Abstract
Peripheral neuropathies are highly prevalent and are most often associated with chronic disease, side effects from chemotherapy, or toxic-metabolic abnormalities. Neuropathies are less commonly caused by genetic mutations, but studies of the normal function of mutated proteins have identified particular vulnerabilities that often implicate mitochondrial dynamics and axon transport mechanisms. Hereditary sensory and autonomic neuropathies are a group of phenotypically related diseases caused by monogenic mutations that primarily affect sympathetic and sensory neurons. Here, I review evidence to indicate that many genetic neuropathies are caused by abnormalities in axon transport. Moreover, in hereditary sensory and autonomic neuropathies. There may be specific convergence on gene mutations that disrupt nerve growth factor signaling, upon which sympathetic and sensory neurons critically depend.
During development, peripheral neurons extend long axons that make contact with a variety of distant tissues, including muscle fibers and sensory receptors. Although still incompletely defined, the process requires complex interactions between many local and target-tissue–derived growth factors, axon guidance molecules, and morphogens that together orchestrate peripheral nervous system innervation and functional control with astonishing precision. Once innervation is established, signaling from peripheral tissues continues to have an important role in maintaining innervation homeostasis and function throughout life. Long distances between neuron cell bodies (CBs) and peripheral target tissues pose unique challenges for peripheral neurons because they need to integrate molecular cues from distant tissues to the CB, and in a reasonably rapid time frame. Thus, neurons have evolved many complex mechanisms to transport biochemical cargo and signaling complexes toward peripheral tissues (orthograde transport) and from peripheral tissues (retrograde transport) to facilitate changes in gene expression and metabolism that are required to maintain innervation.
Peripheral neuropathies (PNs) are a group of heterogeneous diseases that affect motor, sensory, and autonomic neurons and axons, and they often simultaneously involve multiple systems.1 PN is most simply defined as a neurologic debilitation that results from a loss of peripheral axons that innervate particular target tissues. They are often classified by degrees to which they affect functionally distinct peripheral axons and, when known, the diseases, extrinsic factors, or genetic abnormalities associated with them. PNs can result in poor quality of life, and they pose a substantial health care burden because they are relatively prevalent, extremely difficult to medically manage in part because the precise pathogenesis is often unknown, and effective treatment options are limited. Some estimates suggest that 2.4% of the general population is afflicted to some degree with PN, and this incidence increases to about 8% to 15% of humans >40 years of age.2, 3 PN is most prevalent in patients with diabetes mellitus (DM), which number >20 million in the United States,4 with some estimates indicating that 58% of insulin-dependent diabetics ≥30 years of age5 and 26% of noninsulin dependent diabetics6 are afflicted. A greater understanding of the basic mechanisms that mediate neuron-target tissue signaling interactions and associated axon degeneration will be essential to develop effective strategies that affect the pathogenesis of these debilitating diseases.
Although the precise mechanisms underlying PNs are most often unknown, they are well associated with a wide variety of toxic, metabolic, infectious/postinfectious, and genetic insults. The current status of clinically classified PNs, their principle causative associations, and treatment approaches were recently and comprehensively reviewed.7, 8, 9, 10, 11, 12, 13 Emerging evidence suggests that the effects of many different neuropathy-associated factors may converge on a relatively small number of molecular pathways and processes that are essential for maintaining axon/innervation integrity.14 Here, I review some of the neuropathy-associated cellular processes that appear to be targets of many diverse disease-causing insults and then focus on axon transport and growth factor signaling as processes that are particularly vulnerable. Finally, by way of example, I discuss a rare group of monogenic congenital PNs, known as hereditary sensory and autonomic neuropathy (HSAN), and review some published and unpublished data, indicating that these phenotypically similar diseases may be caused, at least in part, by genetic mutations that disrupt target-derived signaling and axon transport pathways.
Axon Transport and Its Role in Neuron and Axon Homeostasis
DM-associated PN and chemotherapy-induced PN account for most patients with the disease. Depending on the duration and type of chemotherapy used, or the duration and effective management of DM, the incidence and severity of PN can greatly vary. For example, with commonly used chemotherapeutic platinum salts such as cisplatin and oxaliplatin, approximately 28% of patients develop PN, whereas with commonly used taxane chemotherapeutic agents, such as paclitaxel and docetaxel, >60% of patients develop PN.15 Because motor and sensory functions are often affected in a heterogeneous pattern, these agents appear to harm different types of neurons and in particular their axons (axonopathy) in humans with chemotherapy-induced PN16, 17 and in rodent models.18 Moreover, as with DM-associated PN, the longest axons in the body appear to be preferentially vulnerable, and this may be due to their enhanced energy demand for transporting cellular constituents within long axons. Indeed, for >30 years axon transport abnormalities were recognized to have a role in PN caused by DM.19 DM may lead to alterations in cytoskeletal components, such as actin, neurofilament, and tubulin that are essential for normal intracellular (axon) transport, either by direct glycation in the context of hyperglycemia or as a consequence of impaired gene expression.20, 21, 22, 23 For example, tubulin glycosylation in DM impairs polymerization and alters microtubule dynamics which is essential for trafficking intracellular macromolecules and organelles24 along microtubule networks. Similarly, microtubule abnormalities are particularly relevant to taxane-induced chemotherapy-induced PN because taxanes disrupt microtubule function by stabilizing their turnover and potentially altering motor protein binding required for intracellular transport.25 Particular examples of critical processes that may be impaired by altered axon transport include mitochondrial transport and dynamics that are critical for energy homeostasis within neurons and their axons,26 and abnormalities in growth factor signaling, which depends on intact retrograde axon transport to provide the mechanism for target-tissue–derived growth factors to signal to the neuron nucleus far away from target tissues.27, 28
Axon Transport–Mediated PN
Although axon transport can be impaired as a consequence of deranged metabolism in systemic diseases such as DM or by toxic substances that target elements of the intracellular transport machinery, such as chemotherapeutic agents, there are many examples of inherited neuropathy caused by genetic mutations that are associated with some form of aberrant axon transport. Studies focused on understanding how gene mutations alter the function of encoded proteins have provided insight into potential molecular targets for therapy. PNs can primarily affect the neuron CB (neuronopathy), axon myelination (myelinopathy), or axons (axonopathy). Because this brief review focuses on some genetic mutations that are associated with abnormalities in axon transport, the reader is referred to recent comprehensive reviews that present the mutations discussed here and the broader panoply of genetic mutations associated with inherited neuropathies in tabulated form.29, 30
Genetic (inherited) PNs, generally also known as Charcot-Marie-Tooth (CMT) disease or hereditary motor and sensory neuropathy, constitute the most common inherited neuromuscular diseases, and they affect at least 1 in 2500 humans.31 These are a group of heterogeneous diseases caused by many different genetic mutations that lead to neuropathy with involvement of either or both sensory and motor systems and sometimes sensory and autonomic nervous systems. They lead to muscle atrophy, weakness, and sensory abnormalities that are often most severe in distal extremities. Most classification schemes divide them into autosomal dominant (AD), autosomal recessive (AR), and rare sex-linked forms, and also by whether they predominantly affect the motor system (hereditary motor neuropathy), the sensory system (hereditary sensory neuropathy), a combination of sensory and motor systems (hereditary motor and sensory neuropathy), or sensory and autonomic nervous systems (HSAN).32 Genetic mutations that are involved in axon transport appear to alter three major processes that affect target tissue innervation homeostasis and axon integrity.
Mitochondrial Dynamics
Long peripheral axons require efficient energy production to sustain anterograde and retrograde transport of intracellular macromolecules and organelles. Mitochondrial respiration is required all along axons in part to generate ATP to sustain axon transport and to regulate mitochondrial dynamics involved in their transport, size, and number (mitochondrial fission and fusion). The transmembrane GTPase protein mitofusin-2 (MFN2; Online Mendelian Inheritance in Man [OMIM] no. 608507, http://www.ncbi.nlm.nih.gov/omim) is embedded in the outer membrane of mitochondria where it has an essential role in mitochondrial fusion and oxidative phosphorylation during mitochondrial respiration.33 Moreover, MFN2 is an important mediator of mitochondrial binding to kinesin (KIF) plus-end motor proteins via the adaptor proteins Miro/Milton to facilitate mitochondrial trafficking along axon microtubules toward their terminal ends.34, 35 MFN2 is one of the several genes associated with CMT and is associated with the most prevalent AD CMT2A whereby mutations in MFN2 account for approximately 20% of all CMT2 (axonal CMT) cases.36, 37 There are at least 100 sequence variations identified in MFN2. Although most mutations have not been functionally characterized, some common pathogenic mutations (R94Q and H361Y) have a major impact on mitochondrial transport along microtubules by mechanisms that have not yet been fully elucidated. Interestingly, these mutations affect mitochondrial transport not by abrogating direct MFN2 binding to Miro/Milton complexes that link them to motor proteins, but rather by another mechanism that influences mitochondrial transport and binding efficiency to motor proteins and/or microtubules.38 The clinical manifestations of patients with MFN2 mutations can be as complex as the number of allelic mutation variants identified. For example, approximately 10% to 20% of patients with MFN2 mutations develop optic nerve degeneration (CMT6/hereditary motor and sensory neuropathy V) and some have long upper motor axon involvement (CMT6/hereditary motor and sensory neuropathy VI).39, 40 Future studies to identify how particular MFN2 mutations mechanistically relate to differing clinical symptoms will be invaluable for understanding how alterations in this complex protein cause axonal neuropathy.41
Another molecule associated with dominant and recessive inheritance of CMT is ganglioside-induced differentiation-associated protein-1 (GDAP1; OMIM no. 606598, http://www.ncbi.nlm.nih.gov/omim).42, 43 GDAP1 is also a mitochondrial outer membrane protein with interaction domains that bind other mitochondrial membrane proteins FIS1 and DRP1 to regulate mitochondrial fission and their number and localization within axons.44, 45, 46 GDAP1 mutation–associated neuropathies are caused by a variety of different missense, nonsense, and splice site mutations, most of which appear to cause protein loss-of-function.47, 48 As such, most GDAP1 mutations cause CMT with an AR inheritance pattern and with considerable variation in patient symptoms. For example, GDAP1 is mutated in CMT4 (probably the most common autosomal recessive form of CMT) with early and severe onset, whereas other GDAP1 mutations lead to more localized and proximal neuropathy (recessive intermediate-CMTA and AR-CMT2K).49, 50, 51, 52 Moreover, although genotype-phenotype correlations are generally not well defined, there are rare missense mutations of GDAP1 that give rise to AD forms of CMT (AD-CMT2K).53 How the myriad GDAP1 mutations each lead to disease is not completely clear, but AR mutations likely lead to mitochondrial fission abnormalities, and AD mutations may lead to mitochondrial fusion abnormalities. Although these abnormalities may result in altered respiratory capacity and energy homeostasis within axons, it is likely that abnormalities in mitochondrial dynamics lead to altered mitochondrial transport reminiscent of MFN2 mutations.48
An interesting rare disease not part of the CMT disease spectrum per se is dominant optic atrophy which is caused by >200 mutations of the optic atrophy-1 gene (OPA1; OMIM no. 605290, http://www.ncbi.nlm.nih.gov/omim).54, 55 Dominant optic atrophy is characterized by optic nerve degeneration and myopathy. In approximately 20% of patients, mild PN (so called dominant optic atrophy plus syndrome) is also observed that can involve both sensory and motor or just sensory modalities. OPA1 is a dynamin-like membrane scission GTPase protein present in the mitochondrial membrane with a critical function in mitochondrial membrane fusion.56 Altered mitochondrial dynamics and respiration associated with OPA1 mutations likely result in neuropathy, and, although many mutations appear to result in loss of protein function, it is not clear how they specifically lead to axon degeneration.
Cytoskeleton-Dependent Axon Trafficking
Abnormalities in mitochondrial dynamics caused by mutations in MFN2, GDAP1, and OPA1 give rise to PN, at least in part by altering their axon transport. Interestingly, mutations in proteins that directly mediate axon transport of a wide variety of intracellular organelles and signaling endosomes also give rise to PN. For example, axon transport from the CB toward axon terminals (anterograde transport) is largely mediated by motor proteins known as KIFs, and transport from axon terminals to the CB (retrograde transport) is primarily mediated by dyneins (DYNs) along polarized microtubular networks.57 Moreover, neurofilament proteins composed of light, medium, and heavy chain proteins serve as additional scaffolding molecules to stabilize the axon microtubule cytoskeleton. Thus, motor proteins that bind to microtubules, microtubule components themselves, and some additional adaptor proteins are not surprisingly mutated in some forms of CMT that primarily affect long axons. For example, mutations in some anterograde motor proteins such as KIF1A58 (OMIM no. 601255), KIF1B (OMIM no. 605995), and KIF5A (OMIM no. 603821, http://www.ncbi.nlm.nih.gov/omim) can rarely harbor pathogenic mutations that cause PN. KIF5A mutations are associated with spastic paraplegia and PN (SPG10) with long axon degeneration.59 The mutations lead to highly variable symptoms, some of which give rise to AD isolated peripheral axonal neuropathy of CMT2.60, 61 KIF1A mutations are associated with a rare autosomal recessive PN with sensory and autonomic abnormalities known as HSAN2, which is discussed in more detail later. KIF1A is preferentially expressed by neurons and involved in anterograde axoplasmic transport of intracellular cargo other than mitochondria, indicating that axonal neuropathy can be caused by transport abnormalities unrelated to mitochondrial dynamics.58 This was confirmed in KIF1A knockout (KO) mice that have both sensory and motor abnormalities together with impairments in vesicular axon transport.58 Finally, mutations in KIF1B appear to be rare with only a single case report in the literature, but KIF1B mutant mice do have motor abnormalities reminiscent of CMT2A.62
A mutation in proteins that affect retrograde axon transport is involved in hereditary PN more frequently than mutations altering anterograde axon transport. DYN, an essential retrograde transport motor protein, is a hetero-hexameric ATPase microtubule binding complex that consists of two light, two intermediate, and two heavy chain proteins. Cytoplasmic DYN heavy chain 1 (DYNC1H1; OMIM no. 600112, http://www.ncbi.nlm.nih.gov/omim) mutations give rise to an AD form of axonal CMT, known as CMT2O.63, 64 Accordingly, heterozygous mice with an orthologous pathogenic mutation of DYNC1H1 have a marked reduction in dynein microtubule binding affinity and abnormal retrograde axon transport which produces a CMT-like motor and sensory neuropathy.65, 66 In addition, DYN-interacting proteins that function as cofactors or modulators of DYN function are identified to cause neuropathy. For example dynactin, which is a large heteromeric protein complex that interacts with DYN to modulate motor protein binding to cargo organelles for transport, contains a 150-kDa protein (the largest in the complex) that is encoded by the dynactin-1 gene (DCTN1; OMIM no. 601143). Dynactin-1 mutations lead to rare AD motor neuropathy, characterized by early-adult onset and progressive muscle weakness.67 Similarly, bicaudal D homolog-2 (BICD2; OMIM no. 609797), another DYN-interacting protein with an important role in retrograde axon transport, also has a role in PN.68 Mutations associated with BICD2 occur in a dominant form of spinal muscular atrophy that have striking similarity to CMT2O which is associated with DYNC1H1 mutations.69, 70 Finally, dynamin 2 gene (DNM2; OMIM no. 602378), a GTPase with a major role in membrane scission, vesicle formation, and dynein motor protein vesicle loading, is also mutated in a rare dominant intermediate axonal CMT referred to as CMT2M.71
Axon transport of mitochondria and organelles is also impaired by mutations in cytoskeletal proteins that are components of the pathways along which motor proteins transport intracellular cargo. Tubulin β-3 gene (TUBB3; OMIM no. 602661, http://www.ncbi.nlm.nih.gov/omim) is a major constituent of microtubules to which motor proteins directly bind and transport their cargo. Mutations in tubulin β-3 lead to congenital fibrosis of extra-ocular muscles type 3 which has a motor and sensory neuropathy as a component of the disease.72, 73 Similarly, mutations in another cytoskeletal protein also important for axon transport, neurofilament light chain (OMIM no. 162280) lead to complex disease phenotypes that can have either AR or AD inheritance and have normal nerve conduction velocity by electrophysiologic studies (CMT2E) or reduced nerve conduction velocity (CMT1F).74, 75
Growth Factor Signaling
Abnormal axon transport has a clear role in neuropathy pathogenesis. In many cases, however, we have an incomplete understanding of what specific transport pathways are affected and/or which pathways have the most important physiologic impact on axon integrity and innervation homeostasis. HSAN is an interesting example. HSANs may be phenotypically linked by gene mutations that alter different aspects of growth factor signaling and axon transport.
In some classification schemes, HSAN can be considered part of the CMT spectrum that comprises a group of clinically similar but genetically heterogeneous disorders characterized by severe developmental and sometimes juvenile or adult onset sensory and autonomic nervous system dysfunction. They were originally subdivided into five types (HSAN1 to HSAN5) on the basis of distinct clinical signs and symptoms.76 The molecular and clinical classification of these diseases has evolved to include two more recently recognized types (HSAN6 and HSAN7)77, 78 and many pathogenic germline mutations in >16 genes which cause both AD and AR forms of HSAN (recently reviewed in detail79, 80). The mechanisms by which mutations in many functionally diverse genes disrupt neuron development and innervation homeostasis in HSAN are generally poorly understood. Interestingly, however, several mutated proteins involved in HSAN appear to have a role in retrograde NGF signaling and transport, suggesting that there may be at least some functional convergence between axon transport and NGF signaling in HSAN pathogenesis.
Sensory and sympathetic neurons are the most clinically important targets of HSAN. Sympathetic neurons require target tissue–derived NGF for survival and proper target tissue innervation during development. NGF primarily signals through its high-affinity cognate tyrosine kinase receptor TrkA (OMIM no. 191315, http://www.ncbi.nlm.nih.gov/omim), and NGF/TrkA ligand-receptor complexes are endocytosed and transported retrograde to the nucleus along microtubule networks in signaling endosomes that modulate gene expression involved in sympathetic neuron survival and innervation patterning.81, 82, 83 Similarly, small diameter sensory neurons located in the dorsal root ganglia, which subserve pain and temperature sensation (nociception), also express high levels of TrkA receptor and require target tissue–derived NGF for their survival and normal innervation.84 Thus, it is not surprising that abnormalities in proteins mediating NGF/TrkA signaling and/or transport may be involved in neuropathies that preferentially effect sympathetic and nociceptive sensory neurons such as in HSAN.
HSAN4 and HSAN5 are rare autosomal recessive congenital neuropathies characterized by abnormal pain perception and sympathetic abnormalities with (HSAN4) and without (HSAN5) mental retardation. These neuropathies are clearly related to abnormal NGF signaling because they are caused by mutations in TrkA (HSAN4) and NGFβ (HSAN5/congenital insensitivity to pain;) genes that alter TrkA-mediated signaling and NGF binding affinity to TrkA, respectively.85 In other HSAN types, abnormal NGF/TrkA signaling has not been directly investigated, but evidence suggests that abnormalities in this signaling axis may exist. In the AD HSAN1, which is primarily associated with pain and temperature and sometimes with distal motor axon involvement, ras-associated protein 7 (RAB7; OMIM no. 602098, http://www.ncbi.nlm.nih.gov/omim) is mutated in a form of the disease which has distal motor axon involvement and sensory abnormalities.86 Rab7 is essential for NGF signaling–endosome trafficking to the nucleus and for NGF/TrkA signaling during neurite outgrowth in NGF-dependent neurons.87, 88, 89 Although not yet examined in mechanistic detail, mutations in Rab7 could lead to neuropathy by specifically altering or disrupting retrograde endosome trafficking and NGF signaling in sympathetic and nociceptive neurons. Another gene mutation associated with HSAN1 is atlastin-1 (OMIM no. 606439). Atlastin-1 has a role in endoplasmic reticulum network assembly and its interaction with the microtubule cytoskeleton. Mutations in atlastin-1 lead to altered microtubule network organization and are hypothesized to alter protein trafficking (potentially NGF signaling endosomes) along microtubules, but functional analysis of pathogenic mutations has not been performed to date, and whether it has any specific role in NGF/TrkA endosome trafficking is conjecture.90
HSAN2 is another autosomal recessive congenital neuropathy that is associated with gene mutations in i) lysine-deficient protein kinase 1 (WNK1; OMIM no. 605232, http://www.ncbi.nlm.nih.gov/omim), ii) KIF1A, and iii) family with sequence similarity 134, member B (FAM134B; OMIM no. 613114). Although several different mutations of FAM134B are associated with HSAN2, its specific mechanistic role in neuropathy pathogenesis is not well studied and remains a mystery. By contrast KIF1A, a major motor protein involved in anterograde axon transport, likely disrupts anterograde vesicular transport.91 Because normal NGF/TrkA signaling sensitivity depends on TrkA receptor recycling back to axon terminals by anterograde transport (transcytosis), abnormalities in anterograde transport proteins could alter NGF/TrkA signaling in disease-vulnerable neurons by impairing transcytotic TrkA receptor processing.92 Similarly, mutations in WNK1 could also alter NGF/TrkA signaling through its protein kinase activity, although abnormalities in kinase activity for particular substrates have not been examined in detail in mutant proteins that contain the pathogenic exon 8B.93 Nevertheless, WNK1 activates Erk5,94 and Erk5 is a critical mitogen-activated protein kinase required for retrograde NGF/TrkA survival signaling in NGF-dependent neurons.95 Alterations in WNK1-mediated activation of Erk5 could lead to altered sensory and sympathetic neuron survival in HSAN2 caused by WNK1 mutations.
HSAN3 (familial dysautonomia; FD) is caused by mutation of the Elp1/IKBKAP (OMIM no. 603722, http://www.ncbi.nlm.nih.gov/omim) gene, but how it functions in FD pathogenesis is still unclear. However, Elp1 appears to have a novel role in retrograde NGF/TrkA signaling.
FD: A Model of Abnormal Retrograde NGF Signaling Resulting in Neuropathy
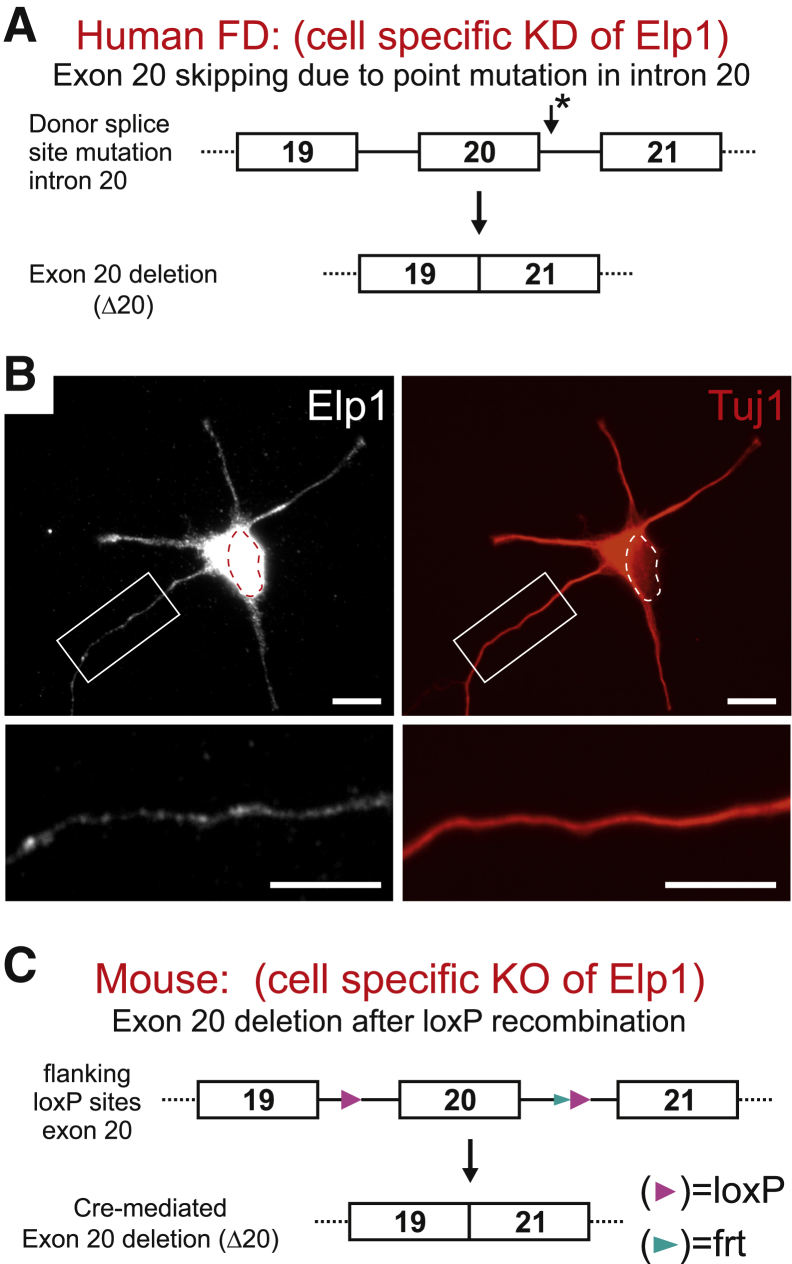
FD is caused by a homozygous splice-site mutation in the Elp1/IKBKAP gene which leads to exon 20 skipping and nonsense-mediated degradation of the truncated protein96, 97 (Figure 1A). It is characterized by debilitating sensory and widespread sympathetic nervous system dysfunction that involves the cardiovascular, renal, gastrointestinal, and pulmonary systems. Most afflicted patients have a poor quality of life and die before age 40, despite aggressive symptomatic treatment.99 Although the mutation occurs in the germline, nociceptive sensory and sympathetic neurons are preferentially affected because Elp1 mis-splicing occurs in these neurons to a greater extent than other cells.100 Little is known about how Elp1 functions in disease-relevant sympathetic and sensory neurons because most studies have focused on nonneuronal cells from FD patients (eg, fibroblasts and leukocytes) or they have involved Elp1 protein modulation in cells not normally involved in disease.101, 102, 103, 104, 105, 106
Figure 1.
Elp1 is localized in a vesicular pattern in neurites and is mutated in humans with FD and a mouse model that emulates the human mutation. A: FD is caused by a point mutation in the intron 20 splice acceptor site of Elp1 (asterisk) which leads to exon 20 skipping (Δ20), introduction of a nonsense mutation and nonsense-mediated degradation of the encoded protein. B: Neurons (primary mouse sympathetic neuron shown here) express Elp1 in the nucleus (dotted ellipse) and the cytoplasm. In the cytoplasm, immunofluorescence for Elp1 (left) shows that it has a vesicular pattern in the neurite cytoplasm (enlarged, bottom left). The cell body and neurites are labeled by immunofluorescence for β-III neuron-specific tubulin (Tuj1, right). C: Elp1-flx mice were generated to mimic the mutation in human FD by enforcing exon 20 skipping in Cre-recombinase expressing cells. The new conditional knockout mouse was recently generated and characterized.98 Scale bar = 10 μm. FD, familial dysautonomia; KD, knockdown; KO, knockout.
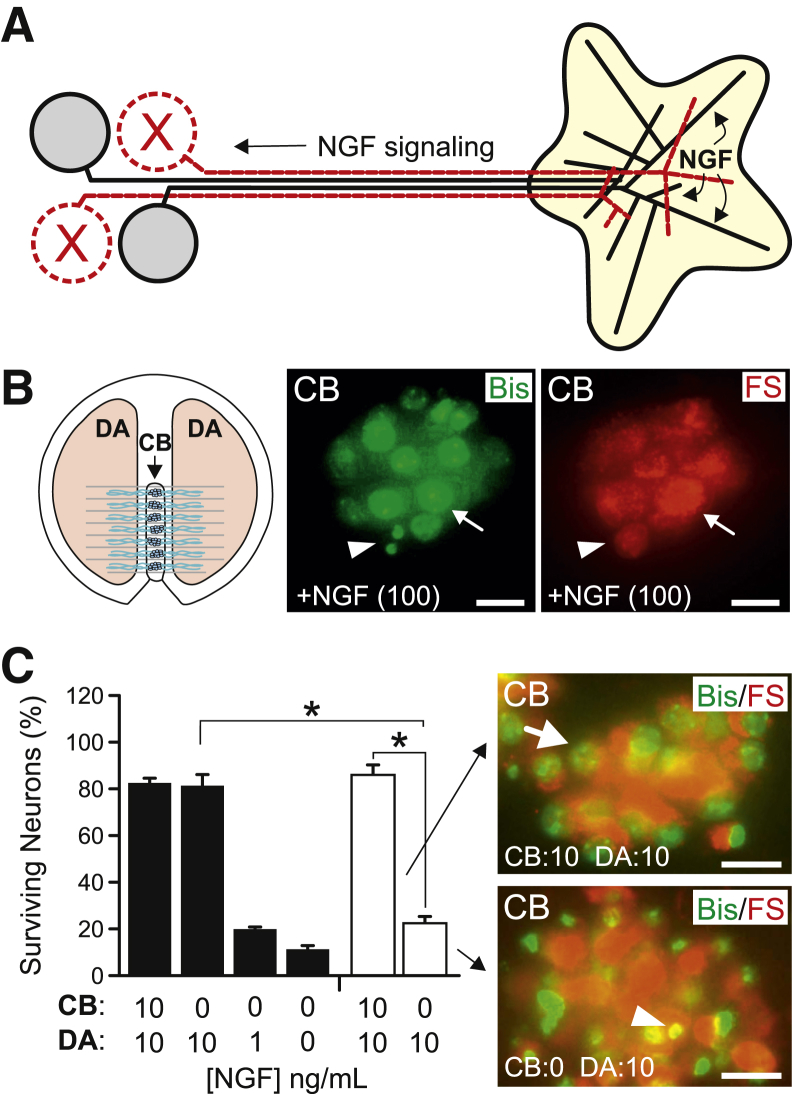
Elp1 is a constituent of the heterohexameric transcriptional elongator complex107; therefore, it may be involved in transcriptional regulation.102, 103, 108 Interestingly, however, a significant amount of Elp1 protein is found in a vesicular pattern in the cytoplasm of both nonneuronal cells104, 109 and neurons (Figure 1B), suggesting that it has a function apart from transcription. Elp1 function in the neuron cytoplasm (axons and dendrites) could be important for signaling processes required for their normal development and innervation homeostasis, but whether this has any relevance to neuron survival and differentiation in FD is not clear. Because loss of Elp1 in the germline (in all cells) leads to early embryonic lethality in mice,110, 111 we developed a conditional Elp1-KO mouse that recapitulates sympathetic and sensory nervous system abnormalities found in FD patients when it is ablated specifically in sympathetic and sensory neurons98 (Figure 1C). These mice have nociceptive and sympathetic target tissue innervation abnormalities that are similar to humans with FD and mice with NGF signaling abnormalities.85, 112, 113, 114 Indeed, our recent studies that used primary sympathetic neurons derived from these mice show that Elp1 is required for normal retrograde NGF signaling. To determine this, we first developed a tamoxifen-inducible system to conditionally ablate Elp1 in cultured neurons because neurons lacking Elp1 die during early development (similar to humans with FD) and therefore are difficult to isolate in newborn mice for in vitro analysis. Tamoxifen treatment of isolated sympathetic neurons ablated Elp1 in Elp1-TcKO neurons, but not in Elp1-TCtl neurons (data not shown). Sympathetic neurons acquire NGF from peripheral target tissues that they innervate, which is signaled retrograde to the neuron CB to mediate gene expression required for their normal innervation patterning and survival115 (Figure 2A). We used sympathetic neurons grown in partitioned chambers that separate distal axons (DAs) from the proximal axons and CBs to emulate the in vivo acquisition of NGF by axons and retrograde signaling to the neuron body. Elp1-TCtl and Elp1-TcKO sympathetic neurons were plated in the CB compartment of the chambers and differentiated in the presence of 100 ng/mL NGF in both CB and DA compartments (Figure 2B). Neurons that projected axons into the DA compartment were retrogradely labeled with fluorescent nanospheres (FSs) added to the DA compartment, and tamoxifen was added to the CB compartments for 24 hours to ablate Elp1 in Elp1-TcKO neurons. The neurons were then treated with differing amounts of NGF in the CB and/or DA compartments for 48 hours. FS+ neurons were scored dead if they had condensed, fragmented, or absent nuclei as previously published116 (Figure 2B). Tamoxifen-treated Elp1-TCtl neurons were able to transport NGF signaling from DAs similar to wild-type neurons.117 Elp1-TCtl neurons treated with 10 ng/mL NGF in both CB and DA compartments or with 10 ng/mL NGF only in the DA compartment showed statistically indistinguishable 82% FS+ neuron survival after 48 hours (Figure 2C). In addition, when NGF was lowered to physiologically limiting levels in the DA compartment (to emulate limiting quantities of target-derived NGF which are present in vivo), such as 1 ng/mL and 0 ng/mL, only 19% and 11% of the FS+ Elp1-TCtl neurons survived after 48 hours, respectively (Figure 2C). However, we found that FS+ Elp1-TcKO neurons had comparable survival to Elp1-TCtl neurons when 10 ng/mL NGF was present in both CB and DA compartments (Figure 2C), consistent with our previously published results that used dissociated Elp1-deficient neurons that were completely immersed in NGF.98 By contrast, when NGF was restricted to distal axons in the DA compartment only 22% of FS+ Elp1-TcKO neurons survived. Elp1-TcKO neurons with 10 ng/mL in the DA compartment survived similar to Elp1-TCtl neurons with 1 ng/mL in the DA compartment; a 10-fold decreased sensitivity in their signaling response to target-derived NGF. Thus, sympathetic dysautonomia in FD is likely explained by impairment in retrograde NGF signaling which leads to survival and target tissue innervation abnormalities in the context of limited NGF produced by target tissues.98
Figure 2.
Retrograde NGF survival signaling is impaired in Elp1-deficient neurons. A: Target-derived NGF is retrogradely transported from target tissues by sympathetic neurons to induce gene expression required for their survival and differentiation. B: In compartment cultures, FSs added to the DA compartment label neurons that project axons into the DA compartment. FS+ neurons (red, right) that are live with normal Bis (green, middle) chromatin staining (arrows) and dead with fragmented chromatin (arrowheads) are shown. C: Elp1-TcKO neurons (white bars) have significant impairment in retrograde survival signaling compared with Elp1-TCtl neurons (black bars). Data are expressed as means ± SEM of percentage of survival 48 hours after treatment. n = 3 to 5 replicates for each condition. ∗P < 0.05. Scale bar = 10 μm. Bis, bisbenzamide; DA, distal axon; FS, fluorescent nanosphere; KO, knockout; NGF, nerve growth factor.
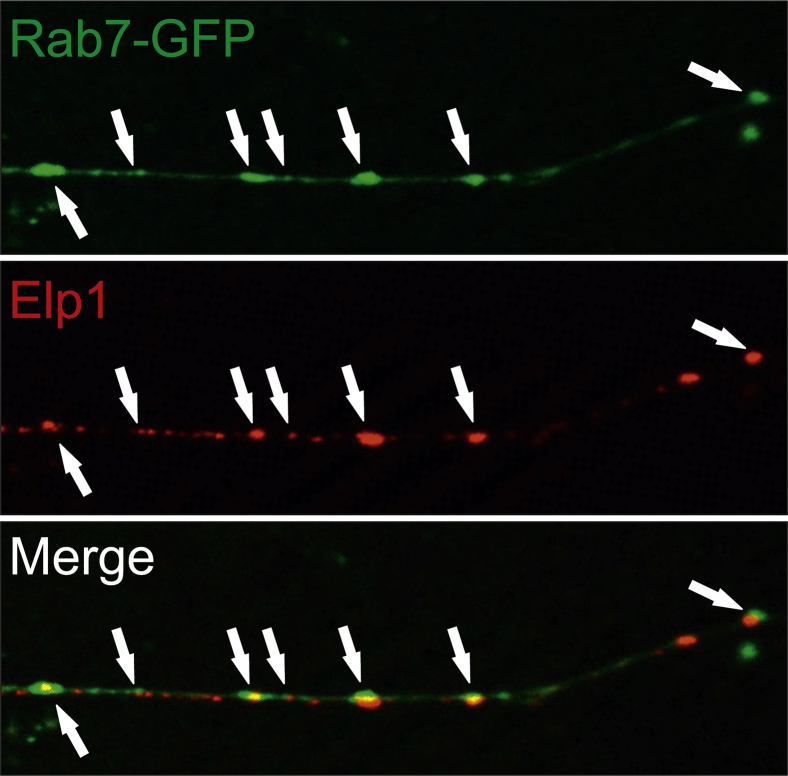
How does Elp1 function in retrograde NGF signaling? Little is currently known about how Elp1 functions in axons, but its role as a component of the transcriptional elongator complex within the nucleus may provide some clues. Elp1 is one of six different proteins (Elp1 to Elp6) in the transcriptional elongator complex which functions in transcription primarily through the acetyl-transferase activity of its binding partner Elp3.104 Elp1 has no detectable enzymatic activity, and it appears to act as a binding protein that stabilizes the heterohexameric protein complex through its WD40-like β-propeller protein-protein interaction domains. Thus, Elp1 may function in the axon in a similar way to facilitate important protein-protein interactions required for retrograde NGF signaling. This hypothesis appears to have some merit, considering recent in vitro imaging studies performed in my laboratory. We found that Elp1 protein colocalizes with Rab7+ late endosomes in NGF-stimulated sympathetic neurons, which is not observed in NGF-deprived neurons (Figure 3). Retrograde NGF signaling is mediated by Rab7+ late endosomes that are retrogradely transported to the nucleus, and disruption of Rab7 expression or function alters NGF signaling efficiency.87, 88, 89 These results strongly suggest that NGF-mediated Elp1 binding to Rab7+ endosomes, either directly or through other binding partners, may be an essential step in Elp1-dependent retrograde NGF signaling. Detailed mechanistic insights for how Elp1 functions in retrograde endosome binding, transport, and signaling must await results from studies that are currently in progress.
Figure 3.
Elp1 and Rab7 colocalize in axons of NGF-treated sympathetic neurons. Elp1-TCtl dissociated sympathetic neurons were nucleofected with Rab7-GFP fusion protein expression plasmid, differentiated, starved, and challenged with NGF for 20 minutes. Elp1 has a vesicular staining pattern in axons that closely localizes with many Rab7+ late endosomes (arrows). GFP, green fluorescent protein; NGF, nerve growth factor.
Conclusions
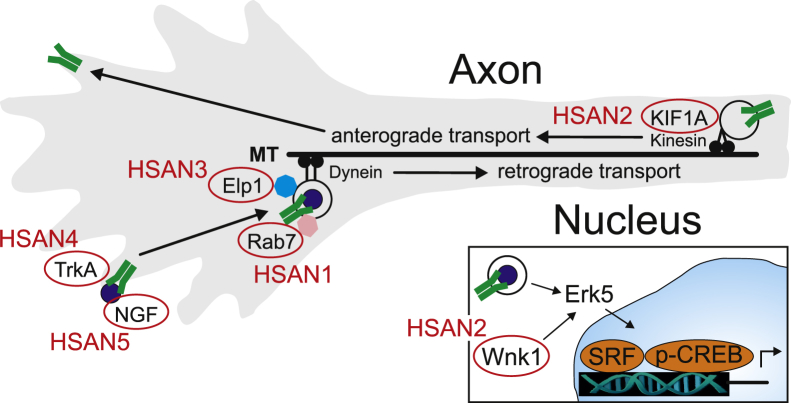
Many inherited motor and sensory neuropathies are caused by abnormalities in mitochondrial dynamics which may involve abnormalities in mitochondrial respiration and their transport along axons. As reviewed here, many additional inherited motor and sensory neuropathies are associated with gene mutations that more directly involve axon transport, suggesting that other cellular organelles and signaling pathways unrelated to mitochondrial dynamics may be involved. HSAN is an example of a group of phenotypically related neuropathies with pathogenesis that appears to converge on alterations in axon transport-dependent NGF signaling which is essential for the development and innervation of sensory and sympathetic neurons. In each of the recognized HSAN types, mutations in proteins involved in some aspect of NGF signaling were identified (Figure 4). Although these inherited neuropathies are rare, a greater understanding of how neuropathy-associated proteins function should help to identify critical vulnerabilities that may be exploited as targets for disease therapy. Improved understanding of the basic mechanisms involved in these discrete neuropathies may help to better understand the basis for the prevalent and poorly treatable neuropathies associated with chronic diseases such as DM.
Figure 4.
Gene mutations in HSAN converge on proteins associated with NGF signaling. HSAN4 (TrkA) and HSAN5 (NGF) alter signaling at the level of receptor affinity and its ligand. In HSAN1, Rab7 mutations may alter NGF/TrkA signaling-endosome loading and retrograde transport along MTs. Elp1, which is mutated in HSAN3, is associated with Rab7+ signaling endosomes, but its exact function and whether it binds directly or indirectly to them is not yet known. In HSAN2, WNK1 may have a role in Erk5 activation which is critical for signaling-endosome activation of NGF-mediated gene expression through activation of SRF and CREB-dependent gene regulation. In addition, KIF1A mutations are also associated with HSAN2 which may alter anterograde transport that is essential for TrkA receptor recycling to the axon terminal in a process known as transcytosis. CREB, cAMP response-element binding protein; HSAN, hereditary sensory and autonomic neuropathy; MT, microtubule; NGF, nerve growth factor; SRF, serum response factor.
Acknowledgments
I thank Marisa Jackson, Katherine Gruner, Laurie Eldredge, David Quach, Michelle Gao, and Lin Li for thoughtful discussions and contributions in the laboratory to our research on familial dysautonomia and sympathetic nervous system development.
Neuropathology Theme Issue
Footnotes
Supported in part by NIH grants R21-HD063078, K02-NS046468, K26-OD026099, R56-NS089626, and P30-CA060553-20-7341 [Mouse Histology and Phenotyping Laboratory (MHPL)].
Disclosures: None declared.
This article is part of a review series on neuropathology.
References
- 1.Hughes R.A. Peripheral neuropathy. Br Med J. 2002;324:466–469. doi: 10.1136/bmj.324.7335.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martyn C.N., Hughes R.A. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310–318. doi: 10.1136/jnnp.62.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg E.W., Sorlie P., Paulose-Ram R., Gu Q., Eberhardt M.S., Wolz M., Burt V., Curtin L., Engelgau M., Geiss L., 1999-2000 national health and nutrition examination survey Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 4.CDC . US Department of Health and Human Services; Atlanta, GA: 2014. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States. [Google Scholar]
- 5.Maser R.E., Steenkiste A.R., Dorman J.S., Nielsen V.K., Bass E.B., Manjoo Q. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes. 1989;38:1456–1461. doi: 10.2337/diab.38.11.1456. [DOI] [PubMed] [Google Scholar]
- 6.Franklin G.M., Kahn L.B., Baxter J., Marshall J.A., Hamman R.F. Sensory neuropathy in non-insulin-dependent diabetes mellitus. The San Luis Valley Diabetes Study. Am J Epidemiol. 1990;131:633–643. doi: 10.1093/oxfordjournals.aje.a115547. [DOI] [PubMed] [Google Scholar]
- 7.Baets J., De Jonghe P., Timmerman V. Recent advances in Charcot-Marie-Tooth disease. Curr Opin Neurol. 2014;27:532–540. doi: 10.1097/WCO.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 8.Hehir M.K., 2nd, Logigian E.L. Infectious neuropathies. Continuum (Minneap Minn) 2014;20:1274–1292. doi: 10.1212/01.CON.0000455881.83803.a9. [DOI] [PubMed] [Google Scholar]
- 9.Saporta M.A., Shy M.E. Inherited peripheral neuropathies. Neurol Clin. 2013;31:597–619. doi: 10.1016/j.ncl.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staff N.P., Windebank A.J. Peripheral neuropathy due to vitamin deficiency, toxins, and medications. Continuum (Minneap Minn) 2014;20:1293–1306. doi: 10.1212/01.CON.0000455880.06675.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vilholm O.J., Christensen A.A., Zedan A.H., Itani M. Drug-induced peripheral neuropathy. Basic Clin Pharmacol Toxicol. 2014;115:185–192. doi: 10.1111/bcpt.12261. [DOI] [PubMed] [Google Scholar]
- 12.Watson J.C., Dyck P.J. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940–951. doi: 10.1016/j.mayocp.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Saporta M.A. Charcot-Marie-Tooth disease and other inherited neuropathies. Continuum (Minneap Minn) 2014;20:1208–1225. doi: 10.1212/01.CON.0000455885.37169.4c. [DOI] [PubMed] [Google Scholar]
- 14.Cashman C.R., Hoke A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci Lett. 2015;596:33–50. doi: 10.1016/j.neulet.2015.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrier J., Pereira V., Busserolles J., Authier N., Balayssac D. Emerging trends in understanding chemotherapy-induced peripheral neuropathy. Curr Pain Headache Rep. 2013;17:364. doi: 10.1007/s11916-013-0364-5. [DOI] [PubMed] [Google Scholar]
- 16.McDonald E.S., Randon K.R., Knight A., Windebank A.J. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18:305–313. doi: 10.1016/j.nbd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Gilardini A., Avila R.L., Oggioni N., Rodriguez-Menendez V., Bossi M., Canta A., Cavaletti G., Kirschner D.A. Myelin structure is unaltered in chemotherapy-induced peripheral neuropathy. Neurotoxicology. 2012;33:1–7. doi: 10.1016/j.neuro.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Boyette-Davis J., Xin W., Zhang H., Dougherty P.M. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medori R., Autilio-Gambetti L., Monaco S., Gambetti P. Experimental diabetic neuropathy: impairment of slow transport with changes in axon cross-sectional area. Proc Natl Acad Sci U S A. 1985;82:7716–7720. doi: 10.1073/pnas.82.22.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown M.R., Keith T.J., Knull H.R. Nonenzymatic incorporation of glucose and galactose into brain cytoskeletal proteins in vitro. Neurochem Int. 1992;21:177–183. doi: 10.1016/0197-0186(92)90144-g. [DOI] [PubMed] [Google Scholar]
- 21.Cullum N.A., Mahon J., Stringer K., McLean W.G. Glycation of rat sciatic nerve tubulin in experimental diabetes mellitus. Diabetologia. 1991;34:387–389. doi: 10.1007/BF00403175. [DOI] [PubMed] [Google Scholar]
- 22.Pekiner C., Cullum N.A., Hughes J.N., Hargreaves A.J., Mahon J., Casson I.F., McLean W.G. Glycation of brain actin in experimental diabetes. J Neurochem. 1993;61:436–442. doi: 10.1111/j.1471-4159.1993.tb02143.x. [DOI] [PubMed] [Google Scholar]
- 23.Mohiuddin L., Fernyhough P., Tomlinson D.R. Reduced levels of mRNA encoding endoskeletal and growth-associated proteins in sensory ganglia in experimental diabetes. Diabetes. 1995;44:25–30. doi: 10.2337/diab.44.1.25. [DOI] [PubMed] [Google Scholar]
- 24.Williams S.K., Howarth N.L., Devenny J.J., Bitensky M.W. Structural and functional consequences of increased tubulin glycosylation in diabetes mellitus. Proc Natl Acad Sci U S A. 1982;79:6546–6550. doi: 10.1073/pnas.79.21.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gornstein E., Schwarz T.L. The paradox of paclitaxel neurotoxicity: mechanisms and unanswered questions. Neuropharmacology. 2014;76 Pt A:175–183. doi: 10.1016/j.neuropharm.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Pareyson D., Saveri P., Sagnelli A., Piscosquito G. Mitochondrial dynamics and inherited peripheral nerve diseases. Neurosci Lett. 2015;596:66–77. doi: 10.1016/j.neulet.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Cheng H.T., Dauch J.R., Hayes J.M., Hong Y., Feldman E.L. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J Neuropathol Exp Neurol. 2009;68:1229–1243. doi: 10.1097/NEN.0b013e3181bef710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernyhough P., Diemel L.T., Hardy J., Brewster W.J., Mohiuddin L., Tomlinson D.R. Human recombinant nerve growth factor replaces deficient neurotrophic support in the diabetic rat. Eur J Neurosci. 1995;7:1107–1110. doi: 10.1111/j.1460-9568.1995.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 29.Mathis S., Goizet C., Tazir M., Magdelaine C., Lia A.S., Magy L., Vallat J.M. Charcot-Marie-Tooth diseases: an update and some new proposals for the classification. J Med Genet. 2015;52:681–690. doi: 10.1136/jmedgenet-2015-103272. [DOI] [PubMed] [Google Scholar]
- 30.Rossor A.M., Evans M.R., Reilly M.M. A practical approach to the genetic neuropathies. Pract Neurol. 2015;15:187–198. doi: 10.1136/practneurol-2015-001095. [DOI] [PubMed] [Google Scholar]
- 31.Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth's disease. Clin Genet. 1974;6:98–118. doi: 10.1111/j.1399-0004.1974.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 32.Reilly M.M., Murphy S.M., Laura M. Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 2011;16:1–14. doi: 10.1111/j.1529-8027.2011.00324.x. [DOI] [PubMed] [Google Scholar]
- 33.Nasrallah C.M., Horvath T.L. Mitochondrial dynamics in the central regulation of metabolism. Nat Rev Endocrinol. 2014;10:650–658. doi: 10.1038/nrendo.2014.160. [DOI] [PubMed] [Google Scholar]
- 34.Baloh R.H. Mitochondrial dynamics and peripheral neuropathy. Neuroscientist. 2008;14:12–18. doi: 10.1177/1073858407307354. [DOI] [PubMed] [Google Scholar]
- 35.Fransson S., Ruusala A., Aspenstrom P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- 36.Baloh R.H., Schmidt R.E., Pestronk A., Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci. 2007;27:422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banchs I., Casasnovas C., Montero J., Martinez-Matos J.A., Volpini V. Two Spanish families with Charcot-Marie-Tooth type 2A: clinical, electrophysiological and molecular findings. Neuromuscul Disord. 2008;18:974–978. doi: 10.1016/j.nmd.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Misko A., Jiang S., Wegorzewska I., Milbrandt J., Baloh R.H. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30:4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung K.W., Suh B.C., Cho S.Y., Choi S.K., Kang S.H., Yoo J.H., Hwang J.Y., Choi B.O. Early-onset Charcot-Marie-Tooth patients with mitofusin 2 mutations and brain involvement. J Neurol Neurosurg Psychiatry. 2010;81:1203–1206. doi: 10.1136/jnnp.2009.181669. [DOI] [PubMed] [Google Scholar]
- 40.Zuchner S., De Jonghe P., Jordanova A., Claeys K.G., Guergueltcheva V., Cherninkova S., Hamilton S.R., Van Stavern G., Krajewski K.M., Stajich J., Tournev I., Verhoeven K., Langerhorst C.T., de Visser M., Baas F., Bird T., Timmerman V., Shy M., Vance J.M. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol. 2006;59:276–281. doi: 10.1002/ana.20797. [DOI] [PubMed] [Google Scholar]
- 41.Verhoeven K., Claeys K.G., Zuchner S., Schroder J.M., Weis J., Ceuterick C. MFN2 mutation distribution and genotype/phenotype correlation in Charcot-Marie-Tooth type 2. Brain. 2006;129:2093–2102. doi: 10.1093/brain/awl126. [DOI] [PubMed] [Google Scholar]
- 42.Baxter R.V., Ben Othmane K., Rochelle J.M., Stajich J.E., Hulette C., Dew-Knight S., Hentati F., Ben Hamida M., Bel S., Stenger J.E., Gilbert J.R., Pericak-Vance M.A., Vance J.M. Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot-Marie-Tooth disease type 4A/8q21. Nat Genet. 2002;30:21–22. doi: 10.1038/ng796. [DOI] [PubMed] [Google Scholar]
- 43.Cuesta A., Pedrola L., Sevilla T., Garcia-Planells J., Chumillas M.J., Mayordomo F., LeGuern E., Marin I., Vilchez J.J., Palau F. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat Genet. 2002;30:22–25. doi: 10.1038/ng798. [DOI] [PubMed] [Google Scholar]
- 44.Niemann A., Huber N., Wagner K.M., Somandin C., Horn M., Lebrun-Julien F., Angst B., Pereira J.A., Halfter H., Welzl H., Feltri M.L., Wrabetz L., Young P., Wessig C., Toyka K.V., Suter U. The Gdap1 knockout mouse mechanistically links redox control to Charcot-Marie-Tooth disease. Brain. 2014;137:668–682. doi: 10.1093/brain/awt371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cassereau J., Chevrollier A., Gueguen N., Desquiret V., Verny C., Nicolas G., Dubas F., Amati-Bonneau P., Reynier P., Bonneau D., Procaccio V. Mitochondrial dysfunction and pathophysiology of Charcot-Marie-Tooth disease involving GDAP1 mutations. Exp Neurol. 2011;227:31–41. doi: 10.1016/j.expneurol.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Marco A., Cuesta A., Pedrola L., Palau F., Marin I. Evolutionary and structural analyses of GDAP1, involved in Charcot-Marie-Tooth disease, characterize a novel class of glutathione transferase-related genes. Mol Biol Evol. 2004;21:176–187. doi: 10.1093/molbev/msh013. [DOI] [PubMed] [Google Scholar]
- 47.Cassereau J., Chevrollier A., Bonneau D., Verny C., Procaccio V., Reynier P., Ferre M. A locus-specific database for mutations in GDAP1 allows analysis of genotype-phenotype correlations in Charcot-Marie-Tooth diseases type 4A and 2K. Orphanet J Rare Dis. 2011;6:87. doi: 10.1186/1750-1172-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niemann A., Wagner K.M., Ruegg M., Suter U. GDAP1 mutations differ in their effects on mitochondrial dynamics and apoptosis depending on the mode of inheritance. Neurobiol Dis. 2009;36:509–520. doi: 10.1016/j.nbd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Azzedine H., Ruberg M., Ente D., Gilardeau C., Perie S., Wechsler B., Brice A., LeGuern E., Dubourg O. Variability of disease progression in a family with autosomal recessive CMT associated with a S194X and new R310Q mutation in the GDAP1 gene. Neuromuscul Disord. 2003;13:341–346. [PubMed] [Google Scholar]
- 50.Claramunt R., Pedrola L., Sevilla T., Lopez de Munain A., Berciano J., Cuesta A., Sanchez-Navarro B., Millan J.M., Saifi G.M., Lupski J.R., Vilchez J.J., Espinos C., Palau F. Genetics of Charcot-Marie-Tooth disease type 4A: mutations, inheritance, phenotypic variability, and founder effect. J Med Genet. 2005;42:358–365. doi: 10.1136/jmg.2004.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crimella C., Tonelli A., Airoldi G., Baschirotto C., D'Angelo M.G., Bonato S., Losito L., Trabacca A., Bresolin N., Bassi M.T. The GST domain of GDAP1 is a frequent target of mutations in the dominant form of axonal Charcot Marie Tooth type 2K. J Med Genet. 2010;47:712–716. doi: 10.1136/jmg.2010.077909. [DOI] [PubMed] [Google Scholar]
- 52.Nelis E., Erdem S., Van Den Bergh P.Y., Belpaire-Dethiou M.C., Ceuterick C., Van Gerwen V., Cuesta A., Pedrola L., Palau F., Gabreels-Festen A.A., Verellen C., Tan E., Demirci M., Van Broeckhoven C., De Jonghe P., Topaloglu H., Timmerman V. Mutations in GDAP1: autosomal recessive CMT with demyelination and axonopathy. Neurology. 2002;59:1865–1872. doi: 10.1212/01.wnl.0000036272.36047.54. [DOI] [PubMed] [Google Scholar]
- 53.Cassereau J., Chevrollier A., Gueguen N., Malinge M.C., Letournel F., Nicolas G., Richard L., Ferre M., Verny C., Dubas F., Procaccio V., Amati-Bonneau P., Bonneau D., Reynier P. Mitochondrial complex I deficiency in GDAP1-related autosomal dominant Charcot-Marie-Tooth disease (CMT2K) Neurogenetics. 2009;10:145–150. doi: 10.1007/s10048-008-0166-9. [DOI] [PubMed] [Google Scholar]
- 54.Alexander C., Votruba M., Pesch U.E., Thiselton D.L., Mayer S., Moore A., Rodriguez M., Kellner U., Leo-Kottler B., Auburger G., Bhattacharya S.S., Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 55.Delettre C., Lenaers G., Griffoin J.M., Gigarel N., Lorenzo C., Belenguer P., Pelloquin L., Grosgeorge J., Turc-Carel C., Perret E., Astarie-Dequeker C., Lasquellec L., Arnaud B., Ducommun B., Kaplan J., Hamel C.P. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 56.Alavi M.V., Fuhrmann N. Dominant optic atrophy, OPA1, and mitochondrial quality control: understanding mitochondrial network dynamics. Mol Neurodegener. 2013;8:32. doi: 10.1186/1750-1326-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kevenaar J.T., Hoogenraad C.C. The axonal cytoskeleton: from organization to function. Front Mol Neurosci. 2015;8:44. doi: 10.3389/fnmol.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yonekawa Y., Harada A., Okada Y., Funakoshi T., Kanai Y., Takei Y., Terada S., Noda T., Hirokawa N. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J Cell Biol. 1998;141:431–441. doi: 10.1083/jcb.141.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reid E., Kloos M., Ashley-Koch A., Hughes L., Bevan S., Svenson I.K., Graham F.L., Gaskell P.C., Dearlove A., Pericak-Vance M.A., Rubinsztein D.C., Marchuk D.A. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10) Am J Hum Genet. 2002;71:1189–1194. doi: 10.1086/344210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crimella C., Baschirotto C., Arnoldi A., Tonelli A., Tenderini E., Airoldi G., Martinuzzi A., Trabacca A., Losito L., Scarlato M., Benedetti S., Scarpini E., Spinicci G., Bresolin N., Bassi M.T. Mutations in the motor and stalk domains of KIF5A in spastic paraplegia type 10 and in axonal Charcot-Marie-Tooth type 2. Clin Genet. 2012;82:157–164. doi: 10.1111/j.1399-0004.2011.01717.x. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y.T., Laura M., Hersheson J., Horga A., Jaunmuktane Z., Brandner S., Pittman A., Hughes D., Polke J.M., Sweeney M.G., Proukakis C., Janssen J.C., Auer-Grumbach M., Zuchner S., Shields K.G., Reilly M.M., Houlden H. Extended phenotypic spectrum of KIF5A mutations: from spastic paraplegia to axonal neuropathy. Neurology. 2014;83:612–619. doi: 10.1212/WNL.0000000000000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao C., Takita J., Tanaka Y., Setou M., Nakagawa T., Takeda S., Yang H.W., Terada S., Nakata T., Takei Y., Saito M., Tsuji S., Hayashi Y., Hirokawa N. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 2001;105:587–597. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]
- 63.Weedon M.N., Hastings R., Caswell R., Xie W., Paszkiewicz K., Antoniadi T., Williams M., King C., Greenhalgh L., Newbury-Ecob R., Ellard S. Exome sequencing identifies a DYNC1H1 mutation in a large pedigree with dominant axonal Charcot-Marie-Tooth disease. Am J Hum Genet. 2011;89:308–312. doi: 10.1016/j.ajhg.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hafezparast M., Klocke R., Ruhrberg C., Marquardt A., Ahmad-Annuar A., Bowen S. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 65.Chen X.J., Levedakou E.N., Millen K.J., Wollmann R.L., Soliven B., Popko B. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic Dynein heavy chain 1 gene. J Neurosci. 2007;27:14515–14524. doi: 10.1523/JNEUROSCI.4338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ori-McKenney K.M., Xu J., Gross S.P., Vallee R.B. A cytoplasmic dynein tail mutation impairs motor processivity. Nat Cell Biol. 2010;12:1228–1234. doi: 10.1038/ncb2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puls I., Jonnakuty C., LaMonte B.H., Holzbaur E.L., Tokito M., Mann E., Floeter M.K., Bidus K., Drayna D., Oh S.J., Brown R.H., Jr., Ludlow C.L., Fischbeck K.H. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 68.Rossor A.M., Oates E.C., Salter H.K., Liu Y., Murphy S.M., Schule R., Gonzalez M.A., Scoto M., Phadke R., Sewry C.A., Houlden H., Jordanova A., Tournev I., Chamova T., Litvinenko I., Zuchner S., Herrmann D.N., Blake J., Sowden J.E., Acsadi G., Rodriguez M.L., Menezes M.P., Clarke N.F., Auer Grumbach M., Bullock S.L., Muntoni F., Reilly M.M., North K.N. Phenotypic and molecular insights into spinal muscular atrophy due to mutations in BICD2. Brain. 2015;138:293–310. doi: 10.1093/brain/awu356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neveling K., Martinez-Carrera L.A., Holker I., Heister A., Verrips A., Hosseini-Barkooie S.M., Gilissen C., Vermeer S., Pennings M., Meijer R., te Riele M., Frijns C.J., Suchowersky O., MacLaren L., Rudnik-Schoneborn S., Sinke R.J., Zerres K., Lowry R.B., Lemmink H.H., Garbes L., Veltman J.A., Schelhaas H.J., Scheffer H., Wirth B. Mutations in BICD2, which encodes a golgin and important motor adaptor, cause congenital autosomal-dominant spinal muscular atrophy. Am J Hum Genet. 2013;92:946–954. doi: 10.1016/j.ajhg.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oates E.C., Rossor A.M., Hafezparast M., Gonzalez M., Speziani F., MacArthur D.G., Lek M., Cottenie E., Scoto M., Foley A.R., Hurles M., Houlden H., Greensmith L., Auer-Grumbach M., Pieber T.R., Strom T.M., Schule R., Herrmann D.N., Sowden J.E., Acsadi G., Menezes M.P., Clarke N.F., Zuchner S., UK10K. Muntoni F., North K.N., Reilly M.M. Mutations in BICD2 cause dominant congenital spinal muscular atrophy and hereditary spastic paraplegia. Am J Hum Genet. 2013;92:965–973. doi: 10.1016/j.ajhg.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zuchner S., Noureddine M., Kennerson M., Verhoeven K., Claeys K., De Jonghe P., Merory J., Oliveira S.A., Speer M.C., Stenger J.E., Walizada G., Zhu D., Pericak-Vance M.A., Nicholson G., Timmerman V., Vance J.M. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat Genet. 2005;37:289–294. doi: 10.1038/ng1514. [DOI] [PubMed] [Google Scholar]
- 72.Demer J.L., Clark R.A., Tischfield M.A., Engle E.C. Evidence of an asymmetrical endophenotype in congenital fibrosis of extraocular muscles type 3 resulting from TUBB3 mutations. Invest Ophthalmol Vis Sci. 2010;51:4600–4611. doi: 10.1167/iovs.10-5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tischfield M.A., Baris H.N., Wu C., Rudolph G., Van Maldergem L., He W. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fabrizi G.M., Cavallaro T., Angiari C., Cabrini I., Taioli F., Malerba G., Bertolasi L., Rizzuto N. Charcot-Marie-Tooth disease type 2E, a disorder of the cytoskeleton. Brain. 2007;130:394–403. doi: 10.1093/brain/awl284. [DOI] [PubMed] [Google Scholar]
- 75.Jordanova A., De Jonghe P., Boerkoel C.F., Takashima H., De Vriendt E., Ceuterick C., Martin J.J., Butler I.J., Mancias P., Papasozomenos SCh, Terespolsky D., Potocki L., Brown C.W., Shy M., Rita D.A., Tournev I., Kremensky I., Lupski J.R., Timmerman V. Mutations in the neurofilament light chain gene (NEFL) cause early onset severe Charcot-Marie-Tooth disease. Brain. 2003;126:590–597. doi: 10.1093/brain/awg059. [DOI] [PubMed] [Google Scholar]
- 76.Dyck P.J., Ohta M. Neuronal atrophy and degeneration predominantly affecting peripheral sensory neurons. In: Dyck P.J., Thomas P.K., Lamgert E.H., editors. Peripheral Neuropathy. W.B. Suanders Company; Philadelphia, PA: 1975. p. 791. [Google Scholar]
- 77.Edvardson S., Cinnamon Y., Jalas C., Shaag A., Maayan C., Axelrod F.B., Elpeleg O. Hereditary sensory autonomic neuropathy caused by a mutation in dystonin. Ann Neurol. 2012;71:569–572. doi: 10.1002/ana.23524. [DOI] [PubMed] [Google Scholar]
- 78.Leipold E., Liebmann L., Korenke G.C., Heinrich T., Giesselmann S., Baets J., Ebbinghaus M., Goral R.O., Stodberg T., Hennings J.C., Bergmann M., Altmuller J., Thiele H., Wetzel A., Nurnberg P., Timmerman V., De Jonghe P., Blum R., Schaible H.G., Weis J., Heinemann S.H., Hubner C.A., Kurth I. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat Genet. 2013;45:1399–1404. doi: 10.1038/ng.2767. [DOI] [PubMed] [Google Scholar]
- 79.Haga N., Kubota M., Miwa Z., Japanese Research Group on Congenital Insensitivity to Pain Hereditary sensory and autonomic neuropathy types IV and V in Japan. Pediatr Int. 2015;57:30–36. doi: 10.1111/ped.12538. [DOI] [PubMed] [Google Scholar]
- 80.Auer-Grumbach M. Hereditary sensory and autonomic neuropathies. Handb Clin Neurol. 2013;115:893–906. doi: 10.1016/B978-0-444-52902-2.00050-3. [DOI] [PubMed] [Google Scholar]
- 81.Glebova N.O., Ginty D.D. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- 82.Harrington A.W., Ginty D.D. Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci. 2013;14:177–187. doi: 10.1038/nrn3253. [DOI] [PubMed] [Google Scholar]
- 83.Marlin M.C., Li G. Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol. 2015;314:239–257. doi: 10.1016/bs.ircmb.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bothwell M. NGF, BDNF, NT3, and NT4. Handb Exp Pharmacol. 2014;220:3–15. doi: 10.1007/978-3-642-45106-5_1. [DOI] [PubMed] [Google Scholar]
- 85.Capsoni S. From genes to pain: nerve growth factor and hereditary sensory and autonomic neuropathy type V. Eur J Neurosci. 2014;39:392–400. doi: 10.1111/ejn.12461. [DOI] [PubMed] [Google Scholar]
- 86.Houlden H., King R.H., Muddle J.R., Warner T.T., Reilly M.M., Orrell R.W., Ginsberg L. A novel RAB7 mutation associated with ulcero-mutilating neuropathy. Ann Neurol. 2004;56:586–590. doi: 10.1002/ana.20281. [DOI] [PubMed] [Google Scholar]
- 87.Saxena S., Bucci C., Weis J., Kruttgen A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci. 2005;25:10930–10940. doi: 10.1523/JNEUROSCI.2029-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang K., Chowdary P.D., Cui B. Visualizing directional Rab7 and TrkA cotrafficking in axons by pTIRF microscopy. Methods Mol Biol. 2015;1298:319–329. doi: 10.1007/978-1-4939-2569-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang K., Fishel Ben Kenan R., Osakada Y., Xu W., Sinit R.S., Chen L., Zhao X., Chen J.Y., Cui B., Wu C. Defective axonal transport of Rab7 GTPase results in dysregulated trophic signaling. J Neurosci. 2013;33:7451–7462. doi: 10.1523/JNEUROSCI.4322-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guelly C., Zhu P.P., Leonardis L., Papić L., Zidar J., Schabhüttl M., Strohmaier H., Weis J., Strom T.M., Baets J., Willems J., De Jonghe P., Reilly M.M., Fröhlich E., Hatz M., Trajanoski S., Pieber T.R., Janecke A.R., Blackstone C., Auer-Grumbach M. Targeted high-throughput sequencing identifies mutations in atlastin-1 as a cause of hereditary sensory neuropathy type I. Am J Hum Genet. 2011;88:99–105. doi: 10.1016/j.ajhg.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riviere J.B., Ramalingam S., Lavastre V., Shekarabi M., Holbert S., Lafontaine J., Srour M., Merner N., Rochefort D., Hince P., Gaudet R., Mes-Masson A.M., Baets J., Houlden H., Brais B., Nicholson G.A., Van Esch H., Nafissi S., De Jonghe P., Reilly M.M., Timmerman V., Dion P.A., Rouleau G.A. KIF1A, an axonal transporter of synaptic vesicles, is mutated in hereditary sensory and autonomic neuropathy type 2. Am J Hum Genet. 2011;89:219–230. doi: 10.1016/j.ajhg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ascano M., Richmond A., Borden P., Kuruvilla R. Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. J Neurosci. 2009;29:11674–11685. doi: 10.1523/JNEUROSCI.1542-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shekarabi M., Girard N., Riviere J.B., Dion P., Houle M., Toulouse A., Lafreniere R.G., Vercauteren F., Hince P., Laganiere J., Rochefort D., Faivre L., Samuels M., Rouleau G.A. Mutations in the nervous system–specific HSN2 exon of WNK1 cause hereditary sensory neuropathy type II. J Clin Invest. 2008;118:2496–2505. doi: 10.1172/JCI34088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu B.E., Stippec S., Lenertz L., Lee B.H., Zhang W., Lee Y.K., Cobb M.H. WNK1 activates ERK5 by an MEKK2/3-dependent mechanism. J Biol Chem. 2004;279:7826–7831. doi: 10.1074/jbc.M313465200. [DOI] [PubMed] [Google Scholar]
- 95.Watson F.L., Heerssen H.M., Bhattacharyya A., Klesse L., Lin M.Z., Segal R.A. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- 96.Anderson S.L., Coli R., Daly I.W., Kichula E.A., Rork M.J., Volpi S.A., Ekstein J., Rubin B.Y. Familial dysautonomia is caused by mutations of the IKAP gene. Am J Hum Genet. 2001;68:753–758. doi: 10.1086/318808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Slaugenhaupt S.A., Blumenfeld A., Gill S.P., Leyne M., Mull J., Cuajungco M.P., Liebert C.B., Chadwick B., Idelson M., Reznik L., Robbins C., Makalowska I., Brownstein M., Krappmann D., Scheidereit C., Maayan C., Axelrod F.B., Gusella J.F. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet. 2001;68:598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jackson M.Z., Gruner K.A., Qin C., Tourtellotte W.G. A neuron autonomous role for the familial dysautonomia gene ELP1 in sympathetic and sensory target tissue innervation. Development. 2014;141:2452–2461. doi: 10.1242/dev.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Axelrod F.B. Familial dysautonomia. Muscle Nerve. 2004;29:352–363. doi: 10.1002/mus.10499. [DOI] [PubMed] [Google Scholar]
- 100.Cuajungco M.P., Leyne M., Mull J., Gill S.P., Lu W., Zagzag D., Axelrod F.B., Maayan C., Gusella J.F., Slaugenhaupt S.A. Tissue-specific reduction in splicing efficiency of IKBKAP due to the major mutation associated with familial dysautonomia. Am J Hum Genet. 2003;72:749–758. doi: 10.1086/368263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheishvili D., Maayan C., Cohen-Kupiec R., Lefler S., Weil M., Ast G., Razin A. IKAP/Elp1 involvement in cytoskeleton regulation and implication for familial dysautonomia. Hum Mol Genet. 2011;20:1585–1594. doi: 10.1093/hmg/ddr036. [DOI] [PubMed] [Google Scholar]
- 102.Cheishvili D., Maayan C., Smith Y., Ast G., Razin A. IKAP/hELP1 deficiency in the cerebrum of familial dysautonomia patients results in down regulation of genes involved in oligodendrocyte differentiation and in myelination. Hum Mol Genet. 2007;16:2097–2104. doi: 10.1093/hmg/ddm157. [DOI] [PubMed] [Google Scholar]
- 103.Close P., Hawkes N., Cornez I., Creppe C., Lambert C.A., Rogister B., Siebenlist U., Merville M.P., Slaugenhaupt S.A., Bours V., Svejstrup J.Q., Chariot A. Transcription impairment and cell migration defects in elongator-depleted cells: implication for familial dysautonomia. Mol Cell. 2006;22:521–531. doi: 10.1016/j.molcel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 104.Creppe C., Malinouskaya L., Volvert M.L., Gillard M., Close P., Malaise O., Laguesse S., Cornez I., Rahmouni S., Ormenese S., Belachew S., Malgrange B., Chapelle J.P., Siebenlist U., Moonen G., Chariot A., Nguyen L. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136:551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 105.Rubin B.Y., Anderson S.L. The molecular basis of familial dysautonomia: overview, new discoveries and implications for directed therapies. Neuromolecular Med. 2008;10:148–156. doi: 10.1007/s12017-007-8019-5. [DOI] [PubMed] [Google Scholar]
- 106.Gold-von Simson G., Leyne M., Mull J., Rolnitzky L.M., Goldberg J.D., Berlin D., Axelrod F.B., Slaugenhaupt S.A. IKBKAP mRNA in peripheral blood leukocytes: a molecular marker of gene expression and splicing in familial dysautonomia. Pediatr Res. 2008;63:186–190. doi: 10.1203/PDR.0b013e31815ef74b. [DOI] [PubMed] [Google Scholar]
- 107.Winkler G.S., Petrakis T.G., Ethelberg S., Tokunaga M., Erdjument-Bromage H., Tempst P., Svejstrup J.Q. RNA polymerase II elongator holoenzyme is composed of two discrete subcomplexes. J Biol Chem. 2001;276:32743–32749. doi: 10.1074/jbc.M105303200. [DOI] [PubMed] [Google Scholar]
- 108.Cohen-Kupiec R., Pasmanik-Chor M., Oron-Karni V., Weil M. Effects of IKAP/hELP1 deficiency on gene expression in differentiating neuroblastoma cells: implications for familial dysautonomia. PLoS One. 2011;6:e19147. doi: 10.1371/journal.pone.0019147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Holmberg C., Katz S., Lerdrup M., Herdegen T., Jaattela M., Aronheim A., Kallunki T. A novel specific role for I kappa B kinase complex-associated protein in cytosolic stress signaling. J Biol Chem. 2002;277:31918–31928. doi: 10.1074/jbc.M200719200. [DOI] [PubMed] [Google Scholar]
- 110.Chen Y.T., Hims M.M., Shetty R.S., Mull J., Liu L., Leyne M., Slaugenhaupt S.A. Loss of mouse Ikbkap, a subunit of elongator, leads to transcriptional deficits and embryonic lethality that can be rescued by human IKBKAP. Mol Cell Biol. 2009;29:736–744. doi: 10.1128/MCB.01313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dietrich P., Yue J., E S., Dragatsis I. Deletion of exon 20 of the Familial Dysautonomia gene Ikbkap in mice causes developmental delay, cardiovascular defects, and early embryonic lethality. PLoS One. 2011;6:e27015. doi: 10.1371/journal.pone.0027015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smeyne R.J., Klein R., Schnapp A., Long L.K., Bryant S., Lewin A., Lira S.A., Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 113.Crowley C., Spencer S.D., Nishimura M.C., Chen K.S., Pitts-Meek S., Armanini M.P. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 114.Ashwin D.P., Chandan G.D., Jasleen H.K., Rajkumar G.C., Rudresh K.B., Prashanth R. Hereditary sensory and autosomal peripheral neuropathy-type IV: case series and review of literature. Oral Maxillofac Surg. 2015;19:117–123. doi: 10.1007/s10006-015-0486-5. [DOI] [PubMed] [Google Scholar]
- 115.Glebova N.O., Ginty D.D. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci. 2004;24:743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ye H., Kuruvilla R., Zweifel L.S., Ginty D.D. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron. 2003;39:57–68. doi: 10.1016/s0896-6273(03)00266-6. [DOI] [PubMed] [Google Scholar]
- 117.Kuruvilla R., Zweifel L.S., Glebova N.O., Lonze B.E., Valdez G., Ye H., Ginty D.D. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]