Abstract
Leptin regulates food intake and energy expenditure (EE) and is produced in adipocytes, the pituitary, and several other tissues. Animals that are leptin or leptin receptor deficient have major metabolic complications, including obesity. This study tests the hypothesis that the pituitary somatotrope may contribute a source of leptin that maintains some of these metabolic functions. We created 2 different tissue-specific leptin knockout animals: a Somatotrope-Lep-null model and an Adipocyte-Lep-null model. Metabolic analysis of both models, along with a global deletion model, was performed. The Somatotrope-Lep-null animals had fewer somatotropes, and females had a 76% decrease in serum prolactin. During the dark (feeding) phase, females had a 35% increase in ambulation coupled with a 4% increase in EE. Mutants showed no change in food intake or weight gain and EE was unchanged in males. During the light (sleep) phase, Somatotrope-Lep-null mutant males had lower EE and females continued to have higher EE. The respiratory quotients (RQs) of mutants and littermate controls were decreased in males and increased in females; all were within the range that indicates predominant carbohydrate burning. The massively obese Adipocyte-Lep-null animals, however, had significant increases in food intake, sleep, and increased EE, with decreased activity. Changes in RQ were sexually dimorphic, with female mutants having higher RQ and males having decreased RQ. We conclude that both adipocyte and somatotrope leptin contribute to the metabolic homeostasis of the mouse, and that extraadipocyte sources of leptin cannot overcome the major metabolic challenges seen in these animals.
Leptin is a cytokine that is produced by white fat cells, as well as the brain, skeletal muscle, the placenta, and subsets of specific pituitary cells (among others) (1–5). Leptin receptors in the hypothalamus transmit signals for appetite suppression and energy regulation, as leptin is produced in proportion to white fat cells (6–10). The leptin signal in the hypothalamus suppresses neuropeptide Y release and increases α-melanocyte-stimulating hormone production, thus leading to decreased food intake (11–14).
Mice with a single point mutation in the leptin gene (ob/ob mice) have no detectable circulating leptin, are obese by puberty, have significant hormonal disturbances, and have significantly impaired fertility (15–20). Ob/ob females and most ob/ob males cannot reproduce, but this sterility can be corrected upon leptin administration or diet restriction (21, 22).
In view of the significant metabolic consequences of the loss of leptin or leptin signals, it is surprising that there are only a few studies that detail the metabolic changes with comprehensive metabolic cage studies. A study by Badman et al showed that male ob/ob mice have significantly higher daily caloric intake compared with lean controls, with a lower core body temperature (23). The same study and Breslow et al found a decrease in oxygen intake in ob/ob mice with no apparent differences in heat production or respiratory quotient (RQ) (24), in agreement with Hwa et al (25). Male 6-week-old ob/ob mice sleep more than lean controls, an effect seen in the dark (but not light) phase (26). This increase in overall sleep time is in spite of increased arousals from sleep and decreased duration of sleep episodes. In the same study, ob/ob males also had increased wheel-running activity in the light phase as compared with controls, with significantly decreased wheel-running activity in the dark phase. However, Morton et al showed overall decreased activity in young ob/ob mice, including decreased wheel running (27). Although the studies did not agree about the direction or extent of the changes, overall, the point mutation in leptin has serious metabolic consequences in the mouse. Some of the deficits like activity, energy expenditure (EE), and oxygen consumption can be corrected by leptin treatment (24, 27).
Our lab has shown that somatotropes partner with leptin in optimizing metabolic activity. We reported recently that leptin signaling through the long-form leptin receptor (LEPRb) is crucial to the maintenance and function of the GH proteins in pituitary somatotropes (28, 29). When somatotropes lack the signaling portion of LEPRb, mice display adult-onset obesity and GH deficiency. When studied in metabolic cages, these mice lacking LEPRb signaling capability on somatotropes had increased RQs and decreased EE (30). Activity levels were sexually dimorphic, as female mutants were less active than controls, whereas male mutants had significantly higher activity levels compared with controls. The results from these studies indicate a strong role for the leptin signal to somatotropes in the regulation of the pituitary as well as overall metabolic function. Our most recent studies of a more complete deletion of the leptin signal in somatotropes, achieved by deleting all leptin receptor isoforms, showed mutant mice with more severe GH deficiency, increased abdominal adiposity, and decreased EE with increased RQ (29).
The links between leptin, GH, and metabolism are well established. During fasting, a state in which leptin levels are low, circulating GH values are increased in humans and mice (31, 32). This makes sense, because GH acts directly on adipocytes to cause lipolysis and decrease leptin production, which would break down fat for nutrients in a state of fasting (33, 34). Fasting also increases Ghrh mRNA levels in the hypothalamus and Ghrhr mRNA in the pituitary (31). In the obese state, GH levels are decreased and do not respond to secretion stimuli (31, 35). Adult-onset somatotrope-specific GH deficiency (in which somatotropes are ablated) causes preferential carbohydrate burning in the absence of major alterations in food intake or activity (36).
We previously reported that somatotropes are the most abundant source of pituitary leptin (37, 38). The strong correlation between leptin and metabolism, as well as leptin's effects on somatotropes, led us to question whether the somatotrope source of leptin is important for the overall metabolic functioning of the mouse. Although our recent studies showed that the adipocyte is the source of circulating leptin in the mouse (39), we hypothesized that somatotrope leptin might also influence (directly or indirectly) activity levels, EE, and sleep patterns of mice.
Therefore, we developed the first floxed leptin mouse model and used Cre-LoxP technology to ablate leptin in cells expressing cre recombinase under the control of the growth hormone-releasing hormone receptor (rGHRHr-cre) (40), which are predominantly somatotropes. After evaluating pituitary hormone function, we then used a metabolic cage system to compare the metabolic activity of somatotrope leptin knockout animals with mice lacking adipocyte leptin (previously described by our laboratory) (39). In this study, we provide the first comprehensive view of metabolic activity in the massively obese Adipocyte- or Global-Lep-null mice, which shows the full impact of the total loss of leptin. In addition, we show that the loss of somatotrope leptin has consequences resulting in lower pituitary GH levels, serum prolactin, and changes in metabolic activity, which are sexually dimorphic.
Materials and Methods
Experimental animals
All animal care protocols have been approved by the Institutional Animal Care and Use Committee. All animals were weaned at 21 days of age. Animals were housed no more than 5 animals/cage at 27°C on a 14-hour light, 10-hour dark cycle. Animals were fed a diet of 22% crude protein, 5% crude fat, and 4.5% crude fiber (Teklad 8640; Harlan).
To create a pituitary-specific, somatotrope-targeted leptin knockout animal, we used our floxed leptin animal model (Lepfl/fl; FVB/NTac; Taconic) (39) and a mouse model in which Cre-recombinase is produced under the control of the rat GHRH receptor promoter (Cre-GHRHr; FVB(Cg)-Tg(Ghrhr-cre)3242Lsk/J; Lawrence Kirschner) (40). Animals from this line have Cre-recombinase activity in at least 40% of pituitary cells, most of which occurs in somatotropes and lactotropes. Another report using the same promoter has also shown activity in a small percentage of thyrotropes (41). The specificity of this promoter is limited to the pituitary with the exception of limited expression in hair follicles of the skin. Because extraadipocyte leptin does not contribute to the circulating pool of leptin, the expression of Cre-recombinase in the hair follicles does not complicate our study (39). We first crossed Lepfl/fl females with males carrying one allele of Cre-GHRHr (Cre-GHRHrTg/0). We then backcrossed Cre-GHRHrTg/0 LepΔ/wt males with Lepfl/fl females to obtain our Cre-GHRHrTg/0 LepΔ/Δ deletion mutants (referred to in this report as Somatotrope-Lep-null) and Lepfl/fl littermate controls. To avoid potential complications during pregnancy from a deletion mutant female, we passed the Cre-recombinase only through the males. Adipocyte-specific and global leptin knockout mice were created as previously described (39). Briefly, to obtain adipocyte-specific leptin knockout animals, we bred Lepfl/fl females with Adipoq-CreTg/0 LepΔ/wt males. Homozygous deletion animals could not breed, because they were not fertile. These deletion mutants, called Adipocyte-Lep-null, do not produce leptin in white fat cells (42). The third model is a whole-body leptin knockout model, used to validate the deletion of exon 3 and act as a positive control for the previous 2 models. This model, called Global-Lep-null, does not allow for any leptin production in any tissues (43). These animals were created by breeding Lepfl/fl females with EIIa-CreTg/0 LepΔ/wt males, as neither homozygous deletion males nor females were fertile.
Phenotypic characterization of the Somatotrope-Lep-null mouse pituitary
Male and female control (Leptinfl/fl) and Somatotrope-Lep-null animals were weighed weekly from weaning until 6 months of age. To determine local consequences of deleting somatotrope leptin, pituitaries and sera were collected after decapitation for pituitary cell dispersion, immunocytochemistry, and serum hormone assays as described previously. The pituitary phenotypes of the Adipocyte-Lep-null and Global-Lep-null animals have been previously described (39) (for antibodies, please see Table 1).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| Leptin | Amino acids 22–40 | Antileptin antibody produced in rabbit | Sigma, L3410 | Rabbit | 1:10 000 |
| GH | Rat GH | A. F. Parlow, Hormone Distribution Program | Rabbit | 1:200 000 |
Comprehensive lab animal monitoring system
For each individual metabolic cage experiment, up to 4 control and 4 deletion mutant animals from each line were tested. Six-month-old male and female animals from each line were used, with at least 5 animals analyzed for each group (for example, at least 10 Somatotrope-Lep-null female mutants and 9 controls were analyzed). Animals were weighed before and after each experiment, and those losing more than 10% body weight during the testing period were excluded from the analysis. Each experiment lasted 72 hours: 24 hours of acclimation and 48 hours of recording.
The CLAMS (Columbus Instruments) was used to determine RQ, EE, activity levels, sleep measurements, and food intake as previously described (30). Briefly, all animals were fed a finely ground version of their regular food, accessible by a spring-loaded feeder in the bottom of the cage. The feeders sat on scales, which communicated information about feeding amounts and times to the computer. Food and water were available ad libitum, and the metabolic cage system was in the same room where the mice were housed (27°C, 14 h light/10 h dark). Activity was measured with infrared beams in the x-axis (to measure grooming and ambulation) as well as the z-axis (to measure rearing and jumping). Every beam break is logged as an activity “count,” and successive counts in the x-axis are considered ambulatory. Sleep calculations were made by the system based on inactivity data. Oxygen and carbon dioxide sensors measured O2 and CO2 levels going into the cage as well as the levels coming out of each cage, based on air samples taken at each experimental interval. This allows the system to continuously calculate the RQ (VCO2/VO2) and EE of each animal. RQ values that approach or reach 1.0 indicate that carbohydrates are being used as the main source of energy, whereas RQ values closer to 0.7 indicate that more fats are being used for fuel. Values shown for energy expenditure, O2, CO2, and RQ have been normalized to lean body mass according to Klieber's law (44, 45) as previously described (29, 30). Sera from Somatotrope-Lep-null males and females were analyzed for triglyceride content using a calorimetric assay (Cayman Chemical).
Statistical analysis
Changes in weight, serum hormone levels, and differences in cell numbers and mRNA transcript levels were analyzed using Student's t test. Individual metabolic values were calculated by the CLAMS software output program CLAX (Columbus Instruments) and analyzed using Prism software (GraphPad, Inc). Most of the metabolic parameters were analyzed using Student's t test to determine differences between control and deletion animals within sexes. Percent relative cumulative frequency was analyzed using Mann-Whitney t test with D'Agostino and Pearson omnibus normality test as used in our previous report (30). The EE analysis of covariance (ANCOVA) was provided by the National Institute of Diabetes and Digestive and Kidney Diseases Mouse Metabolic Phenotyping Centers using their Energy Expenditure Analysis page (http://www.mmpc.org/shared/regression.aspx).
Results
Characterization of the Somatotrope-Lep-null mouse
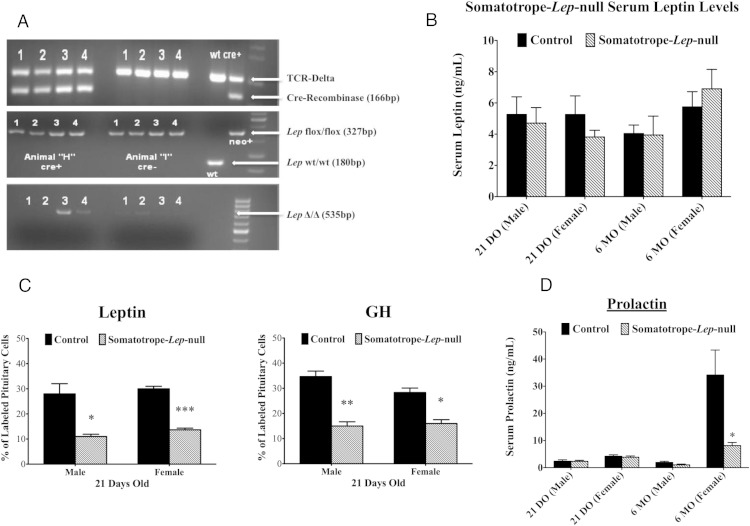
Organ genotyping showed that Cre-recombinase activity in the Somatotrope-Lep-null mutant was found in the pituitary (Figure 1A) and, as reported previously for this rGHRHr-cre-bearing model, the skin (40). Serum levels of leptin, GH, and all other pituitary hormones were normal (Figure 1 and Supplemental Figure 1) in males, but serum prolactin levels were significantly reduced by 76% in adult Somatotrope-Lep-null females (Figure 1D). The Somatotrope-Lep-null animals had significantly lower percentages of immunolabeled GH cells (by 43.5–56.7%) (Figure 1C). Serum triglycerides were not different between Somatotrope-Lep-null mutants and controls for either males (control: 135.5 ± 17.26 mg/dL, n = 8; deletion: 150.0 ± 23.95 mg/dL, n = 8) or females (control: 100.6 ± 14.56 mg/dL, n = 8; deletion: 101.1 ± 12.75 mg/dL, n = 8).
Figure 1.
Characterization of the Somatotrope-Lep-null line. A, Sample organ genotyping from a deletion mutant (left) and a control (right) male of the Somatotrope-Lep-null line. Organs numbered 1–4 are: abdominal fat (1), skeletal muscle (2), pituitary (3), and skin (4). Deleted bands are seen only in the pituitary and skin (third gel). Presence of Cre-recombinase is detected in the first gel, and the second gel shows that all organs from both animals have 2 alleles of floxed Lep (with no wild-type Lep). B, Chart of serum leptin levels in 21 day-old and 6-month-old male and female controls and Somatotrope-Lep-null mutants. C, Somatotrope-Lep-null control and mutant pituitary cells were labeled in weaning-aged animals for leptin (left) and GH (right). D, Serum prolactin levels are shown for male and female Somatotrope-Lep-null control and mutant animals at 21 days and 6 months of age. Student's t tests were used to determine significant differences within sexes and age groups with significance set at P < .05.
Food intake and body weight
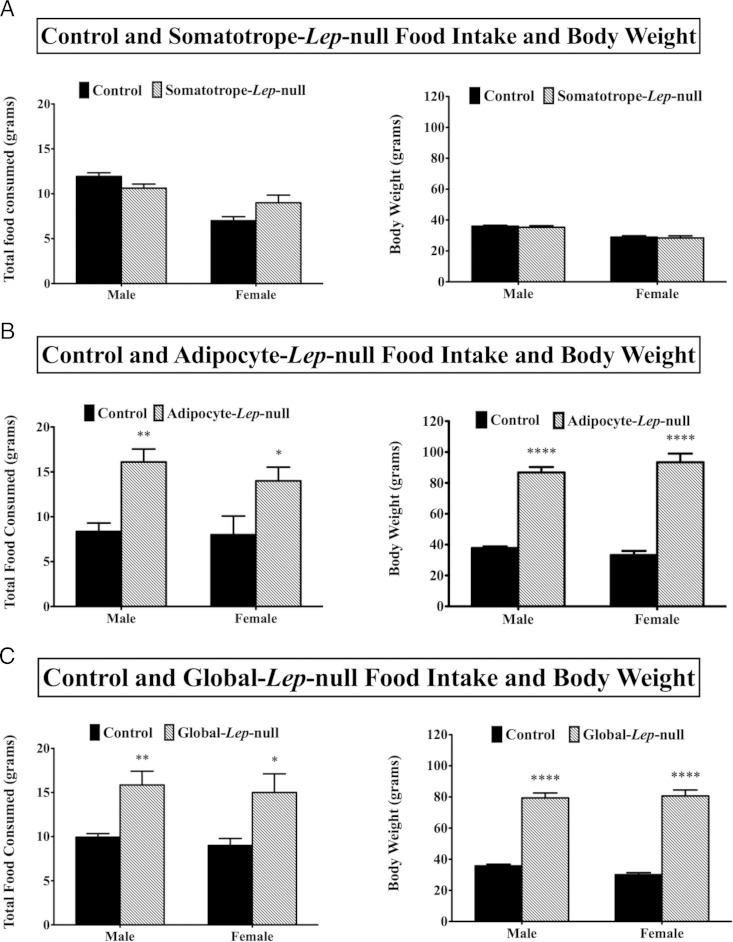
Neither male nor female Somatotrope-Lep-null mutants had any significant change in food intake or body weight (Figure 2A). As expected from their undetectable serum leptin levels (39), mutant males and females from the Adipocyte-Lep-null line weighed at least twice as much as controls and ate 39.5%–48.1% more food throughout the experiment (Figure 2B). Similar results were seen in the Global-Lep-null line (Figure 2C).
Figure 2.
Food intake and body weights. Total amount of food consumed during the 48-hour experimental period is shown, along with weights at the beginning of the experiments, for the Somatotrope-Lep-null line (A), the Adipocyte-Lep-null line (B), and the Global-Lep-null line (C). Student's t tests were used to determine differences between groups within sexes. Significance was set at P < .05.
Sleep and activity
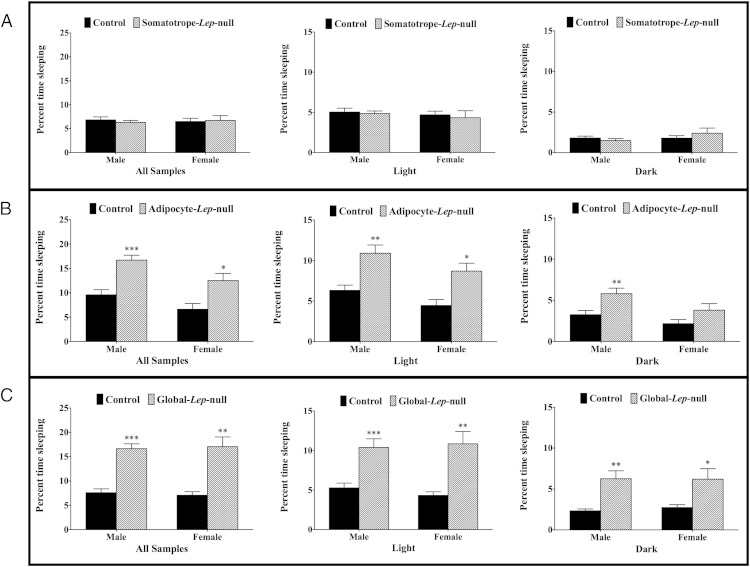
The total sleep measurements in Figure 3 are a summation of 240 seconds of inactivity throughout the entire experiment, reflecting the percentage of total time in the metabolic cage spent sleeping. The well-established CLAMS method of evaluating sleep via inactivity as measured by infrared beam breaks has been compared with electroencephalography and video monitoring and is highly comparable with both methods (46). Neither male nor female mutants from the Somatotrope-Lep–null line had any significant differences in total percent time spent sleeping (Figure 3A). Adipocyte-Lep-null males (control 9.6 ± 1.0%, deletion 16.7 ± 1.3%; P < .0005) and females (control 7.0 ± 1.0%, deletion 13.8 ± 2.6%; P < .05) spent more of the experimental period sleeping as compared with controls (Figure 3B). Male mutants slept more than controls in both the light (P < .005) and dark (P < .05) phases, whereas female mutants slept significantly more than controls in the light (P < .05) but not dark (P = .13) phase (Figure 3B). Much like the Adipocyte-Lep-null mutants, the Global-Lep–null mutants had significant differences in percent time spent sleeping during the light and dark phases, as well as overall (Figure 3C).
Figure 3.
Total time spent sleeping for all lines. This figure displays the percentage of experimental time spent sleeping by Somatotrope-Lep-null animals (A), Adipocyte-Lep-null animals (B), and Global-Lep-null animals (C). Analysis was done for the entire experimental period (first graph in each series) as well as light and dark phases (second and third graphs in each series). Statistical analysis was performed using Student's t tests, with significance at P < .05.
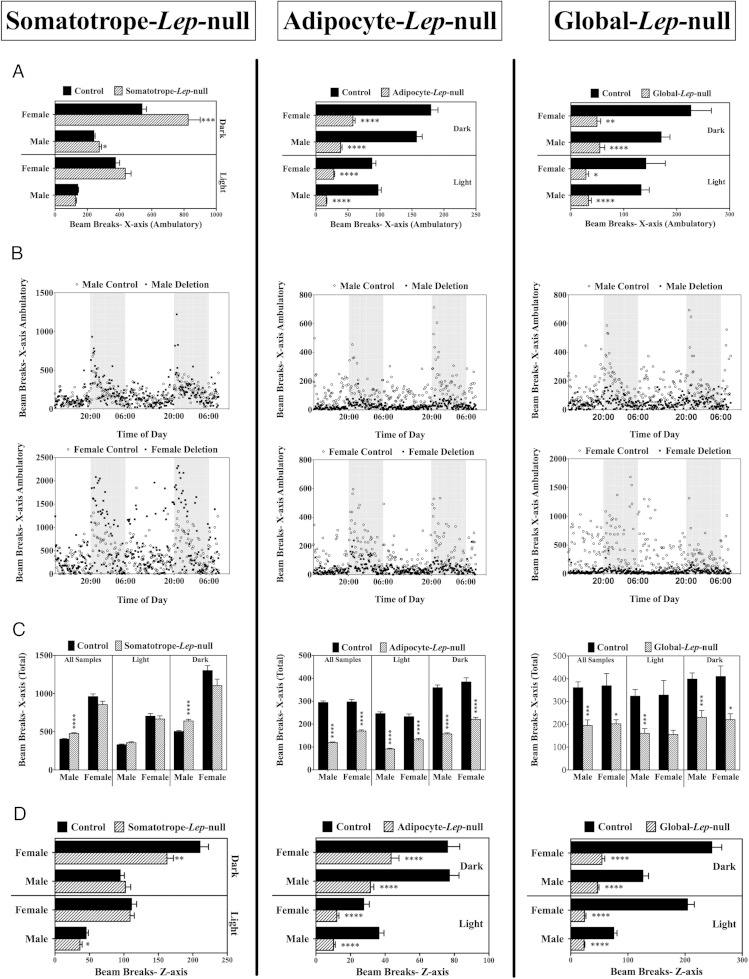
Activity measured in the x-axis is interpreted as walking (ambulation) and/or grooming. During the dark (feeding) phase when the mice are normally more active, Somatotrope-Lep-null mice showed higher levels of walking, especially the females, which showed a significant 34.7% increase in walking/ambulation (P < .0001) (Figure 4A), although total activity (including grooming) was not greater (Figure 4C). Males also showed slightly higher ambulation (P < .0001) (Figure 4A) and total activity (P < .0001) (Figure 4C) during the dark phase. Neither sex showed significant increased activity during the light (sleep) phase, which correlates with their normal sleep levels. However, both male and female Somatotrope-Lep-null mutants had decreased z-axis activity (Figure 4D), which accounts for rearing and jumping movements. In males, this was significant (20% lower) in the light phase (P < .05), and in females, this was significant (22.8% lower) only in the dark phase (P < .005).
Figure 4.
Activity levels, all lines. Activity levels are displayed for all lines, from left to right: Somatotrope-Lep-null, Adipocyte-Lep-null, and Global-Lep-null control and mutant males and females. A, C, and D, Each bar is an average of the activity levels of at least 5 animals (per sex, per genotype), during either the light or dark phases. Differences between genotypes (within the sexes) were determined by Student's t tests, with significance set at P < .05. A, Graphs show the total number of beam breaks along the x-axis, which are representative of walking (detected by consecutive beam breaks). B, X-ambulatory activity over the experimental period, with the dark phases shaded in gray. The top line of graphs shows male data, and the bottom line shows female data. Each point represents the average value at that time for at least 5 animals. C, Average values for total x-axis beam breaks (ambulatory as well as other x-axis activity events) are shown in light and dark phases, as well as average for entire experimental period. D, Beam breaks in the z-axis indicate vertical movements, like rearing. Average z-axis values are shown for males and females in dark and light phases.
A significant reduction in activity was seen in the obese, 6-month-old Adipocyte-Lep-null and Global-Lep-null mutant males and females during both light and dark phases (Figure 4). In contrast, somatotrope-Lep-null mice showed 200- to 400-fold higher levels of beam breaks compared with the other 2 deletion mutant groups.
Respiratory quotient
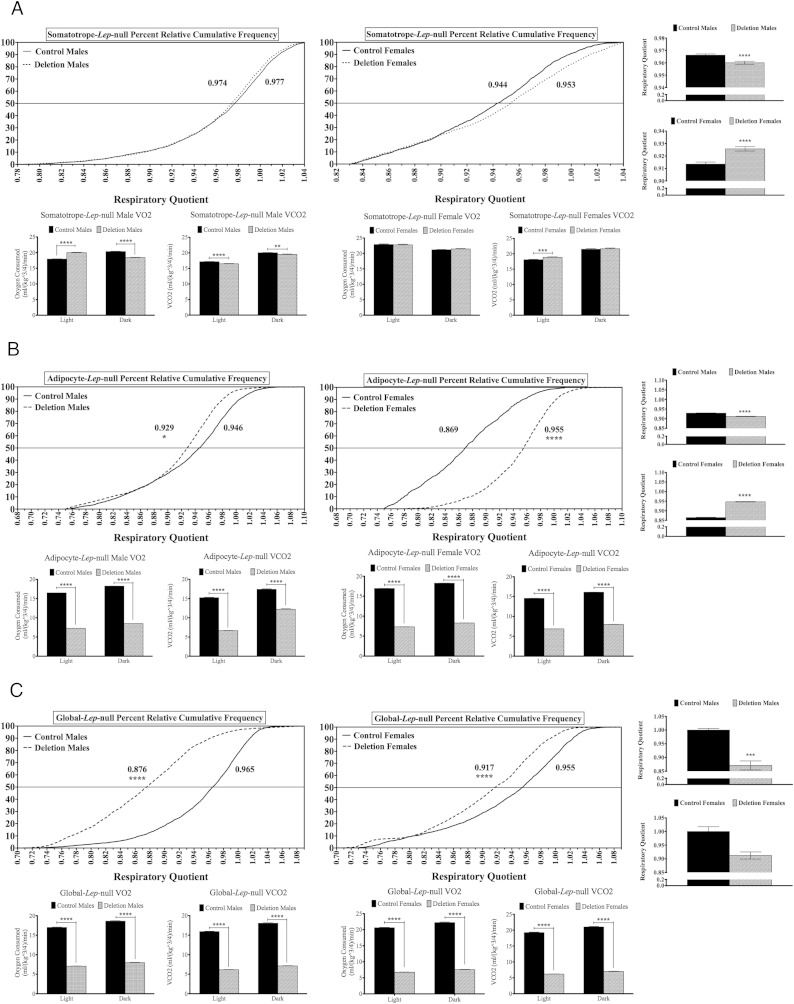
The RQ was measured based on gas values (VCO2/VO2) and normalized to lean body mass. A number near 0.7 reflects fat oxidation and a number near 1 reflects primarily carbohydrate oxidation. The RQ values were plotted and expressed as percent relative cumulative frequency distributions. When the value at the 50th percentile was compared, for male or female Somatotrope-Lep-null mutants, there were no significant differences (Figure 5A). Averages of RQ values indicated that male Somatotrope-Lep-null males had significantly decreased RQ (P < .0005) (with significant changes in both O2 and CO2), and female mutants had significantly increased RQ (P < .0001) (which was the result of increased CO2 in the light phase). However, because values for both controls and mutants were near 1, the predominance of carbohydrate oxidation over fat oxidation exists in both mutants and controls.
Figure 5.
Percent relative cumulative frequency analysis of RQ. The PRCF analyses for males (left) and females (right) of the Somatotrope-Lep-null line (A), the Adipocyte-Lep-null line (B), and the Global-Lep–null line (C) are shown, with controls from each line represented by solid lines and mutants represented by dashed lines. Mann-Whitney tests were used to determine significant differences. On the far right of each set of figures is the average RQ for males (top) and females (bottom). A breakdown of VO2 and VCO2 for each sex during the light and dark phases is displayed below the PRCF graphs. For the averaged RQ, VO2, and VCO2 values, differences were determined using Student's t tests, with significance set at P < .05.
Among the Adipocyte-Lep-null females, control RQ values indicated that these mice oxidized a mixture fat and carbohydrates; however, the mutants lacking leptin in adipocytes showed a striking significant shift to carbohydrate burning (P < .0001). In contrast, the mutant and control males both show the predominance of carbohydrate burning in their RQ values with a slight, but significant (P < .05), decrease in the mutants (Figure 5B). The mutant and control male values were similar to those from the Somatotrope-Lep-null mutants and their littermate controls as well as the Global-Lep-null female mutants and controls. Control males from the Global-Lep-null group showed a more normal pattern of mixed fat and carbohydrate burning and the mutant group showed a striking significant shift to RQ values that indicate carbohydrate burning (P < .0005) (Figure 5C). Both Adipocyte-Lep-null and Global-Lep-null males and females have significant decreases in O2 and CO2 (Figure 5, B and C).
Energy expenditure
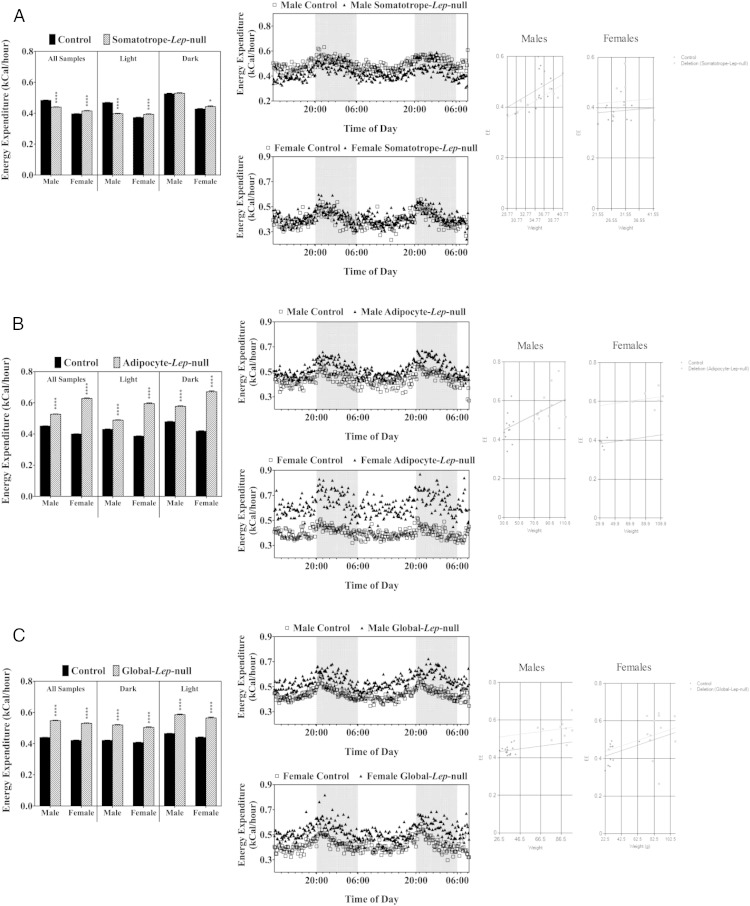
In the Somatotrope–Lep-null mice, during the dark (active) phase, there were no changes in EE in males, and only a 4% increase in the more active females (Figure 6A). During the light phase, males had a slight reduction in EE (P < .0001) and females continued to have a slight, but significant increase (P < .0001). In contrast, both groups of obese mutants had significantly increased EE (P < .0001) during both light and dark phases (Figure 6, B and C). The values, however, were similar to those reported by Morton et al, who studied ob/ob mice and the impact of leptin on EE (27). To determine whether these gross differences in EE are due to the loss of adipocyte or global leptin, or whether they are due to the major differences in body weights between controls and mutants within these lines, we performed an ANCOVA analysis for each group (47). Neither Adipocyte-Lep-null nor Global-Lep-null EE differences were found to be due to the increased fat mass seen in these mutants (Figure 6). As expected, Somatotrope-Lep-null males and females also had no differences due to weight.
Figure 6.
Energy expenditure. A, The bar graph shows a breakdown of average EE values throughout the different phases for the Somatotrope-Lep-null males and females. The middle panels (males, top and females, bottom) show EE throughout the experimental period with dark phases (8 pm to 6 am) indicated by shading. The figures on the far right show ANCOVA analysis of male and female energy expenditure data, with weight as the covariant. The same analyses were performed for the Adipocyte-Lep-null line (B) and the Global-Lep-null line (C). Student's t tests were performed to determine differences with significance set at P < .05.
Discussion
The role of leptin as a satiety signal and a regulator of EE is well known (7, 48–50). Increased leptin levels in obese individuals may lead to leptin resistance, whereas decreased levels in starved individuals impair reproductive function (49, 51–53). Our laboratory has shown that leptin is also an important regulator of the GH-producing cells of the pituitary (somatotropes). Using a mouse model in which the signaling portion of the leptin receptor is deleted in somatotropes, we have previously shown that the leptin signal is necessary to maintain the somatotrope population (28). Without this signal, the animals have decreased GH secretion and display adult-onset obesity. Preobese animals from this line have increased RQs, which, in combination with low EE, predispose the animals to the fat accumulation seen in older animals (30). A more complete model, in which all isoforms of the leptin receptor were deleted in somatotropes, has been recently characterized by our lab (29). These animals are severely GH deficient, and both males and females have decreased EE and increased RQ, as well as increased abdominal adiposity and adipose density.
In this report, we tested the hypothesis that the somatotropes may contribute to the leptin signal important for maintaining metabolic status in the mouse. By creating 2 different leptin knockout mouse models, we provided a comprehensive view of the metabolic activity of mice in which either the somatotrope source or the adipocyte source of leptin is ablated. In addition, we compared both models with a global knockout using the same floxed leptin model. It is critical to note that the deletion of Lep exon 3 ablates most the Lep gene, thus making this deletion more severe than the point mutation seen in the ob/ob mouse line (54).
Deletion of Lep in the pituitary: Prolactin deficiency
Reports from the development of this Ghrhr-cre bearing model indicate that it can also be expressed by a small subset of thyrotropes and lactotropes. This would be expected because GHRH-R binding sites are expressed with prolactin in 10%–15% of pituitary cells (depending on the physiological state) and with TSH in 3% of pituitary cells (54). The overlap in expression of TSH, GH, and prolactin in the GHRH-R target cells suggests that the TSH and prolactin are likely being expressed by multihormonal somatotropes.
By ablating leptin selectively in cells bearing rGHRHr cre, we have eliminated leptin from the predominant leptin producer in the pituitary (during most physiological states). However, we recognize that there is considerable plasticity in leptin production by different pituitary cell types, with significant increases in corticotropes after the stress of fasting (55) and the predominant production of leptin by gonadotropes in pregnant and lactating females (which represent 60% leptin-bearing cells) (37). Thus, it should be noted that this selective knockout was not expected to remove all pituitary sources of leptin. Our counts of leptin-bearing cells in mutant male or diestrous female mice showed a 70% reduction in pituitary cells containing leptin protein (from 36% in controls to 11% in male or female mutants). Further studies of these other physiological states are needed to determine whether the deletion in somatotropes affects leptin production by somatogonadotropes.
The GH cells in the Somatotrope-Lep-null mice are reduced at weaning; however, this does not cause reductions in serum GH levels. Neither male nor female mutants from this line have any significant changes in body weight at the time of experimentation, nor do they eat significantly more or less food than controls. This is not surprising, given the fact that the serum leptin levels in these mice were normal. We also found in that deletion of leptin in the GHRH-R-expressing cells does not lead to major changes in other circulating pituitary hormones, with the exception of a significant 76% decrease in circulating prolactin. This could reflect the fact that leptin was ablated in the somatolactotropes, or that somatotrope leptin is important for normal secretion of prolactin. Prolactin is also known to be low in ob/ob mice, and we have also shown decreased prolactin in our Adipocyte-Lep-null females (19, 39). This suggests that both circulating and pituitary leptin regulate pituitary prolactin release, although these sources of leptin most likely act at multiple points of the hypothalamic-pituitary axis.
The loss in prolactin levels stimulated a breeding study to determine whether female deletion mutants showed compromised fertility (Supplemental Figure 2). After several litters in 4 different breeding cages, it was clear that the loss of leptin in GHRH-R-expressing cells and the consequential significant reduction in prolactin did not alter the reproductive competence of the mutant females based on litter size and length of time between litters. Competent nutrition (lactation) was assumed based on normal weanling weights, compared with offspring of male deletion mutants, as well as visual inspection for a milk spot in the early postnatal days. Leptin is known to increase prolactin secretion in vivo and has been shown to specifically up-regulate prolactin the lactating mammary gland (56, 57). Thus, local sources of prolactin, perhaps stimulated by the normal serum leptin levels in our Somatotrope-Lep-null mice, may support reproduction and lactation in the face of decreased serum prolactin. Future studies of the prolactin cell population in our Somatotrope-Lep–null animals will determine whether the low serum prolactin is due to defects in stores or responses to secretagogues.
Metabolic activity of Somatotrope-Lep-null mutants
During the dark (feeding) phase, the higher ambulatory activity of the female mutant Somatotrope-Lep-null mice was accompanied by a slight 3.6% increase in EE. Males were also slightly more active. The increased activity of mutants did not affect food intake, weight gain, or mutant male EE. During the light (sleep) phase, mutants showed no major changes in ambulatory movements or sleep, although mutant males had 15% lower EE and females continued to have slightly (5.4%) higher EE. The RQs of mutants and littermate controls were not different and suggested that both groups oxidized carbohydrates preferentially. Collectively, their normal serum GH, serum leptin, and normal food intake prevented their weight gain, indicating that somatotrope leptin plays little role in regulating these aspects of metabolism.
However, the Somatotrope-Lep-null females have significantly decreased serum prolactin. Prolactin receptors have been found in the hypothalamus, and prolactin is known to play a myriad of roles in metabolism (58). Prolactin receptors have been identified on adipocytes in mice, suggesting that the hormone acts directly on adipose tissue to affect metabolism (59). Male mice lacking prolactin receptors have increased EE and changes in beige adipose tissue, rendering them resistant to the weight-gain caused by a high-fat diet (60). However, the prolactin knockout mouse has very minor metabolic effects (61). Thus, the specifics of prolactin's role in the control of food intake and EE are still to be refined. It is possible that decreased prolactin levels in our Somatotrope-Lep-null animals could be playing a role in the increased activity seen in the females during the dark phase, which could relate to food-seeking behavior.
Deletion of Lep in adipocytes
Detailed metabolic analyses of the global leptin knockout, the ob/ob mouse, are found in only a few publications, each of which focuses on one or 2 aspects of the metabolic response. Therefore, this is the first report of a comprehensive monitoring of a massively obese, adult model, studying both males and females that have nearly all leptin deleted in adipocytes or in all cells. As expected from their undetectable serum leptin (39), Adipocyte-Lep-null animals had significant increases in food intake (39%–48%) and sleep (43%–47%) coupled with decreased activity of all types (43%–79%). They were massively obese with a 56%–64% increase in body weight. The Global-Lep-null mice were similar to Adipocyte-Lep-null mice in their 37%–40% increased food intake, 46%–59% increased sleep, 55%–63% body weight gain, and 45%–71% decreased activity. It is interesting to note that the activity levels seen in the Adipocyte-Lep-null line were similar to the low numbers seen for ob/ob mice that were much younger and half as obese (27). With regard to feeding and weight, Adipocyte-Lep-null animals appeared to be very similar to our positive control Global-Lep-null animals. Both male and female mutants had increased food consumption and weighed significantly more than controls. These animals also spent a greater percentage of the experimental period sleeping, with the exception of females in the dark phase. Activity levels, both ambulatory/grooming and rearing/jumping, were significantly decreased in males and females during light and dark phases. Collectively, these similarities indicate that adipocyte leptin is the most important source of leptin for regulation of metabolic function. Other sources of leptin, which are presumably intact in the Adipocyte-Lep-null mice, play little role in leptin's regulation of sleep, appetite, and body composition.
An unexpected finding was the fact that both Global- and Adipocyte-Lep-null deletion mutants had a striking 14%–36% increase in EE compared with controls. However, Morton et al had examined ob/ob mice for EE with the use of the same type of equipment employed in this study, including the formula for EE (27). The values for the ob/ob mice were in the same range as those seen in the Adipocyte-Lep-null or Global-Lep-null mutants. Furthermore, Morton et al reported that leptin stimulated a further increase in EE in a dose-dependent manner. No data from wild type mice were given in that study of C57Bl6 mice. We can conclude that our littermate control FVB mice bearing only 2 alleles of floxed leptin simply have lower EE than the littermate deletion mutants and that the latter is similar to the other obese model (ob/ob).
Finally, the RQ values for these obese mutants were similar to those reported for ob/ob mice. The male and female Adipocyte-Lep-null mutants and the male littermate controls was more than 0.92, which indicated predominant oxidation of carbohydrates rather than fat. Only the control females in this line showed an RQ indicative of more fat burning. Similarly, RQ values of Global-Lep-null females and males (and control females) were also in the range for carbohydrate burning. Only the control males showed a tendency for more fat burning. An analysis of RQ values in ob/ob mice from 2 separate studies showed that they were in the carbohydrate burning range. In one of the studies, the controls showed similar RQ values (23). The other study showed that leptin treatment reduced RQ values to fat-burning ranges (25). It is unclear why control mice have higher RQ values. This tendency to burn more carbohydrates did not lead to obesity in the controls of either of the Lep-null lines.
These few differences in the Adipocyte-Lep-null and Global-Lep-null mice are difficult to explain. The Adipocyte-Lep-null animals have no circulating leptin (39) and thus are assumed to only maintain local leptin production. We found that the pituitaries of these males actually produce greater amounts of leptin, but clearly our comparative studies show that this source of leptin does not appear to be secreted, as serum levels remain undetectable. The overall phenotype of this line indicates that somatotrope leptin does not support appetite control or other general metabolic functions of this hormone. Thus, the combination of a lack of circulating leptin with pituitary dysfunction leads to the obesity phenotype seen in these animals.
Summary and conclusions
We produced 2 different tissue-specific leptin knockout animal models to compare the metabolic activity of the somatotrope source of leptin with the adipocyte source as 2 potential functional sources of leptin. We hypothesized that, in the absence of adipocyte leptin, local sources of leptin may act to retain some metabolic control in the context of obesity. We also hypothesized that the deletion of somatotrope leptin in particular may have metabolic consequences because of its involvement with metabolic functions. The Somatotrope-Lep-null animals did not have major metabolic disturbances, other than slight changes in activity and EE that could relate to food-seeking behavior. However, these changes could have been the result of the local GH deficiency (low GH stores) and/or the low serum prolactin levels. Although the changes indicate some role for local leptin in regulating GH and/or prolactin expression, they do not translate to gross variations in metabolism. In contrast, the Adipocyte-Lep-null animals had significant metabolic disturbances, indicating that the pituitary source of leptin is not able to rescue the animals from the severe consequences of deleting circulating leptin. Most of the changes are similar to those seen in the ob/ob mouse and reflect the vital role that adipocyte leptin plays in maintaining metabolic balance and nutritional status. This study begins to clarify the role of the pituitary source of leptin as maintaining local regulatory control for 2 important hormones, and it is significant that the loss in somatotrope leptin leads to a dramatic reduction in serum prolactin, which is believed to also play a role in metabolic homeostasis. It is clear that extraadipocyte sources of leptin, although potentially vital in a paracrine or autocrine sense, cannot overcome the major metabolic challenges of deleting circulating leptin.
Acknowledgments
We would like to thank Drs Lawrence Kirschner and Chris Bradfield for the generous gift of the rHGRHr-cre mice.
This work was supported by the National Institutes of Health Grant R03 HD059066, core facilities of the Center for Translational Neuroscience were funded by Institutional Development Award P20 GM103425, and by the Sturgis Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANCOVA
- analysis of covariance
- CLAMS
- comprehensive lab animal monitoring system
- EE
- energy expenditure
- LEPRb
- long-form leptin receptor
- rGHRHr-cre
- cre recombinase under the control of the growth hormone-releasing hormone receptor
- RQ
- respiratory quotient
- VCO2
- maximum rate of carbon dioxide consumption
- VO2
- maximum rate of oxygen consumption.
References
- 1. Cioffi JA, Van Blerkom J, Antczak M, Shafer A, Wittmer S, Snodgrass HR. The expression of leptin and its receptors in pre-ovulatory human follicles. Mol Hum Reprod. 1997;3:467–472. [DOI] [PubMed] [Google Scholar]
- 2. Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. [DOI] [PubMed] [Google Scholar]
- 3. Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. [DOI] [PubMed] [Google Scholar]
- 4. Sobhani I, Bado A, Vissuzaine C, et al. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47:178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. [DOI] [PubMed] [Google Scholar]
- 6. Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113–116. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz MW, Baskin DG, Bukowski TR, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. [DOI] [PubMed] [Google Scholar]
- 8. Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. [DOI] [PubMed] [Google Scholar]
- 10. Woods AJ, Stock MJ. Leptin activation in hypothalamus. Nature. 1996;381:745. [DOI] [PubMed] [Google Scholar]
- 11. Balthasar N, Coppari R, McMinn J, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. [DOI] [PubMed] [Google Scholar]
- 12. Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. [DOI] [PubMed] [Google Scholar]
- 13. Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. [DOI] [PubMed] [Google Scholar]
- 14. Korner J, Savontaus E, Chua SC, Jr, Leibel RL, Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol. 2001;13:959–966. [DOI] [PubMed] [Google Scholar]
- 15. Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. [DOI] [PubMed] [Google Scholar]
- 16. Barash IA, Cheung CC, Weigle DS, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. [DOI] [PubMed] [Google Scholar]
- 17. Swerdloff RS, Batt RA, Bray GA. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology. 1976;98:1359–1364. [DOI] [PubMed] [Google Scholar]
- 18. Luque RM, Kineman RD. Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology. 2006;147:2754–2763. [DOI] [PubMed] [Google Scholar]
- 19. Sinha YN, Salocks CB, Vanderlaan WP. Prolactin and growth hormone secretion in chemically induced and genetically obese mice. Endocrinology. 1975;97:1386–1393. [DOI] [PubMed] [Google Scholar]
- 20. Garthwaite TL, Kalkhoff RK, Guansing AR, Hagen TC, Menahan LA. Plasma free tryptophan, brain serotonin, and an endocrine profile of the genetically obese hyperglycemic mouse at 4–5 months of age. Endocrinology. 1979;105:1178–1182. [DOI] [PubMed] [Google Scholar]
- 21. Lane PW, Dickie MM. Fertile, obese male mice: relative sterility in obese males corrected by dietary restriction. J Hered. 1954;45:2. [Google Scholar]
- 22. Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. [DOI] [PubMed] [Google Scholar]
- 23. Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab. 2009;297:E1197–E1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Breslow MJ, Min-Lee K, Brown DR, Chacko VP, Palmer D, Berkowitz DE. Effect of leptin deficiency on metabolic rate in ob/ob mice. Am J Physiol. 1999;276:E443–E449. [DOI] [PubMed] [Google Scholar]
- 25. Hwa JJ, Fawzi AB, Graziano MP, et al. Leptin increases energy expenditure and selectively promotes fat metabolism in ob/ob mice. Am J Physiol. 1997;272:R1204–R1209. [DOI] [PubMed] [Google Scholar]
- 26. Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R894–R903. [DOI] [PubMed] [Google Scholar]
- 27. Morton GJ, Kaiyala KJ, Fisher JD, Ogimoto K, Schwartz MW, Wisse BE. Identification of a physiological role for leptin in the regulation of ambulatory activity and wheel running in mice. Am J Physiol Endocrinol Metab. 2011;300:E392–E401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Childs GV, Akhter N, Haney A, et al. The somatotrope as a metabolic sensor: deletion of leptin receptors causes obesity. Endocrinology. 2011;152:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allensworth-James ML, Odle A, Haney A, Childs G. Sex differences in somatotrope dependency on leptin receptors in young mice: ablation of LEPR causes severe growth hormone deficiency and abdominal obesity in males. Endocrinology. 2015;156:3253–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akhter N, Odle AK, Allensworth-James ML, et al. Ablation of leptin signaling to somatotropes: changes in metabolic factors that cause obesity. Endocrinology. 2012;153:4705–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luque RM, Park S, Kineman RD. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: potential role of neuropeptide Y and corticotropin-releasing hormone. Endocrinology. 2007;148:300–309. [DOI] [PubMed] [Google Scholar]
- 32. Nørrelund H. The metabolic role of growth hormone in humans with particular reference to fasting. Growth Horm IGF Res. 2005;15:95–122. [DOI] [PubMed] [Google Scholar]
- 33. Fain JN, Kovacev VP, Scow RO. Effect of growth hormone and dexamethasone on lipolysis and metabolism in isolated fat cells of the rat. J Biol Chem. 1965;240:3522–3529. [PubMed] [Google Scholar]
- 34. Ottosson M, Lönnroth P, Björntorp P, Edén S. Effects of cortisol and growth hormone on lipolysis in human adipose tissue. J Clin Endocrinol Metab. 2000;85:799–803. [DOI] [PubMed] [Google Scholar]
- 35. Lanzi R, Luzi L, Caumo A, et al. Elevated insulin levels contribute to the reduced growth hormone (GH) response to GH-releasing hormone in obese subjects. Metabolism. 1999;48:1152–1156. [DOI] [PubMed] [Google Scholar]
- 36. Luque RM, Lin Q, Cordoba-Chacon J, et al. Metabolic impact of adult-onset, isolated, growth hormone deficiency (AOiGHD) due to destruction of pituitary somatotropes. PLoS One. 2011;6:e15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akhter N, Johnson BW, Crane C, et al. Anterior pituitary leptin expression changes in different reproductive states: stimulation, in vitro, by gonadotropin-releasing hormone. J Histochem Cytochem. 2007;55:151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McDuffie IA, Akhter N, Childs GV. Regulation of leptin mRNA and protein expression in pituitary somatotropes. J Histochem Cytochem. 2004;52:263–273. [DOI] [PubMed] [Google Scholar]
- 39. Odle AK, Haney A, Allensworth-James M, Akhter N, Childs GV. Adipocyte versus pituitary leptin in the regulation of pituitary hormones: somatotropes develop normally in the absence of circulating leptin. Endocrinology. 2014;155:4316–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yin Z, Williams-Simons L, Rawahneh L, Asa S, Kirschner LS. Development of a pituitary-specific cre line targeted to the Pit-1 lineage. Genesis. 2008;46:37–42. [DOI] [PubMed] [Google Scholar]
- 41. Yin Z, Williams-Simons L, Parlow AF, Asa S, Kirschner LS. Pituitary-specific knockout of the Carney complex gene Prkar1a leads to pituitary tumorigenesis. Mol Endocrinol. 2008;22:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eguchi J, Wang X, Yu S, et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lakso M, Pichel JG, Gorman JR, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–351. [Google Scholar]
- 45. Kleiber M. Body size and metabolic rate. Physiol Rev. 1947;27:511–541. [DOI] [PubMed] [Google Scholar]
- 46. Pack AI, Galante RJ, Maislin G, et al. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28:232–238. [DOI] [PubMed] [Google Scholar]
- 47. Tschöp MH, Speakman JR, Arch JR, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2012;9:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. [DOI] [PubMed] [Google Scholar]
- 49. Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. [DOI] [PubMed] [Google Scholar]
- 50. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. [DOI] [PubMed] [Google Scholar]
- 51. Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. [DOI] [PubMed] [Google Scholar]
- 52. Hamilton BS, Paglia D, Kwan AY, Deitel M. Increased obese mRNA expression in omental fat cells from massively obese humans. Nat Med. 1995;1:953–956. [DOI] [PubMed] [Google Scholar]
- 53. Lönnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med. 1995;1:950–953. [DOI] [PubMed] [Google Scholar]
- 54. Childs GV, Unabia G, Miller BT, Collins TJ. Differential expression of gonadotropin and prolactin antigens by GHRH target cells from male and female rats. J Endocrinol. 1999;162:177–187. [DOI] [PubMed] [Google Scholar]
- 55. Crane C, Akhter N, Johnson BW, et al. Fasting and glucose effects on pituitary leptin expression: is leptin a local signal for nutrient status? J Histochem Cytochem. 2007;55:1059–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Feuermann Y, Shamay A, Mabjeesh SJ. Leptin up-regulates the lactogenic effect of prolactin in the bovine mammary gland in vitro. J Dairy Sci. 2008;91:4183–4189. [DOI] [PubMed] [Google Scholar]
- 57. Feuermann Y, Mabjeesh SJ, Shamay A mammary fat can adjust prolactin effect on mammary epithelial cells via leptin and estrogen. Int J Endocrinol. 2009;2009:427260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ben-Jonathan N, Hugo E. Prolactin (PRL) in adipose tissue: regulation and functions. Adv Exp Med Biol. 2015;846:1–35. [DOI] [PubMed] [Google Scholar]
- 59. Ling C, Hellgren G, Gebre-Medhin M, et al. Prolactin (PRL) receptor gene expression in mouse adipose tissue: increases during lactation and in PRL-transgenic mice. Endocrinology. 2000;141:3564–3572. [DOI] [PubMed] [Google Scholar]
- 60. Auffret J, Viengchareun S, Carré N, et al. Beige differentiation of adipose depots in mice lacking prolactin receptor protects against high-fat-diet-induced obesity. FASEB J. 2012;26:3728–3737. [DOI] [PubMed] [Google Scholar]
- 61. LaPensee CR, Horseman ND, Tso P, Brandebourg TD, Hugo ER, Ben-Jonathan N. The prolactin-deficient mouse has an unaltered metabolic phenotype. Endocrinology. 2006;147:4638–4645. [DOI] [PubMed] [Google Scholar]