Abstract
Background
Broadly neutralizing antibodies (bNAbs) targeting conserved epitopes on the HIV envelope glycoprotein have been identified in blood from HIV-1 infected women. We investigated whether antibodies in the genital tract from these women share similar epitope specificities and functional profiles as those in blood.
Methods
IgG and IgA antibodies were isolated from cervicovaginal lavages or Softcups from 13 HIV-infected women in the CAPRISA 002 cohort using Protein G and Peptide M respectively. Binding antibodies to envelope antigens were quantified by ELISA and binding antibody multiplex assay. Neutralizing antibody titers and epitope targets were measured using the TZM-bl assay with Env-pseudotyped wild-type and mutated viruses.
Results
HIV-specific IgG, but not IgA, was detected in genital secretions and the ratio of total IgG to HIV-specific IgG was similar to plasma. HIV-specific IgG reacted with multiple envelope antigens, including V1V2, gp120, gp140 and gp41. Two women had high plasma titers of HIV-specific IgG3 which was also detected in their genital tract samples. IgG from the genital tract had neutralizing activity against both Tier 1 and Tier 2 primary HIV-isolates. Antibodies targeting well-known glycan epitopes and the membrane proximal region of gp41 were detected in genital secretions, and matched specificities in plasma.
Conclusions
Women with HIV-specific plasma bNAbs have overlapping specificities in their genital secretions, indicating that these predominantly IgG isotype antibodies may transudate from blood to the genital tract. These data provide evidence that induction of systemic HIV-specific bNAbs can lead to antiviral immunity at the portal of entry.
INTRODUCTION
Sexual transmission of HIV remains the most common route of infection, with young women especially at risk [1, 2]. Mucosal surfaces of the genital tract are the principal and initial sites of infection, and therefore local mucosal antibody immunity is crucial in the control of HIV replication before systemic dissemination [3]. Broadly neutralizing antibodies (bNAbs) are able to inhibit the majority of HIV strains and, if elicited by an HIV vaccine, are likely to be effective at blocking infection at the site of entry. In non-human primates, passively-infused bNAbs have been shown to inhibit simian-human immunodeficiency virus (SHIV) infection using the high-dose vaginal challenge model [4-7]. In addition, vaginally applied bNAbs protected macaques from SHIV vaginal challenge [8, 9]. An HIV vaccine may therefore be required to elicit potent, long-lasting HIV-specific antibodies in blood and at the genital mucosa, where the virus is first encountered. In the RV144 human vaccine trial that showed moderate efficacy, HIV-specific V1V2 binding antibodies, particularly of the IgG3 subclass, were found to correlate with a reduced risk of HIV infection [10-12]. However, since no mucosal sampling was done in this vaccine trial, the presence of these potentially protective antibodies in the genital tract could not be assessed.
HIV-specific binding and neutralizing antibodies have been described in the genital tract of HIV-infected women [13-15], and in highly-exposed but persistently HIV seronegative (HEPS) women [16-18]. HIV-specific antibodies from lower genital tract secretions have been shown to be predominantly IgG rather than IgA, suggesting that transudation of systemic HIV-specific IgG antibodies contributes to IgG dominance at this mucosal surface [13, 19-21]. The neonatal receptor (FcRn) is involved in IgG transport across polarised epithelial cells lining mucosal surfaces such as the single-layered columnar epithelial cells of the endocervical canal, in a pH-dependent manner [22]. B cells have also been identified in tissue from the genital tract of HIV-infected women [23-25], suggesting that there is potentially also local production of antibodies from resident B cells in addition to transudation of antibodies from blood.
Natural HIV infection studies have shown that a proportion of HIV-infected individuals develop bNAbs in their plasma, generally after many years of infection [26-30]. The targets of these bNAbs on the HIV envelope have been mapped to the CD4bs, the glycan at 332, the V1V2 domain, the membrane proximal external (MPER) region, and the gp120-gp41 interface [31, 32]. Approximately 20% of HIV-infected individuals in the CAPRISA 002 cohort developed plasma bNAbs after 2-4 years of infection [27]. In this study, we investigated whether HIV-specific bNAbs are present in genital secretions from these HIV-infected women who developed breadth systemically, and whether these antibodies recognized common binding and neutralization epitopes.
METHODS
Study participants
Plasma and genital secretions collected by cervicovaginal lavages (CVLs) and/or Softcups were obtained from 13 women in the CAPRISA 002 and CAPRISA 004 cohorts, from Kwa-Zulu Natal, South Africa [33-35] (Supplementary Table 1). This study was approved by the Human Research Ethics Committees of the University of Witwatersrand, University of KwaZulu-Natal and University of Cape Town. All participants provided written informed consent.
Collection of genital secretions
CVL samples were collected as previously described [36]. Each woman underwent a speculum examination during which her cervix was irrigated with a lavage of 10 ml sterile saline. Aspirated saline was transferred to a clean 15 ml tube and centrifuged at 2,300 rpm for 10 minutes to remove cells. Supernatants were harvested and stored at −80°C. In addition, cervical secretions were collected using a Softcup® Menstrual cup (EuroFemPro, Netherlands) and processed as previously described with modifications [37]. For this, the Softcup was inserted into the vagina for a minimum of 1 hour by a clinician, placed into a 50 ml tube and centrifuged [38]. The fluid phase was collected into an eppendorf tube and the volume measured. The pellet was resuspended and centrifuged in 300μl PBS to increase antibody recovery and the fluid phases combined to a total volume of 500μl. This was used for IgG purification on the same day.
Immunoglobulin isolation and quantification
IgG antibodies were isolated using Protein G (Pierce/Thermo Scientific) while IgA was isolated by Peptide M (InvivoGen) as previously described [39]. The final fractions were concentrated using 50,000 MW concentrators (Millipore). Total IgG and IgA concentrations in plasma, CVLs and Softcup secretions were quantitated by ELISA, as previously described [40]. High protein-binding 96-well microplates (Nalge Nunc International, Rochester, NY) were coated with 4μg/ml goat polyclonal anti-human IgA or IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), and then blocked with 5% goat serum in PBS-0.05% Tween 20. Duplicates of 2-fold serially diluted samples and a human Immunoglobulin (Ig) reference serum (Human Immunoglobulin Calibrator; Binding Site, Birmingham, U.K.) were added to the plates and incubated for 1 hr at 37°C. The captured Ig was detected after consecutive incubations with biotin-labelled goat F(ab’)2 specific for human IgA or IgG (BioSource, Camarillo, CA) and horseradish (HRP)-labelled avidin (Sigma, St. Louis, MO). The ELISA was developed with TMB substrate (Thermo Fisher Scientific, Rockford, IL) and stopped with a 1M H2SO4 solution. The plates were read at 450 nm on a microplate reader (Molecular Devices, Sunnyvale, CA). A standard curve was run on each plate with serial dilutions of a commercial human IgG (Sigma-Aldrich) or IgA (Jackson Immunoresearch, PA) to calculate antibody concentrations.
Binding antibody multiplex assay (BAMA)
Binding antibodies to HIV proteins and peptides were measured using a customized HIV BAMA, as previously described [10, 41]. The HIV antigens used included: consensus gp120 Env (Con6 gp120), clade C gp140 Env trimer (1086 Trimer), clade C TV1 gp140, Group M consensus gp140 Env (ConS gp140), clade B gp70 V1V2 fusion protein (gp70_V1V2), a clade C V1V2 fusion protein (1086_V1V2), clade B MPER tetramer (MPR.03) and gp41 (Immunodiagnostics, Woburn MA). Carboxylated fluorescent beads (Luminex Corp,USA) were covalently coupled to purified HIV antigens and incubated with clinical samples at various dilutions. CVL supernatants (not purified for IgG) were used at a 1:5 dilution while plasma antibodies were measured from a titrated dilution (1:100 to 1:312,500). HIV-specific total IgG and IgG3 were detected using PE-conjugated mouse anti-human IgG and IgG3 (both Southern Biotech, USA), respectively. The beads were washed and acquired on a Bio-Plex instrument (Bio-Rad) and results expressed as mean fluorescence intensity (MFI). Responders had MFI≥100. The specific activity (MFI*dilution/antibody concentration) was calculated for genital tract IgG binding to account for variability in antibody levels recovered from different women. Responders had specific activity> 0.01.
HIV Neutralization assays
Neutralization was measured using HIV-Env pseudotyped viruses in the TZM-bl assay. Envelope genes were either cloned previously in our laboratory [42] or obtained from the NIH AIDS Research and Reference Reagent Program. This included one Tier 1 virus (SF162.LS) and three Tier 2 viruses (ConC, CAP45.G3 and TRO.11). MuLV (Murine Leukemia Virus) was included in the assay as a negative control. Neutralization was measured as a reduction in luciferase gene expression after a single round of infection in JC53bl-13 cells, also known as TZM-bl cells, with Env-pseudotyped viruses [43]. IC50 was calculated as the IgG concentration causing a 50% reduction of relative light units.
Epitope mapping
For epitope mapping via ELISA, plates were coated with ConC gp120 proteins or with an MPER peptide (MPR.03).The ELISA was performed using purified IgG, as described above. Antibody binding to wild-type gp120 but not mutant gp120 (containing an N332A mutation), indicated dependence on the glycan at position 332. Direct antibody binding to the MPR.03 peptide was used to demonstrate the presence of MPER-specific IgG. For mapping via neutralization, viruses containing mutations at key epitopes were used in the neutralization assay, described above. This included ConC N160A, CAP45.G3 K169E and TRO.11 N332A mutants, used to map bNAb epitopes targeting sites in the V2 and V3 regions, shown using monoclonal antibodies targeting these sites (data not shown). A reduction in IC50 of the mutant virus compared to the wild-type virus was used to identify the epitope specificities of the isolated IgGs.
Statistical Analysis
The Mann–Whitney test was used for independent sample comparisons and a Wilcoxon matched-pairs signed rank test was performed for dependent sample comparisons, using GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA). All tests were two-tailed and P-values of <0.05 were considered significant.
RESULTS
Higher concentrations of IgG than IgA isotypes in genital secretions
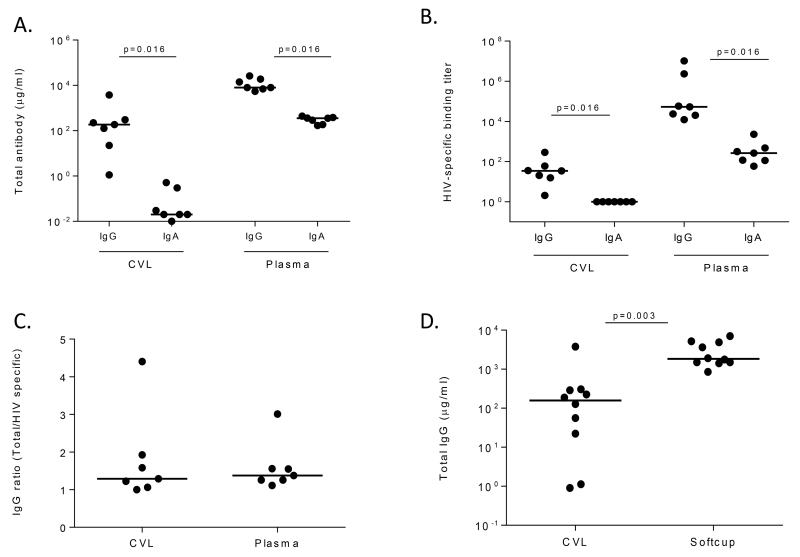
The quantity of total IgG and IgA antibodies isolated from matched plasma and genital samples of women, previously identified as having plasma bNAbs, was investigated. CVL samples were obtained from 7 ART-naïve women in the CAPRISA 002 cohort at 3 years post –infection [24]. The concentration of total IgA was negligible in genital secretions with a mean value of less than 1 μg/ml, while in plasma the mean value was 359 μg/ml. Overall, IgG concentrations were 2 logs greater and significantly higher than IgA concentrations in both plasma (p=0.016) and CVL (p=0.016) (Figure 1A).
Figure 1. Total and HIV-specific immunoglobulins in the female genital tract and plasma are predominantly of the IgG isotype.
(A) Quantification of IgG and IgA concentrations in CVL and plasma of the 7 CAPRISA 002 women with broadly cross neutralizing activity. (B) gp120-specific binding of IgG and IgA antibodies purified from CVL and plasma from the same women. (C) Ratio of total IgG to HIV-specific IgG concentrations in CVL and plasma from the 7 CAPRISA 002 women, based on 17b mAb standard curve for gp120 IgG. (D) Comparison of total IgG yields from CVL original data (from CAPRISA 002) and genital secretions collected using Softcup from 10 women in the CAPRISA 002 and 004 cohorts.
To determine whether these antibodies were HIV-specific, we tested them in a gp120 ELISA (Figure 1B). CVL IgG antibodies bound gp120 at a midpoint titer of 1:34 while the plasma HIV-specific IgG binding titer was 3 logs higher at 1:53,697. HIV-specific IgA antibodies in plasma were detected at a median titer of 1:266. None of the individuals had HIV-specific IgA detected in CVL, with binding titers at the same level as background. Given this, we focused the rest of the study on IgG antibodies. In order to accurately compare HIV-specific IgG concentrations in plasma and CVL, we calculated the ratio of HIV-specific to total IgG. Even though plasma consistently had higher titers of IgG than CVL, the median HIV-specific activity was similar in CVL and plasma (Figure 1C).
In order to optimize sample collection for neutralizing antibody assays, we explored the use of the Softcup for collecting genital secretions. Since this method requires fresh sample processing we recruited 4 of the women with bNAbs from the CAPRISA 002 cohort who were by that time on antiretroviral therapy (ART), but still had detectable neutralization titers (Mkhize, unpublished) plus an additional 6 chronically HIV-infected women from the CAPRISA 004 cohort who also developed plasma bNAbs (Madzivhandila, unpublished). We compared the yields of total IgG obtained from CVL to those recovered from the genital tract via the Softcup in all 10 women. The total IgG recovered from CVL varied amongst the participants with a range of 0.9-3,773 μg/ml, while the total IgG collected via Softcup was more consistent with a range of 1,060-6,752 μg/ml (Figure 1D). Compared to CVL, the Softcup method resulted in a 13-fold higher level of total IgG (p=0.003).
HIV-specific antibodies in CVL recognize multiple HIV envelope antigens
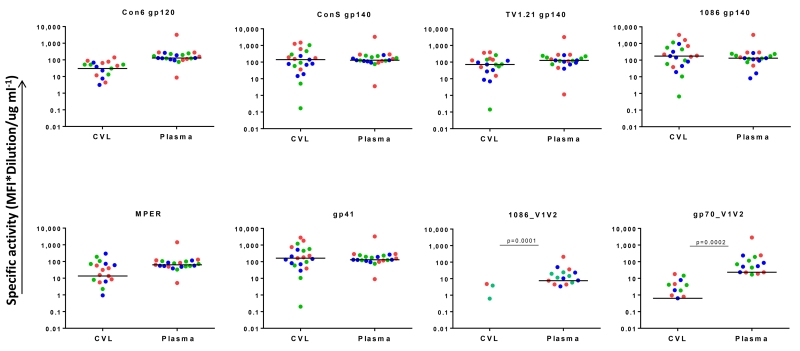
The kinetics, magnitude and specificities of antibodies in CVL and plasma collected from women at 1, 2 and 3 years post-infection were compared using BAMA. HIV-specific IgG binding reactivity to all 8 HIV Env antigens was detected in CVLs and plasma, and since there was little difference between time-points, the data were analysed together (Figure 2). Antibodies to gp120, gp140, gp41 and MPER were present at all time-points at similar levels in CVL and plasma. Only antibodies to the V1V2 antigens differed, with genital tract IgG binding levels significantly lower than plasma (1086_V1V2 (p=0.0001); gp70_V1V2 (p=0.0002). IgG responses to gp70 _V1V2 were better than to 1086_V1V2 and these were detected in 4/7 women at ≥1 time-points. Overall, these data suggest that HIV-specific IgG antibody specificities in the genital compartment generally match those found in blood plasma, even though levels were generally lower in the genital compartment.
Figure 2. HIV-specific activity of IgG antibodies in CVL and plasma.
IgG binding antibodies were measured in CVL and plasma from the 7 CAPRISA 002 women who developed broadly cross neutralizing antibodies. Dots represent CVL and plasma samples from 1 year (red), 2 years (green) and 3 years (blue) post HIV infection. Reactivity to 8 antigens was evaluated via a customized binding antibody multiplex assay (BAMA). The median fluorescence intensity (MFI) values were normalized to total IgG concentration (specific activity). Samples that had MF1<100 were considered non-responders therefore the points are not reflected on the plots. Responders had a specific activity >0.01 for all antigens.
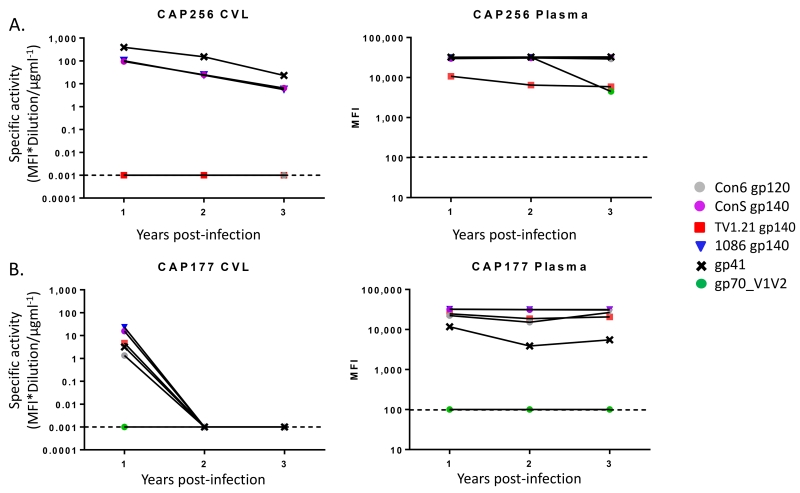
Considering the important role of the IgG3 subclass in HIV-1 vaccine studies [10, 11] and bNAbs [44, 45], we further examined this subclass binding response in the genital compartment. Two individuals, CAP256 and CAP177, were found to have detectable HIV-specific IgG3 responses in both plasma and genital compartments (Figure 3). For CAP256, plasma IgG3 antibodies to all antigens remained at high titers over time although responses to gp70_V1V2 decayed slightly by 3 years post infection. Declining responses to all antigens were also observed in CVL although V1V2 IgG3 responses were not detected at any time-point. Plasma from CAP177 contained HIV-specific IgG3 to all antigens except gp70_V1V2. In this individual, low titers of these antibodies were also found in the CVL at 1 year post-infection but declined to below the levels of detection at later time points. This is likely due to the low overall concentrations of IgG antibodies recovered from this participant at the 2 and 3 year time points (not shown).
Figure 3. IgG3 binding to HIV-specific antigens.
IgG3 responses were measured against Env antigens at 1, 2 and 3 years post HIV-infection. Responses were detected in CAP256 (A) and CAP177 (B), represented as specific activity for CVL (left panel) and MFI for plasma samples (right panel). Full data is presented in supplementary Table 1.
HIV-specific neutralizing antibody activity in the female genital tract
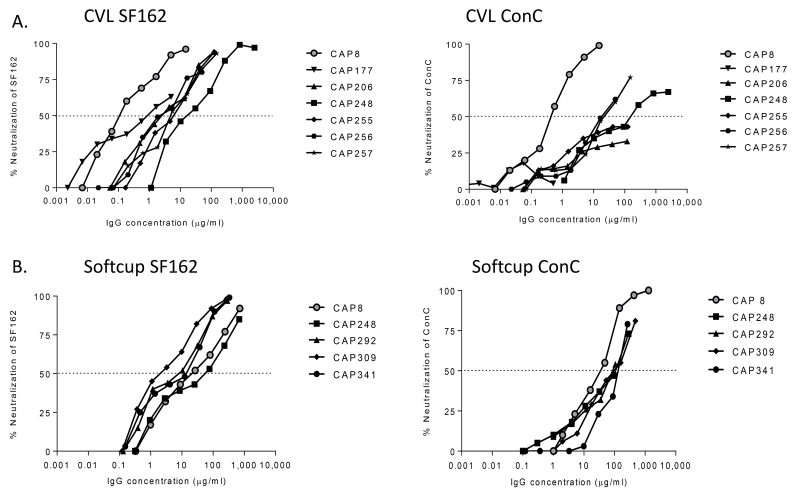
Next, we determined whether HIV-specific antibodies in CVLs had neutralizing activity. All 7 HIV-infected women previously shown to have bNAbs [27] had HIV-specific IgG antibodies that neutralized SF162, an easy-to-neutralize Tier 1 virus (Figure 4A). Furthermore, they also all showed dose-dependent activity against ConC, a Tier 2 virus, although this did not reach 50% neutralization in 3/7 women. The potent neutralization of the more sensitive SF162 suggested that Tier 1 neutralizing antibodies were more prevalent in CVL, as is also seen in plasma. However, there was a wide range in IC50 values among these samples with CAP8 showing the highest level of activity against both viruses.
Figure 4. Neutralization of HIV by purified IgG from the genital tract.
SF162 (Tier 1) and ConC (Tier 2) viruses were tested in a TZM-bl neutralization assay using IgG isolated from CVL supernatant (A) and Softcup (B) from women in the CAPRISA 002 and 004 cohorts. The dotted line represents the IC50.
Because antibody concentrations were generally low in CVLs, we next tested IgG antibodies freshly collected from these women using cervical Softcups to improve IgG recovery [38] and allow for more neutralization assays to be performed (Supplementary Table 1). This included two CAPRISA 002 women (CAP8 and CAP248) who were receiving ART at the time of Softcup sampling, as well as three additional women from the CAPRISA 004 cohort who were ART-naive. Softcup-derived IgGs isolated from 5 of these women were able to neutralize both SF162 and ConC (Figure 4B). In particular, the ability of all samples to neutralize ConC with measurable IC50 titers was a notable improvement over the CVL preparations. This is despite the fact that some of these women were on ART that is known to reduce antibody titers [46-48]. Overall the IC50 values of the curves suggested that IgG recovered from the Softcup provided a more consistent preparation compared to CVL.
Mapping of genital tract IgG antibody specificities
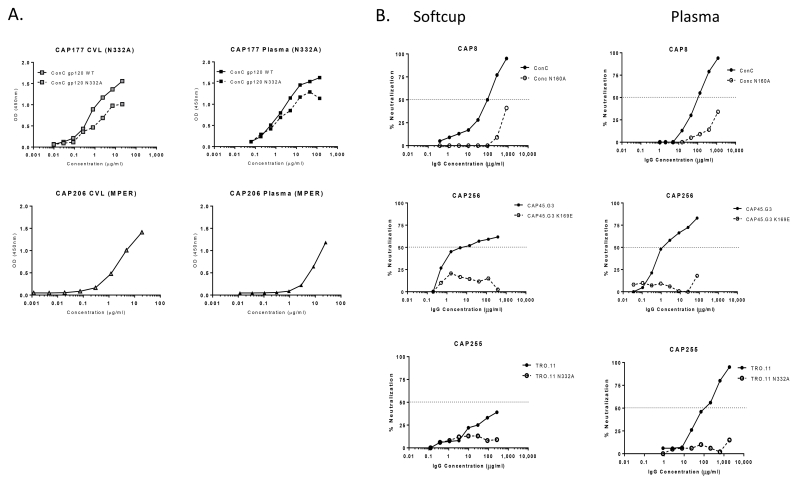
We previously showed that the plasma bNAbs in CAP177 were dependent on the glycan at position 332 in the V3 region of gp120 [27]. To determine whether genital tract antibodies shared the same specificity, we used a wild-type gp120 and an N332A mutant protein that lacked this glycan, in an ELISA assay. PGT128, a mAb that is highly dependent on N332 [49] showed significantly less binding to this mutant protein (data not shown). Comparison of IgG binding levels revealed a 4-fold reduction in binding to the mutant protein by IgG from CVL and a 3-fold reduction for plasma IgG (Figure 5A). This indicated that a subset of antibodies isolated from the CVL of CAP177 targeted the N332 glycan epitope on gp120 similar to the plasma antibodies.
Figure 5. Epitope mapping of antibody specificities by ELISA (A) and neutralization (B).
CVL and plasma IgG antibody specificities in CAP177 at 3 years post infection were tested by binding to wild-type and N332A mutant gp120 while MPER IgG activity in CAP206 was detected at 3 years post infection in both genital tract and plasma (A). Plasma and genital tract IgG antibody from CAP8, CAP256 and CAP255 were mapped by measuring neutralization of wild-type virus and mutant viruses. The dotted lines represent IC50 values (B).
Plasma bNAbs in CAP206 were previously shown to target the MPER of gp41 and bind the MPR.03 peptide in an ELISA assay [45, 50]. We found that IgG antibodies isolated from both plasma and CVL of CAP206 bound the MPR.03 peptide with comparable levels (Figure 5A) indicating that antibodies with a similar specificity were present in both compartments.
We next mapped antibodies by neutralization assay using epitope-ablating mutants. For this we used the more abundant IgG collected via Softcup which allowed us to test multiple mutants (Figure 5B). For CAP8, purified IgG antibodies from cervical softcups and plasma were sensitive to the N160A mutation in ConC that is characteristic of many V2-dependent antibodies [51, 52]. A V2 antibody was previously isolated from blood of donor CAP256 that is sensitive to the K169E mutation [53]. Here we show IgG antibodies from the genital tract of CAP256 were also sensitive to K169E in the CAP45.G3 virus. The presence of N332-dependent antibodies in both genital secretions and plasma from CAP255 was demonstrated using the TRO.11 virus. Although the level of neutralization against the wild-type virus was <50% inhibition (probably due to low levels of recoverable HIV-specific antibody), there was almost complete knock-out with the N332A mutant. Overall, these data reliably confirmed the presence of identical HIV-specific antibody specificities in both genital secretions and plasma.
DISCUSSION
We have detected HIV-specific binding and neutralizing IgG antibodies in the female genital tract of HIV-infected women previously shown to have bNAbs in plasma. Overall, these IgG antibodies matched those in plasma, targeting major neutralizing antibody epitopes including V2 and V3 glycans and the MPER of gp41. Binding antibodies to the V1V2 region, previously shown to correlate with protection in the RV144 HIV vaccine trial [12], were also present in the genital tract. The similarity of the antibody specificities detected in genital secretions and plasma suggest that the HIV-specific antibodies (or B cells) are able to transude across the genital mucosa. This further suggests that antibodies elicited by systemic vaccination (or transferred through passive immunization) are likely to reach mucosal surfaces and could contribute to preventing the sexual transmission of HIV.
This study has confirmed earlier reports that HIV-specific responses in genital secretions are almost exclusively of the IgG isotype [13, 14, 21, 54]. The paucity of HIV-specific IgA despite abundant levels of total IgA in lavages of HIV-infected women has also been noted by others [19 , 55-57]. A similar lack of HIV-specific IgA is seen in other mucosal external secretions including breast milk although in digestive mucosal secretions, such as saliva and intestinal surfaces, HIV-specific IgA antibodies predominate [58-60]. Even though IgG is the dominant isotype in the genital tract, the levels of total IgG have been reported to vary during the phases of the menstrual cycle [61, 62]. In this study, sampling of genital secretions was not normalised according to cycle as our focus was on examining the functional properties of genital antibodies which are unlikely to be affected by the menstrual cycle. Interestingly, HIV-specific IgG could be recovered from patients on long-term ART indicating that removal of antigenic stimulation did not ablate the IgG response in the genital compartment.
Mucosal sampling is particularly challenging for measuring antibody neutralization because of the low levels of IgG in genital secretions as well as interfering components [63]. We therefore isolated and quantified IgG prior to performing neutralization assays. This resulted in variable HIV-specific IgG content (the ratio of HIV specific IgG to total IgG) probably because large and variable volumes recovered by CVL. Antibody recovery was significantly improved through the use of the cervical Softcup [64, 65], which provided more consistent data and higher IgG recovery from genital fluids. The Softcup collects undiluted samples that are processed immediately [38], and thus are not exposed to freeze-thaw cycles, which may reduce the concentration of IgG antibodies in CVL [66]. Furthermore, other studies have shown that the endocervix, which is more specifically sampled by Softcup, has more concentrated antibodies than the vagina [60] which is a wash-out mainly of the vaginal vault. Another possible explanation for the improved recovery of genital IgG is that the Softcup method retains mucus during processing, which has been shown to harbour antibodies and other antiviral proteins [67], while during CVL processing mucus is spun down and discarded. The Softcup thus provides a significant improvement on CVL sample collection for the analysis of the humoral immunity to HIV.
The HIV-specific IgG binding activity to most Env antigens tested was similar for plasma and CVL, except for V1V2 antigens where lower concentrations of IgG at the genital tract made it difficult to detect epitope-specific responses. While HIV Env-specific IgG reactivity, which encompasses IgG1-4 isotypes, was observed in all individuals, only 2 had detectable IgG3 responses. These normally short-lived antibodies [68, 69] were detected up to 3 years post-infection in plasma and CVL. V1V2-specific IgG3 antibodies were shown to correlate with decreased risk of HIV infection in the RV144 trial [10-12]. While we were unable to detect V1V2 IgG3 at the genital tract, we suspect this is simply due to lower concentrations in CVL.
The finding of identical specificities among genital and plasma neutralizing IgG antibodies strongly supports a link between the female genital mucosa and blood. This suggests transudation of antibody from blood across the genital mucosa; or trafficking of B cells from the systemic compartment to become resident mucosal antibody secreting cells [60]. A recent study showed recruitment of IgG-secreting plasma cells to the genital tract of vaccinated rhesus macaques as well as a distinct set of resident cervical reserve epithelial cells that delivered IgG to the lumen via the neonatal Fc receptor [70]. The high expression of FcRn reserve cells in the epithelial layer offers a mechanism by which IgG from the circulation is transferred to the lumen and genital tract external surfaces. Several vaccine studies in humans and primate models have shown HIV or SIV-specific IgG antibodies in the genital tract [71, 72], providing further evidence of transudation of antibodies either from the circulation or migration of plasma cells to the mucosa.
Even though it was not possible to test large panels of viruses, this study suggests that HIV-specific broadly neutralizing antibodies are present in the female genital tract, and overlap in specificity with those present systemically. This study of women naturally infected with HIV provides pertinent insight into which plasma-derived protective antibodies can transduce to the genital tract. Our data suggest that systemic vaccine induced or passively infused antibodies would migrate or transudate to the genital compartment where they could protect against invading HIV particles.
Supplementary Material
Acknowledgments
We thank the women in the CAPRISA cohorts and the clinical and laboratory teams at CAPRISA for providing samples. Dr Elin Gray provided guidance at the start up of this project and Mary Phoswa provided technical assistance. All gp140, gp120, V1V2 glycoproteins and MPER tetramers were provided by Drs. B. Haynes, H. Liao and M.A. Moody (Duke Human Vaccine Institute, USA). We thank the Technology Innovation Agency (TIA) of the Department of Science and Technology (DST) of South Africa, U.S. National Institutes of Health for its Comprehensive International Program of Research on AIDS (CIPRA grant AI51794), NIH/NIAID (UM1AI068618), the Duke Center for AIDS Research (AI064518) and the Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP grant D43TW00231) for the research infrastructure and training that made the CAPRISA 002 acute infection study possible. NNM was funded by the Clinical Infectious Diseases Research Initiative (CIDRI), PRF and the Fogarty AITRP to visit Dr Georgia Tomaras laboratory to perform BAMA assays. PLM is a Wellcome Trust Intermediate Fellow in Public Health and Tropical Medicine (grant 089933/Z/09/Z).
REFERENCES
- 1.UNAIDS . UNAIDS Gap report 2013. 2013. [Google Scholar]
- 2.Abdool Karim SS, Abdool Karim Q, Baxter C. Antibodies for HIV prevention in young women. Curr Opin HIV AIDS. 2015;10:183–189. doi: 10.1097/COH.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 7.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 8.Moog C, Dereuddre-Bosquet N, Teillaud JL, Biedma ME, Holl V, Van Ham G, et al. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2014;7:46–56. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 9.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 10.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, et al. Vaccine-Induced Env V1–V2 IgG3 Correlates with Lower HIV-1 Infection Risk and Declines Soon After Vaccination. Sci Transl Med. 2014;6:228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, et al. Polyfunctional Fc-Effector Profiles Mediated by IgG Subclass Selection Distinguish RV144 and VAX003 Vaccines. Science Translational Medicine. 2014;6:228ra238. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 12.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-Correlates Analysis of an HIV-1 Vaccine Efficacy Trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Q, Moldoveanu Z, Huang WQ, Alexander RC, Goepfert PA, Mestecky J. Comparative Evaluation of HIV-1 Neutralization in External Secretions and Sera of HIV-1-Infected Women. Open AIDS J. 2012;6:293–302. doi: 10.2174/1874613601206010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright PF, Kozlowski PA, Rybczyk GK, Goepfert P, Staats HF, VanCott TC, et al. Detection of mucosal antibodies in HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 2002;18:1291–1300. doi: 10.1089/088922202320886334. [DOI] [PubMed] [Google Scholar]
- 15.Mestecky J, Wei Q, Alexander R, Raska M, Novak J, Moldoveanu Z. Humoral immune responses to HIV in the mucosal secretions and sera of HIV-infected women. Am J Reprod Immunol. 2014;71:600–607. doi: 10.1111/aji.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broliden K, Hinkula J, Devito C, Kiama P, Kimani J, Trabbatoni D, et al. Functional HIV-1 specific IgA antibodies in HIV-1 exposed, persistently IgG seronegative female sex workers. Immunol Lett. 2001;79:29–36. doi: 10.1016/s0165-2478(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 17.Devito C, Hinkula J, Kaul R, Kimani J, Kiama P, Lopalco L, et al. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J Acquir Immune Defic Syndr. 2002;30:413–420. doi: 10.1097/00042560-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Seaton KE, Ballweber L, Lan A, Donathan M, Hughes S, Vojtech L, et al. HIV-1 Specific IgA Detected in Vaginal Secretions of HIV Uninfected Women Participating in a Microbicide Trial in Southern Africa Are Primarily Directed Toward gp120 and gp140 Specificities. PLoS One. 2014;9:e101863. doi: 10.1371/journal.pone.0101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mestecky J, Jackson S, Moldoveanu Z, Nesbit LR, Kulhavy R, Prince SJ, et al. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res Hum Retroviruses. 2004;20:972–988. doi: 10.1089/aid.2004.20.972. [DOI] [PubMed] [Google Scholar]
- 20.Buchacz K, Parekh BS, Padian NS, van der Straten A, Phillips S, Jonte J, et al. HIV-specific IgG in cervicovaginal secretions of exposed HIV-uninfected female sexual partners of HIV-infected men. AIDS Res Hum Retroviruses. 2001;17:1689–1693. doi: 10.1089/08892220152741388. [DOI] [PubMed] [Google Scholar]
- 21.Belec L, Dupre T, Prazuck T, Tevi-Benissan C, Kanga JM, Pathey O, et al. Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. J Infect Dis. 1995;172:691–697. doi: 10.1093/infdis/172.3.691. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trifonova RT, Lieberman J, van Baarle D. Distribution of immune cells in the human cervix and implications for HIV transmission. Am J Reprod Immunol. 2014;71:252–264. doi: 10.1111/aji.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SK, Kim CJ, Kim DJ, Kang JH. Immune cells in the female reproductive tract. Immune Netw. 2015;15:16–26. doi: 10.4110/in.2015.15.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nkwanyana NN, Gumbi PP, Roberts L, Denny L, Hanekom W, Soares A, et al. Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology. 2009;128:e746–757. doi: 10.1111/j.1365-2567.2009.03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS (London, England) 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol. 2011;85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sather DN, Stamatatos L. Epitope Specificities of Broadly Neutralizing Plasmas from HIV-1 Infected Subjects. Vaccine. 2010;28S2:B8–B12. doi: 10.1016/j.vaccine.2009.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doria-Rose NA, Connors M. Antibody-secreting B cells in HIV infection. Curr Opin HIV AIDS. 2009;4:426–430. doi: 10.1097/COH.0b013e32832d9fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomaras GD, Binley JM, Gray ES, Crooks ET, Osawa K, Moore PL, et al. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J Virol. 2011;85:11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wibmer CK, Moore PL, Morris L. HIV broadly neutralizing antibody targets. Curr Opin HIV AIDS. 2015 doi: 10.1097/COH.0000000000000153. DOI:10.1097/COH.0000000000000153:1746-1630X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. Antibodies in HIV-1 Vaccine Development and Therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Loggerenberg F, Mlisana K, Williamson C, Auld SC, Morris L, Gray CM, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS One. 2008;3:e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mlisana K, Werner L, Garrett NJ, McKinnon LR, van Loggerenberg F, Passmore JA, et al. Rapid disease progression in HIV-1 subtype C-infected South African women. Clin Infect Dis. 2014;59:1322–1331. doi: 10.1093/cid/ciu573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrett NJ, Werner L, Naicker N, Naranbhai V, Sibeko S, Samsunder N, et al. HIV disease progression in seroconvertors from the CAPRISA 004 tenofovir gel pre-exposure prophylaxis trial. J Acquir Immune Defic Syndr. 2015;68:55–61. doi: 10.1097/QAI.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bebell LM, Passmore JA, Williamson C, Mlisana K, Iriogbe I, van Loggerenberg F, et al. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis. 2008;198:710–714. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 37.Shukair SA, Allen SA, Cianci GC, Stieh DJ, Anderson MR, Baig SM, et al. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 2013;6:427–434. doi: 10.1038/mi.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Archary D, Liebenberg LJ, Werner L, Tulsi S, Majola N, Naicker N, et al. Randomized Cross-Sectional Study to Compare HIV-1 Specific Antibody and Cytokine Concentrations in Female Genital Secretions Obtained by Menstrual Cup and Cervicovaginal Lavage. PLoS ONE. 2015;10:e0131906. doi: 10.1371/journal.pone.0131906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A. 2013;110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray ES, Moody MA, Wibmer CK, Chen X, Marshall D, Amos J, et al. Isolation of a monoclonal antibody that targets the alpha-2 helix of gp120 and represents the initial autologous neutralizing-antibody response in an HIV-1 subtype C-infected individual. J Virol. 2011;85:7719–7729. doi: 10.1128/JVI.00563-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray ES, Moore PL, Choge IA, Decker JM, Bibollet-Ruche F, Li H, et al. Neutralizing Antibody Responses in Acute Human Immunodeficiency Virus Type 1 Subtype C Infection. J Virol. 2007;81:6187–6196. doi: 10.1128/JVI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol. 2005 doi: 10.1002/0471142735.im1211s64. Chapter 12:Unit 12 11. [DOI] [PubMed] [Google Scholar]
- 44.Scharf O, Golding H, King LR, Eller N, Frazier D, Golding B, et al. Immunoglobulin G3 from Polyclonal Human Immunodeficiency Virus (HIV) Immune Globulin Is More Potent than Other Subclasses in Neutralizing HIV Type 1. Journal of Virology. 2001;75:6558–6565. doi: 10.1128/JVI.75.14.6558-6565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray ES, Madiga MC, Moore PL, Mlisana K, Abdool Karim SS, Binley JM, et al. Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region. J Virol. 2009;83:11265–11274. doi: 10.1128/JVI.01359-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris L, Binley JM, Clas BA, Bonhoeffer S, Astill TP, Kost R, et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falkensammer B, Freissmuth D, Hubner L, Speth C, Dierich MP, Stoiber H. Changes in HIV-specific antibody responses and neutralization titers in patients under ART. Front Biosci. 2007;12:2148–2158. doi: 10.2741/2218. [DOI] [PubMed] [Google Scholar]
- 48.Gach JS, Achenbach CJ, Chromikova V, Berzins B, Lambert N, Landucci G, et al. HIV-1 specific antibody titers and neutralization among chronically infected patients on long-term suppressive antiretroviral therapy (ART): a cross-sectional study. PLoS One. 2014;9:e85371. doi: 10.1371/journal.pone.0085371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, et al. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS One. 2011;6:e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonsignori M, Montefiori DC, Wu X, Chen X, Hwang KK, Tsao CY, et al. Two distinct broadly neutralizing antibody specificities of different clonal lineages in a single HIV-1-infected donor: implications for vaccine design. J Virol. 2012;86:4688–4692. doi: 10.1128/JVI.07163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moldoveanu Z, Mestecky J. Mucosal antibody responses to HIV. Methods Mol Biol. 2009;485:333–345. doi: 10.1007/978-1-59745-170-3_22. [DOI] [PubMed] [Google Scholar]
- 55.Mestecky J, Wright PF, Lopalco L, Staats HF, Kozlowski PA, Moldoveanu Z, et al. Scarcity or absence of humoral immune responses in the plasma and cervicovaginal lavage fluids of heavily HIV-1-exposed but persistently seronegative women. AIDS Res Hum Retroviruses. 2011;27:469–486. doi: 10.1089/aid.2010.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McKinley SA, Chen A, Shi F, Wang S, Mucha PJ, Forest MG, et al. Modeling Neutralization Kinetics of HIV by Broadly Neutralizing Monoclonal Antibodies in Genital Secretions Coating the Cervicovaginal Mucosa. PLoS One. 2014;9:e100598. doi: 10.1371/journal.pone.0100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexander R, Mestecky J. Neutralizing antibodies in mucosal secretions: IgG or IgA? Curr HIV Res. 2007;5:588–593. doi: 10.2174/157016207782418452. [DOI] [PubMed] [Google Scholar]
- 58.Fouda GG, Yates NL, Pollara J, Shen X, Overman GR, Mahlokozera T, et al. HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies. J Virol. 2011;85:9555–9567. doi: 10.1128/JVI.05174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mestecky J. Humoral immune responses to the human immunodeficiency virus type-1 (HIV-1) in the genital tract compared to other mucosal sites. J Reprod Immunol. 2007;73:86–97. doi: 10.1016/j.jri.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Bard E, Riethmuller D, Biichle S, Meillet D, Pretet JL, Mougin C, et al. Validation of a high sensitive immunoenzymatic assay to establish the origin of immunoglobulins in female genital secretions. J Immunoassay Immunochem. 2002;23:145–162. doi: 10.1081/IAS-120003658. [DOI] [PubMed] [Google Scholar]
- 61.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kutteh WH, Prince SJ, Hammond KR, Kutteh CC, Mestecky J. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin Exp Immunol. 1996;104:538–542. doi: 10.1046/j.1365-2249.1996.36742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mestecky J, Moldoveanu Z, Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am J Reprod Immunol. 2005;53:208–214. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 64.Boskey ER, Moench TR, Hees PS, Cone RA. A self-sampling method to obtain large volumes of undiluted cervicovaginal secretions. Sex Transm Dis. 2003;30:107–109. doi: 10.1097/00007435-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donadoni C, Bisighini C, Scotti L, Diomede L, Ngyen M, Nouhin J, et al. Setting of Methods for Analysis of Mucosal Antibodies in Seminal and Vaginal Fluids of HIV Seropositive Subjects from Cambodian and Italian Cohorts. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saltzman WM, Radomsky ML, Whaley KJ, Cone RA. Antibody diffusion in human cervical mucus. Biophys J. 1994;66:508–515. doi: 10.1016/s0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yates NL, Lucas JT, Nolen TL, Vandergrift NA, Soderberg KA, Seaton KE, et al. Multiple HIV-1-specific IgG3 responses decline during acute HIV-1: implications for detection of incident HIV infection. AIDS. 2011;25:2089–2097. doi: 10.1097/QAD.0b013e32834b348e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Q, Zeng M, Duan L, Voss JE, Smith AJ, Pambuccian S, et al. Live simian immunodeficiency virus vaccine correlate of protection: local antibody production and concentration on the path of virus entry. J Immunol. 2014;193:3113–3125. doi: 10.4049/jimmunol.1400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 72.Pavot V, Rochereau N, Lawrence P, Girard MP, Genin C, Verrier B, et al. Recent progress in HIV vaccines inducing mucosal immune responses. AIDS. 2014;28:1701–1718. doi: 10.1097/QAD.0000000000000308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.