Abstract
Midbrain dopaminergic (DA) neurons in the substantia nigra pars compacta and ventral tegmental area regulate extrapyramidal movement and important cognitive functions, including motivation, reward associations, and habit learning. Dysfunctions in DA neuron circuitry have been implicated in several neuropsychiatric disorders, including addiction and schizophrenia, whereas selective degeneration of DA neurons in substantia nigra pars compacta is a key neuropathological feature in Parkinson disease. Efforts to understand these disorders have focused on dissecting the underlying causes, as well as developing therapeutic strategies to replenish dopamine deficiency. In particular, the promise of cell replacement therapies for clinical intervention has led to extensive research in the identification of mechanisms involved in DA neuron development. It is hoped that a comprehensive understanding of these mechanisms will lead to therapeutic strategies that improve the efficiency of DA neuron production, engraftment, and function. This review provides a comprehensive discussion on how Wnt/β-catenin and sonic hedgehog–Smoothened signaling mechanisms control the specification and expansion of DA progenitors and the differentiation of DA neurons. We also discuss how mechanisms involving transforming growth factor-β and transcriptional cofactor homeodomain interacting protein kinase 2 regulate the survival and maturation of DA neurons in early postnatal life. These results not only reveal fundamental mechanisms regulating DA neuron development, but also provide important insights to their potential contributions to neuropsychiatric and neurodegenerative diseases.
Midbrain dopaminergic (DA) neurons are located in three major nuclei, including the substantia nigra pars compacta (SNpc; A9 group), the ventral tegmental area (VTA; A10 group), and the retrorubral field (A8 group) (Figure 1A). DA neurons in SNpc project to dorsal striatum via the nigrostriatal pathway, and regulate voluntary movement control as part of the basal ganglia circuitry. In contrast, DA neurons in VTA project to nucleus accumbens (ventral striatum), limbic systems, and the prefrontal cortex via the mesolimbic and mesocortical pathways, respectively (Figure 1A). Dopamine released from the nigrostriatal pathway modulates corticostriatal transmission in medium spiny neurons expressing dopamine D1 or D2 receptors, which leads to movement activation or suppression, respectively.1, 2, 3 In Parkinson disease, loss of DA neurons from SNpc is thought to result in overall motor inhibition because of differential effects on D1 and D2 receptor–expressing neurons.1, 2 In addition to its role in motor control, recent studies have identified DA input from SNpc to be important for goal-directed behaviors and habit learning.4, 5, 6, 7, 8 Moreover, the mode of DA neuron firing (tonic or phasic) appears to predict or modulate distinct aspects of behavior, with abolishment of phasic firing selectively impairing acquisition of cue-dependent learning and leaving other DA-dependent behaviors intact.9, 10, 11 In contrast to DA neurons in SNpc, DA neurons in the VTA and retrorubral field regulate cognitive functions, including emotion, motivation, reward, and addictive behaviors. Dysfunctions in these neurons have been implicated in psychiatric disorders.
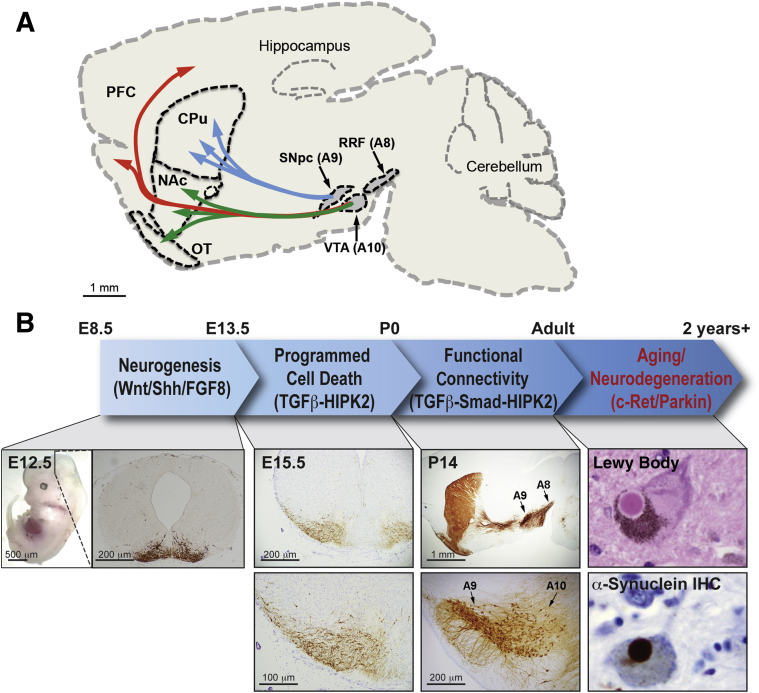
Figure 1.
Mechanisms controlling neurogenesis and survival of dopaminergic (DA) neurons. A: A schematic diagram showing DA neuron clusters in substantia nigra pars compacta (SNpc; A9), ventral tegmental area (VTA; A10), and retrorubral field (RRF; A8), and dopaminergic axonal projects from SNpc to caudate/putamen (CPu; blue arrows), from VTA to nucleus accumbens (NAc) and olfactory tubercle (OT; green arrows), and from VTA to prefrontal cortex (PFC; red arrows). B: The embryonic ventral midbrain undergoes patterning and neurogenesis from embryonic day (E) 8.5 to E13.5, wherein extrinsic factors like sonic hedgehog (Shh), Wnt, and fibroblast growth factor (FGF) 8 activate transcriptional programs required for the specification of neural progenitor cells and determination of their dopaminergic fate. A coronal section taken from an E12.5 midbrain shows newly born tyrosine hydroxlase (TH)–positive DA neurons migrating from the ventricular zone, where DA progenitors are specified, to their final locations in the marginal zone. From E13.5 to P0, DA neurons undergo further expansion and maturation as well as a period of programmed cell death, which peaks at P0 in mice. The transforming growth factor (TGF)-β pathway, signaling through homeodomain interacting protein kinase 2 (HIPK2), supports the survival of DA neurons during PCD. Formation of functional connectivity occurs from late embryonic to early postnatal stages. At this time, afferent dopaminergic pathways terminating in various cortical and striatal regions are established, as shown in the P14 sagittal view of TH staining. In the ventral midbrain, local circuitry is also established by the extension of TH-positive dendrites into substantia nigra pars reticulate and the formation of synaptic contacts from input neurons to DA neurons. This foundation of connectivity is also TGF-β dependent and appears to dictate the excitation-inhibition balance of DA neurons. As aging progresses, multiple mechanisms, including c-Ret/Parkin pathways, contribute to the degeneration of DA neurons. IHC, immunohistochemistry.
Despite the physiological and clinical relevance of midbrain DA neurons and the associated neural circuitry, the mechanisms involved in its establishment and maintenance are not well elucidated. To provide a more comprehensive view of the DA neural circuit at structural and functional levels, a major task has been to uncover the cellular and molecular mechanisms that govern the development of DA neurons and the formation of functional DA neural circuits. Regarding the development of DA neurons, there have been intense interests in the identification of both intrinsic and extrinsic cues that determine cell fate specification, progenitor expansion, and differentiation of DA neurons12 (Figure 1B). The goal is to apply these mechanisms to reprogram fibroblasts and generate large amounts of DA neurons that can be used in cell replacement therapy in Parkinson disease. If successful, these approaches will circumvent many technical and ethical issues related to using human fetal midbrain tissue for transplantation. In addition, investigations into trophic factor signaling in DA neurons have emerged as a promising route of inquiry because of their potential roles in early specification of DA progenitors, the neuroprotective effect of trophic factors against toxic insults, and their concerted activity in the maintenance of the nigrostriatal circuit.13, 14
In this review, we summarize our recent studies on the role of Wingless-type MMTV integration site family (Wnt)/β-catenin and sonic hedgehog (Shh)–Smoothened pathways in the development of DA progenitors and DA neurons. We also discuss how trophic support from transforming growth factor (TGF)-β regulates the survival and maturation of DA neurons, and how TGF-β and its downstream signaling mechanisms might have broad implications on the development and maintenance of neural circuits involving DA neurons (Figure 1B). These discussions summarize the emerging recognition of a holistic approach to investigate DA neurons in the context of circuit functions, and provide an important framework for future studies to further elucidate additional mechanisms that control the assembly, connectivity, maintenance, and degeneration in the important neural circuits established by DA neurons.
Development of DA Progenitors and DA Neurons during Early Embryogenesis
Concerted Effects of Intrinsic and Extrinsic Factors Regulate DA Neurogenesis
It is now established that midbrain DA neurons are originated from a neurogenic niche in the ventricular zone of the ventral midbrain (vMB), where neurogenesis of DA neurons occurs from approximately embryonic day (E) 9.5 to E14.5 in the mouse embryo. Within this neurogenic niche, the expression of several cell type–specific transcription factors, including Nurr1, Pitx3, En1/2, Otx2, Foxa1/2, Ngn2, and Lmx1a, enable neural stem and progenitor cells to establish proper cell identity, and control a cascade of transcriptional machinery that regulates cell fate and differentiation of DA neurons at the early stages of neurogenesis.15, 16 DA progenitors that fail to express these transcription factors show aberrant cell identity or undergo accelerated degeneration because of cell death. Conversely, overexpression of Nurr1, Pitx3, and Lmx1a in embryonic stem cells promotes a robust generation of DA neurons with molecular profiles and functional properties that resemble DA neurons in vivo.17, 18, 19
In addition to the intrinsic transcriptional programs, extrinsic factors, such as Wnts and Shh, can activate distinct transcriptional cascades necessary for the induction of the DA phenotype. Wnt signaling through the canonical β-catenin pathway controls the activation of Otx2 and Lmx1a/b20, 21, 22; however, Shh exerts its effects through Gli transcription factor–mediated induction of Foxa2.23 Although distinct, these extrinsic signals and their intrinsic transcriptional targets functionally interact at multiple levels. At the ligand level, Wnt1 antagonizes Shh signaling to cause down-regulation of Shh expression and its downstream target, Foxa2.24, 25 However, forced expression of Wnt1 transcription targets, Lmx1a and Otx2, acts synergistically with Foxa2 to enhance differentiation of DA neurons.20, 26 The interplay between these distinct, yet interdependent, mechanisms and their effects on DA neurogenesis in vivo and in vitro is still not well-elucidated.
Patterning of DA Progenitor Domains in vMB
Several lines of evidence indicate that the ventral region of the developing neural tube contains progenitors that can be divided into a distinct domain on the basis of the expression of cell type–specific transcription factors, which are required for the development of different groups of neurons in the ventral neural tube.27, 28, 29 Consistent with this idea, several studies have shown that a combinatorial code of cell type–specific transcription factors defines discrete progenitor domains in vMB that are distinctly different from the ventral progenitors in the spinal cord. As early as E8 to E8.5, the expression of transcription factors Lmx1a, Foxa2, Nkx2.1, and Nkx6.1 can be identified in a group of progenitors along the midline of vMB (Figure 2, A and B). As neurogenesis progresses, the DA niche undergoes medial to lateral expansion from E8.5 to E11.5. Unlike the developing spinal cord, the Foxa2+ progenitor domain (D1 and D2 domains) in vMB undergoes a tremendous expansion and shows a transient coexpression with Nkx2.2 at E9.5, followed by a persistent and extensive coexpression with Nkx6.1 from E10.5 to E12.5.26, 30, 31 Together, these results delineate a dynamic expansion of the vMB progenitor domains that are distinctively different from those in the spinal cord.
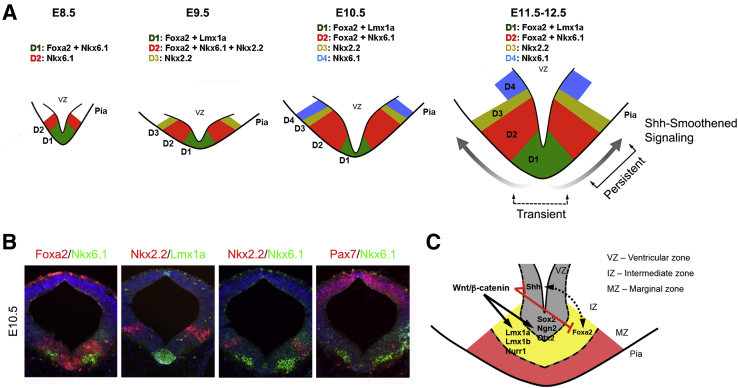
Figure 2.
Wnt/β-catenin and sonic hedgehog (Shh)–Smoothened signaling mechanisms regulate the dopaminergic neurogenic niche in ventral midbrain (vMB). A: Schematic diagrams illustrating the D1 to D4 progenitor domains in vMB defined by a combinatorial code of transcription factors from embryonic day (E) 8.5 to E12.5. At E8.5, Foxa2 and Nkx6.1 double-positive progenitors define the D1 domain and expression of Nkx6.1-positive progenitors defines the D2 domain in vMB. At E9.5, the D1 domain expresses Foxa2 and Lmx1a, whereas the D2 domain expresses Foxa2, Nkx6.1, and Nkx2.2. A more lateral D3 domain expresses Nkx2.2 only. At E10.5, D1 domain continues to express the same combination of Foxa2 and Lmx1a, but D2 domain expresses only Foxa2 and Nkx6.1. At E11.5 to 12.5, D1 to D4 domains continue to express the same combination of transcription factors. Genetic analyses show that Shh-Smoothened signaling exerts spatial and temporal influences on the progenitors in vMB at E11.5 to E12.5. B: Representative images of coexpression of transcription factors in the vMB at E10.5. C: A schematic diagram showing that Wnt/β-catenin antagonizes Shh/Smoothened signaling in the DA progenitors in the neurogenic niche of vMB. The image in A was adapted and modified from Tang et al.30 The image in B was adapted and modified from Tang et al,21 with permission from Company of Biologists. The image in C was adapted and modified from Tang et al,24 with permission from Society for Neuroscience.
Role of Wnt/β-Catenin and Shh-Smoothened Signaling in DA Neuron Development
Wnt/β-Catenin Signaling
Several members of the Wnt family have been shown to regulate distinct aspects of the development of vMB DA neurons. For instance, the canonical Wnt signaling mechanisms, mediated by Wnt1, Wnt2, and Wnt3a, control the patterning of midbrain-hindbrain boundary and the initial generation of DA progenitors in vMB, whereas Wnt5a regulates the differentiation of DA neurons.32, 33, 34 Results from mouse genetic studies further reveal a network of genetic interactions controlled by Wnt1 to regulate the early expansion of DA progenitors and the differentiation of DA neurons.22 Consistent with these results, removal of β-catenin in vMB using Shh-Cre or in DA progenitors and DA neurons using TH-IRES-Cre reveals a critical role of Wnt/β-catenin signaling in cell cycle progression and differentiation during DA neurogenesis.21, 25 In addition, β-catenin is prominently expressed in the radial glia processes and shows extensive colocalization with N-cadherin in the adherens junction of DA progenitors.21 Consistent with the important role of β-catenin in the maintenance of adherens junctions, DA progenitors lacking β-catenin show severe disruption of the radial glia process. These results are similar to those reported in mice lacking N-cadherin.35 Given the important functions of radial glia in neuronal migration during development,36 these results support the idea that the interaction between β-catenin and N-cadherin may regulate the migration and final segregation of DA neurons to their final destination in SNpc and VTA.
Consistent with the role of Wnt/β-catenin in regulating cell proliferation, constitutive activation of Wnt/β-catenin by stabilizing β-catenin in DA progenitors shortens cell cycle progression and leads to a marked expansion of early DA progenitors that express Sox2, Ngn2, and Otx2, as well as committed DA progenitors that express Lmx1a, Lmx1b, and Nurr1. However, DA progenitors with stabilized β-catenin exhibit reduced, rather than increased, production of mature DA neurons. This phenotype may be because of the perturbations in cell cycle progression in DA progenitors. Alternatively, it is possible that the imbalance in canonical and noncanonical Wnt signaling pathways in DA progenitors may suppress their ability to become mature DA neurons. In support of the latter scenario, DA progenitors expressing stabilized β-catenin are fully capable of differentiating into mature DA neurons in the presence of Wnt5a using dissociated cultures.24 In addition, cell type–specific activation of Wnt/β-catenin in a few Nurr1+ committed DA progenitors using TH-IRES-Cre leads to an increase in Nurr1+ cells and mature DA neurons in prenatal and perinatal brains.24 Together, these results are in agreement with the data from mouse embryonic stem cells that forced expression of Wnt target gene Lmx1a alone can induce a robust up-regulation of Nurr1 and Pitx3, but only a limited number of DA progenitors can differentiate into DA neurons.20 Collectively, these results support that Wnt/β-catenin signaling provides critical permissive cues to support the expansion, cell cycle progression, and differentiation of DA progenitors.
Antagonistic Interactions between Wnt/β-Catenin and Shh-Smoothened
Unlike the diffuse and nondiscrete expression of Wnt ligands, Shh expression is intense in vMB and defines an enriched region that produces a diverse group of neurons, including DA neurons, serotoninergic neurons, and neurons in the red nucleus.30, 37, 38, 39, 40 Consistent with these results, Shh signaling effectors, including Shh receptor Smoothened, Patched, and Gli1, show dynamic changes in vMB from E9.5 to E12.5, whereas the expression of Gli2 and Gli3 is more restricted to the dorsal midbrain. Interestingly, despite the broad expression of Shh signaling effectors in vMB, removal of Smoothened using Shh-Cre results in only a transient reduction in DA progenitors at E10.5 in Shh-Cre;Smofl/fl mutants30 (Figure 2). The modest and transient loss of DA progenitors and DA neurons in Shh-Cre;Smofl/fl mutants is different from the more severe DA neuron deficits seen in En1-Cre;Smofl/fl and En1-Cre;Shhfl/fl mutants,38, 41 most likely because of the broader patterning defects caused by En1-Cre in the dorsal midbrain and vMB, which can disrupt the expression of another important patterning gene, FGF8, in the midbrain and hindbrain boundary.
Using in vitro cultures and mouse genetics, several groups have shown that the canonical Wnt signaling antagonizes Shh expression during the neurogenesis of DA neurons.21, 24, 25 Similar effects of Wnt and Shh have also been confirmed for the generation of DA neurons from stem cells.20 Interestingly, analyses of the phenotypes in loss-of-function Shh-Cre;Smofl/fl mutants or the gain-of-function Shh-Cre;SmoM2 mutants indicate that Shh-Smoothened activity has a more dominant effect in regulating the development of DA progenitors at early embryonic stages before E10.5, whereas the effects of the canonical Wnt–β-catenin signaling are much more robust after E12.5. Together, these results support the model that Shh-Smoothened and Wnt–β-catenin signaling has sequential and antagonistic effects in regulating the development of DA neurons.
Mechanisms Supporting DA Neuron Survival in Postnatal Life
Developmental PCD in DA Neurons
After neurogenesis, the number of DA neurons progressively increases from postnatal day (P) 0 and reaches its maximum at P14. Similar to sensory and spinal motor neurons, DA neurons undergo programmed cell death (PCD) after neurogenesis is complete (Figure 1). In rats, two waves of PCD in DA neurons have been described, with most PCD occurring between P0 and P4 and a much smaller number detected at P14.42 Similar findings of PCD have also been identified in mice at perinatal stages from E17.5 to P0, but the smaller peak of PCD in rats at P14 has not been confirmed in mice.43
Several studies have investigated the underlying mechanisms contributing to PCD in DA neurons. On the basis of the timing of PCD in DA neurons, it has been proposed that, similar to PCD in sensory neurons and spinal motor neurons, competition for limited neurotrophic supports may contribute to PCD in DA neurons. Indeed, several members of the TGF-β superfamily, including glial cell line–derived neurotrophic factor (GDNF), TGF-β, and bone morphogenic proteins, have been shown to mediate a spectrum of developmental processes from gastrulation, epithelial-to-mesenchymal transition, to vascular and neuronal development.44, 45 For instance, GDNF receptors c-Ret and GDNF family receptor (GFR)α1 are expressed in DA neurons during development and in postnatal life, whereas GDNF mRNA is present in high abundance in the striatum. Interestingly, ectopic expression of GDNF in the striatum reduces the extent of PCD in DA neurons at perinatal stages.46, 47 In contrast, injection of GDNF neutralizing antibody in the striatum enhances PCD at perinatal stages.47 Despite these results, however, most studies indicate that mice lacking GDNF, GFRα1, or c-Ret in DA neurons show no evidence of deficits in DA neurons during perinatal stages,48, 49, 50, 51, 52 although one study argues that removal of GDNF in adult brains is sufficient to compromise the survival of DA neurons.53, 54 Interestingly, removal of c-Ret appears to affect the maintenance of mature DA neurons during the aging process via the prosurvival phosphoinositide-3-kinase–NF-κB pathway and Parkin-dependent regulation of mitochondrial functions.55 These results reveal surprising and previously unrecognized roles of GDNF–c-Ret signaling in the maintenance of DA neurons during neurodegeneration processes, and suggest the presence of additional trophic factors that could support the survival and functions of DA neurons in perinatal and early postnatal stages.
Signaling Mechanisms of TGF-β in Neural Development
Three different TGF-β ligands, TGF-β1, TGF-β2, and TGF-β3, bind to the serine/threonine receptor kinase TGF-β type II receptor (TβRII) to activate a myriad of signal transduction pathways. Engagement of TGF-β ligands with TβRII causes the recruitment and phosphorylation of the TGF-β type I receptor, which, in turn, phosphorylates receptor-regulated Smads (R-Smads, including Smads 2 and 3), allowing them to form complexes with co-Smads (Smad 4). The activated Smad complex translocates to the nucleus, where it regulates the transcription of target genes by targeting specific enhancer elements.56, 57, 58 The final outcome of this canonical TGF-β signaling depends on the chromatin state of the target genes, and can be further modulated by the recruitment of transcriptional cofactors that promote or inhibit transcription.58 Depending on the cellular context, TGF-β signaling can also be activated through Smad-independent (noncanonical) pathways, mediated through the activation of kinases like Erk, stress-activated protein kinase/c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase.44, 45 Such diverse signaling mechanisms contribute to the context-dependent and unique roles of TGF-β during brain development, as well as maintenance, and aging processes.
Several lines of evidence indicate that TGF-β has diverse and highly evolutionarily conserved roles in regulating induction, specification, and maintenance of the neural phenotype that is highly context dependent on spatial, temporal, and cell type specificity. For instance, inhibition of TGF-β signaling leads to induction of neural tissue in the ectoderm during gastrulation59, 60 and conversion of human ES and iPS cells to a neural fate.61 In Drosophila, TGF-β signaling is important in regulating synaptic growth, as well as axonal remodeling during metamorphosis.62, 63 In addition, loss of TGF-β1 leads to neuronal apoptosis, whereas neutralization of TGF-βs prevents ontogenetic neuron death.64, 65 To circumvent indirect or non–cell-autonomous effects of loss-of-function in TGF-β, several studies use more conditional mutagenesis approaches to directly address the cell-type specificity of TGF-β signaling. For instance, loss of TGF-β signaling from neocortical neurons prevented axon specification through interaction with the Par3/6 polarity complex.66 Furthermore, deletion of TβRII from retinal ganglion cells during development of the visual system resulted in synaptic pruning defects in the lateral geniculate nucleus.67
TGF-β–Homeodomain Interacting Protein Kinase 2 Signaling in DA Neuron Development
Several studies have shown TGF-β signaling can provide trophic supports for DA neurons during development (Figure 1). The neurotrophic effect of TGF-β is further underlined by the routine use of TGF-β ligands to derive DA neurons from mouse and human embryonic stem cells.68 Addition of TGF-β1 to embryo-derived vMB neurospheres along with GDNF family ligands also induces the expression of transcription factors like Nurr1 and Pitx3, critical regulators of mDA neurons.69 vMB astrocytes have also been shown to induce DA neurogenesis from rat vMB precursors through release of TGF-β3.70, 71 A series of genetic studies indicate that TGF-β signaling critically regulates the survival of DA neurons. For instance, lack of TGF-β3 results in increased apoptosis of TH+ neurons in SNpc and VTA during the period of PCD at P043; however, these mutant mice did not show a detectable difference in DA neurons at E12.5, suggesting that TGF-β is required for the survival, but not neurogenesis, of these neurons. Mice with single null mutations of TGF-β isoforms also do not display severe phenotypes in midbrain DA neurons during embryonic development, suggesting that TGF-β isoforms may functionally compensate for each other.71, 72, 73 In support of this idea, double knockout of TGF-β2 and TGF-β3 leads to a loss of DA neurons at E14.5 in mouse embryos.74 Together, these results support that a different TGF-β isoform may be functionally redundant in regulating the survival of DA neurons during embryonic development.
Despite the well-documented effects of TGF-β in the survival of DA neurons in culture and during embryonic development,75 the perinatal or early postnatal lethality of TGF-β null mice precludes investigation into the functions of TGF-β in the maturation and postnatal development of the nigrostriatal and mesocortical system. Some recent studies suggest a role for TGF-β in postnatal development of vMB DA neurons. It was determined that TGF-β2 haploinsufficient mice have reductions in DA neurons and striatal dopamine at 6 weeks of age.76 Smad3 (a downstream mediator of TGF-β signaling) deficient mice were also reported to lose nigrostriatal neurons at approximately 2 to 3 months of age.77 However, these studies have inherent limitations, such as the failure to account for cell type specificity and the lack of distinction between a neurodevelopmental and a neurodegenerative phenotype.
Several transcriptional cofactors have been identified to further diversify the cell type–specific and context-dependent modifications of the outcome of TGF-β signaling. Among these, homeodomain interacting protein kinase 2 (HIPK2) can directly interact with R-Smads, including Smad1, Smad2, and Smad3, and thereby enhance the suppressor functions of Smad1 on activation by bone morphogenic proteins.78 In addition, interaction between HIPK2 and Smad2/3 promotes their transcriptional activity and regulates the expression of several TGF-β target genes.43, 78, 79 Interestingly, HIPK2 expression is prominent in the developing nervous system and HIPK2 has been implicated in neuronal survival and cell cycle regulation in stem and progenitor cells.80, 81 In the developing vMB, HIPK2 can be detected in nascent DA neurons from E15.5 to P0, but not in DA progenitors at earlier embryonic stages. Consistent with the role of HIPK2 in regulating the prosurvival function of TGF-β signaling, analyses of Hipk2−/− mutants show that loss of HIPK2 does not affect DA neurogenesis, but leads to a significant increase in cell death in DA neurons during perinatal stages that coincide with PCD. DA neurons lacking HIPK2 fail to respond to the prosurvival signals from TGF-β, but not to GDNF or fibroblast growth factor 8.43 Because of the increase in PCD, Hipk2−/− mutants show approximately 40% of DA neuron loss in SNpc and VTA throughout the postnatal life. Interestingly, unlike the DA neuron–specific c-Ret conditional mutants, Hipk2−/− mutants do not show further reduction of DA neurons during the aging process (J. Zhang, unpublished data). These results underline the critical, yet stage-dependent, role of TGF-β–HIPK2 signaling supporting DA neurons during PCD. Because of the significant loss of DA neurons, Hipk2−/− mutants exhibit several parkinsonian symptoms, including resting tremor and difficulty in initiating movement, especially at early postnatal stages. Although these phenotypes subsequently subside, adult Hipk2−/− mutants continue to show psychomotor behavioral deficits, including reduced activity in a novel environment, which can be corrected by injections of psychomimetic agents, such as amphetamine and cocaine.43 Together, these results support the important role of TGF-β–HIPK2 signaling in DA neuron survival and maturation at perinatal and early postnatal stages (Figure 3). Given the persistent neurological phenotypes in adult Hipk2−/− mutants, it is possible that the same mechanism may further contribute to DA neural circuits by regulating synaptic connectivity and functions.
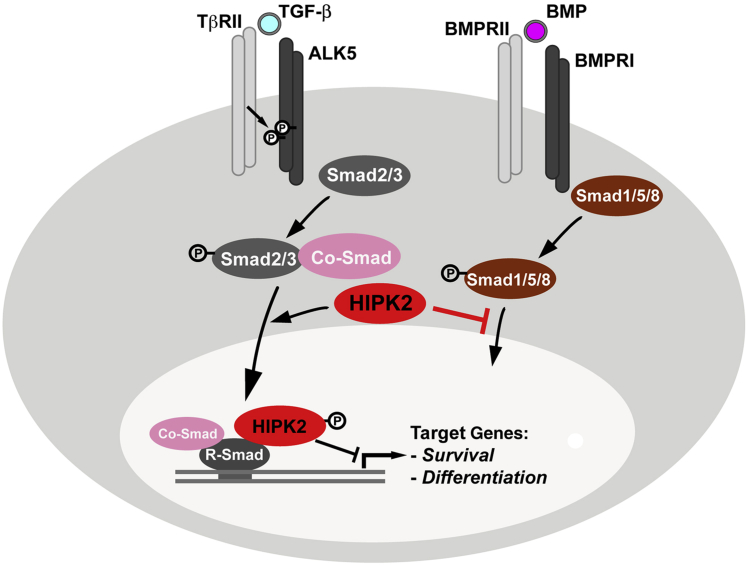
Figure 3.
A working model for homeodomain interacting protein kinase 2 (HIPK2) in the downstream signaling pathways of transforming growth factor (TGF)-β and bone morphogenetic protein (BMP). In dopaminergic (DA) neurons, HIPK2 interacts with R-Smads to regulate the transcription of R-Smad downstream target genes that promote the survival of DA neurons. In contrast, within the enteric nervous system, HIPK2 appears to inhibit phosphorylation of Smad1/5/8 in enteric neurons through unknown mechanisms to regulate BMP signaling. TβRII, TGF-β type II receptor.
In addition to the essential role of TGF-β–HIPK2 in supporting the survival of vMB DA neurons during PCD, loss of HIPK2 also affects the postnatal development of DA neurons in the enteric nervous system (ENS). The mechanism of HIPK2 in the enteric DA neurons, however, is slightly different from that in vMB DA neurons. Unlike vMB DA neurons, DA neurons in the ENS show no detectable canonical TGF-β signals, as revealed by the lack of p-Smad2/3 staining. Instead, enteric DA neurons and their neighboring glia receive robust trophic factor supports from bone morphogenic protein signaling to regulate the diversity and maturation of these cells.82, 83, 84 Interestingly, loss of HIPK2 does not affect the embryonic development of ENS, but leads to a significant reduction in the number of DA neurons in ENS in early postnatal life.85 Most enteric neuron Hipk2−/− mutants show features of growth and maturation arrest, including reduced ganglion size, interganglionic fiber density, and synaptic density within the ENS. These phenotypes can be detected as early as P0 and persist in adult mice. Curiously, enteric neurons in Hipk2−/− mutants show a significant increase in p-Smad1/5/8 expression and autophagosome formation. These results, together with the increase in glia density in the ENS of Hipk2−/− mutants, indicate that HIPK2 most likely has cell context–dependent functions in regulating synapse formation, autophagy pathway in enteric neurons, and the neuron-glia decision in ENS (Figure 3).
Mechanisms Regulating Circuit Assembly in DA Neurons
Neurotrophic Factors and Maturation of DA Neurons
The perinatal and early postnatal stages define critical periods when DA neurons undergo tremendous growth and maturation, as evidenced by the extensive neurite outgrowth, synaptic connections, and the acquisition of unique rhythmic firing properties. Output firing patterns of SNpc midbrain DA neurons are determined by the integration of synaptic inputs from diverse regions in the brain.86, 87 Glutamatergic and cholinergic afferents from somatosensory/motor cortex and the subthalamic nucleus likely drive phasic firing, whereas GABAergic input from areas like dorsal striatum and globus pallidus likely mediate suppression of firing.86, 87, 88 More recently, local inhibitory microcircuits in the substantia nigra pars reticulata were shown to directly attenuate phasic firing of SNpc DA neurons in an associative learning behavioral paradigm.89 Consistent with this idea, the extent of DA dendritic innervation into substantia nigra pars reticulata dictated GABAergic input and predicted inhibitory responses to aversive stimuli.90
Despite these characteristic properties of DA neurons, there is limited information regarding the molecular mechanisms that regulate the formation of these properties. Several lines of evidence support that exogenous factors, such as Shh, Wnt, GDNF, and TGF-β, may continue to modulate the maturation and synaptic connectivity in DA neurons in postnatal life. These results underline the stage-dependent, multimodal functions of these factors. For instance, amperometric techniques measuring the quantal release of dopamine from presynaptic terminals also support that GDNF can significantly increase quantal size.91 Consistent with these results, GDNF treatment in rat DA neurons isolated from VTA leads to an increase in excitatory synaptic terminals and thereby promotes dopamine release from the terminals.92 Although the exact mechanism of GDNF in regulating synaptic transmission has not been formally investigated at the structural level, one possibility is that GDNF may promote the growth of local excitatory synaptic inputs to DA neurons.
Unexpected Role of Shh in Adult DA Neurons
Another developmentally critical factor that continues to modulate adult DA neuron functions is Shh, which when exogenously applied to the striatum increases the resistance of DA neurons to toxicity caused by neurotoxins 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and hydroxydopamine.93, 94 Interestingly, Shh and Shh receptor Smoothened are expressed in adult DA neurons, whereas Shh coreceptors Patched 1 and Patched 2 are expressed in neighboring cells. Removal of Shh from DA neurons causes progressive loss of DA neurons. In contrast, removing Smoothened from DA neurons does not lead to any detectable defects in DA neurons. These results support a non–cell-autonomous mode of Shh signaling in supporting the maintenance of adult DA neurons.95 Characterizations of DA neurons lacking Shh further reveal that Shh is required to maintain the expression of GDNF in striatum. As a consequence, loss of Shh in DA neurons further leads to subtype-specific dysregulation of gene expression in striatal neurons. Together, these results reveal surprising roles of Shh, which is produced and released by DA neurons, in regulating the structural and functional integrity of the nigrostriatal neural circuits.
Relevance to Neurodegenerative and Neurodevelopmental Disorders
By investigating Wnt/β-catenin, Shh-Smoothened, and TGF-β–HIPK2 signaling mechanisms in the development of DA neurons, we and others have uncovered several fundamental principles that govern neurogenesis, survival, and maintenance of DA neurons. Not only do these results provide unprecedented clarity regarding the cellular and molecular mechanisms that are essential to control the development of DA neurons within the neurogenic niche and support the survival of DA neurons once they become fully mature, they also lead to many unanswered questions regarding additional mechanisms that can potentially regulate connectivity, synaptic functions, and degeneration in DA neurons. For instance, in addition to the unexpected roles of GDNF and Shh in regulating synaptic functions and neural circuit integrity in adult DA neurons, one recent study shows that GDNF receptor c-Ret can work synergistically with Parkin, which is mutated in the autosomal recessive form of Parkinson disease, to improve mitochondrial function and the survival of DA neurons.55 These results provide important insights to the potential roles of GDNF–c-Ret in maintaining long-term survival of DA neurons in the aging brain. In a similar vein, it is possible that a TGF-β–Smad–HIPK2 signaling mechanism may regulate synapse formation and homeostasis in mature DA neurons, similar to those reported in the invertebrate nervous system.96, 97 Indeed, our unpublished results indicate that TGF-β signaling in postnatal DA neurons appears to regulate dendritic growth and the balance of excitatory and inhibitory synaptic inputs. As a consequence, conditional mutants lacking TβRII in DA neurons exhibit reversal learning deficits and hyperactivity, resembling patients with attention-deficit/hyperactivity disorder (S.X.L., unpublished data). In addition to its role in regulating synaptic balance, it will be important to investigate how TGF-β–HIPK2 signaling in DA neurons affects our understanding of the pathogenesis of Parkinson disease. Specifically, does HIPK2 continue to regulate survival of DA neurons when these neurons are exposed to factors that trigger neurodegeneration? Furthermore, it is now well-established that patients with Parkinson disease show Lewy body pathology in the ENS at the early stage of disease progression.98, 99 Consistent with these findings, transgenic mice expressing mutant α-synuclein show reduced DA neurons in the ENS.100 The similar ENS phenotypes in α-synuclein transgenic mice and Hipk2−/− mutants raise the possibility that HIPK2 might be mechanistically linked to the pathogenesis of α-synuclein proteinopathy. These intriguing questions will provide important guidance for future research that will lead to discovery of disease mechanisms and therapeutics.
In conclusion, investigations to the molecular and cellular mechanisms of DA neuron development have uncovered many fundamental principles that govern the patterning, specification, differentiation, survival, and circuit formation in DA neurons. Intriguingly, there is increasing evidence that perturbations to these mechanisms may also contribute to the pathogenesis of neurodegenerative and neurodevelopmental diseases, such as Parkinson disease and attention-deficit/hyperactivity disorder. These results will provide important guidance for future exploration of more disease mechanisms and potential therapeutic targets.
Neuropathology Theme Issue
Footnotes
Supported by NIH grants OD010927 and OD011915; the Department of Veterans Affairs grants BX001108 and BX001625 (E.J.H.); the Singapore Agency for Science, Technology, and Research (A*STAR) Scholar Program (S.X.L.); and the University of California San Francisco Graduate Education in Medical Sciences (GEMS) Scholar Award (S.X.L.).
Disclosures: None declared.
This article is part of a review series on neuropathology.
References
- 1.Kravitz A.V., Freeze B.S., Parker P.R., Kay K., Thwin M.T., Deisseroth K., Kreitzer A.C. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calabresi P., Picconi B., Tozzi A., Ghiglieri V., Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- 3.Tritsch N.X., Sabatini B.L. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faure A., Haberland U., Conde F., El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redgrave P., Rodriguez M., Smith Y., Rodriguez-Oroz M.C., Lehericy S., Bergman H., Agid Y., DeLong M.R., Obeso J.A. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromberg-Martin E.S., Matsumoto M., Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seger C.A., Spiering B.J. A critical review of habit learning and the basal ganglia. Front Syst Neurosci. 2011;5:66. doi: 10.3389/fnsys.2011.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L.P., Li F., Wang D., Xie K., Shen X., Tsien J.Z. NMDA receptors in dopaminergic neurons are crucial for habit learning. Neuron. 2011;72:1055–1066. doi: 10.1016/j.neuron.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin X., Costa R.M. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–462. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cagniard B., Beeler J.A., Britt J.P., McGehee D.S., Marinelli M., Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Cagniard B., Balsam P.D., Brunner D., Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- 12.Arenas E., Denham M., Villaescusa J.C. How to make a midbrain dopaminergic neuron. Development. 2015;142:1918–1936. doi: 10.1242/dev.097394. [DOI] [PubMed] [Google Scholar]
- 13.Aron L., Klein R. Repairing the parkinsonian brain with neurotrophic factors. Trends Neurosci. 2011;34:88–100. doi: 10.1016/j.tins.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Andressoo J.O., Saarma M. Signalling mechanisms underlying development and maintenance of dopamine neurons. Curr Opin Neurobiol. 2008;18:297–306. doi: 10.1016/j.conb.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Prakash N., Wurst W. Genetic networks controlling the development of midbrain dopaminergic neurons. J Physiol. 2006;575:403–410. doi: 10.1113/jphysiol.2006.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smidt M.P., Burbach J.P. How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci. 2007;8:21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- 17.Andersson E., Tryggvason U., Deng Q., Friling S., Alekseenko Z., Robert B., Perlmann T., Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 18.Chung S., Hedlund E., Hwang M., Kim D.W., Shin B.S., Hwang D.Y., Kang U.J., Isacson O., Kim K.S. The homeodomain transcription factor Pitx3 facilitates differentiation of mouse embryonic stem cells into AHD2-expressing dopaminergic neurons. Mol Cell Neurosci. 2005;28:241–252. doi: 10.1016/j.mcn.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Wagner J., Akerud P., Castro D.S., Holm P.C., Canals J.M., Snyder E.Y., Perlmann T., Arenas E. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nat Biotechnol. 1999;17:653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- 20.Chung S., Leung A., Han B.S., Chang M.Y., Moon J.I., Kim C.H., Hong S., Pruszak J., Isacson O., Kim K.S. Wnt1-lmx1a forms a novel autoregulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell. 2009;5:646–658. doi: 10.1016/j.stem.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang M., Miyamoto Y., Huang E.J. Multiple roles of beta-catenin in controlling the neurogenic niche for midbrain dopamine neurons. Development. 2009;136:2027–2038. doi: 10.1242/dev.034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prakash N., Brodski C., Naserke T., Puelles E., Gogoi R., Hall A., Panhuysen M., Echevarria D., Sussel L., Weisenhorn D.M., Martinez S., Arenas E., Simeone A., Wurst W. A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development. 2006;133:89–98. doi: 10.1242/dev.02181. [DOI] [PubMed] [Google Scholar]
- 23.Ferri A.L., Lin W., Mavromatakis Y.E., Wang J.C., Sasaki H., Whitsett J.A., Ang S.L. Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development. 2007;134:2761–2769. doi: 10.1242/dev.000141. [DOI] [PubMed] [Google Scholar]
- 24.Tang M., Villaescusa J.C., Luo S.X., Guitarte C., Lei S., Miyamoto Y., Taketo M.M., Arenas E., Huang E.J. Interactions of Wnt/beta-catenin signaling and sonic hedgehog regulate the neurogenesis of ventral midbrain dopamine neurons. J Neurosci. 2010;30:9280–9291. doi: 10.1523/JNEUROSCI.0860-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joksimovic M., Yun B.A., Kittappa R., Anderegg A.M., Chang W.W., Taketo M.M., McKay R.D., Awatramani R.B. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat Neurosci. 2009;12:125–131. doi: 10.1038/nn.2243. [DOI] [PubMed] [Google Scholar]
- 26.Nakatani T., Kumai M., Mizuhara E., Minaki Y., Ono Y. Lmx1a and Lmx1b cooperate with Foxa2 to coordinate the specification of dopaminergic neurons and control of floor plate cell differentiation in the developing mesencephalon. Dev Biol. 2010;339:101–113. doi: 10.1016/j.ydbio.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Jessell T.M. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 28.Dessaud E., Ribes V., Balaskas N., Yang L.L., Pierani A., Kicheva A., Novitch B.G., Briscoe J., Sasai N. Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen sonic hedgehog. PLoS Biol. 2010;8:e1000382. doi: 10.1371/journal.pbio.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dessaud E., Yang L.L., Hill K., Cox B., Ulloa F., Ribeiro A., Mynett A., Novitch B.G., Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- 30.Tang M., Luo S.X., Tang V., Huang E.J. Temporal and spatial requirements of Smoothened in ventral midbrain neuronal development. Neural Dev. 2013;8:8. doi: 10.1186/1749-8104-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakatani T., Minaki Y., Kumai M., Ono Y. Helt determines GABAergic over glutamatergic neuronal fate by repressing Ngn genes in the developing mesencephalon. Development. 2007;134:2783–2793. doi: 10.1242/dev.02870. [DOI] [PubMed] [Google Scholar]
- 32.Danielian P.S., McMahon A.P. Engrailed-1 as a target of the Wnt-1 signalling pathway in vertebrate midbrain development. Nature. 1996;383:332–334. doi: 10.1038/383332a0. [DOI] [PubMed] [Google Scholar]
- 33.Castelo-Branco G., Wagner J., Rodriguez F.J., Kele J., Sousa K., Rawal N., Pasolli H.A., Fuchs E., Kitajewski J., Arenas E. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci U S A. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson E.R., Prakash N., Cajanek L., Minina E., Bryja V., Bryjova L., Yamaguchi T.P., Hall A.C., Wurst W., Arenas E. Wnt5a regulates ventral midbrain morphogenesis and the development of A9-A10 dopaminergic cells in vivo. PLoS One. 2008;3:e3517. doi: 10.1371/journal.pone.0003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadowaki M., Nakamura S., Machon O., Krauss S., Radice G.L., Takeichi M. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Kriegstein A., Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaess S., Bodea G.O., Kabanova A., Chanet S., Mugniery E., Derouiche A., Stephen D., Joyner A.L. Temporal-spatial changes in Sonic Hedgehog expression and signaling reveal different potentials of ventral mesencephalic progenitors to populate distinct ventral midbrain nuclei. Neural Dev. 2011;6:29. doi: 10.1186/1749-8104-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaess S., Corrales J.D., Joyner A.L. Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development. 2006;133:1799–1809. doi: 10.1242/dev.02339. [DOI] [PubMed] [Google Scholar]
- 39.Hayes L., Zhang Z., Albert P., Zervas M., Ahn S. Timing of Sonic hedgehog and Gli1 expression segregates midbrain dopamine neurons. J Comp Neurol. 2011;519:3001–3018. doi: 10.1002/cne.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joksimovic M., Anderegg A., Roy A., Campochiaro L., Yun B., Kittappa R., McKay R., Awatramani R. Spatiotemporally separable Shh domains in the midbrain define distinct dopaminergic progenitor pools. Proc Natl Acad Sci U S A. 2009;106:19185–19190. doi: 10.1073/pnas.0904285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Balaguer A., Puelles E., Wurst W., Martinez S. Shh dependent and independent maintenance of basal midbrain. Mech Dev. 2009;126:301–313. doi: 10.1016/j.mod.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Jackson-Lewis V., Vila M., Djaldetti R., Guegan C., Liberatore G., Liu J., O'Malley K.L., Burke R.E., Przedborski S. Developmental cell death in dopaminergic neurons of the substantia nigra of mice. J Comp Neurol. 2000;424:476–488. doi: 10.1002/1096-9861(20000828)424:3<476::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J., Pho V., Bonasera S.J., Holtzman J., Tang A.T., Hellmuth J., Tang S., Janak P.H., Tecott L.H., Huang E.J. Essential function of HIPK2 in TGFbeta-dependent survival of midbrain dopamine neurons. Nat Neurosci. 2007;10:77–86. doi: 10.1038/nn1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massague J., Chen Y.G. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 45.Schmierer B., Hill C.S. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 46.Kholodilov N., Yarygina O., Oo T.F., Zhang H., Sulzer D., Dauer W., Burke R.E. Regulation of the development of mesencephalic dopaminergic systems by the selective expression of glial cell line-derived neurotrophic factor in their targets. J Neurosci. 2004;24:3136–3146. doi: 10.1523/JNEUROSCI.4506-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oo T.F., Kholodilov N., Burke R.E. Regulation of natural cell death in dopaminergic neurons of the substantia nigra by striatal glial cell line-derived neurotrophic factor in vivo. J Neurosci. 2003;23:5141–5148. doi: 10.1523/JNEUROSCI.23-12-05141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cacalano G., Farinas I., Wang L.C., Hagler K., Forgie A., Moore M., Armanini M., Phillips H., Ryan A.M., Reichardt L.F., Hynes M., Davies A., Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enomoto H., Araki T., Jackman A., Heuckeroth R.O., Snider W.D., Johnson E.M., Jr., Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- 50.Enomoto H., Heuckeroth R.O., Golden J.P., Johnson E.M., Milbrandt J. Development of cranial parasympathetic ganglia requires sequential actions of GDNF and neurturin. Development. 2000;127:4877–4889. doi: 10.1242/dev.127.22.4877. [DOI] [PubMed] [Google Scholar]
- 51.Kramer E.R., Aron L., Ramakers G.M., Seitz S., Zhuang X., Beyer K., Smidt M.P., Klein R. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 2007;5:e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kopra J., Vilenius C., Grealish S., Harma M.A., Varendi K., Lindholm J., Castren E., Voikar V., Bjorklund A., Piepponen T.P., Saarma M., Andressoo J.O. GDNF is not required for catecholaminergic neuron survival in vivo. Nat Neurosci. 2015;18:319–322. doi: 10.1038/nn.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pascual A., Hidalgo-Figueroa M., Piruat J.I., Pintado C.O., Gomez-Diaz R., Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- 54.Pascual A., Lopez-Barneo J. Reply to “GDNF is not required for catecholaminergic neuron survival in vivo”. Nat Neurosci. 2015;18:322–323. doi: 10.1038/nn.3942. [DOI] [PubMed] [Google Scholar]
- 55.Meka D.P., Muller-Rischart A.K., Nidadavolu P., Mohammadi B., Motori E., Ponna S.K., Aboutalebi H., Bassal M., Annamneedi A., Finckh B., Miesbauer M., Rotermund N., Lohr C., Tatzelt J., Winklhofer K.F., Kramer E.R. Parkin cooperates with GDNF/RET signaling to prevent dopaminergic neuron degeneration. J Clin Invest. 2015;125:1873–1885. doi: 10.1172/JCI79300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Attisano L., Wrana J.L. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 57.Massague J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 58.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang C., Harland R.M. Neural induction requires continued suppression of both Smad1 and Smad2 signals during gastrulation. Development. 2007;134:3861–3872. doi: 10.1242/dev.007179. [DOI] [PubMed] [Google Scholar]
- 60.Sasai N., Yakura R., Kamiya D., Nakazawa Y., Sasai Y. Ectodermal factor restricts mesoderm differentiation by inhibiting p53. Cell. 2008;133:878–890. doi: 10.1016/j.cell.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 61.Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng X., Wang J., Haerry T.E., Wu A.Y., Martin J., O'Connor M.B., Lee C.H., Lee T. TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell. 2003;112:303–315. doi: 10.1016/s0092-8674(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 63.Sweeney S.T., Davis G.W. Unrestricted synaptic growth in spinster: a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron. 2002;36:403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 64.Krieglstein K., Richter S., Farkas L., Schuster N., Dunker N., Oppenheim R.W., Unsicker K. Reduction of endogenous transforming growth factors beta prevents ontogenetic neuron death. Nat Neurosci. 2000;3:1085–1090. doi: 10.1038/80598. [DOI] [PubMed] [Google Scholar]
- 65.Brionne T.C., Tesseur I., Masliah E., Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40:1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- 66.Yi J.J., Barnes A.P., Hand R., Polleux F., Ehlers M.D. TGF-beta signaling specifies axons during brain development. Cell. 2010;142:144–157. doi: 10.1016/j.cell.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bialas A.R., Stevens B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Perrier A.L., Tabar V., Barberi T., Rubio M.E., Bruses J., Topf N., Harrison N.L., Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roussa E., Oehlke O., Rahhal B., Heermann S., Heidrich S., Wiehle M., Krieglstein K. Transforming growth factor beta cooperates with persephin for dopaminergic phenotype induction. Stem Cells. 2008;26:1683–1694. doi: 10.1634/stemcells.2007-0805. [DOI] [PubMed] [Google Scholar]
- 70.Li K., Xue B., Wang Y., Wang X., Wang H. Ventral mesencephalon astrocytes are more efficient than those of other regions in inducing dopaminergic neurons through higher expression level of TGF-beta3. J Mol Neurosci. 2009;37:288–300. doi: 10.1007/s12031-008-9146-7. [DOI] [PubMed] [Google Scholar]
- 71.Kaartinen V., Voncken J.W., Shuler C., Warburton D., Bu D., Heisterkamp N., Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 72.Sanford L.P., Ormsby I., Gittenberger-de Groot A.C., Sariola H., Friedman R., Boivin G.P., Cardell E.L., Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shull M.M., Ormsby I., Kier A.B., Pawlowski S., Diebold R.J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D., Annunziata N., Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roussa E., Wiehle M., Dunker N., Becker-Katins S., Oehlke O., Krieglstein K. Transforming growth factor beta is required for differentiation of mouse mesencephalic progenitors into dopaminergic neurons in vitro and in vivo: ectopic induction in dorsal mesencephalon. Stem Cells. 2006;24:2120–2129. doi: 10.1634/stemcells.2005-0514. [DOI] [PubMed] [Google Scholar]
- 75.Krieglstein K., Suter-Crazzolara C., Fischer W.H., Unsicker K. TGF-beta superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J. 1995;14:736–742. doi: 10.1002/j.1460-2075.1995.tb07052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andrews Z.B., Zhao H., Frugier T., Meguro R., Grattan D.R., Koishi K., McLennan I.S. Transforming growth factor beta2 haploinsufficient mice develop age-related nigrostriatal dopamine deficits. Neurobiol Dis. 2006;21:568–575. doi: 10.1016/j.nbd.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 77.Tapia-Gonzalez S., Giraldez-Perez R.M., Cuartero M.I., Casarejos M.J., Mena M.A., Wang X.F., Sanchez-Capelo A. Dopamine and alpha-synuclein dysfunction in Smad3 null mice. Mol Neurodegener. 2011;6:72. doi: 10.1186/1750-1326-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harada J., Kokura K., Kanei-Ishii C., Nomura T., Khan M.M., Kim Y., Ishii S. Requirement of the co-repressor homeodomain-interacting protein kinase 2 for ski-mediated inhibition of bone morphogenetic protein-induced transcriptional activation. J Biol Chem. 2003;278:38998–39005. doi: 10.1074/jbc.M307112200. [DOI] [PubMed] [Google Scholar]
- 79.Shang Y., Doan C.N., Arnold T.D., Lee S., Tang A.A., Reichardt L.F., Huang E.J. Transcriptional corepressors HIPK1 and HIPK2 control angiogenesis via TGF-beta-TAK1-dependent mechanism. PLoS Biol. 2013;11:e1001527. doi: 10.1371/journal.pbio.1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei G., Ku S., Ma G.K., Saito S., Tang A.A., Zhang J., Mao J.H., Appella E., Balmain A., Huang E.J. HIPK2 represses beta-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:13040–13045. doi: 10.1073/pnas.0703213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wiggins A.K., Wei G., Doxakis E., Wong C., Tang A.A., Zang K., Luo E.J., Neve R.L., Reichardt L.F., Huang E.J. Interaction of Brn3a and HIPK2 mediates transcriptional repression of sensory neuron survival. J Cell Biol. 2004;167:257–267. doi: 10.1083/jcb.200406131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chalazonitis A., D'Autreaux F., Guha U., Pham T.D., Faure C., Chen J.J., Roman D., Kan L., Rothman T.P., Kessler J.A., Gershon M.D. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J Neurosci. 2004;24:4266–4282. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chalazonitis A., Pham T.D., Li Z., Roman D., Guha U., Gomes W., Kan L., Kessler J.A., Gershon M.D. Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. J Comp Neurol. 2008;509:474–492. doi: 10.1002/cne.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Faure C., Chalazonitis A., Rheaume C., Bouchard G., Sampathkumar S.G., Yarema K.J., Gershon M.D. Gangliogenesis in the enteric nervous system: roles of the polysialylation of the neural cell adhesion molecule and its regulation by bone morphogenetic protein-4. Dev Dyn. 2007;236:44–59. doi: 10.1002/dvdy.20943. [DOI] [PubMed] [Google Scholar]
- 85.Chalazonitis A., Tang A.A., Shang Y., Pham T.D., Hsieh I., Setlik W., Gershon M.D., Huang E.J. Homeodomain interacting protein kinase 2 regulates postnatal development of enteric dopaminergic neurons and glia via BMP signaling. J Neurosci. 2011;31:13746–13757. doi: 10.1523/JNEUROSCI.1078-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee C.R., Tepper J.M. Basal ganglia control of substantia nigra dopaminergic neurons. J Neural Transm Suppl. 2009:71–90. doi: 10.1007/978-3-211-92660-4_6. [DOI] [PubMed] [Google Scholar]
- 87.Watabe-Uchida M., Zhu L., Ogawa S.K., Vamanrao A., Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 88.Lobb C.J., Wilson C.J., Paladini C.A. A dynamic role for GABA receptors on the firing pattern of midbrain dopaminergic neurons. J Neurophysiol. 2010;104:403–413. doi: 10.1152/jn.00204.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan W.X., Brown J., Dudman J.T. Neural signals of extinction in the inhibitory microcircuit of the ventral midbrain. Nat Neurosci. 2013;16:71–78. doi: 10.1038/nn.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henny P., Brown M.T., Northrop A., Faunes M., Ungless M.A., Magill P.J., Bolam J.P. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat Neurosci. 2012;15:613–619. doi: 10.1038/nn.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pothos E.N., Davila V., Sulzer D. Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J Neurosci. 1998;18:4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bourque M.J., Trudeau L.E. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 93.Dass B., Iravani M.M., Huang C., Barsoum J., Engber T.M., Galdes A., Jenner P. Sonic hedgehog delivered by an adeno-associated virus protects dopaminergic neurones against 6-OHDA toxicity in the rat. J Neural Transm. 2005;112:763–778. doi: 10.1007/s00702-004-0227-7. [DOI] [PubMed] [Google Scholar]
- 94.Dass B., Iravani M.M., Jackson M.J., Engber T.M., Galdes A., Jenner P. Behavioural and immunohistochemical changes following supranigral administration of sonic hedgehog in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated common marmosets. Neuroscience. 2002;114:99–109. doi: 10.1016/s0306-4522(02)00214-2. [DOI] [PubMed] [Google Scholar]
- 95.Gonzalez-Reyes L.E., Verbitsky M., Blesa J., Jackson-Lewis V., Paredes D., Tillack K., Phani S., Kramer E.R., Przedborski S., Kottmann A.H. Sonic hedgehog maintains cellular and neurochemical homeostasis in the adult nigrostriatal circuit. Neuron. 2012;75:306–319. doi: 10.1016/j.neuron.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCabe B.D., Marques G., Haghighi A.P., Fetter R.D., Crotty M.L., Haerry T.E., Goodman C.S., O'Connor M.B. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 97.West R.J., Lu Y., Marie B., Gao F.B., Sweeney S.T. Rab8, POSH, and TAK1 regulate synaptic growth in a Drosophila model of frontotemporal dementia. J Cell Biol. 2015;208:931–947. doi: 10.1083/jcb.201404066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singaram C., Ashraf W., Gaumnitz E.A., Torbey C., Sengupta A., Pfeiffer R., Quigley E.M. Dopaminergic defect of enteric nervous system in Parkinson's disease patients with chronic constipation. Lancet. 1995;346:861–864. doi: 10.1016/s0140-6736(95)92707-7. [DOI] [PubMed] [Google Scholar]
- 99.Wakabayashi K., Takahashi H., Ohama E., Ikuta F. Parkinson's disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 100.Kuo Y.M., Li Z., Jiao Y., Gaborit N., Pani A.K., Orrison B.M., Bruneau B.G., Giasson B.I., Smeyne R.J., Gershon M.D., Nussbaum R.L. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet. 2010;19:1633–1650. doi: 10.1093/hmg/ddq038. [DOI] [PMC free article] [PubMed] [Google Scholar]