Abstract
Background
Parkinson disease (PD) has detrimental effects on swallowing function. Treatment options are largely behavioral; thus, patients would benefit from an earlier start to therapy. Early swallowing changes in PD are not well-known, so patients do not typically receive swallowing treatment until later in the progression of PD.
Objective
We used predictive modeling to determine what quantitative swallowing variables best differentiate individuals with early to mid-stage PD from healthy controls.
Methods
Participants included twenty-six individuals with early to mid-stage PD and 26 healthy, age- and sex-matched controls. Swallowing was evaluated by simultaneous high-resolution manometry and videofluoroscopy as well as the Sydney Swallow Questionnaire (SSQ). Binomial logistic regression was performed on 4 sets of data: 1) high-resolution manometry only; 2) videofluoroscopy only; 3) SSQ only; and 4) all data combined.
Results
A model from a combined data set had the highest accuracy in differentiating individuals with PD from controls. The model included maximum pressure in the velopharynx (soft palate), pressure variability in the velopharynx, and the SSQ item concerning difficulty with saliva swallowing. No significant models could be generated using the videofluoroscopy data.
Conclusions
Individuals with PD show quantitative changes in pressure generation and are able to self-assess aspects of swallowing function in the early and mid-stages of PD, even in the absence of swallowing changes seen on videofluoroscopy. A multimodal approach for the assessment of swallowing may be more accurate for determining subtle swallowing changes that occur in the early stages of PD.
Keywords: Parkinson disease, deglutition, high-resolution manometry, videofluoroscopy
INTRODUCTION
Parkinson disease (PD) affects approximately 1% of the world population, with increasing incident rates with age [1]. Difficulty swallowing (dysphagia) can occur in up to 100% of patients with PD [2] and is a risk factor for aspiration pneumonia [3], the leading cause of death in individuals with PD [4]. Other sequalae of dysphagia include malnutrition, dehydration, and diminished quality of life [5]. Dopaminergic therapy and deep-brain stimulation do not improve signs and symptoms of dysphagia in individuals with PD [6–11], so standard of care for dysphagia treatment in these patients generally includes diet modifications and behavioral therapy. However, treatment is often not initiated until the patient is seen by a speech-language pathologist; this may not occur until late in the progression of the disease, as many patients do not report symptoms despite having dysphagia [2]. Early identification of swallowing deficits in individuals with PD may allow for earlier, preventative intervention and thus improved health and quality of life outcomes [12]. Nonetheless, swallowing changes in the early stages of PD are not well described.
Clinical evaluation tools are needed for the identification of subtle, sub-clinical changes in swallowing function. Self-report measures may not be reliable in detecting changes in swallowing function, especially those that ask only whether or not the individual experiences difficulty swallowing [2, 13]. The standard for oropharyngeal swallowing evaluation is videofluoroscopy, a video x-ray of the mouth and throat. A standard videofluroscopic swallowing evaluation involves imaging the patient while chewing and/or swallowing a bolus of food or liquid mixed with a contrast agent (typically barium sulfate). Analyses of videofluoroscopy can include qualitative and/or quantitative measurements of: 1) bolus trajectory, including airway invasion; 2) food or liquid residue in the oral or pharyngeal spaces after swallowing: amounts and location; 3) patient sensitivity to airway invasion or residue; 4) kinematics of oropharyngeal structures, such as the tongue, hyoid bone, larynx, and upper esophageal sphincter; and 5) changes to swallowing function with modifications to bolus texture, head position, or compensatory behaviors [5]. Evaluation with videofluoroscopy in the clinic is mostly subjective with the exception of a few validated methods [14–16], and even objective analysis may not be sensitive enough to reveal subtle differences in swallowing function in patients with early to mid-stage PD [17].
High-resolution manometry (HRM) is a clinical procedure in which a thin, flexible catheter is placed in the pharynx and esophagus for measurement of swallowing-related contact pressure against the catheter’s closely-spaced pressure sensors. The output of HRM is objective pressure data with high temporal and spatial resolution. HRM has the capability to determine objective changes in swallowing pressure in patients with dysphagia [18–20]. HRM data is objective with good temporal and spatial resolution and thus can reveal subtle changes in swallowing-related pressure and timing events in patients in the early stages of PD, before signs or symptoms of overt dysphagia appear. To date, no investigations of oropharyngeal swallowing pressures using HRM have been completed in patients with PD.
Given the radiation exposure associated with videofluoroscopy and the relative invasiveness of HRM, swallowing-specific questionnaires may be useful in screening for swallowing disorders by healthcare providers not otherwise trained to evaluate swallowing function. Questionnaires with items concerning swallowing have also been used in epidemiologic studies of PD [21–23], and a patient’s perspective of his or her own swallowing function is an important clinical outcome measure [24–28]. When asked a single question about swallowing, such as on the Unified Parkinson Disease Rating Scale [29], patients with PD do not always report difficulty swallowing that matches with an instrumented swallowing evaluation [2, 30–32]. However, when asked specific questions regarding different aspects of swallowing, questionnaires may be better able to detect symptoms of dysphagia [33–36].
A multimodal swallowing evaluation, combining elements of videofluoroscopy, HRM, and explicit self-report measures may be a more robust method for the identification of subtle changes in swallowing function than each evaluation independently. The purpose of this study was to use predictive modeling to determine what quantitative swallowing variables differentiate individuals with early and mid-stage PD from healthy controls using videofluoroscopy, HRM, and a swallowing-specific questionnaire. We hypothesized that individuals with PD would exhibit quantitative differences in swallowing physiology measurable by HRM and that a combination of a multimodal swallowing evaluation would improve the predictive power of the models generated.
MATERIALS AND METHODS
Participants
Participants with PD included 26 individuals in the early and mid-stages of the disease (modified Hoehn & Yahr 1–3 [37]), 13 male, ages 50–88 years (mean age: 69 ± 10.6 years for males & females). All participants with PD were in the ON stage of medication during participation in study activities. Health and demographic information for each participant with PD can be found in Supplementary Table 1. Diagnosis of PD was confirmed by neurologist report and staging was completed by a physician member of the study team. Participants with PD were without any comorbidities that may have impacted swallowing function (e.g., dementia, stroke, head & neck cancer), confirmed by medical chart review and participant interview. Healthy control participants included 26 age and sex-matched individuals, 13 male, ages 49–86 years (mean age: 69.8 ± 10.7 years for males and females). Healthy participants were without swallowing, respiratory, neurological, or gastrointestinal disorders, based on participant interview. Power was calculated from results from Sung, et al. (2010). For an effect size and power of 0.8, 16 subjects per group are needed. Given the heterogeneity of the PD population, we decided to include additional subjects per group. All participants gave informed consent under the approval of the University of Wisconsin – Madison Institutional Review Board, and the study was conducted in accordance with the Helsinki Declaration of 1975.
Equipment and Procedure
Sydney Swallowing Questionnaire
Prior to the swallowing evaluation, each participant completed the Sydney Swallow Questionnaire (SSQ). The SSQ is a 17-item, visual-analog scale questionnaire intended to elicit an individual’s perception on many areas of their swallowing performance (e.g., difficulty swallowing thin liquids, thick liquids, pastes, dry solids, etc.) [33]. The values from each question range from 0–100 and are summed for a maximum possible score of 1,700. Higher scores indicate a higher level of perceived dysfunction. The SSQ has been validated for use in patients with PD [33], and the range for healthy is between 10–235 [38].
High-resolution manometry
High-resolution manometry and videofluoroscopy were performed simultaneously. Swallowing pressures were recorded with a high-resolution manometry system (ManoScan360 High-Resolution Manometry System, Given Imaging, Atlanta, GA). The manometric catheter is comprised of 36 solid-state, circumferential pressure sensors, spaced 1 cm apart. The outer diameter of the manometric catheter is 2.75 mm. The system records pressures at 50 Hz from −20 mmHg to 600 mmHg with 2 mmHg fidelity. The manometric catheter was calibrated before each use according to manufacturer specifications. Participants were seated upright for the entire procedure. The participant’s nasal passage was lightly anesthetized with topical 2% viscous lidocaine hydrochloride (< 0.5 mL). The manometric catheter was placed through the nasal cavity and pharynx into the cervical esophagus. Videofluoroscopy was used for confirmation of correct placement and identification of the manometric sensors that fell in each pharyngeal region of interest [39]. Participants rested for 5 minutes in order to adjust to the manometric catheter before performing experimental swallows.
Videofluoroscopy
Continuous videofluoroscopy was recorded in the lateral plane (OEC 9900, General Electric, Fairfield, CT), with the following boundaries: incisors anteriorly, cervical spine posteriorly, nasal edge of the soft palate superiorly, and the cervical esophagus inferiorly. Video was digitized and recorded on a DVD+RW at 30 frames/second (DVO-1000MD, Sony, Park Ridge, NJ). Videofluoroscopic and manometric data were captured simultaneously with the use of a time code embedded into the videofluoroscopic signal (UTG-50, Horita, Mission Viejo, CA). With the head in a neutral position, participants swallowed 10 trials of 10 mL thin-liquid barium at a 40% weight-to-volume ratio (Varibar, Bracco Diagnostics, Monroe Township, NJ). Boluses were presented via syringe, and participants were instructed to hold the bolus in the mouth until hearing a cue to swallow from the examiner. A 10 mL thin-liquid bolus was chosen due to its tolerability and due to previous reports of its sufficiency to differentiate healthy swallowers from those with PD [9, 40–45]. In order to reduce exposure to radiation, videofluoroscopy was cued on and off by a study team member, ensuring that the entire swallow was captured without any extra exposure.
Data Analysis
The SSQ was scored according to the developers’ instructions [33]: the participant’s marking on the line was measured with a clear, plastic ruler from the left edge of the line and recorded in mm. Markings that fell in between mm lines on the ruler were rounded up to the nearest mm.
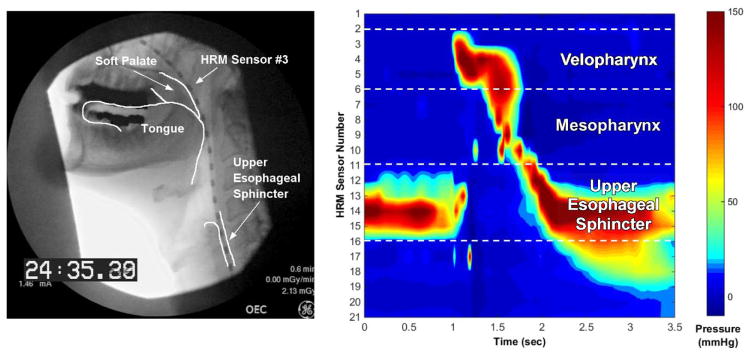
Manometric data were analyzed off-line using customized Matlab programs (The MathWorks, Natick, MA); the user was masked to condition. A validated, semi-automatic program was used to calculate the pressure durations, maximum amplitudes, and pressure integrals (areas under the curve) in the velopharynx and mesopharynx, as well as pressure nadir durations, minimum opening pressure, and maximum closing pressure in the upper esophageal sphincter [46] (Figure 1). The velopharynx is a region of swallowing-related pressure that spans 2–3 cm and is related to the soft palate closing against the contracting pharyngeal walls [39]. The upper esophageal sphincter is a region of high pressure at baseline, a decrease to a nadir pressure during opening, and then an increase in pressure following bolus flow with a gradual return to baseline pressure [47]. The mesopharynx is the region of pressure between the velopharynx and upper esophageal sphincter, with pressure contributions from the tongue base, pharyngeal walls, and laryngeal structures [46]. Pressure variability was measured in the following manner: 1) a zero time-point was assigned to the pressure peak on the most superior sensor in the velopharynx for each swallow; 2) pressure waveforms from each sensor were aligned across swallows according to the zero time-point; 3) a coefficient of variation (CV = mean/standard deviation) was calculated on each sensor from the onset to offset of swallowing-related pressure change; 4) CVs were averaged over each sensor in the following regions: velopharynx, mesopharynx, and upper esophageal sphincter; and 5) CVs averaged over each region were summed into a single, overall pressure variability parameter. Reliability analysis was not performed for this study, but we have shown that our methods for extracting HRM measures is reliable between expert users, speech-language pathologists, and undergraduate research assistants [48].
Figure 1.
Simultaneous videofluoroscopy with anatomical referents (left) and high-resolution manometry with pressure-related regions of interest (right). Velopharyngeal pressure relates to pressure generated from the soft palate and pharyngeal constriction. Hypopharyngeal pressure relates to pressure generated in between the tongue base and upper esophageal sphincter. Upper esophageal sphincter may cover up to 6 pressure sensors due to movement of pharyngeal and laryngeal structures during swallowing.
A modified version of the Modified Barium Swallow Impairment Profile (MBSImP) [14] was used to analyze videofluoroscopy. Each swallow was scored on the following MBSImP parameters: initiation of pharyngeal swallow, laryngeal elevation, anterior hyoid excursion, epiglottic movement, pharyngeal stripping wave, pharyngoesophageal sphincter opening, tongue base retraction, and pharyngeal residue. Additionally, a Penetration-Aspiration Scale [49] score was given for each swallow. Each swallow was scored by 2 trained raters masked to condition and discrepancies were re-scored by the first author.
Statistical Analysis
Binomial logistic regression was used to determine which swallowing parameters are most influential in determining the difference between our two groups: PD and healthy control. All continuous variables that did not follow a normal distribution were transformed using log or rank functions. A forward/backward model selection process was run using the Bayesian Information Criterion (BIC) to choose the best-fitting model; a low BIC indicates a better fit with fewer parameters in the model. Once a model was selected, parameters in the model were tested for multicollinearity using the Pearson product-moment correlation. Parameters that were significantly correlated with other parameters were removed from the model, leaving the model with the lowest BIC. Accuracy of the model to determine group membership was calculated by measuring the area under the receiver operating characteristic (ROC) curve. All statistical analyses were completed using R software packages (R Foundation, Austria).
RESULTS
Four models were generated, with varying parameters available for model selection: 1) HRM parameters only; 2) videofluoroscopy parameters only; 3) swallowing questionnaire parameters only; and 4) all parameters available. Parameter estimates and goodness of fit data for each model can be found in Table 1. Group data for all measures are in Table 2.
Table 1.
Logistic model estimates and goodness of fit.
| I. High Resolution Manometry Parameters Only: Group ~ VP maximum pressure + VP Coefficient of Variation | ||||
|---|---|---|---|---|
| Estimate | Standard Error | Z-value | P-value | |
| Intercept | 3.18 | 1.26 | 2.54 | 0.012 |
| VP max pressure | −0.02 | 0.01 | −2.35 | 0.019 |
| VP CV | 0.99 | 0.47 | 2.10 | 0.035 |
| BIC | 73.08 | |||
| Overall p-value | 0.004 | |||
| Area under ROC curve | 0.747 (95% CI: 0.612 – 0.882) | |||
| II. Videofluoroscopy Parameters Only: Group ~ (intercept) | ||||
|---|---|---|---|---|
| Estimate | Standard Error | Z-value | P-value | |
| Intercept | 0 | 0.28 | 0 | 1 |
| BIC | 76.04 | |||
| Overall p-value | 1 | |||
| Area under ROC curve | 0.5 (95% CI: 0.5 – 0.5) | |||
| III. Questionnaire Parameters Only: Group ~ SSQ #17 | ||||
|---|---|---|---|---|
| Estimate | Standard Error | Z-value | P-value | |
| Intercept | −2.72 | 0.78 | −3.48 | < 0.001 |
| SSQ #17 | 0.10 | 0.03 | 3.79 | < 0.001 |
| BIC | 59.64 | |||
| Overall p-value | < 0.001 | |||
| Area under ROC curve | 0.828 (95% CI: 0.720 – 0.937) | |||
| IV. High Resolution Manometry, Videofluoroscopy, and Questionnaire Parameters Group ~ VP maximum pressure + VP Coefficient of Variation + SSQ # 7 | ||||
|---|---|---|---|---|
| Estimate | Standard Error | Z-value | P-value | |
| Intercept | 2.38 | 1.66 | 1.43 | 0.15 |
| VP max pressure | −0.03 | 0.01 | −2.81 | 0.005 |
| VP CV | 1.64 | 0.70 | 2.35 | 0.019 |
| SSQ #7 | 0.16 | 0.05 | 3.27 | 0.001 |
| BIC | 52.90 | |||
| Overall p-value | < 0.001 | |||
| Area under ROC curve | 0.919 (95% CI: 0.848 – 0.990) | |||
VP = velopharynx; CV = coefficient of variation; BIC = Bayesian Information Criterion; ROC = receiver operator characteristic; SSQ = Sydney Swallow Questionnaire.
Table 2.
Group data for each parameter measured, reported as mean ± standard error of the mean. Videofluoroscopy parameters were measured on an ordinal scale, with higher values indicating a greater degree of impairment [14, 49]. The Sydney Swallow Questionnaire is a visual-analog scale ranging from 0–100 with higher values indicating a greater degree of impairment, with a highest possible score of 1700 [33].
| Healthy Controls | Parkinson Disease | |
|---|---|---|
| High Resolution Manometry Parameters | ||
| VP pressure duration (sec) | 0.86 ± 0.04 | 0.85 ± 0.05 |
| VP maximum pressure (mmHg)** | 183.9 ± 9.7 | 150.2 ± 10.1 |
| VP integral (mmHg*sec) | 143.6 ± 12.4 | 133.2 ± 15.6 |
| VP coefficient of variation** | 0.64 ± 0.11 | 1.02 ± 0.21 |
| MP pressure duration (sec) | 0.74 ± 0.04 | 0.77 ± 0.05 |
| MP maximum pressure (mmHg) | 163.5 ± 8.6 | 149.7 ± 12.63 |
| MP integral (mmHg*sec) | 134.56 ± 10.0 | 143.5 ± 23.9 |
| MP coefficient of variation | 0.97 ± 0.14 | 1.6 ± 0.28 |
| UES minimum pressure duration (sec) | 0.53 ± 0.04 | 0.56 ± 0.03 |
| UES minimum pressure (mmHg) | −5.9 ± 0.9 | −3.1 ± 1.0 |
| UES maximum pressure (mmHg) | 223.0 ± 13.1 | 200.1 ± 8.5 |
| UES coefficient of variation | 0.63 ± 0.06 | 1.06 ± 0.23 |
| Total coefficient of variation | 4.66 ± 0.42 | 7.62 ± 1.07 |
| Videofluoroscopy Parameters | ||
| Initiation of pharyngeal swallow (0–4 scale) | 0.8 ± 0.2 | 0.9 ± 0.2 |
| Laryngeal elevation (0–3 scale) | 0.2 ± 0.1 | 0.1 ± 0.1 |
| Anterior hyoid excursion (0–2 scale) | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Epiglottic movement (0–2 scale) | 0.1 ± 0.1 | 0.2 ± 0.1 |
| Pharyngeal stripping wave (0–2 scale) | 0.2 ± 0.1 | 0.2 ± 0.1 |
| UES opening (0–3 scale) | 0.2 ± 0.1 | 0.3 ± 0.1 |
| Tongue base retraction (0–4 scale) | 0.8 ± 0.1 | 1.1 ± 0.1 |
| Pharyngeal residue (0–4 scale) | 1.3 ± 0.1 | 1.5 ± 0.1 |
| Penetration-aspiration scale (1–8 scale) | 1.2 ± 0.1 | 1.5 ± 0.1 |
| Sydney Swallow Questionnaire | ||
| 1: Difficulty with swallowing at present | 4.4 ± 1.7 | 15.3 ± 2.5 |
| 2: Difficulty swallowing thin liquids | 5.0 ± 3.9 | 4.5 ± 1.0 |
| 3: Difficulty swallowing thick liquids | 3.2 ± 2.2 | 9.0 ± 2.8 |
| 4: Difficulty swallowing soft foods | 1.1 ± 0.5 | 7.0 ± 1.9 |
| 5: Difficulty swallowing hard foods | 5.7 ± 2.6 | 20.5 ± 4.0 |
| 6: Difficulty swallowing dry foods | 5.2 ± 2.6 | 19.5 ± 4.0 |
| 7: Difficulty swallowing saliva** | 0.8 ± 0.4 | 4.9 ± 1.1 |
| 8: Difficulty starting a swallow | 2.2 ± 0.8 | 7.92 ± 1.9 |
| 9: Feeling of food stuck in throat | 10.8 ± 3.4 | 21.5 ± 3.7 |
| 10: Coughing/choking when swallowing solids | 7.0 ± 2.2 | 15.3 ± 3.2 |
| 11: Coughing/choking when swallowing liquids | 8.7 ± 2.7 | 11.0 ± 2.7 |
| 12: Duration of an average meal | 12.7 ± 2.2 | 17.7 ± 2.3 |
| 13: Food or liquid goes into or comes out of nose | 0.8 ± 0.4 | 6.0 ± 2.0 |
| 14: Need to swallow more than once per bite | 5.1 ± 1.8 | 21.0 ± 3.9 |
| 15: Cough or spit out food during a meal | 2.5 ± 0.9 | 9.3 ± 2.5 |
| 16: Rate the severity of swallowing problem today | 3.2 ± 1.2 | 14.0 ± 2.4 |
| 17: How much swallowing problem interferes with enjoyment or quality of life** | 1.4 ± 0.5 | 16.6 ± 4.8 |
| Total Score | 79.7 ± 20.2 | 251.2 ± 34.4 |
VP = velopharynx; MP = mesopharynx; UES = upper esophageal sphincter;
indicates parameter included in the one of the models from Table 1.
With only HRM parameters available for model selection, a significant model was generated with two predictor variables: maximum pressure in the velopharynx, and pressure CV in the velopharynx (p = 0.004). No significant models were able to be generated with only videofluoroscopic parameters (p = 1.0), indicating that the videofluoroscopic parameters in this study were not adequate to sufficiently differentiate between patients with PD and healthy controls (additionally, see Table 2). With only questionnaire data available for model selection, a significant model was generated from Question #17: “How much does your swallowing problem interfere with your enjoyment or quality of life?” (p < 0.001) [33].
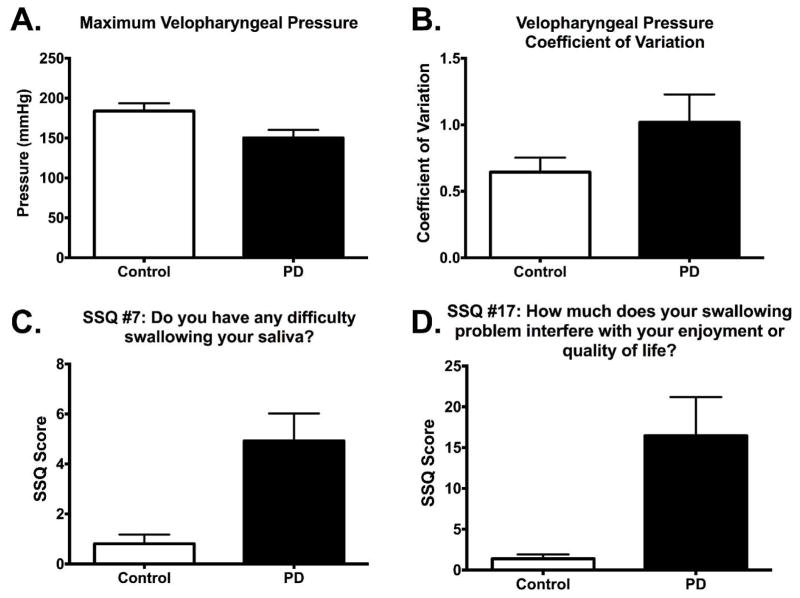
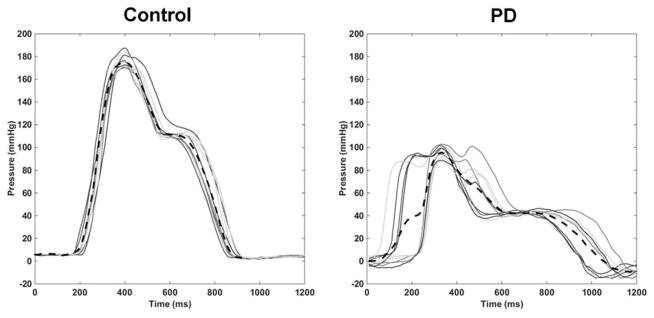
With all parameters available for model selection, a significant model was generated with three predictor variables: maximum pressure in the velopharynx, pressure CV in the velopharynx, and Question #7 on the Sydney Swallow Questionnaire: “Do you have any difficulty swallowing your saliva?” (p < 0.001) [33]. The statistical software had originally generated this model with 6 SSQ items: questions 5, 6, 7, 8, 10, 12, and 13 (see Table 2 for definitions); however, all of the SSQ parameters were correlated with each other, and inclusion of only SSQ #7 generated the model with the lowest BIC. Group data for parameters included in the models from Table 1 are presented in Figure 2, and an illustration of pressure variability (CV) in the velopharynx is in Figure 3.
Figure 2.
Box plots of parameters included in logistic models. Parameters shown in A and B were included in models I and IV in Table 1. Parameter shown in C was included in model IV from Table 1, and parameter shown in D was included in model III from Table 1. SSQ = Sydney Swallow Questionnaire.
Figure 3.
Example of velopharyngeal pressure variation from one participant from the control group and one participant with PD. Each solid line represents a pressure wave from a single swallow, and the black dashed line represents the mean over 10 swallows of 10 mL thin liquid. Coefficient of variation was calculated from the time the first pressure wave rose from baseline until the last pressure wave returned to baseline.
DISCUSSION
This is the first study to evaluate swallowing pressures in patients with PD using high-resolution manometry. While no videofluoroscopy parameters sufficiently differentiated between patients with PD and healthy controls, both high-resolution manometry and the Sydney Swallow Questionnaire parameters were included in significant logistic models. In the absence of videofluoroscopic signs of dysphagia, HRM and targeted self-report (SSQ) may be able to detect subtle changes in swallowing function as dysphagia emerges in PD. Despite the inherent limitations to statistical modeling and the relatively small sample size, HRM and the SSQ shows promise to illuminate subtle changes in the swallowing mechanism in a heterogeneous group of patients with PD.
Videofluoroscopy may not be the most sensitive tool for the evaluation of swallowing-related changes in the earlier stages of PD. A significant logistic model differentiating patients with PD and healthy, age and sex-matched controls could not be generated given only the MBSImP parameters measured in this study. A similar lack of differentiation is reflected in another study of objective timing and movement data from videofluoroscopy in a group of patients with PD in stages similar to that in the present study [50]. That study revealed, out of 30 parameters evaluated, only a difference existed in timing of velopharyngeal closure between patients with early to mid-stage PD and healthy, age and sex-matched controls [50]. A similar objective analysis, if performed in the present study, may have resulted in similar findings. A limitation of the present study is that we only evaluated the pharyngeal stage of 10 mL swallows of thin liquid; it is possible that larger or thicker consistencies that challenged the oral phase of swallowing may have revealed group differences not otherwise seen. It is likely that, in the early and mid-stages of PD, the amplitude of pharyngeal kinematic movement has not been greatly affected by PD neuropathology, but that subtle changes in motor planning and force generation are some of the first areas of swallowing function to be affected.
Between-individual pharyngeal swallowing variability is well-recognized [51–61], but within-individual pharyngeal swallowing pressure variability during a repeated task appears to be a novel measurement parameter that may be powerful in distinguishing healthy behavior from dysphagia. Within-individual motor variability in patients with PD has been described in limb forces and movements [62–69] and in gait [70–76], but this is the first study to describe swallowing pressure variability in the same patient population (CV in the velopharynx). Although the model with the best fit in this study only included pressure variability in the velopharyngeal region, pressure variability appears increased in all regions of interest in the group of patients with PD (Table 2). It is currently unclear how pharyngeal pressure variability relates to overall swallowing function. At the very least, identification of increased pharyngeal pressure variability in a patient with PD may result in periodic follow-up by a speech-language pathologist until more overt dysphagia signs and symptoms arise. Future work may reveal predictive components from pharyngeal pressure variability that may identify patients who could benefit from early, preventive swallowing therapy. Further investigations of swallowing pressure variability should also include evaluations of esophageal pressures, given the prevalence of esophageal dysmotility in PD [77]. Given the sensitive and objective nature of HRM, swallowing pressure variability may be used to identify subtle changes in swallowing function before overt signs of dysphagia are present on videofluoroscopy.
Complete velopharyngeal closure during swallowing prevents food and liquid from entering the nasal cavity and allows for a closed system for applying pressure to the bolus [78]. In the participants with PD, pressure in the velopharynx was less than that of age-matched controls. However, the pressure range seen in this group does not deviate from mean velopharyngeal pressure previously reported in groups of younger healthy swallowers: 124–169 mmHg [19, 39, 79–82]. It is worthwhile to note that while there was a difference seen in maximum amplitude of pressure generated in this region, there does not appear to be a large difference between groups in the velopharyngeal pressure integral (Table 2), which represent a measure of total pressure generated in this region. The difference in these two measurements may reflect a compensatory mechanism by the participants with PD to generate adequate pressure in the region while not achieving the same maximum pressure.
The single item from the Sydney Swallow Questionnaire that best differentiated between people with PD and healthy controls was in regards to swallowing-related quality of life. This has very important clinical implications; patients with PD may notice and may be personally affected by changes in their swallowing function before such changes are evident on videofluoroscopy. Such complaints should be addressed by healthcare providers at any stage of the disease process, even in the absence of overt signs of dysphagia. When combined with swallowing pressure data, the one item from the SSQ that differentiated between the two groups in the present study concerned difficulty swallowing saliva. Drooling is a common occurrence in PD, as well as other aspects of saliva production [83], and although the scores for this item from the group with PD were not particularly high on the SSQ scale, the combination of these scores and the HRM parameters in the model sufficiently differentiated between groups. Feelings of difficulty swallowing saliva and reduced swallowing-related quality of life may indicate early, subjective identification of a common problem in PD and should be addressed early in disease management. The mean total score for our healthy control group matches that of another study using the SSQ in healthy individuals throughout the age range, and the mean total score of the individuals with PD fell slightly above the upper limit of the normal range [38]. Miller and colleagues [13] report that an unspecific, binary question such as “Do you have any difficulty swallowing?” in patients with PD may not be a reliable indicator of swallowing function. Given the findings in the present study, it may be the case that more directed questions with a visual-analog scale answering system, such as with the Sydney Swallow Questionnaire, can be sensitive enough to determine differences between groups of individuals with PD and healthy controls.
CONCLUSION
Swallowing changes in the early and mid-stages of PD may be subtle and thus difficult to identify. Our study investigated swallowing pressures, pharyngeal kinematics, and swallowing self-assessment and found that maximum pressure and pressure variability in the velopharynx, along with patient-reported accounts of saliva swallowing was sufficient to differentiate a group of individuals with early to mid-stage PD from healthy age and sex-matched controls. A combination of pressure and questionnaire data more accurately differentiated between groups than either method alone. The lack of significant group differences using videofluoroscopy occurred because it may not be sensitive enough to detect early, sub-clinical changes in swallowing function within thin liquid swallows. The detection of swallowing pressure and self-assessment changes in the early stages of PD has the potential to improve swallowing-related treatment strategies by intervening at an earlier time point, allowing for maintenance of function (prevention) and thus potentially improving long-term outcomes.
Supplementary Material
Acknowledgments
Funding sources: National Institutes of Health grant DC011130
CAJ was supported by National Institutes of Health grants DC011130 and T32 GM007507 and a scholarship from the American Speech-Language-Hearing Foundation (New Century Scholars Doctoral Scholarship). MRC was supported by National Institutes of Health grant DC011130. We would like to acknowledge Timothy McCulloch, MD, FACS for research support and comments on the final draft of this manuscript, biostatistician Glen Leverson, PhD for assistance with statistical design and Suzan Abdelhalim, MD, MPH; Chelsea Walczak, BS; Will Bleifuss, BS; and Alyssa Mitchell, BS for assistance with data collection and analysis.
Footnotes
Financial disclosure: National Institutes of Health grants DC011130 and T32 GM007507. Internal grant from Department of Surgery, University of Wisconsin-Madison.
Author roles
CAJ was responsible for conception, organization, and execution of the research project, design and execution of the statistical analysis, writing the first draft of the manuscript, and review and critique of the manuscript. MRC was responsible for conception and organization of the research project, design, review and critique of the statistical analysis, and review and critique of the manuscript.
References
- 1.de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63:1240–1244. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- 2.Bird MR, Woodward MC, Gibson EM, Phyland DJ, Fonda D. Asymptomatic swallowing disorders in elderly patients with Parkinson’s disease: a description of findings on clinical examination and videofluoroscopy in sixteen patients. Age and Ageing. 1994;23:251–254. doi: 10.1093/ageing/23.3.251. [DOI] [PubMed] [Google Scholar]
- 3.Langmore SE, Terpenning MS, Schork A, Chen Y, Murray JT, Lopatin D, Loesche WJ. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13:69–81. doi: 10.1007/PL00009559. [DOI] [PubMed] [Google Scholar]
- 4.Pennington S, Snell K, Lee M, Walker R. The cause of death in idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:434–437. doi: 10.1016/j.parkreldis.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Logemann J. Pro-Ed; Austin, TX: 1998. [Google Scholar]
- 6.Melo A, Monteiro L. Swallowing improvement after levodopa treatment in idiopathic Parkinson’s disease: lack of evidence. Parkinsonism and Related Disorders. 2013;19:279–281. doi: 10.1016/j.parkreldis.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Troche MS, Brandimore AE, Foote KD, Morishita T, Chen D, Hegland KW, Okun MS. Swallowing Outcomes Following Unilateral STN vs. GPi Surgery: A Retrospective Analysis. Dysphagia. 2014 doi: 10.1007/s00455-014-9522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troche MS, Brandimore AE, Foote KD, Okun MS. Swallowing and deep brain stimulation in Parkinson’s disease: a systematic review. Parkinsonism and Related Disorders. 2013;19:783–788. doi: 10.1016/j.parkreldis.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciucci MR, Barkmeier-Kraemer JM, Sherman SJ. Subthalamic nucleus deep brain stimulation improves deglutition in Parkinson’s disease. Movement Disorders. 2008;23:676–683. doi: 10.1002/mds.21891. [DOI] [PubMed] [Google Scholar]
- 10.Lengerer S, Kipping J, Rommel N, Weiss D, Breit S, Gasser T, Plewnia C, Kruger R, Wachter T. Deep-brain-stimulation does not impair deglutition in Parkinson’s disease. Parkinsonism and Related Disorders. 2012;18:847–853. doi: 10.1016/j.parkreldis.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Kulneff L, Sundstedt S, Olofsson K, van Doorn J, Linder J, Nordh E, Blomstedt P. Deep brain stimulation - effects on swallowing function in Parkinson’s disease. Acta Neurologica Scandinavica. 2013;127:329–336. doi: 10.1111/ane.12019. [DOI] [PubMed] [Google Scholar]
- 12.Ciucci MR, Grant LM, Rajamanickam ES, Hilby BL, Blue KV, Jones CA, Kelm-Nelson CA. Early identification and treatment of communication and swallowing deficits in Parkinson disease. Seminars in Speech and Language. 2013;34:185–202. doi: 10.1055/s-0033-1358367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller N, Allcock L, Hildreth AJ, Jones D, Noble E, Burn DJ. Swallowing problems in Parkinson disease: frequency and clinical correlates. Journal of Neurology, Neurosurgery, and Psychiatry. 2009;80:1047–1049. doi: 10.1136/jnnp.2008.157701. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, Maxwell R, Blair J. MBS Measurement Tool for Swallow Impairment-MBSImp: Establishing a Standard. Dysphagia. 2008;23:392–405. doi: 10.1007/s00455-008-9185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard R, Belafsky PC, Rees CJ. Relationship between fluoroscopic and manometric measures of pharyngeal constriction: The pharyngeal constriction ratio. Annals of Otology Rhinology and Laryngology. 2006;115:897–901. doi: 10.1177/000348940611501207. [DOI] [PubMed] [Google Scholar]
- 16.Thompson TZ, Obeidin F, Davidoff AA, Hightower CL, Johnson CZ, Rice SL, Sokolove RL, Taylor BK, Tuck JM, Pearson WG., Jr Coordinate mapping of hyolaryngeal mechanics in swallowing. Journal of Visualized Experiments. 2014 doi: 10.3791/51476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baijens L, Barikroo A, Pilz W. Intrarater and interrater reliability for measurements in videofluoroscopy of swallowing. European Journal of Radiology. 2013;82:1683–1695. doi: 10.1016/j.ejrad.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman MR, Mielens JD, Omari TI, Rommel N, Jiang JJ, McCulloch TM. Artificial neural network classification of pharyngeal high-resolution manometry with impedance data. Laryngoscope. 2013;123:713–720. doi: 10.1002/lary.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mielens JD, Hoffman MR, Ciucci MR, McCulloch TM, Jiang JJ. Application of classification models to pharyngeal high-resolution manometry. Journal of Speech Language and Hearing Research. 2012 doi: 10.1044/1092-4388(2011/11-0088). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman MR, Jones CA, Geng Z, Abelhalim SM, Walczak CC, Mitchell AR, Jiang JJ, McCulloch TM. Classification of high-resolution manometry data according to videofluoroscopic parameters using pattern recognition. Otolaryngology Head & Neck Surgery. 2013;149:126–133. doi: 10.1177/0194599813489506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartelius L, Svensson P. Speech and swallowing symptoms associated with Parkinson’s disease and multiple sclerosis: a survey. Folia Phoniatrica et Logopaedica. 1994;46:9–17. doi: 10.1159/000266286. [DOI] [PubMed] [Google Scholar]
- 22.Dickson JM, Grunewald RA. Somatic symptom progression in idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2004;10:487–492. doi: 10.1016/j.parkreldis.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Müller J, Wenning GK, Verny M, McKee A, Chaudhuri KR, Jellinger K, Poewe W, Litvan I. Progression of dysarthria and dysphagia in postmortem-confirmed parkinsonian disorders. Archives of Neurology. 2001;58:259–264. doi: 10.1001/archneur.58.2.259. [DOI] [PubMed] [Google Scholar]
- 24.Skeat J, Perry A. Outcome measurement in dysphagia: not so hard to swallow. Dysphagia. 2005;20:113–122. doi: 10.1007/s00455-004-0028-z. [DOI] [PubMed] [Google Scholar]
- 25.Miller N, Noble E, Jones D, Burn D. Hard to swallow: dysphagia in Parkinson’s disease. Age and Ageing. 2006;35:614–618. doi: 10.1093/ageing/afl105. [DOI] [PubMed] [Google Scholar]
- 26.Ward EC, Bishop B, Frisby J, Stevens M. Swallowing outcomes following laryngectomy and pharyngolaryngectomy. Archives of Otolaryngology - Head & Neck Surgery. 2002;128:181–186. doi: 10.1001/archotol.128.2.181. [DOI] [PubMed] [Google Scholar]
- 27.Argolo N, Sampaio M, Pinho P, Melo A, Nobrega AC. Do swallowing exercises improve swallowing dynamic and quality of life in Parkinson’s disease? NeuroRehabilitation. 2013;32:949–955. doi: 10.3233/NRE-130918. [DOI] [PubMed] [Google Scholar]
- 28.Athukorala RP, Jones RD, Sella O, Huckabee ML. Skill Training for Swallowing Rehabilitation in Patients With Parkinson’s Disease. Arch Phys Med Rehabil. 2014 doi: 10.1016/j.apmr.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement Disorders. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 30.Potulska A, Friedman A, Krolicki L, Spychala A. Swallowing disorders in Parkinson’s disease. Parkinsonism & Related Disorders. 2003;9:349–353. doi: 10.1016/s1353-8020(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 31.Sung HY, Kim JS, Lee KS, Kim YI, Song IU, Chung SW, Yang DW, Cho YK, Park JM, Lee IS, Kim SW, Chung IS, Choi MG. The prevalence and patterns of pharyngoesophageal dysmotility in patients with early stage Parkinson’s disease. Movement Disorders. 2010;25:2361–2368. doi: 10.1002/mds.23290. [DOI] [PubMed] [Google Scholar]
- 32.Volonte MA, Porta M, Comi G. Clinical assessment of dysphagia in early phases of Parkinson’s disease. Neurological Science. 2002;23(Suppl 2):S121–122. doi: 10.1007/s100720200099. [DOI] [PubMed] [Google Scholar]
- 33.Wallace KL, Middleton S, Cook IJ. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678–687. doi: 10.1016/s0016-5085(00)70137-5. [DOI] [PubMed] [Google Scholar]
- 34.Simons JA, Fietzek UM, Waldmann A, Warnecke T, Schuster T, Ceballos-Baumann AO. Development and validation of a new screening questionnaire for dysphagia in early stages of Parkinson’s disease. Parkinsonism and Related Disorders. 2014;20:992–998. doi: 10.1016/j.parkreldis.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Manor Y, Giladi N, Cohen A, Fliss DM, Cohen JT. Validation of a swallowing disturbance questionnaire for detecting dysphagia in patients with Parkinson’s disease. Movement Disorders. 2007;22:1917–1921. doi: 10.1002/mds.21625. [DOI] [PubMed] [Google Scholar]
- 36.Leow LP, Huckabee ML, Anderson T, Beckert L. The impact of dysphagia on quality of life in ageing and Parkinson’s disease as measured by the swallowing quality of life (SWAL-QOL) questionnaire. Dysphagia. 2010;25:216–220. doi: 10.1007/s00455-009-9245-9. [DOI] [PubMed] [Google Scholar]
- 37.Goetz CG, Poewe W, Rascol O, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations The Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Movement Disorders. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 38.Szczesniak MM, Maclean J, Zhang T, Liu R, Cook IJ. The normative range for and age and gender effects on the Sydney Swallow Questionnaire (SSQ) Dysphagia. 2014;29:535–538. doi: 10.1007/s00455-014-9541-x. [DOI] [PubMed] [Google Scholar]
- 39.McCulloch TM, Hoffman MR, Ciucci MR. High-resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Annals of Otology, Rhinology, and Laryngology. 2010;119:369–376. doi: 10.1177/000348941011900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YH, Oh BM, Jung IY, Lee JC, Lee GJ, Han TR. Spatiotemporal characteristics of swallowing in Parkinson’s disease. Laryngoscope. 2015;125:389–395. doi: 10.1002/lary.24869. [DOI] [PubMed] [Google Scholar]
- 41.Troche MS, Rosenbek JC, Okun MS, Sapienza CM. Detraining outcomes with expiratory muscle strength training in Parkinson disease. Journal of Rehabilitation Research and Development. 2014;51:305–310. doi: 10.1682/JRRD.2013.05.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umemoto G, Tsuboi Y, Kitashima A, Furuya H, Kikuta T. Impaired food transportation in Parkinson’s disease related to lingual bradykinesia. Dysphagia. 2011;26:250–255. doi: 10.1007/s00455-010-9296-y. [DOI] [PubMed] [Google Scholar]
- 43.Fuh JL, Lee RC, Wang SJ, Lin CH, Wang PN, Chiang JH, Liu HC. Swallowing difficulty in Parkinson’s disease. Clinical Neurology and Neurosurgery. 1997;99:106–112. doi: 10.1016/s0303-8467(97)00606-9. [DOI] [PubMed] [Google Scholar]
- 44.Monte FS, da Silva-Junior FP, Braga-Neto P, Nobre e Souza MA, de Bruin VM. Swallowing abnormalities and dyskinesia in Parkinson’s disease. Movement Disorders. 2005;20:457–462. doi: 10.1002/mds.20342. [DOI] [PubMed] [Google Scholar]
- 45.Stroudley J, Walsh M. Radiological assessment of dysphagia in Parkinson’s disease. The British Journal of Radiology. 1991;64:890–893. doi: 10.1259/0007-1285-64-766-890. [DOI] [PubMed] [Google Scholar]
- 46.Geng Z, Hoffman MR, Jones CA, McCulloch TM, Jiang JJ. Three-dimensional analysis of pharyngeal high-resolution manometry data. The Laryngoscope. 2013;123:1746–1753. doi: 10.1002/lary.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones CA, Hammer MJ, Hoffman MR, McCulloch TM. Quantifying contributions of the cricopharyngeus to upper esophageal sphincter pressure changes by means of intramuscular electromyography and high-resolution manometry. Annals of Otology, Rhinology, and Laryngology. 2014;123:174–182. doi: 10.1177/0003489414522975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones CA, Hoffman MR, Geng Z, Abdelhalim SM, Jiang JJ, McCulloch TM. Reliability of an Automated High-Resolution Manometry Analysis Program Across Expert Users, Novice Users, and Speech-Language Pathologists. Journal of Speech, Language, and Hearing Research. 2014 doi: 10.1044/2014_JSLHR-S-13-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 50.Baijens LW, Speyer R, Passos VL, Pilz W, Roodenburg N, Clave P. Swallowing in Parkinson Patients versus Healthy Controls: Reliability of Measurements in Videofluoroscopy. Gastroenterology Research and Practice. 2011;2011:380682. doi: 10.1155/2011/380682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molfenter SM, Leigh C, Steele CM. Event sequence variability in healthy swallowing: building on previous findings. Dysphagia. 2014;29:234–242. doi: 10.1007/s00455-013-9501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molfenter SM, Steele CM. Temporal variability in the deglutition literature. Dysphagia. 2012;27:162–177. doi: 10.1007/s00455-012-9397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steele CM, Hung D, Sejdic E, Chau T, Fraser S. Variability in execution of the chin-down maneuver by healthy adults. Folia Phoniatrica et Logopaedica. 2011;63:36–42. doi: 10.1159/000319737. [DOI] [PubMed] [Google Scholar]
- 54.Galen S, Jost-Brinkmann PG. B-mode and M-mode Ultrasonography of Tongue Movements during Swallowing. Journal of Orofacial Orthopedics. 2010;71:125–135. doi: 10.1007/s00056-010-9928-8. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy D, Kieser J, Bolter C, Swain M, Singh B, Waddell JN. Tongue Pressure Patterns During Water Swallowing. Dysphagia. 2010;25:11–19. doi: 10.1007/s00455-009-9223-2. [DOI] [PubMed] [Google Scholar]
- 56.Martin-Harris B, Brodsky MB, Michel Y, Lee FS, Walters B. Delayed initiation of the pharyngeal swallow: normal variability in adult swallows. Journal of Speech, Language, and Hearing Research. 2007;50:585–594. doi: 10.1044/1092-4388(2007/041). [DOI] [PubMed] [Google Scholar]
- 57.Mishellany A, Woda A, Labas R, Peyron MA. The challenge of mastication: preparing a bolus suitable for deglutition. Dysphagia. 2006;21:87–94. doi: 10.1007/s00455-006-9014-y. [DOI] [PubMed] [Google Scholar]
- 58.Kendall KA, Leonard RJ, McKenzie SW. Sequence variability during hypopharyngeal bolus transit. Dysphagia. 2003;18:85–91. doi: 10.1007/s00455-002-0086-z. [DOI] [PubMed] [Google Scholar]
- 59.Kendall KA. Oropharyngeal swallowing variability. Laryngoscope. 2002;112:547–551. doi: 10.1097/00005537-200203000-00025. [DOI] [PubMed] [Google Scholar]
- 60.Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2001;280:G354–360. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- 61.Dodds WJ, Taylor AJ, Stewart ET, Kern MK, Logemann JA, Cook IJ. Tipper and dipper types of oral swallows. American Journal of Roentgenology. 1989;153:1197–1199. doi: 10.2214/ajr.153.6.1197. [DOI] [PubMed] [Google Scholar]
- 62.Longstaff MG, Mahant PR, Stacy MA, Van Gemmert AW, Leis BC, Stelmach GE. Discrete and dynamic scaling of the size of continuous graphic movements of parkinsonian patients and elderly controls. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74:299–304. doi: 10.1136/jnnp.74.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaillancourt DE, Slifkin AB, Newell KM. Inter-digit individuation and force variability in the precision grip of young, elderly, and Parkinson’s disease participants. Motor Control. 2002;6:113–128. doi: 10.1123/mcj.6.2.113. [DOI] [PubMed] [Google Scholar]
- 64.Alberts JL, Saling M, Adler CH, Stelmach GE. Disruptions in the reach-to-grasp actions of Parkinson’s patients. Experimental Brain Research. 2000;134:353–362. doi: 10.1007/s002210000468. [DOI] [PubMed] [Google Scholar]
- 65.Arias P, Robles-Garcia V, Espinosa N, Corral Y, Cudeiro J. Validity of the finger tapping test in Parkinson’s disease, elderly and young healthy subjects: is there a role for central fatigue? Clinical Neurophysiology. 2012;123:2034–2041. doi: 10.1016/j.clinph.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Haaxma CA, Bloem BR, Overeem S, Borm GF, Horstink MW. Timed motor tests can detect subtle motor dysfunction in early Parkinson’s disease. Movement Disorders. 2010;25:1150–1156. doi: 10.1002/mds.23100. [DOI] [PubMed] [Google Scholar]
- 67.O’Boyle DJ, Freeman JS, Cody FW. The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson’s disease. Brain. 1996;119(Pt 1):51–70. doi: 10.1093/brain/119.1.51. [DOI] [PubMed] [Google Scholar]
- 68.Robichaud JA, Pfann KD, Leurgans S, Vaillancourt DE, Comella CL, Corcos DM. Variability of EMG patterns: a potential neurophysiological marker of Parkinson’s disease? Clinical Neurophysiology. 2009;120:390–397. doi: 10.1016/j.clinph.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torres EB, Cole J, Poizner H. Motor output variability, deafferentation, and putative deficits in kinesthetic reafference in Parkinson’s disease. Frontiers in Human Neuroscience. 2014;8:823. doi: 10.3389/fnhum.2014.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. Journal of the Neurological Sciences. 2003;212:47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 71.Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM. Effect of gait speed on gait rhythmicity in Parkinson’s disease: variability of stride time and swing time respond differently. Journal of Neuroengineering and Rehabilitation. 2005;2:23. doi: 10.1186/1743-0003-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hausdorff JM, Lowenthal J, Herman T, Gruendlinger L, Peretz C, Giladi N. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. European Journal of Neuroscience. 2007;26:2369–2375. doi: 10.1111/j.1460-9568.2007.05810.x. [DOI] [PubMed] [Google Scholar]
- 73.Hausdorff JM. Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos. 2009;19:026113. doi: 10.1063/1.3147408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirchner M, Schubert P, Liebherr M, Haas CT. Detrended Fluctuation Analysis and Adaptive Fractal Analysis of Stride Time Data in Parkinson’s Disease: Stitching Together Short Gait Trials. PLoS One. 2014:9. doi: 10.1371/journal.pone.0085787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roemmich RT, Nocera JR, Vallabhajosula S, Amano S, Naugle KM, Stegemoller EL, Hass CJ. Spatiotemporal variability during gait initiation in Parkinson’s disease. Gait & Posture. 2012;36:340–343. doi: 10.1016/j.gaitpost.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blin O, Ferrandez AM, Serratrice G. Quantitative analysis of gait in Parkinson patients: increased variability of stride length. Journal of the Neurological Sciences. 1990;98:91–97. doi: 10.1016/0022-510x(90)90184-o. [DOI] [PubMed] [Google Scholar]
- 77.Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Movement Disorders. 2008;23:1065–1075. doi: 10.1002/mds.22051. [DOI] [PubMed] [Google Scholar]
- 78.Finkelstein Y, Talmi YP, Rubel Y, Zohar Y. Nasopharyngeal pressure gradients during non-phonetic activities of the velopharyngeal valve. Part I. International journal of pediatric otorhinolaryngology. 1990;18:197–209. doi: 10.1016/0165-5876(90)90143-f. [DOI] [PubMed] [Google Scholar]
- 79.Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high resolution manometry. The Laryngoscope. 2010;120:2367–2373. doi: 10.1002/lary.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoffman MR, Mielens JD, Ciucci MR, Jones CA, Jiang JJ, McCulloch TM. High-resolution manometry of pharyngeal swallow pressure events associated with effortful swallow and the Mendelsohn maneuver. Dysphagia. 2012;27:418–426. doi: 10.1007/s00455-011-9385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsubara K, Kumai Y, Samejima Y, Yumoto E. Swallowing pressure and pressure profiles in young healthy adults. Laryngoscope. 2014;124:711–717. doi: 10.1002/lary.24311. [DOI] [PubMed] [Google Scholar]
- 82.Takasaki K, Umeki H, Enatsu K, Tanaka F, Sakihama N, Kumagami H, Takahashi H. Investigation of Pharyngeal Swallowing Function Using High-Resolution Manometry. Laryngoscope. 2008;118:1729–1732. doi: 10.1097/MLG.0b013e31817dfd02. [DOI] [PubMed] [Google Scholar]
- 83.Srivanitchapoom P, Pandey S, Hallett M. Drooling in Parkinson’s disease: a review. Parkinsonism and Related Disorders. 2014;20:1109–1118. doi: 10.1016/j.parkreldis.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.