfamiliar (i.e., unknown) stimuli—in contrast to the more commonly observed repetition suppression of repeated familiar stimuli (e.g., objects, words, famous faces)—reflects additional processing of novel components of the unknown stimulus that did not occur during its initial encoding (e.g., fluent semantic retrieval). This interpretation is consistent with the finding of PRC activations during the fluent processing of novel semantic associations (Reber et al., 2014; Henke et al., 2003) and unfamiliar faces (Mundy et al., 2013) and is also consistent within the context of our study: Participants lacked knowledge about the statements (i.e., no previous representations existed) and, critically, were not required to assess truth during the initial exposure phase. Thus, activity increases as a function of perceived truth for repeated statements may reflect the fluent semantic processing of these newly created representations. On the other hand, PRC activity decreased as a function of perceived truth for new statements, perhaps reflecting semantic fluency (Kuperberg, Sitnikova, & Lakshmanan, 2008; Rossell, Price, & Nobre, 2003) and/or incidental encoding of new statements whose veracity seemed uncertain.
As mentioned earlier, repetition uniquely induces both perceptual and conceptual fluency. Previous work indicates that conceptual fluency leads to a greater illusory truth effect compared with perceptual fluency (Parks & Toth, 2006), that PRC activity changes are related to conceptual fluency (Wang et al., 2014; Dew & Cabeza, 2013; Heusser et al., 2013; Voss et al., 2012; O’Kane et al., 2005), and that PRC lesions affect conceptual fluency (Wang et al., 2010; Bowles et al., 2007; Davies et al., 2004). Thus, although the current study does not distinguish between conceptual and perceptual fluency, it is likely that conceptual fluency contributed more to our observed effects, particularly given our use of trivia statements. Future work assessing the neural correlates of illusory truth should manipulate conceptual and perceptual (rather than repetition) fluency to confirm the role of PRC in conceptual fluency. Additionally, although it is unlikely that explicit recollection differentially biased the perceived truth of repeated unknown statements—given that illusory truth can be elicited without repetition (e.g., Parks & Toth, 2006; McGlone & Tofighbakhsh, 2000; Reber & Schwarz, 1999) and given that repeated statements were encoded twice and homogenously recognizable2 (thus, it is unlikely that some repeated sentences were perceived as more true than others because of explicit memory)—assessing the neural correlates of illusory truth by manipulating perceptual and conceptual fluency will also rule out the influence of explicit memory in our illusory truth effect.
More broadly, future work should seek to replicate our finding to shed light on the directionality of fluency-related PRC effects. As mentioned above, we observed an interaction between repetition and perceived truth, whereas most previous studies of fluency or implicit memory simply report an effect of repetition suppression. We expect that other effects influenced by fluency, such as the mere exposure effect for unfamiliar (as opposed to familiar) stimuli, would yield similar interactions between repetition and behavior in PRC, with increasing activity for repeated (i.e., fluent) trials as a function of increasing ratings of likability (or frequency or fame). Although our pattern of results could be explained by the unfamiliar nature of our stimuli, other factors may also play a role. For example, truth ratings were unrelated to episodic memory, unlike most studies of recognition confidence, and constituted a novel decision not performed during the initial encoding, unlike most studies of repetition priming. Unfortunately, as discussed above, the ceiling performance in our episodic memory task prevented us from assessing activity changes as a function of recognition confidence. Thus, future studies should seek to investigate the role of the task performed on repeated stimuli. Such manipulations, as well as those examining fluency with and without repetition, will illuminate circumstances under which patterns of PRC activity (i.e., enhancement or suppression) may differ during fluent processing (Segaert, Weber, de Lange, Petersson, & Hagoort, 2013).
To summarize, the current study provides evidence for the role of PRC in illusory truth. We observed increasing PRC activity as a function of perceived truth of repeated (i.e., fluent) statements, in conjunction with decreasing PRC activity as a function of perceived truth of new statements. We propose that these different effects may reflect the sensitivity of PRC to both mnemonic and nonmnemonic forms of fluency. Our results offer important evidence for a common mechanism that can account for multiple types of fluency-based decisions (Unkelbach & Greifeneder, 2013; Alter & Oppenheimer, 2009) and have significant implications for understanding the process by which repeated information, even if false or suspect in nature, can seamlessly become integrated into our knowledge base.
Acknowledgments
We thank Shaina Garrison, Elena Lagon, and Rachel James for their help with developing stimuli, recruiting participants, and collecting data. This work was supported by a NSF Graduate Research Fellowship (N. M. B.), and fellowship F32 AG049574 (W. C. W.) and grant R01 AG034580 (R. C.) from the National Institute on Aging.
Footnotes
The entire cluster fell within an anterior parahippocampal mask derived from the Harvard–Oxford Cortical Structural Atlas (fsl.fmrib.ox.ac.uk/fsl) and, using 3dClustSim (afni.nimh.nih.gov/afni), survived correction for multiple comparisons (p < .05) within this mask using a voxel-wide threshold of p < .001 and a cs of no less than six contiguous voxels.
Recognition memory was near ceiling in the episodic recognition task. The mean hit rates for repeated unknown statements were 90% (SEM = 2%) and 79% (SEM = 3%) when restricted to high-confidence old responses (M memory confidence = 5.43 out of 6, SEM = 0.10), whereas the false alarm rates were 10% (SEM = 2%) and 2% (SEM = 1%) when restricted to high-confidence new responses (M memory confidence = 1.64 out of 6, SEM = 0.13).
REFERENCES
- Alter AL, Oppenheimer DM. Uniting the tribes of fluency to form a metacognitive nation. Personality and Social Psychology Review. 2009;13:219–235. doi: 10.1177/1088868309341564. [DOI] [PubMed] [Google Scholar]
- Begg IM, Anas A, Farinacci S. Dissociation of processes in belief: Source recollection, statement familiarity, and the illusion of truth. Journal of Experimental Psychology: General. 1992;121:446–458. [Google Scholar]
- Belke B, Leder H, Strobach T, Carbon CC. Cognitive fluency: High-level processing dynamics in art appreciation. Psychology of Aesthetics, Creativity, and the Arts. 2010;4:214–222. [Google Scholar]
- Betsch C, Renkewitz F, Betsch T, Ulshöfer C. The influence of vaccine-critical websites on perceiving vaccination risks. Journal of Health Psychology. 2010;15:446–455. doi: 10.1177/1359105309353647. [DOI] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, et al. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruffaerts R, Dupont P, Peeters R, De Deyne S, Storms G, Vandenberghe R. Similarity of fMRI activity patterns in left perirhinal cortex reflects semantic similarity between words. Journal of Neuroscience. 2013;33:18597–18607. doi: 10.1523/JNEUROSCI.1548-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danckert S, Gati J, Menon R, Köhler S. Perirhinal and hippocampal contributions to visual recognition memory can be distinguished from those of occipito-temporal structures based on conscious awareness of prior occurrence. Hippocampus. 2007;17:1081–1092. doi: 10.1002/hipo.20347. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza RE. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Davies RR, Graham KS, Xuereb JH, Williams GB, Hodges JR. The human perirhinal cortex and semantic memory. European Journal of Neuroscience. 2004;20:2441–2446. doi: 10.1111/j.1460-9568.2004.03710.x. [DOI] [PubMed] [Google Scholar]
- Dechene A, Stahl C, Hansen J, Wanke M. The truth about the truth: A meta-analytic review of the truth effect. Personality and Social Psychology Review. 2010;14:238–257. doi: 10.1177/1088868309352251. [DOI] [PubMed] [Google Scholar]
- Dew ITZ, Cabeza R. The porous boundaries between explicit and implicit memory: Behavioral and neural evidence. Annals of the New York Academy of Sciences. 2011;1224:174–190. doi: 10.1111/j.1749-6632.2010.05946.x. [DOI] [PubMed] [Google Scholar]
- Dew ITZ, Cabeza R. A broader view of perirhinal function: From recognition memory to fluency-based decisions. Journal of Neuroscience. 2013;33:14466–14474. doi: 10.1523/JNEUROSCI.1413-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Gruber T, Supp GG. Neuronal mechanisms of repetition priming in occipitotemporal cortex: Spatiotemporal evidence from functional magnetic resonance imaging and electroencephalography. Journal of Neuroscience. 2005;25:3414–3422. doi: 10.1523/JNEUROSCI.4107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnepain P, Chételat G, Landeau B, Dayan J, Eustache F, Lebreton K. Spoken word memory traces within the human auditory cortex revealed by repetition priming and functional magnetic resonance imaging. Journal of Neuroscience. 2008;28:5281–5289. doi: 10.1523/JNEUROSCI.0565-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles J. Giving life to a lie. New Scientist. 2010;206:42–43. [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: Multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Habib R, Nyberg L. Neural correlates of availability and accessibility in memory. Cerebral Cortex. 2008;18:1720–1726. doi: 10.1093/cercor/bhm201. [DOI] [PubMed] [Google Scholar]
- Hall SA, Rubin DC, Miles A, Davis SW, Wing EA, Cabeza R, et al. The neural basis of involuntary episodic memories. Journal of Cognitive Neuroscience. 2014;26:2385–2399. doi: 10.1162/jocn_a_00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Goldstein D, Toppino T. Frequency and the conference of referential validity. Journal of Verbal Learning and Verbal Behavior. 1977;16:107–112. [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nature Reviews Neuroscience. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Henke K, Mondadori CR, Treyer V, Nitsch RM, Buck A, Hock C. Nonconscious formation and reactivation of semantic associations by way of the medial temporal lobe. Neuropsychologia. 2003;41:863–876. doi: 10.1016/s0028-3932(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Henkel LA, Mattson ME. Reading is believing: The truth effect and source credibility. Consciousness and Cognition. 2011;20:1705–1721. doi: 10.1016/j.concog.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Henson RNA. Repetition effects for words and nonwords as indexed by event-related fMRI: A preliminary study. Scandinavian Journal of Psychology. 2001;42:179–186. doi: 10.1111/1467-9450.00229. [DOI] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Cansino S, Herron JE, Robb WGK, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:259–262. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Herzog SM, Hertwig R. The ecological validity of fluency. In: Unkelbach C, Greifeneder R, editors. The experience of thinking: How the fluency of mental processes influences cognition and behaviour. New York: Psychology Press; 2013. pp. 190–219. [Google Scholar]
- Heusser AC, Awipi T, Davachi L. The ups and downs of repetition: Modulation of the perirhinal cortex by conceptual repetition predicts priming and long-term memory. Neuropsychologia. 2013;51:2333–2343. doi: 10.1016/j.neuropsychologia.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL, Whitehouse K. An illusion of memory: False recognition influenced by unconscious perception. Journal of Experimental Psychology: General. 1989;118:126–135. [Google Scholar]
- Jacoby LL, Woloshyn V, Kelley C. Becoming famous without being recognized: Unconscious influences of memory produced by dividing attention. Journal of Experimental Psychology: General. 1989;118:115–125. [Google Scholar]
- Kelley CM, Lindsay DS. Remembering mistaken for knowing: Ease of retrieval as a basis for confidence in answers to general knowledge questions. Journal of Memory and Language. 1993;32:1–24. [Google Scholar]
- Kuperberg GR, Sitnikova T, Lakshmanan BM. Neuroanatomical distinctions within the semantic system during sentence comprehension: Evidence from functional magnetic resonance imaging. Neuroimage. 2008;40:367–388. doi: 10.1016/j.neuroimage.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: Re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay DS, Kelley CM. Creating illusions of familiarity in a cued recall remember/know paradigm. Journal of Memory and Language. 1996;35:197–211. [Google Scholar]
- Marsh EJ, Meade ML, Roediger HL., III Learning facts from fiction. Journal of Memory and Language. 2003;49:519–536. [Google Scholar]
- McGlone MS, Tofighbakhsh J. Birds of a feather flock conjointly (?): Rhyme as reason in aphorisms. Psychological Science. 2000;11:424–428. doi: 10.1111/1467-9280.00282. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Dodson CS, Schacter DL. fMRI evidence for the role of recollection in suppressing misattribution errors: The illusory truth effect. Journal of Cognitive Neuroscience. 2005;17:800–810. doi: 10.1162/0898929053747595. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Turner BO, Ashby FG, Poldrack RA. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. Neuroimage. 2012;59:2636–2643. doi: 10.1016/j.neuroimage.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy ME, Downing PE, Dwyer DM, Honey RC, Graham KS. A critical role for the hippocampus and perirhinal cortex in perceptual learning of scenes and faces: Complementary findings from amnesia and fMRI. Journal of Neuroscience. 2013;33:10490–10502. doi: 10.1523/JNEUROSCI.2958-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kane G, Insler RZ, Wagner AD. Conceptual and perceptual novelty effects in human medial temporal cortex. Hippocampus. 2005;15:326–332. doi: 10.1002/hipo.20053. [DOI] [PubMed] [Google Scholar]
- Parks CM, Toth JP. Fluency, familiarity, aging, and the illusion of truth. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology, and Cognition. 2006;13:225–253. doi: 10.1080/138255890968691. [DOI] [PubMed] [Google Scholar]
- Reber R, Schwarz N. Effects of perceptual fluency on judgments of truth. Consciousness and Cognition. 1999;8:338–342. doi: 10.1006/ccog.1999.0386. [DOI] [PubMed] [Google Scholar]
- Reber R, Unkelbach C. The epistemic status of processing fluency as source for judgments of truth. Review of Philosophy and Psychology. 2010;1:563–581. doi: 10.1007/s13164-010-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber TP, Luechinger R, Boesiger P, Henke K. Detecting analogies unconsciously. Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41:550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Segaert K, Weber K, de Lange FP, Petersson KM, Hagoort P. The suppression of repetition enhancement: A review of fMRI studies. Neuropsychologia. 2013;51:59–66. doi: 10.1016/j.neuropsychologia.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Soldan A, Zarahn E, Hilton HJ, Stern Y. Global familiarity of visual stimuli affects repetition-related neural plasticity but not repetition priming. Neuroimage. 2008;39:515–526. doi: 10.1016/j.neuroimage.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KI, Moss HE, Stamatakis EA, Tyler LK. Binding crossmodal object features in perirhinal cortex. Proceedings of the National Academy of Sciences, U.S.A. 2006;103:8239–8244. doi: 10.1073/pnas.0509704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Availability: A heuristic for judging frequency and probability. Cognitive Psychology. 1973;5:207–232. [Google Scholar]
- Unkelbach C, Greifeneder R. A general model of fluency effects in judgment and decision making. The Experience of Thinking. 2013:11–32. [Google Scholar]
- Voss JL, Federmeier KD, Paller KA. The potato chip really does look like Elvis! Neural hallmarks of conceptual processing associated with finding novel shapes subjectively meaningful. Cerebral Cortex. 2012;22:2354–2364. doi: 10.1093/cercor/bhr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Lazzara MM, Ranganath C, Knight RT, Yonelinas AP. The medial temporal lobe supports conceptual implicit memory. Neuron. 2010;68:835–842. doi: 10.1016/j.neuron.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Ranganath C, Yonelinas AP. Activity reductions in perirhinal cortex predict conceptual priming and familiarity-based recognition. Neuropsychologia. 2014;52:19–26. doi: 10.1016/j.neuropsychologia.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Yonelinas AP. Familiarity and conceptual implicit memory: Individual differences and neural correlates. Cognitive Neuroscience. 2012;3:213–214. doi: 10.1080/17588928.2012.689968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittlesea BW. Illusions of familiarity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19:1235–1253. [Google Scholar]
- Zaragoza MS, Mitchell KJ. Repeated exposure to suggestion and the creation of false memories. Psychological Science. 1996;7:294–300. [Google Scholar]