Abstract
Background
Routine postoperative intensive care unit (ICU) observation of patients undergoing cytoreductive surgery (CRS) and heated intraperitoneal chemotherapy (HIPEC) is driven by historically reported morbidity and mortality data. The validity of this practice and the criteria for ICU admission have not been elucidated.
Methods
A prospectively maintained database of 1146 CRS/HIPEC procedures performed from December 1991 to 2014 was retrospectively analyzed. Patients with routine postoperative ICU admission were compared with patients sent directly to the surgical floor. To test the safety of non-ICU care practice, patients with less than 48 h ICU admission were compared with patients directly admitted to the floor. Demographics, primary tumor site, comorbidities, estimated blood loss (EBL), extent of CRS, Eastern Cooperative Oncology Group (ECOG) status, and overall survival were analyzed.
Results
Complete data were available for 1064 CRS/HIPEC procedures, of which 244 cases (22.93 %) did not require ICU admission. Multivariate logistic regression identified age [odds ratio (OR) 1.024; p = 0.02], EBL (OR 1.002; p < 0.0001), number of resected organs (OR 1.308; p = 0.01) and ECOG > 2 (OR 6.387; p = 0.003) as predictive variables of postoperative ICU admission. The cohort directly admitted to the floor demonstrated less minor grade I/II morbidity (29 vs. 47 %; p < 0.0001) and similar grade III/IV major morbidity (16.5 vs. 13.4 %; p = 0.3) than the patients admitted to the ICU for less than 48 h.
Conclusions
ICU observation is not routinely required for all patients treated with CRS/HIPEC. Selective ICU admission based on ECOG status, nutritional status, age, EBL, and CRS extent is safe, with potential implications for hospitalization cost for these complex cases.
Cytoreductive surgery (CRS) and heated intraperitoneal chemotherapy (HIPEC) is a well-established treatment modality for selected patients with peritoneal dissemination from a number of epithelial malignancies. Several factors, including patient age, Eastern Cooperative Oncology Group (ECOG) performance status, nutritional status, extent of disease, and cytoreduction, determine morbidity, mortality, and overall survival of these patients.1–4 Traditionally, higher rates of complications and death reported from this surgery have prompted routine postoperative admission to the intensive care unit (ICU), either for initial observation or for continued management.5–7 However, with improvement in patient selection, surgical technique, advancements in anesthesia and perioperative fluid management, and increasing experience gained by high-volume centers, many of these patients likely do not require a level of care that needs routine ICU admission after surgery.8–12 The decision to send patients to the ICU after CRS/HIPEC is physician or institution driven, with no clearly defined criteria that could help guide this process. Additionally, with the rising costs of healthcare, changes in the pattern of reimbursement, and the demand for healthcare systems to move towards value-based practice, there is an increasing need to maximize the efficient utilization of available resources and minimize costs.13–17
The primary aim of this study was to determine the factors that are associated with selective ICU admission and to assess the safety of non-ICU management of CRS/HIPEC patients.
METHODS
A prospectively maintained single-institution database of all CRS/HIPEC procedures performed from 30 December 1991 to 2014 was retrospectively analyzed. Eighty-two cases from 30 December 1991 to June 1996 were excluded due to incomplete chart data. Institutional IRB approval was obtained for the study.
The eligibility criteria for CRS/HIPEC were histologic or cytological diagnosis of peritoneal carcinomatosis, complete recovery from prior systemic chemotherapy or radiation, primary lesion resected or amenable to resection, debulkable peritoneal disease, and no extraperitoneal spread. The presence of peripheral liver metastases, if readily resectable, was not considered a contraindication. Patients with medical comorbidities were included only after clearance by cardiology and anesthesia staff members familiar with CRS/HIPEC procedures. In addition, a 4- to 6-week long post-treatment break was used to ensure recovery of performance status and blood counts preferably to pre-chemotherapy levels. All patients had a complete history and physical examination, tumor markers, and computed tomography (CT) of the chest, abdomen, and pelvis before CRS/HIPEC procedures.
The CRS/HIPEC procedure was performed with the closed technique, as previously described by our group.2
Postoperatively, the decision to admit patients to the ICU or floor was left to the discretion of the surgeon, with input from the anesthesiologist. Patients with routine postoperative ICU admission were compared with patients sent directly to the surgical floor. Additionally, to test the safety of post CRS/HIPEC non-ICU care practice, patients who were admitted directly to the floor were compared with the best of the ICU patient cohort, which was empirically defined as those patients who were admitted to the ICU for 48 h or less with no subsequent readmissions to the ICU. We used 48 h to account for routine variability in availability of floor beds. Hospitalization entailed admission to a standard surgical ward with 3–1 nursing staff and no use of step-down or intermediate care units. Care was additionally provided by residents and experienced midlevel providers. Demographics, primary tumor site, comorbidities, estimated blood loss (EBL), extent of CRS, ECOG status, and overall survival were analyzed. Postoperative complications within 30 days were graded according to the Clavien–Dindo classification system.18 R0 and R1 resections were grouped together as complete cytoreductions. Cytoreductions with residual macroscopic disease were characterized as R2 and subdivided based on the size of residual disease as follows: R2a (≤5 mm), R2b (≤2 cm), R2c (>2 cm).
Statistical Analysis
Analysis of variance (ANOVA) and χ2 tests were used to compare patients admitted to the ICU after surgery with patients who were not admitted to the ICU. To determine characteristics predictive of admission to the ICU after surgery, multivariate logistic regression was implemented. This regression model was adjusted for race, sex, smoking, ECOG status, preoperative body mass index (BMI), pre-operative albumin, age at surgery, EBL, total number of organs resected, number of comorbidities and Clavien– Dindo grade. Overall survival was summarized using Kaplan–Meier methods, overall and by ICU after surgery. Differences in overall survival were assessed using the log-rank test. A multivariate Cox proportional hazards model was used to determine factors associated with better overall survival in patients who were admitted to the ICU; the model was adjusted for race, sex, smoking, ECOG status, preoperative BMI, preoperative albumin, age at surgery, EBL, total number of organs resected, number of comorbidities, and Clavien–Dindo grade.
The same analysis was then conducted in the subset of patients who stayed in the ICU for less than 48 h and did not return to the ICU. All analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and a 0.05 significance level was used throughout this analysis.
RESULTS
Patients Admitted Directly to the Intensive Care Unit (ICU) Versus Those Admitted to the Floor
Complete data for analysis were available for 1064 of 1146 CRS/HIPEC procedures. Of those, 244/1064 cases (22.93 %) were admitted postoperatively to a surgical floor, while 820/1064 (77.06 %) patients were admitted to the ICU. Demographic and clinicopathological characteristics of both groups are represented in Table 1.
TABLE 1.
Demographic and clinicopathological characteristics of patients admitted to the floor and ICU
| Overall | No ICU post op (n = 244) | ICU post op (n = 820) | P value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Race | |||||||
| Black | 113 | 10.7 | 18 | 7.44 | 95 | 11.67 | 0.1199 |
| Other | 26 | 2.46 | 8 | 3.31 | 18 | 2.21 | |
| White | 917 | 86.84 | 216 | 89.26 | 701 | 86.12 | |
| Sex | |||||||
| Female | 579 | 54.42 | 142 | 58.2 | 437 | 53.29 | 0.1769 |
| Male | 485 | 45.58 | 102 | 41.8 | 383 | 46.71 | |
| Diabetes | |||||||
| No | 939 | 90.29 | 216 | 92.7 | 723 | 89.59 | 0.1575 |
| Yes | 101 | 9.71 | 17 | 7.3 | 84 | 10.41 | |
| Heart disease | |||||||
| No | 948 | 91.15 | 213 | 91.03 | 735 | 91.19 | 0.9575 |
| Yes | 92 | 8.85 | 21 | 8.97 | 71 | 8.81 | |
| Lung disease | |||||||
| No | 999 | 96.15 | 229 | 98.28 | 770 | 95.53 | 0.0547 |
| Yes | 40 | 3.85 | 4 | 1.72 | 36 | 4.47 | |
| Smoking | |||||||
| Current | 144 | 14.19 | 34 | 14.66 | 110 | 14.05 | 0.9378 |
| Never | 689 | 67.88 | 158 | 68.1 | 531 | 67.82 | |
| Past | 182 | 17.93 | 40 | 17.24 | 142 | 18.14 | |
| Clavien-Dindo grade | |||||||
| None | <.0001 | ||||||
| 0 | 380 | 35.78 | 140 | 57.38 | 240 | 29.34 | |
| Minor | |||||||
| I | 86 | 8.1 | 21 | 8.61 | 65 | 7.95 | |
| II | 285 | 26.84 | 42 | 17.21 | 243 | 29.71 | |
| Major | |||||||
| III | 197 | 18.55 | 36 | 14.75 | 161 | 19.68 | |
| IV | 51 | 4.8 | 2 | 0.82 | 49 | 5.99 | |
| Death | |||||||
| V | 63 | 5.93 | 3 | 1.23 | 60 | 7.33 | |
| ECOG | |||||||
| 0 | 481 | 46.12 | 146 | 61.09 | 335 | 41.67 | <.0001 |
| 1 | 416 | 39.88 | 85 | 35.56 | 331 | 41.17 | |
| 2 | 114 | 10.93 | 7 | 2.93 | 107 | 13.31 | |
| 3+ | 32 | 3.07 | 1 | 0.42 | 31 | 3.86 | |
| Primary site | |||||||
| Colorectal | 229 | 21.52 | 45 | 18.44 | 184 | 22.44 | 0.077 |
| Mesothelioma | 81 | 7.61 | 14 | 5.74 | 67 | 8.17 | |
| Ovarian | 80 | 7.52 | 28 | 11.48 | 52 | 6.34 | |
| Appendix | 537 | 50.47 | 128 | 52.46 | 409 | 49.88 | |
| Gastric | 33 | 3.1 | 7 | 2.87 | 26 | 3.17 | |
| Other | 104 | 9.77 | 22 | 9.02 | 82 | 10 | |
| Mean | SD | Mean | SD | Mean | SD | P value | |
| Pre-op BMI | 27.94 | 6.07 | 27.83 | 5.40 | 27.97 | 6.26 | 0.7535 |
| Pre-op Albumin | 3.77 | 0.58 | 3.91 | 0.50 | 3.73 | 0.59 | <.0001 |
| HIPEC age | 52.71 | 12.34 | 50.12 | 12.17 | 53.47 | 12.30 | 0.0002 |
| EBL | 757.65 | 732.49 | 381.39 | 312.41 | 872.40 | 783.78 | <.0001 |
| Total number of resected organs | 2.76 | 1.53 | 2.03 | 1.16 | 2.94 | 1.55 | <.0001 |
| Length of ICU stay | N/A | 4.10 | 9.80 | ||||
| Number of comorbidities | 0.22 | 0.51 | 0.18 | 0.44 | 0.24 | 0.52 | 0.1302 |
ECOG Eastern Cooperative Oncology Group, BMI body mass index, EBL estimated blood loss
Bold values are statistically significant (p < 0.05)
Predictors of Direct ICU Admission
Univariate analysis showed significant differences between the two groups in ECOG performance status (p < 0.0001), Clavien–Dindo complication grade (p < 0.0001), EBL (p < 0.0001), age (p < 0.0002), preoperative albumin level (p < 0.0001) and number of organs resected (p < 0.0001) (Table 1).
Multivariate analysis demonstrated that predictors of ICU admission were ECOG ≥ 2 [odds ratio (OR) 5.3, confidence interval (CI) 1.7–16.3; p = 0.0033], higher age (OR 1.021, CI 1.0–1.04; p = 0.02), increased EBL (OR 1.002, CI 1.001–1.003, p < 0.0001), number of organs resected (OR 1.24, CI 1.04–1.46; p = 0.01) and Clavien–Dindo grade ≥ II (Table 2).
TABLE 2.
Multivariate logistic regression model predicting admission to the ICU
| OR | Lower 95 % CI | Upper 95 % CI | P value | |
|---|---|---|---|---|
| Race | ||||
| Black | 1.098 | 0.526 | 2.295 | 0.8033 |
| Other | 1.091 | 0.352 | 3.382 | 0.8803 |
| White | Ref | |||
| Sex | ||||
| Female | 1.253 | 0.814 | 1.927 | 0.3052 |
| Male | Ref | |||
| Primary site | ||||
| Colorectal | 1.25 | 0.717 | 2.182 | 0.4315 |
| Gastric | 0.662 | 0.15 | 2.913 | 0.5849 |
| Mesothelioma | 1.224 | 0.507 | 2.953 | 0.6526 |
| Other | 1.539 | 0.714 | 3.317 | 0.271 |
| Ovarian | 0.764 | 0.341 | 1.712 | 0.5126 |
| Appendix | ||||
| Smoking | ||||
| Current | 1.517 | 0.806 | 2.855 | 0.1968 |
| Never | ||||
| Past | 0.883 | 0.52 | 1.501 | 0.4626 |
| ECOG | ||||
| 0 | Ref | |||
| 1 | 1.199 | 0.761 | 1.888 | 0.4343 |
| 2+ | 5.354 | 1.751 | 16.375 | 0.0033 |
| Pre-op BMI | 1.017 | 0.98 | 1.057 | 0.3716 |
| Pre-op Albumin | 0.733 | 0.465 | 1.158 | 0.183 |
| HIPEC age | 1.021 | 1.002 | 1.04 | 0.0263 |
| EBL | 1.002 | 1.001 | 1.003 | <.0001 |
| Total number of resected organs | 1.235 | 1.043 | 1.463 | 0.0144 |
| Number of comorbidities | 0.951 | 0.612 | 1.478 | 0.8239 |
| Clavien-Dindo grade | ||||
| 0 | Ref | |||
| I | 1.497 | 0.752 | 2.979 | 0.2504 |
| II | 2.111 | 1.231 | 3.619 | 0.0066 |
| III | 1.9 | 1.066 | 3.385 | 0.0295 |
| IV | 3.831 | 0.803 | 18.283 | 0.0921 |
| V | 3.954 | 0.847 | 18.458 | 0.0803 |
ECOG Eastern Cooperative Oncology Group, BMI body mass index, EBL estimated blood loss
Bold values are statistically significant (p < 0.05)
Survival Analysis Based on Status of ICU Admission
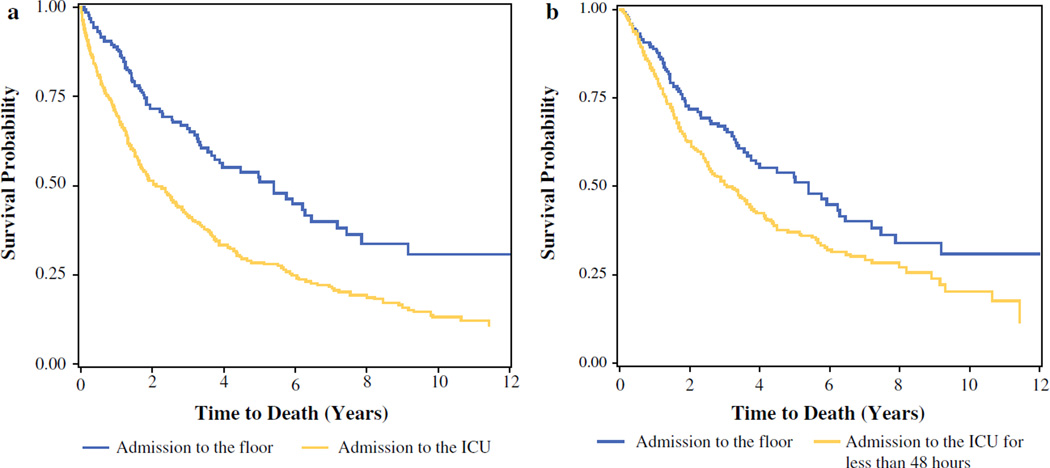
Patients who were admitted directly to the floor had a better median survival (5.4 vs. 2.12; p < 0.0001) as well as 3- and 5-year survival (0.67 vs. 0.49 and 0.52 vs. 0.43, respectively) compared with those who were admitted to the ICU (Fig. 1a). This difference was most notable for patients with colorectal (p = 0.0386), ovarian (p = 0.0054), and appendiceal (p < 0.0001) primaries.
FIG. 1.
Survival plot of patients by admission status. a Admission to floor versus ICU. b Admission to floor versus ICU for <48 h
For patients who were admitted to the ICU, survival was poorer in current smokers [hazard ratio (HR) 1.45, 95 % CI 1.05–2.00], patients with ECOG ≥ 2 (HR 2.63, 95 % CI 1.88–3.69), patients with colorectal (HR 2.68, 95 % CI 2.00–3.61), gastric (HR 2.33, 95 % CI 0.93–5.81) and ovarian (HR 2.14, 95 % CI 1.28–3.56) primaries, and patients with a Clavien–Dindo grade of IV (HR 3.66, 95 % CI 2.48–5.41).
Need for ICU Transfer After Initial Admission to the Floor
Twelve of the 244 patients (4.92 %) were transferred to ICU after initial admission to the general floor. Of these 12 patients, three were transferred to the ICU due to respiratory insufficiency from hemopneumothorax and pulmonary edema, two patients due to issues pertaining to narcotic use (one overdose, one intractable pain), two patients due to tachyarrhythmia, two patients for bowel perforation, two patients for hypotension from under-resuscitation, and one patient due to postoperative hemorrhage. The pneumothoraces required chest tube placement, the bowel perforations and postoperative hemorrhage required re-exploration, and one case of tachyarrhythmia required cardioversion. Death occurred in two of these 12 patients within 90 days as a result of sepsis (secondary to enteric leak) and multiple organ failure.
Patients Admitted to the ICU for Less Than 48 Hours Versus Those Admitted to the Floor
In order to evaluate the safety of postoperative admission to the floor, we compared the direct floor admission cohort with the 465/820 (56.8 %) patients who were routinely admitted postoperatively to the ICU for less than 48 h without subsequent ICU readmission.
Predictors of Less Than 48-Hour ICU Admission
Significant differences were noted between the two groups of patients with respect to age (0.0293), preoperative albumin level (0.0203), EBL (p < 0.0001), total number of organs resected (p < 0.0001), number of comorbidities (p < 0.0001), ECOG status (p = 0.0006), and Clavien–Dindo complication grade (p < 000.1) (Table 3). Multivariate logistic regression showed ECOG ≥ 2 (OR 4.9, 95 % CI 1.3–18.8; p = 0.018), age (OR 1.023, 95 %CI 1.001–1.044; p = 0.03), EBL (OR 1.002, 95 % CI 1.001–1.002; p < 0.0001), and total number of organs resected (OR 1.29, 95 % CI 1.056–1.573; p = 0.0125) to be independent predictors of admission to the ICU for less than 48 h (Table 4).
TABLE 3.
Descriptive statistics of patients admitted to the floor and those admitted to the ICU for less than 48 h
| Overall | No ICU post op (n = 244) | ICU post op (n = 465) | P value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Race | |||||||
| Black | 80 | 11.4 | 18 | 7.44 | 62 | 13.48 | 0.0485 |
| Other | 19 | 2.71 | 8 | 3.31 | 11 | 2.39 | |
| White | 603 | 85.9 | 216 | 89.26 | 387 | 84.13 | |
| Sex | |||||||
| Female | 402 | 56.7 | 142 | 58.2 | 260 | 55.91 | 0.56 |
| Male | 307 | 43.3 | 102 | 41.8 | 205 | 44.09 | |
| Diabetes | |||||||
| No | 631 | 90.92 | 216 | 92.7 | 415 | 90.02 | 0.2454 |
| Yes | 63 | 9.08 | 17 | 7.3 | 46 | 9.98 | |
| Heart disease | |||||||
| No | 640 | 92.09 | 213 | 91.03 | 427 | 92.62 | 0.4605 |
| Yes | 55 | 7.91 | 21 | 8.97 | 34 | 7.38 | |
| Lung disease | |||||||
| No | 668 | 96.25 | 229 | 98.28 | 439 | 95.23 | 0.0453 |
| Yes | 26 | 3.75 | 4 | 1.72 | 22 | 4.77 | |
| Smoking | |||||||
| Current | 93 | 13.66 | 34 | 14.66 | 59 | 13.14 | 0.8507 |
| Never | 467 | 68.58 | 158 | 68.1 | 309 | 68.82 | |
| Past | 121 | 17.77 | 40 | 17.24 | 81 | 18.04 | |
| Clavien-Dindo grade | |||||||
| None | |||||||
| 0 | 276 | 44.23 | 110 | 53.4 | 166 | 39.71 | <.0001 |
| Minor | |||||||
| I | 64 | 10.26 | 18 | 8.74 | 46 | 11 | |
| II | 191 | 30.61 | 41 | 19.9 | 150 | 35.89 | |
| Major | |||||||
| III | 87 | 13.94 | 32 | 15.53 | 55 | 13.16 | |
| IV | 3 | 0.48 | 2 | 0.97 | 1 | 0.24 | |
| Death | |||||||
| V | 3 | 0.48 | 3 | 1.46 | |||
| ECOG | |||||||
| 0 | 371 | 53.3 | 146 | 61.09 | 225 | 49.23 | 0.0006 |
| 1 | 263 | 37.79 | 85 | 35.56 | 178 | 38.95 | |
| 2 | 52 | 7.47 | 7 | 2.93 | 45 | 9.85 | |
| 3+ | 10 | 1.44 | 1 | 0.42 | 9 | 1.97 | |
| Primary site | |||||||
| Colorectal | 151 | 21.3 | 45 | 18.44 | 106 | 22.8 | 0.2437 |
| Mesothelioma | 47 | 6.63 | 14 | 5.74 | 33 | 7.1 | |
| Ovarian | 59 | 8.32 | 28 | 11.48 | 31 | 6.67 | |
| Appendix | 366 | 51.62 | 128 | 52.46 | 238 | 51.18 | |
| Gastric | 15 | 2.12 | 7 | 2.87 | 9 | 1.94 | |
| Other | 71 | 10.01 | 22 | 9.02 | 48 | 10.32 | |
| Mean | SD | Mean | SD | Mean | SD | P value | |
| Pre-op BMI | 28.06 | 5.94 | 27.83 | 5.40 | 28.18 | 6.20 | 0.4731 |
| Pre-op Albumin | 3.85 | 0.50 | 3.91 | 0.50 | 3.82 | 0.51 | 0.0203 |
| HIPEC age | 51.50 | 12.05 | 50.12 | 12.17 | 52.21 | 11.94 | 0.0293 |
| EBL | 642.11 | 619.93 | 385.10 | 311.64 | 775.48 | 694.10 | <.0001 |
| Total number of resected organs | 0.57 | 0.69 | 0.43 | 0.57 | 0.65 | 0.74 | <.0001 |
| Length of ICU stay | 2.56 | 1.45 | 2.03 | 1.16 | 2.81 | 1.51 | 0.2908 |
| Number of comorbidities | 0.21 | 0.49 | 0.18 | 0.44 | 0.22 | 0.52 | <.0001 |
| Complications | |||||||
| None | 276 | 110 | 53.4 | 166 | 39.71 | 0.0012 | |
| Minor | 255 | 59 | 28.64 | 196 | 46.89 | <0.0001 | |
| Major | 90 | 34 | 16.5 | 56 | 13.4 | 0.2987 | |
| Death | 3 | 3 | 1.46 | 0 | 0 | 0.0356 | |
ECOG Eastern Cooperative Oncology Group, BMI body mass index, EBL estimated blood loss
Bold values are statistically significant (p < 0.05)
TABLE 4.
Multivariate logistic regression model predicting admission to the ICU for less than 48 h
| OR | Lower 95% CI | Upper 95% CI | P value | |
|---|---|---|---|---|
| Race | ||||
| Black | 1.386 | 0.608 | 3.158 | 0.4375 |
| Other | 1.479 | 0.443 | 4.939 | 0.5244 |
| White | Ref | |||
| Sex | ||||
| Female | 1.155 | 0.705 | 1.892 | 0.5673 |
| Male | Ref | |||
| Primary site | ||||
| Colorectal | 1.383 | 0.724 | 2.64 | 0.3264 |
| Gastric | 0.208 | 0.016 | 2.672 | 0.2279 |
| Mesothelioma | 1.351 | 0.461 | 3.963 | 0.5837 |
| Other | 1.586 | 0.696 | 3.617 | 0.2728 |
| Ovarian | 1.029 | 0.387 | 2.738 | 0.9538 |
| Appendix | Ref | |||
| Smoking | ||||
| Current | 1.44 | 0.674 | 3.077 | 0.3461 |
| Never | ||||
| Past | 0.85 | 0.452 | 1.597 | 0.6127 |
| ECOG | ||||
| 0 | Ref | |||
| 1 | 1.113 | 0.663 | 1.871 | 0.6851 |
| 2+ | 4.961 | 1.306 | 18.844 | 0.0187 |
| Pre-op BMI | 1.011 | 0.966 | 1.057 | 0.6451 |
| Pre-op Albumin | 0.972 | 0.57 | 1.657 | 0.9161 |
| HIPEC age | 1.023 | 1.001 | 1.044 | 0.037 |
| EBL | 1.002 | 1.001 | 1.002 | <.0001 |
| Total number of resected organs | 1.289 | 1.056 | 1.573 | 0.0125 |
| Number of comorbities | 0.982 | 0.602 | 1.604 | 0.5821 |
| Clavien-Dindo grade | ||||
| 0 | ||||
| I | 1.407 | 0.649 | 3.05 | 0.3878 |
| II | 1.594 | 0.884 | 2.873 | 0.1213 |
| ≥III | 0.81 | 0.417 | 1.572 | 0.533 |
ECOG Eastern Cooperative Oncology Group, BMI body mass index, EBL estimated blood loss
Bold values are statistically significant (p < 0.05)
Survival Based on Admission Status
Median survival was better in patients admitted to the floor versus those who were admitted to the ICU for less than 48 h (5.4 vs. 3.04 years; p = 0.0027) (Fig. 1b). Amongst patients who were admitted to the ICU for less than 48 h, survival was poorer for those with ECOG ≥ 2 (HR 2.2, 95 % CI 1.31–3.7; p = 0.003), colorectal primary (HR 2.78, 95 % CI 2.08–3.72; p < 0.0001), and ovarian primary (HR 1.9, 95 % CI 1.14–3.15; p = 0.0098).
Morbidity and Mortality
The floor cohort had 53 % of patients being discharged without complications versus 40 % for the less than 48 h ICU group (p = 0.0012). Minor Clavien I and II morbidity occurred in 29 % of the floor cohort versus 47 % of the less than 48 h ICU patients (p < 0.0001), while there was no difference in the major Clavien III/IV morbidity (16.5 % vs. 13.4 %; p = 0.3). The observed difference in mortality between the two groups was expected, since the 48 h ICU cohort included only those patients without ICU readmission.
DISCUSSION
CRS/HIPEC has demonstrated a survival benefit for selected patients with peritoneal carcinomatosis compared with systemic chemotherapy alone.19 Postoperatively, these patients are routinely admitted to the ICU, presumably for the prevention or early detection and therapeutic intervention of complications, which have a direct impact on patient outcome.7 However, no data exist to support routine ICU admission, while ICU care is an expensive and limited resource.15,20 Critical care services constitute a large and increasing proportion of hospital costs (20 %) and account for 1 % of the US gross domestic product.21,22 In 2011, 26.9 % of hospital stays in 29 States involved ICU charges, accounting for 47.5 % of aggregate total hospitalization costs; hospital stays that involved ICU services were 2.5 times more costly than other hospital stays.23 In the changing landscape of healthcare, a major increase in resources is unlikely to occur without significant proof of cost effectiveness. Therefore, judicious use of this resource is highly desirable. The primary aim of this study was to determine factors that are associated with selective postoperative ICU admission, and to assess the safety of non-ICU management of CRS/HIPEC patients.
Factors independently associated with increased risk of ICU admission were worse performance status, Clavien–Dindo complication grade, higher EBL, age, and number of organs resected. Survival analysis showed poorer median survival of the ICU cohort (5.4 vs. 2.1 years) (Fig. 1a). Smoking, ECOG performance status ≥ 2, increased number of organs resected, major complications, and colorectal, gastric and ovarian primaries were associated with poor long-term survival. The difference in survival is multifactorial and includes increased volume of peritoneal disease, multiple prior treatments, comorbidities, and extensive CRS, leading to increased morbidity and mortality. These outcomes are consistent with what we and others have published in the literature.1,2,24–27
Twelve patients (4.9 %) who were initially admitted to the floor returned to the ICU for reasons that would not have been prevented by upfront ICU hospitalization. Likewise, death in 2 of these 12 patients occurred within 90 days from causes likely not preventable with initial admission to the ICU. Studies have shown that variation in hospital mortality rates after major abdominal procedures is associated with failure to rescue, rather than complication rates themselves.28,29 CRS/HIPEC procedures can thus be safely performed with acceptable morbidity and mortality in high-volume centers that have the ability to rescue patients from potential complications.
A prior study of 39 CRS/HIPEC procedures similarly concluded that patient selection for postoperative ICU admission should be employed.30 To assess the safety of non-ICU care of patients, we compared patients who were admitted directly to the floor with those patients who were transferred out of the ICU within 48 h with no subsequent readmission. There was no difference in major grade III/IV morbidity between the two groups, while minor morbidity grade I/II was significantly higher in the less than 48 h ICU cohort. There was no observed mortality in the less than 48 h ICU cohort, but that was expected since these patients were excluded by definition from the specific cohort. Although median survival was better in this group compared with the entire ICU cohort, it was still worse than those patients who directly went to the floor (3.04 vs. 5.4 years) (Fig. 1b).
The type of chemotherapy did influence location of initial postoperative admission. Specifically, mesothelioma patients being perfused with cisplatin are routinely managed in the ICU for close monitoring of renal function and aggressive hydration.
Although outside of the scope of this study, we did preliminarily evaluate the cost impact of ICU versus non-ICU care. We found that, on average, even admission of patients to the ICU for less than 48 h, costs approximately $4000 more than direct floor admission.
This study is limited by several factors, the retrospective nature of the analysis, and inherent selection bias. These data reflect the learning curve and institutional practice over a period of 24 years, during the earlier part of which very few, if any, criteria or guidelines were established for perioperative management of CRS/HIPEC patients. This explains why over the last 3 years 35.2 % of patients went directly to the floor versus 19 % in the years prior. Therefore, although not an independent factor, admission to the ICU potentially represents a surrogate marker for poorer overall survival of CRS/HIPEC patients. In addition, peritoneal cancer index (PCI) and length of operations were not universally available for analysis.
We recognize that our accumulated institutional experience, nursing expertise, and repeated exposure of residents to the care of CRS/HIPEC patients inevitably generates multiple layers of defense that allows for safe postoperative floor admission. Therefore, we do not recommend routine floor admission in centers with scarce resources or early in their institutional experience.
CONCLUSIONS
CRS/HIPEC patients do not routinely require observation in the ICU postoperatively. Appropriate selection of patients for non-ICU care based on ECOG status, nutritional status, age, intraoperative blood loss and CRS extent is safe, with an acceptable rate of late ICU admission. Selective ICU admission should help optimize efficient utilization of resources with a potentially favorable impact on hospitalization cost for these complex cases.
Acknowledgments
This work was supported by Wake Forest University Biostatistics shared resource NCI CCSG P30CA012197.
Footnotes
This work was presented at the 10th International Symposium on Regional Cancer Therapies, Clearwater, FL, USA, 14–16 February 2015 (third best poster award).
DISCLOSURES Nothing to disclose.
REFERENCES
- 1.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 2.Levine EA, Stewart JH, 4th, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218:573–585. doi: 10.1016/j.jamcollsurg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Votanopoulos KI, Russell G, Randle RW, et al. Peritoneal surface disease (PSD) from appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): overview of 481 cases. Ann Surg Oncol. 2015;22:1274–1279. doi: 10.1245/s10434-014-4147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Votanopoulos KI, Newman NA, Russell G, et al. Outcomes of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients older than 70 years; survival benefit at considerable morbidity and mortality. Ann Surg Oncol. 2013;20:3497–3503. doi: 10.1245/s10434-013-3053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franssen B, Tabrizian P, Weinberg A, et al. Outcome of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on patients with diaphragmatic involvement. Ann Surg Oncol. 2015;22(5):1639–1644. doi: 10.1245/s10434-014-4083-x. [DOI] [PubMed] [Google Scholar]
- 6.Votanopoulos KI, Ihemelandu C, Shen P, et al. Outcomes of repeat cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for the treatment of peritoneal surface malignancy. J Am Coll Surg. 2012;215(3):412–417. doi: 10.1016/j.jamcollsurg.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell JC, Rylah BG, Chambers RW, et al. Perioperative management of patients undergoing cytoreductive surgery combined with heated intraperitoneal chemotherapy for peritoneal surface malignancy: a multi-institutional experience. Ann Surg Oncol. 2012;19(13):4244–4251. doi: 10.1245/s10434-012-2496-y. [DOI] [PubMed] [Google Scholar]
- 8.Raspe C, Piso O, Wiesenack C, Bucher M. Anesthetic management in patients undergoing hyperthermic chemotherapy. Curr Opin Anaesthesiol. 2012;25:348–355. doi: 10.1097/ACO.0b013e32835347b2. [DOI] [PubMed] [Google Scholar]
- 9.Donati A, Loggi S, Preiser JC, et al. Goal directed intraoperative therapy reduces morbidity and length of hospital stay in high risk surgical patients. Chest. 2007;132:1817–1824. doi: 10.1378/chest.07-0621. [DOI] [PubMed] [Google Scholar]
- 10.Colantonio L, Claroni C, Fabrizi L, et al. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg. 2015;19(4):722–729. doi: 10.1007/s11605-015-2743-1. [DOI] [PubMed] [Google Scholar]
- 11.Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure? A systematic review of morbidity and mortality. Ann Surg. 2009;249(6):900–907. doi: 10.1097/SLA.0b013e3181a45d86. [DOI] [PubMed] [Google Scholar]
- 12.Kusamura S, Baratti D, Virzì S, et al. Learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies: analysis of two centres. J Surg Oncol. 2013;107(4):312–319. doi: 10.1002/jso.23231. [DOI] [PubMed] [Google Scholar]
- 13.Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303:1371–1372. doi: 10.1001/jama.2010.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wunsch H, Angus DC, Harrison DA, et al. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36:2787–2793. doi: 10.1097/CCM.0b013e318186aec8. [DOI] [PubMed] [Google Scholar]
- 15.Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: an analysis of bed numbers, occupancy rates, payer mix, costs. Crit Care Med. 2010;38:65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 16.Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307:1513–1516. doi: 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]
- 17.Vincent J-L, Rubenfeld GD. Does intermediate care improve patient outcomes or reduce costs? Crit Care. 2015;19(1):89. doi: 10.1186/s13054-015-0813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 20.Halpern NA. Can the costs of critical care be controlled? Curr Opin Crit Care. 2009;15(6):591–596. doi: 10.1097/MCC.0b013e328332f54f. [DOI] [PubMed] [Google Scholar]
- 21.Halpern NA, Bettes L, Greenstein R. Federal and nationwide intensive care units and healthcare costs: 1986–1992. Crit Care Med. 1994;22:2001–2007. [PubMed] [Google Scholar]
- 22.Levit K, Smith C, Cowan C, et al. Trends in U.S. health care spending, 2001. Health Aff (Millwood) 2003;22:154–164. doi: 10.1377/hlthaff.22.1.154. [DOI] [PubMed] [Google Scholar]
- 23.Barrett ML, Smith MW, Elixhauser A, et al. Utilization of intensive care services, 2011. Healthcare Cost and Utilization Project. Statistical brief #185. 2014 Dec; http://www.hcup-us.ahrq.gov/reports/statbriefs/sb185-Hospital-Intensive-Care-Units-2011.pdf.
- 24.Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116(24):5608–5618. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- 25.Baratti D, Kusamura S, Iusco D, et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum. 2014;57(7):858–868. doi: 10.1097/DCR.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 26.Bakrin N, Bereder JM, Decullier E, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013;39(12):1435–1443. doi: 10.1016/j.ejso.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Desantis M, Bernard JL, Casanova V, et al. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC) Langenbecks Arch Surg. 2015;400(1):37–48. doi: 10.1007/s00423-014-1253-z. [DOI] [PubMed] [Google Scholar]
- 28.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in Medicare patients. Ann Surg. 2009;250:1029–1034. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 29.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 30.López-Basave HN, Morales-Vasquez F, Mendez-Herrera C, et al. Intensive care unit admission after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Is it necessary? J Oncol. 2014;2014:307–317. doi: 10.1155/2014/307317. [DOI] [PMC free article] [PubMed] [Google Scholar]