Abstract
Remyelination is the regenerative response to demyelination. Although the oligodendrocyte progenitor is established as the major source of remyelinating cells, there is no conclusive evidence on whether mature, differentiated oligodendrocytes can also contribute to remyelination. Using two different inducible myelin-CreER mouse strains in which mature oligodendrocytes were prelabeled by the expression of membrane-bound Green fluorescent protein, we found that after focal spinal cord demyelination, the surrounding surviving labeled oligodendrocytes did not proliferate but remained at a consistent density. Furthermore, existing (prelabeled) oligodendrocytes showed no evidence of incorporation or migration into the lesioned area, or of process extension from the peripheral margins into the lesion. Thus, mature oligodendrocytes do not normally contribute to remyelination and are therefore not a promising target for regenerative therapy.
The translational challenge of promoting remyelination therapeutically in chronic demyelinating diseases such as multiple sclerosis requires an understanding of the cells that are involved in generating new oligodendrocytes after demyelination. Although it is now firmly established that oligodendrocyte progenitors (OPs), a population of abundant and widely distributed multipotent adult central nervous system progenitors, are the major source of new remyelinating oligodendrocytes,1, 2 uncertainty has surrounded the contribution made by mature oligodendrocytes. It has been suggested that mature myelin-forming oligodendrocytes may be able to undergo cell division, implying that they have the potential to generate new cells for remyelination.3, 4, 5 However, two lines of evidence have strongly suggested that this was not the case.6 The first was the observation that oligodendrocytes that survive demyelination, albeit no longer supporting myelin sheaths, make no contribution to subsequent remyelination.7 A second study showed that culture-derived oligodendrocytes are unable to remyelinate demyelinated axons after transplantation.8 However, the conclusions drawn from both studies can be challenged. In the first, to distinguish oligodendrocyte-derived remyelination from OP-derived remyelination, the lesioned tissue was exposed to very high levels of X-irradiation to suppress OP proliferation, and no remyelination was seen subsequently, suggesting that only proliferating OPs and not postmitotic OLs are capable of remyelinating. However, in the process, not only OPs but also all other cells, including surviving oligodendrocytes, were also exposed to high levels of X-irradiation, perhaps impairing their normal function and rendering them incapable of a potential regenerative response of which they might have been capable under physiological circumstances. In the second, the derivation and maintenance of oligodendrocytes in culture before their experimental introduction into areas of demyelination might have compromised their ability to remyelinate.
To resolve this issue, we used two new mouse strains that express tamoxifen-inducible form of Cre recombinase (iCreER) exclusively in differentiated oligodendrocytes (OLs), under myelin basic protein (Mbp) or Opalin (also known as transmembrane protein 10) transcriptional control.9, 10 These were used in combination with a τ-mGfp-LacZ reporter strain, to prelabel mature OLs with membrane-bound Green fluorescent protein (mGfp) and β-galactosidase. This has enabled us to assess the contribution of oligodendrocytes under normal physiological conditions (exposed to neither X-irradiation nor to tissue culture) to remyelination after toxin-induced demyelination. We found no incidence of prelabeled OLs contributing to remyelination and therefore provide definitive evidence that mature myelinating oligodendrocytes make no significant contribution to their regeneration.
Materials and Methods
Generation of Transgenic Animals
An Mbp-iCreERT2 transgenic mouse strain was made using small plasmid transgenesis. A 5.89-Kbp upstream region of Mbp (promoter fragment) was used for driving iCreERT2 with parts of Plp exons 6 and 7 downstream, followed by a poly(A) sequence. The Plp downstream fragment was purified from plasmid Mβp,11 kindly gifted by Brian Popko, University of Chicago (Chicago, IL). An Mbp promoter fragment (9 Kbp) was gifted by Alan Peterson (McGill University, Montreal, QC, Canada) and reduced to 5.89 Kbp by digest with KpnI. This approximately 6-Kbp fragment lacks the Schwann cell enhancer (M4) located at approximately −9 Kbp relative to the transcriptional start site.12 The iCreERT2 cassette [gifted by Marcus Fruttiger, University College London (London, UK)] was synthesized according to the published sequence. The final Mbp-iCreERT2-Plp plasmid transgene was assembled and purified from the plasmid backbone before pronuclear injection. Two founders were obtained, and as both had similar expression patterns and intensity, one was selected for further characterization.
The Opalin-iCreERT2 mouse was generated via bacterial artificial chromosome transgenesis using the Opalin genomic bacterial artificial chromosome RP24-510M10. Insertion of the iCreERT2-polyA-frt-Kanamycin-frt cassette was achieved by homologous recombination (with no loss of the genomic bacterial artificial chromosome) immediately after the Opalin initiation codon ATG of the open reading frame of the Opalin sequence. The frt-kanamycin-frt was removed using flippase recombinase. The bacterial artificial chromosome was linearized using Nocardia otitidis-caviarum I restriction enzyme before gel purification and pronuclear injection. Four founders were generated, three of which expressed the transgene. The one with highest expression was chosen for further experimentation. Both the Mbp-iCreERT2 and the Opalin-iCreERT2 mice were generated on a C57BL/6 × CBA mixed background.
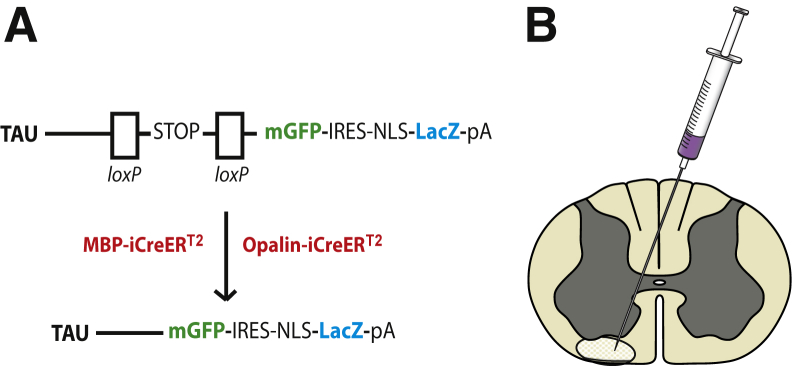
Cell-type specificity of the inducible CreER strains was assessed by crossing them individually to Rosa-Yfp and immunolabeling double-transgenic offspring (after tamoxifen injection) for yellow fluorescent protein and platelet-derived growth factor receptor alpha (OP), sex-determining region Y box 10 (OL lineage cells), or adenomatous polyposis coli antibody hybridoma clone (differentiated OLs). Both CreER strains were highly specific for sex-determining region Y box 10–positive, CC1+ OLs (R.B. Tripathi, I. McKenzie, and W.D. Richardson, manuscript in preparation). Here, to visualize myelin internodes, the CreER strains were crossed to tau-lox-STOP-lox-mGfp-Ires-Nls-LacZ-pA (tau-mGfp/LacZ) mice13 (Figure 1A).
Figure 1.
A: Transgenic mouse strains: A reporter mouse strain, in which membrane-bound Green fluorescent protein (mGfp) and β-galactosidase (LacZ) are under the control of the Tau promoter, was crossed to either of two inducible Cre strains in which Cre recombinase expression was restricted to mature oligodendrocytes using the Mbp or Opalin promoters. Excision of the STOP sequence by tamoxifen-inducible Cre recombination results in expression of mGfp and LacZ. B: Schematic diagram of the lysolecithin microinjection site in the white matter of the spinal cord ventral funiculus. IRES, internal ribosome entry site; NLS, nuclear localisation sequence; pA, polyadenylation.
Genotyping
Mice (both males and females) carrying the Opalin-iCreERT2 and Mbp-iCreERT2 transgenes were confirmed by PCR using the following primers against the iCre region (forward/reverse): 5′-GAGGGACTACCTCCTGTACC-3′/5′-TGCCCAGAGTCATCCTTGGC-3′. τ-mGfp-LacZ mice were genotyped using the following primers (forward/reverse): 5′-CCCTGAAGTTCATCTGCACCAC-3′/5′-TTCTCGTTGGGGTCTTTGCTC-3′.
Tamoxifen Administration
Tamoxifen (10 mg/mL) was prepared in ethanol/sunflower oil (1:9) by sonication for 35 to 45 minutes and administered at a dose of 55 mg/kg via i.p. injection on 4 successive days. Mice used in this study were aged P64 to P99 (mean, P77). The last tamoxifen injection was given 14 days before the demyelinating lesions were generated, to prevent any further ongoing recombination during and after the lesioning procedure.
Focal Spinal Cord Demyelination
Primary demyelination was induced in the caudal thoracic ventral funiculus of the spinal cord by injection of 1% lysolecithin as previously described1 (Figure 1B).
Tissue Processing
Mice were fixed by intracardiac perfusion at 5 (n = 3) or 21 (n = 6) days postlesion induction (dpl) using 4% (w/v) paraformaldehyde in 0.1 mol/L phosphate-buffered saline. Spinal cords were removed and postfixed in 4% paraformaldehyde for 2 hours at room temperature, then cryoprotected with 20% (w/v) sucrose for 24 to 48 hours, and finally embedded and frozen in OCT medium and stored at −80°C. Spinal cords were sectioned at 12-μm nominal thickness and collected onto Polysine-coated glass slides (Thermo Fisher Scientific Inc., Rochester, NY).
Immunocytochemistry and Microscopy
Spinal cord sections were pretreated with blocking solution [8% (v/v) normal goat serum, 0.1% (v/v) Triton X-100 in phosphate-buffered saline], incubated with primary antibodies overnight at 4°C and then secondary antibodies for 2 hours at 20°C to 25°C. Primary antisera were chicken anti-Gfp (1:1000 dilution; Aves Labs, Inc., Tigard, OR), rabbit anti-LacZ (anti–β-galactosidase) (1:2000; MP Biomedicals, Solon, OH), mouse monoclonal anti-CC1 (anti–adenomatous polyposis coli) (Calbiochem, 1:100; EMD Millipore Corp., Billerica, MA), rat anti-Mbp (1:400; AbD Serotec, Raleigh, NC), rabbit anti–Ki-67 (1:1000; Abcam plc, Cambridge, UK), and mouse monoclonal anti–proliferating cell nuclear antigen (1:300; Santa Cruz Biotechnology, Inc., Dallas, TX). Secondary antibodies were goat anti-chicken Alexa Fluor 488, goat anti-rabbit Alexa Fluor 647 (1:500), goat anti-mouse IgG2b Alexa Fluor 568. Alexa Fluor antibodies (InvitroGen, Carlsbad, CA) were used at 1:1000 dilution unless otherwise stated. All antibodies were diluted in the blocking solution. All Gfp expression was detected by immunolabeling. Cell nuclei were visualized by post-staining with Hoechst 33258 (1:1000; Sigma-Aldrich Corp., St. Louis, MO). Sections were mounted under coverslips and examined in a Leica TCS SPE confocal microscope (Leica Microsystems, Buffalo Grove, IL). For cell counts, at least three sections per animal were examined. Data are quoted as means ± SEM (n = 3 to 6).
Statistics
SPSS software version 22 (SPSS Inc., Chicago, IL) was used for confirming normal distribution of the data. The Student's t-test was used for determining significant differences among groups, and P < 0.05 was considered significant.
Results
Mbp+ Oligodendrocytes Show No Significant Contribution to Remyelination
To determine whether mature oligodendrocytes can contribute to the regeneration of an adjacent area of demyelination, we crossed the Mbp-iCreERT2 strain to a reporter mouse strain in which LacZ and mGfp are under the control of the tau-promoter. After tamoxifen administration, mature oligodendrocytes expressing Mbp undergo recombination and activation of expression of LacZ and mGfp. Recombination efficiency was found to be very high in this strain, with 74.4% ± 12% of mature, CC1+ oligodendrocytes successfully labeled.
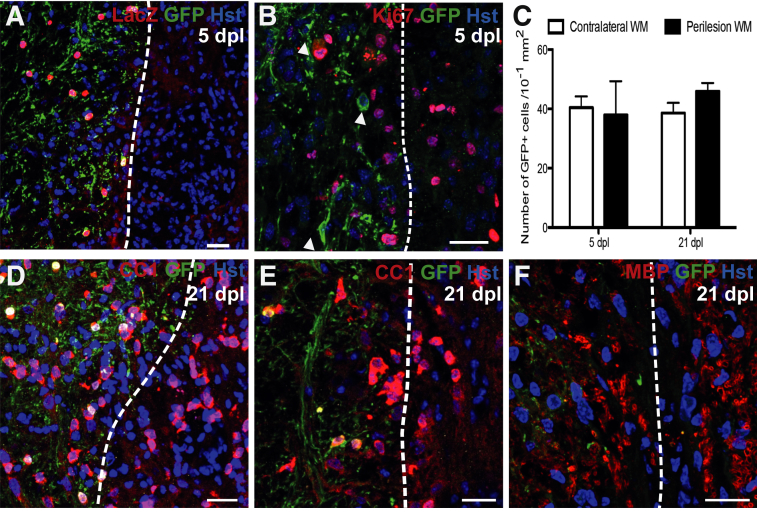
A focal area of demyelination was induced by the injection of lysolecithin, leading to the local depletion of mGfp+ oligodendrocytes by 5 dpl (Figure 2A). By 21 dpl, the lesioned area had been repopulated with CC1+ cells (Figure 2, D and E) and Mbp+ myelin sheaths, confirming remyelination. However, no mGfp+, LacZ+ oligodendrocytes were found within the lesioned area at 5 or 21 dpl (Figure 2, A, B, and D–F). This finding suggests that mature oligodendrocytes are not recruited into the area undergoing remyelination. Furthermore, there was no detectable process extension from surviving mGfp-labeled oligodendrocytes around the lesion periphery into the region undergoing remyelination (Figure 2F). Hence, surrounding oligodendrocytes show no detectable extension of new myelin sheaths to locally demyelinated axons.
Figure 2.
A: Five days postlesion (dpl) in Mbp-iCreERT2:Tau-mGfp-LacZ mouse: dashed line indicates presumed lesion edge (based on increased cell density), with lesioned tissue on the right. B: Ki-67+ cells in the lesion and in perilesion white matter (WM) at 5 dpl: no surrounding Green fluorescent protein (Gfp)-positive oligodendrocytes (OLs) (indicated by arrowheads) costain for Ki-67. C: Number of Gfp+ cells in contralateral and perilesion WM at 5 and 21 dpl: no significant difference was detected. D–F: At 21 dpl, membranous Gfp+ processes do not extend into adjacent areas of remyelination. Adenomatous polyposis coli antibody hybridoma clone CC1–positive OLs: with images from two separate animals (D and E), and myelin basic protein (Mbp)-positive myelin sheaths (F) confirm remyelination of lesioned area. Data are expressed as means ± SEM. P = 0.69 at 5 dpl, P = 0.13 at 21 dpl (Student's t-test). Scale bars = 20 μm. Original magnification: ×20 (A); ×40 (B, D, and E); ×63 (F). Hst, hoechst.
Ki-67 was used as a marker of cells actively replicating at the time of sacrifice. Abundant Ki-67+ cells were detected within the demyelinated lesion at 5 dpl (Figure 2B). Gfp-labeled oligodendrocytes surrounding the lesion periphery did not colabel with Ki-67, suggesting that oligodendrocytes do not proliferate in response to a local area of acute demyelination. In support of this conclusion, we did not find any cells that coexpressed LacZ and proliferating cell nuclear antigen, expressed during the DNA synthesis stage of the cell cycle (data not shown). The number of Gfp+, LacZ+ oligodendrocytes in the normal ventral funiculus was compared to that in the region immediately surrounding the lesion at 5 and 21 dpl; no significant differences were detected (Figure 2C). Therefore, preexisting oligodendrocytes do not divide in response to focal acute demyelination.
We recently discovered that the Mbp-iCreERT2 reporter mouse strain can undergo spontaneous recombination, thereby labeling cells independently of tamoxifen administration, presumably because of the high iCreERT2 expression in this strain. However, this did not appear to be an issue in this study as no labeled cells were identified within the demyelinated area; also we did not detect an increase in labeled cells within the 21-day experiment time frame. However, the high recombination efficiency prevented single-cell analysis. To ensure that spontaneous recombination was not influencing our findings and to enable single-cell visualization, the experiment was repeated using an Opalin-iCreERT2 strain.
Opalin+ Oligodendrocytes Show No Significant Contribution to Remyelination
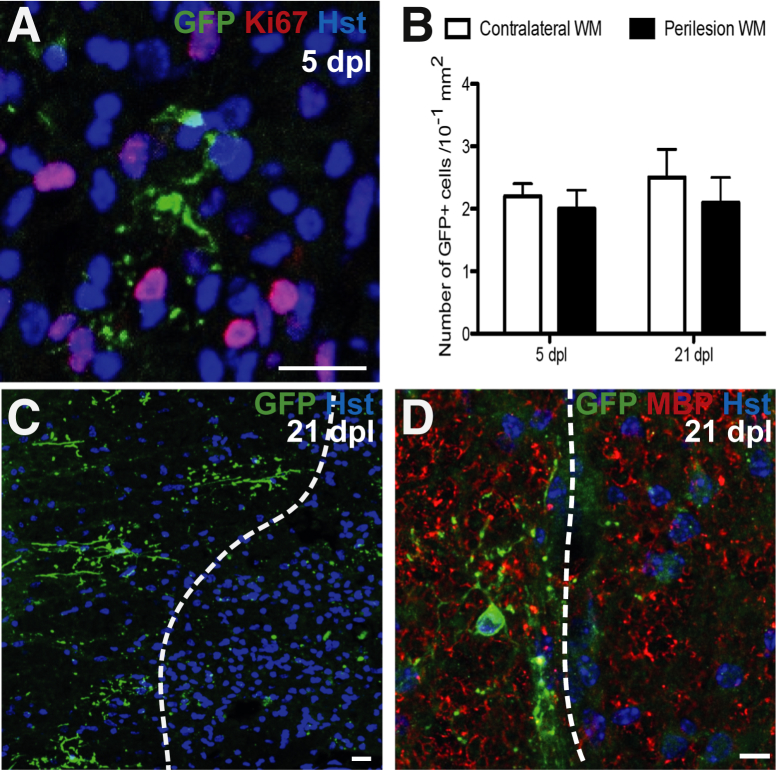
The tau-mGfp reporter mice were crossed to the Opalin-iCreERT2 mice; oligodendrocytes expressing Opalin at the time of tamoxifen administration would be expected to label with mGfp. The extent of recombination was much lower than with the Mbp-Cre strain, resulting in 6.5% ± 0.8% of CC1+ oligodendrocytes expressing mGfp. This relative scarcity enables the visualization of individual cells and their extensive myelinating processes (Figure 3A).
Figure 3.
A: Five days postlesion (dpl) in Opalin-iCreERT2:Tau-mGfp-LacZ mouse: Ki-67–Green fluorescent protein (Gfp)-positive oligodendrocyte (OL) from the presumed lesion edge surrounded by Ki-67+ cells. B: Number of membranous Gfp+cells in contralateral and perilesion white matter (WM) at 5 and 21 dpl: no significant difference is detected. C: Gfp+ OLs surrounding the lesion make no contribution to remyelination at 21 dpl (lesion right of dashed line). Fragments of Gfp+ staining are present within the lesion, but the morphology is not consistent with a myelinating cell and are presumed to be labeled myelin debris generated during demyelination that has not been cleared from the lesion. D: Gfp+ OL adjacent to remyelinated lesion: remyeination shown by myelin basic protein (Mbp)-positive myelin sheaths. Data are expressed as means ± SEM. P = 0.56 at 5 dpl, P = 0.75 at 21 dpl (t-test). Scale bars = 20 μm. Original magnification: ×63 (A and C); ×40 (B). Hst, hoechst.
Analysis of the lesioned region showed an absence of mGfp+ oligodendrocytes at both 5 and 21 dpl. Labeled oligodendrocytes surrounding the lesion periphery showed no detectable process extension into the lesioned region (Figure 3, B and C). Staining for the Ki-67 antigen revealed abundant proliferating, Ki-67+ cells within the demyelinated area at 5 dpl, but there was no costaining of surrounding, mGfp+ oligodendrocytes. Similarly, we did not find any cells that coexpressed LacZ and proliferating cell nuclear antigen (data not shown). Also, the number of Gfp-labeled cells remained constant in the normal ventral funiculus at 5 and 21 dpl (Figure 3D). Therefore, the use of two transgenic mouse strains in which mature oligodendrocytes can be effectively visualized failed to detect oligodendrocyte contribution to the remyelination of a toxin-induced, acutely demyelinated lesion.
Discussion
Areas of remyelination contain a greater number of oligodendrocytes compared to normal white matter, indicating that remyelination requires the generation of new oligodendrocytes.6, 14 This is dependent on cellular proliferation; exposing a demyelinated region of spinal cord to X-irradiation at a dose sufficient to cause the death of dividing cells prevents remyelination.15 The major proliferating cell population in the adult central nervous system is the OP,16 whose activation and recruitment in response to demyelination have been extensively documented.17, 18 However, whether mature oligodendrocytes can also contribute to the remyelination process has remained unclear.
Initial studies suggested that oligodendrocytes could undergo a proliferative response after a demyelinating insult, based on their incorporation of tritiated thymidine.3, 19 Subsequent studies, utilizing various experimental strategies such as irradiation, proliferation assays,7 and cell transplantation,8 have suggested that oligodendrocytes do not proliferate in response to demyelination, nor do they contribute to remyelination. However, each of these strategies has the potential to interfere with the normal cellular physiology of the oligodendrocyte.
In this study, transgenic mouse strains were used in which mature, differentiated oligodendrocytes are labeled by a membrane-bound Gfp after the administration of tamoxifen, thus enabling their assessment in a normal in vivo environment. This technique does not compromise the physiology of the oligodendrocyte and enables the remyelination process to proceed unhindered by any potential adverse effects of irradiation, immunosuppression, or transplantation.
A model of toxin-induced demyelination was used in which a focal area of acute demyelination was generated by the injection of lysolecithin. This lesioned area was depleted of mature oligodendrocytes by 5 dpl and became repopulated with CC1+ oligodendrocytes and Mbp+ myelin sheaths after 21 days. This repopulation was devoid of Gfp-labeled cells, suggesting that there was no significant contribution of the labeled, mature oligodendrocytes to this area of remyelination. There was no detectable proliferative response in the labeled cells surrounding the lesion, whose numbers remained static between normal and lesioned tissue. Gfp-labeled processes were not seen to extend into the area of demyelination, suggesting that closely adjacent oligodendrocytes do not extend new myelin sheaths to adjacent demyelinated axons. However, it remains possible that this occurs at a low level that we were not able to visualize, in part because of the difficulties of accurately defining the lesion border, or that the demyelination model used in our study is not conducive to such an event. Regardless, efficient remyelination was achieved without a significant contribution from mature oligodendrocytes.
In conclusion, mature oligodendrocytes do not appear to contribute to remyelination to any detectable extent. OPs present throughout the adult central nervous system are the source of remyelinating oligodendrocytes, and strategies to enhance endogenous remyelination in the context of clinical disease and aging should focus on enhancement of the OP function, as opposed to promotion of oligodendrocyte survival.
Acknowledgments
We thank Ulla Dennehy for technical help. MβP plasmid was kindly provided by Brian Popko (University of Chicago, Chicago, IL).
Footnotes
Supported by European Research Council grant agreement 293544 (W.D.R.), Wellcome Trust grant WT100269AIA, Medical Research Council grant G0800575, a Royal Society-USA/Canada Exchange Fellowship (I.M.), the UK Multiple Sclerosis Society, and a Wellcome Trust Integrated Veterinary Training Fellowship (A.H.C.).
A.H.C. and R.B.T. contributed equally to this work.
W.D.R. and R.J.M.F. contributed equally to this work as senior authors.
Disclosures: None declared.
References
- 1.Zawadzka M., Rivers L.E., Fancy S.P., Zhao C., Tripathi R., Jamen F., Young K., Goncharevich A., Pohl H., Rizzi M., Rowitch D.H., Kessaris N., Suter U., Richardson W.D., Franklin R.J.M. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripathi R.B., Rivers L.E., Young K.M., Jamen F., Richardson W.D. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci. 2010;30:16383–16390. doi: 10.1523/JNEUROSCI.3411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludwin S.K. Proliferation of mature oligodendrocytes after trauma to the central nervous system. Nature. 1984;308:274–275. doi: 10.1038/308274a0. [DOI] [PubMed] [Google Scholar]
- 4.Ludwin S.K., Bakker D.A. Can oligodendrocytes attached to nuclei proliferate? J Neurosci. 1988;8:1239–1244. doi: 10.1523/JNEUROSCI.08-04-01239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood P.M., Bunge R.P. Evidence that axons are mitogenic for oligodendrocytes isolated from adult animals. Nature. 1986;320:756–758. doi: 10.1038/320756a0. [DOI] [PubMed] [Google Scholar]
- 6.Blakemore W.F., Keirstead H.S. The origin of remyelinating cells in the CNS. J Neuroimmunol. 1999;98:69–76. doi: 10.1016/s0165-5728(99)00083-1. [DOI] [PubMed] [Google Scholar]
- 7.Keirstead H.S., Blakemore W.F. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Targett M.P., Sussman J., Scolding N., O'Leary M.T., Compston D.A., Blakemore W.F. Failure to remyelinate rat axons following transplantation of glial cells obtained from adult human brain. Neuropathol Appl Neurobiol. 1996;22:199–206. [PubMed] [Google Scholar]
- 9.Yoshikawa F., Sato Y., Tohyama K., Akagi T., Hashikawa T., Nagakura-Takagi Y., Sekine Y., Morita N., Baba H., Suzuki Y., Sugano S., Sato A., Furuichi T. Opalin, a transmembrane sialylglycoprotein located in the central nervous system myelin paranodal loop membrane. J Biol Chem. 2008;283:20830–20840. doi: 10.1074/jbc.M801314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aruga J., Yoshikawa F., Nozaki Y., Sakaki Y., Toyoda A., Furuichi T. An oligodendrocyte enhancer in a phylogenetically conserved intron region of the mammalian myelin gene Opalin. J Neurochem. 2007;102:1533–1547. doi: 10.1111/j.1471-4159.2007.04583.x. [DOI] [PubMed] [Google Scholar]
- 11.Gow A., Friedrich V.L., Jr., Lazzarini R.A. Myelin basic protein gene contains separate enhancers for oligodendrocyte and Schwann cell expression. J Cell Biol. 1992;119:605–616. doi: 10.1083/jcb.119.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farhadi H.F., Lepage P., Forghani R., Friedman H.C., Orfali W., Jasmin L., Miller W., Hudson T.J., Peterson A.C. A combinatorial network of evolutionarily conserved myelin basic protein regulatory sequences confers distinct glial-specific phenotypes. J Neurosci. 2003;23:10214–10223. doi: 10.1523/JNEUROSCI.23-32-10214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hippenmeyer S., Vrieseling E., Sigrist M., Portmann T., Laengle C., Ladle D.R., Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prayoonwiwat N., Rodriguez M. The potential for oligodendrocyte proliferation during demyelinating disease. J Neuropathol Exp Neurol. 1993;52:55–63. doi: 10.1097/00005072-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Blakemore W.F., Patterson R.C. Suppression of remyelination in the CNS by X-irradiation. Acta Neuropathol. 1978;42:105–113. doi: 10.1007/BF00690975. [DOI] [PubMed] [Google Scholar]
- 16.Dawson M.R., Polito A., Levine J.M., Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 17.Moyon S., Dubessy A.L., Aigrot M.S., Trotter M., Huang J.K., Dauphinot L., Potier M.C., Kerninon C., Melik Parsadaniantz S., Franklin R.J.M., Lubetzki C. Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J Neurosci. 2015;35:4–20. doi: 10.1523/JNEUROSCI.0849-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin R.J.M., Gallo V. The translational biology of remyelination: past, present, and future. Glia. 2014;62:1905–1915. doi: 10.1002/glia.22622. [DOI] [PubMed] [Google Scholar]
- 19.Arenella L.S., Herndon R.M. Mature oligodendrocytes. Division following experimental demyelination in adult animals. Arch Neurol. 1984;41:1162–1165. doi: 10.1001/archneur.1984.04050220060015. [DOI] [PubMed] [Google Scholar]