Abstract
Elimination of estrogen receptorα (ERα) from kisspeptin (Kiss1) neurons results in premature LH release and pubertal onset, implicating these receptors in 17β-estradiol (E2)-mediated negative feedback regulation of GnRH release during the prepubertal period. Here, we tested the dependency of prepubertal negative feedback on ERα in Kiss1 neurons. Prepubertal (postnatal d 14) and peripubertal (postnatal d 34) wild-type (WT) and Kiss1 cell-specific ERα knockout (KERαKO) female mice were sham operated or ovariectomized and treated with either vehicle- or E2-containing capsules. Plasma and tissues were collected 2 days after surgery for analysis. Ovariectomy increased LH and FSH levels, and E2 treatments completely prevented these increases in WT mice of both ages. However, in prepubertal KERαKO mice, basal LH levels were elevated vs WT, and both LH and FSH levels were not further increased by ovariectomy or affected by E2 treatment. Similarly, Kiss1 mRNA levels in the medial basal hypothalamus, which includes the arcuate nucleus, were suppressed with E2 treatment in ovariectomized prepubertal WT mice but remained unaffected by any treatment in KERαKO mice. In peripubertal KERαKO mice, basal LH and FSH levels were not elevated vs WT and were unaffected by ovariectomy or E2. In contrast to our previous findings in adult animals, these results demonstrate that suppression of gonadotropins and Kiss1 mRNA by E2 in prepubertal animals depends upon ERα activation in Kiss1 neurons. Our observations are consistent with the hypothesis that these receptors play a critical role in restraining GnRH release before the onset and completion of puberty.
Puberty begins with an increase in the pulsatile neurosecretion of GnRH and resultant activation of the hypothalamic-pituitary-gonadal (HPG) axis. In females, elevated GnRH secretion induces an increase in circulating levels of LH and FSH, which act on the ovary to prepare for the first ovulation by increasing steroid production and inducing maturational changes in the ovarian follicles. A combination of increased excitatory and decreased inhibitory input onto GnRH neurons may initiate the increase in GnRH secretion at the onset of puberty in mammals (1).
The neuropeptide kisspeptin (Kiss1), a potent stimulator of GnRH and gonadotropin secretion, likely functions as one excitatory signal that is up-regulated at the onset of puberty. Early activation of Kiss1 signaling leads to precocious puberty in mammals: humans with gain-of-function mutations in the genes encoding KISS1 or its receptor Kiss1 receptor (KISS1R), which is expressed by most GnRH neurons, exhibit an early onset of puberty (2, 3). Moreover, central administration of Kiss1 in prepubertal rodents leads to the early onset of puberty (4), whereas treatment with Kiss1r antagonists delays the onset of puberty in rodents (5).
The central and peripheral signals that may activate or disinhibit Kiss1 neurons at pubertal onset are not well understood. A recent study documented that Kiss1 mRNA expression in the anteroventral periventricular/rostral periventricular nucleus is increased substantially through puberty. By contrast, Kiss1 mRNA expression in the arcuate nucleus (ARC) is reduced from postnatal day (P)15 until the pubertal transition and then modestly increased thereafter (6). Ovarian 17β-estradiol (E2) secretions have been implicated both in stimulating anteroventral periventricular/rostral periventricular nucleus Kiss1 protein expression through the peripubertal period (7), and in restraining ARC Kiss1 expression until the onset of puberty (6, 8). We have recently demonstrated that the deletion of estrogen receptorα (ERα) from all Kiss1 neurons in female mice results in the elevation of prepubertal LH secretion, major advancement of puberty onset and a failure to attain ovulatory cyclicity. Increased Kiss1 expression in the ARC was also noted in these animals (9). A recent study also documented that Kiss1 cell-specific ablation of progesterone receptors, which are known to be induced via ERα activation, results in a moderate advancement of puberty onset (10). Because these findings suggested that ERα in ARC Kiss1 neurons mediates ovarian restraint of GnRH release, we also recently assessed the importance of these receptors in the negative feedback actions of E2 in adult mice (11). Surprisingly, we determined that E2-mediated negative feedback is not attenuated in postpubertal Kiss1 cell-specific ERα knockout (KERαKO) mice, suggesting that ERα in Kiss1 neurons is not required for negative feedback regulation in the adult. It remains possible that ERα plays a role in restraining Kiss1 expression and GnRH release during the prepubertal period, a function that would then be acquired by other ERα-expressing cells in adulthood. In these studies, we test this idea by comparing the effects of E2 on gonadotropin secretion in wild-type (WT) vs KERαKO prepubertal and peripubertal mice. We additionally assess the effects of E2 on Kiss1 mRNA expression to determine any correlation between alterations in Kiss1 mRNA and the presence or absence of E2 effects on LH and/or FSH during the prepubertal period. Our results confirm that ERα in Kiss1 neurons mediates feedback suppression of both Kiss1 expression and gonadotropin secretions during the prepubertal period.
Materials and Methods
Animals
Mice were housed in animal facilities located at the University of Wisconsin-Madison. All experimental procedures adhered to guidelines provided in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at University of Wisconsin-Madison. Mice were maintained on a 12-hour light, 12-hour dark cycle (7 am lights on) and given access to water and standard rodent chow (2019 Teklad; Harlan Laboratories) ad libitum.
To specifically ablate ERα from Kiss1 cells, mice with Cre recombinase knocked-in downstream of the Kiss1 promoter (Kiss-Cre mice) were bred to mice with exon 3 of the ERα gene (Esr1) flanked by loxP sites (ERαlox/lox mice). Cre recombinase-mediated excision of exon 3 of Esr1 results in the absence of ERα protein from almost all Kiss1-expressing neurons (9). All experiments were performed on female Kiss-Cre+/ERαlox/lox (KERαKO) mice and their Kiss-Cre−/ERαlox/lox and Kiss-Cre−/ERαlox/wt (WT) littermates.
Hormone treatment
Experiments were performed on female mice of 2 ages (day of birth designated as P0): P14 (prepubertal) and P34 (peripubertal). Mice were anesthetized by isoflurane inhalation and bilaterally ovariectomized (OVX) or sham operated (sham). Each OVX animal received 1 Silastic capsule (0.058” inner diameter, 0.077” outer diameter) containing sesame oil (vehicle [V]) or E2 dissolved in sesame oil (50 μg/mL) placed sc under one flank. Silastic capsules were prepared according to Gill et al (8). Briefly, capsules were cut to 5 mm (P14) or 9 mm (P34) in length, plugged at both ends with Silastic medical grade adhesive and filled with 1 μL (P14) or 5 μL (P34) of treatment. As a result, E2-containing capsules contained 0.05 μg of E2 (P14) or 0.25 μg (P34) of E2. These E2 doses are effective at suppressing LH secretion in female mice of similar ages (8). For all experimental groups and measurements described below, n = 8–12 per group.
Tissue collection
Mice were anesthetized 2 days after ovariectomy at P16 or P36 between 7:30 and 11:30 am. Blood was withdrawn by cardiac puncture and plasma was stored at −20°C for hormone measurements. Brains and pituitary glands were rapidly removed and fresh frozen on dry ice. Medial basal hypothalami (MBHs), which include the ARC, were dissected from fresh frozen brains. Pituitary glands from both ages and MBHs from P16 mice were processed for quantitative real-time PCR (qPCR) procedures.
Quantitative real-time PCR
Total RNA was extracted from pituitary glands using the RNeasy Mini kit (QIAGEN) according to the manufacturer's protocol. RNA was reverse transcribed with High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). qPCRs were performed with Fast SYBR Green Master Mix (Applied Biosystems) to measure Rn18s, gonadotropin β-subunit (Lhb and Fshb) and GnRH receptor (Gnrhr) mRNA. The primer sets used were designed to span exon-exon junctions and are listed in Table 1.
Table 1.
qPCR Primers for Pituitary Gene Expression
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| Rn18s | TGGTTGATCCTGCCAGTAG | CGACCAAAGGAACCATAACT |
| Lhb | CAGTCTGCATCACCTTCACCA | GGTAGGTGCACACTGGCTGA |
| Fshb | CAGGCAATCTTACGGTCTCG | GTGCGGGCTACTGCTACACT |
| Gnrhr | TTCGCTACCTCCTTTGTCGT | CACGGGTTTAGGAAAGCAAA |
Total RNA was extracted from MBHs using RNAzol reagent (Molecular Research Center) according to the manufacturer's protocol. RNA was reverse transcribed with High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). qPCRs were performed with TaqMan Universal Gene Expression Master Mix II (Applied Biosystems). Kiss1 and Rn18s mRNA were measured using the Gene Expression Assays Mm03058560_m1 and Mm03928990_g1 (Life Technologies), respectively.
All qPCRs were performed on a StepOnePlus Real-Time PCR System (Applied Biosystems). For all analyses, Rn18s was used as the reference gene. Fold changes in relative gene expression were calculated by the 2−Δ(ΔCt) method, where ΔCt = Ct (gene of interest) − Ct (Rn18s) and Δ(ΔCt) = ΔCt − ΔCt (WT sham). To analyze Gnrhr mRNA expression levels across ages, Δ(ΔCt) = ΔCt − ΔCt (P16 WT sham).
Hormone assays
Plasma LH and FSH levels were measured using the MILLIPLEX MAP Mouse Pituitary Magnetic Bead Panel MPTMAG-49K (EMD Millipore). The assay had a lower limit of detection of 0.04 ng/mL for LH and 0.06 ng/mL for FSH, an interassay coefficient of variation of 5.5% for LH and 7.3% for FSH, and an intraassay coefficient of variation of 14% for LH and 9.3% for FSH.
Circulating levels of E2 in prepubertal and peripubertal mice are below the range of values that can be reliably distinguished from OVX counterparts by currently available immunoassays (12). We therefore estimated E2 levels in ovary-intact and E2-treated animals using liquid chromatography and mass spectrometry (LC/MS), as performed by the Assay Services laboratory at the Wisconsin National Primate Research Center. To increase the sensitivity of these measurements, large amounts of plasma sample were required. Therefore, plasma samples from each group were pooled, allowing 1 measurement per group. The assay had a lower limit of detection of 1.9 pg/mL.
Statistical analysis
Data are presented as the mean ± SEM. Two-way ANOVA followed by Bonferroni post hoc tests were performed using SPSS Statistics (IBM) to determine statistical significance in all analyses. Differences were considered significant when P < .05.
Results
Absence of gonadotropin suppression by E2 in prepubertal KERαKO mice
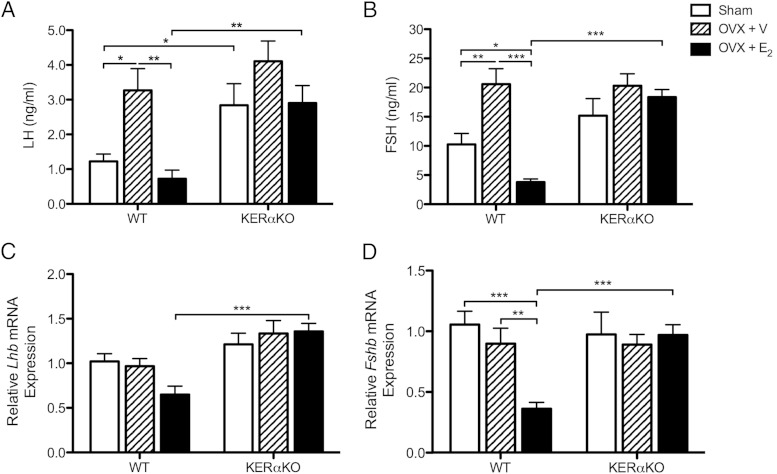
To examine whether E2 acts through ERα in Kiss1 neurons to inhibit Lhb and Fshb mRNA expression and gonadotropin secretion, prepubertal and peripubertal WT and KERαKO mice were OVX and treated with vehicle- or E2-containing capsules. Circulating levels of gonadotropins, as well as Lhb and Fshb mRNA expression levels in the pituitary gland, were measured 2 days after ovariectomy. In P16 WT mice, LH and FSH levels were increased compared with sham levels after ovariectomy (LH, P < .05; FSH, P < .01) and were significantly suppressed by E2 treatment (LH, P < .01; FSH, P < .001 vs WT OVX + V) (Figure 1, A and B). E2 suppressed FSH to levels lower than the basal levels exhibited by sham WT mice (P < .05) (Figure 1B).
Figure 1.
E2 suppresses gonadotropin secretion and Fshb mRNA expression in prepubertal WT mice but has no effect on gonadotropin secretion or Lhb and Fshb mRNA expression in prepubertal KERαKO mice. Shown are mean plasma LH (A) and FSH (B) levels, as well as relative Lhb (C) and Fshb (D) mRNA expression levels, for WT and KERαKO mice that were left intact (sham) or bilaterally OVX and implanted with a capsule containing vehicle (V) or E2 on P14 and then euthanized 2 days later (P16). All Lhb and Fshb mRNA expression levels are normalized to Rn18s and compared relative to expression levels in sham WT mice. Two-way ANOVA analyses indicated a significant interaction between genotype and treatment for FSH and Fshb mRNA expression levels (P < .01) but not LH (P = .50) or Lhb mRNA expression (P = .07) levels. Genotype significantly affected all measurements independent of treatment (P < .05), whereas treatment significantly affected all measurements independent of genotype (P < .05), except for Lhb mRNA expression levels (P = .29). *, P < .05; **, P < .01; ***, P < .001 as determined by post hoc comparisons. All data represented as mean ± SEM.
As seen previously (9), LH levels were elevated in P16 sham KERαKO mice compared with age-matched sham WT mice (P < .05) (Figure 1A). FSH levels did not differ between sham WT and KERαKO mice (Figure 1B). Moreover, LH and FSH levels in P16 KERαKO mice were unaffected by ovariectomy or E2 treatment (Figure 1, A and B). Therefore, circulating gonadotropin levels were higher in E2-treated KERαKO mice compared with E2-treated WT mice (LH, P < .01; FSH, P < .001) but did not differ between vehicle-treated mice (Figure 1, A and B).
Unlike circulating gonadotropin levels, Lhb and Fshb mRNA levels did not increase compared with sham levels after ovariectomy in P16 WT mice (Figure 1, C and D). Fshb mRNA levels were significantly suppressed with E2 treatment compared with sham and vehicle-treated WT mice (P < .001 vs WT sham; P < .01 vs WT OVX + V), whereas Lhb mRNA levels trended lower than sham WT levels with E2 treatment (P = .07) (Figure 1, C and D). Meanwhile, Lhb mRNA levels followed a trend to be slightly increased in P16 sham KERαKO mice compared with their WT littermates (P = .06) (Figure 1C). Fshb mRNA levels did not differ between sham WT and KERαKO mice (Figure 1D). Similar to LH and FSH levels, Lhb and Fshb mRNA levels were unaffected by E2 treatment in P16 KERαKO mice (Figure 1, C and D). As a result, Lhb and Fshb mRNA levels were higher in E2-treated KERαKO mice compared with E2-treated WT mice (P < .001) but did not differ between vehicle-treated groups (Figure 1, C and D).
Absence of gonadotropin suppression by E2 in peripubertal KERαKO mice
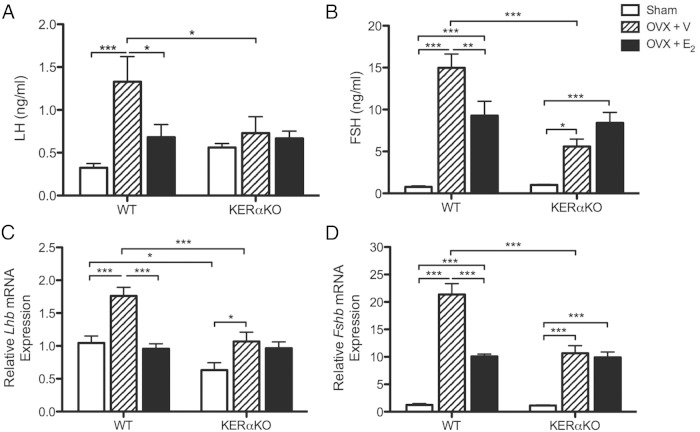
LH and FSH levels, as well as Lhb and Fshb mRNA levels, increased 2 days after ovariectomy in P36 WT mice (P < .001 vs WT sham) (Figure 2). LH and Lhb mRNA levels were fully suppressed to sham WT levels with E2 treatment (LH, P < .05; Lhb, P < .001 vs WT OVX + V) (Figure 2, A and C). However, FSH and Fshb mRNA levels were only partially suppressed in the presence of E2 (FSH, P < .01; Fshb, P < .001 vs WT OVX + V) as they remained significantly higher than sham WT levels (P < .001) (Figure 2, B and D).
Figure 2.
E2 suppresses gonadotropin secretion and Lhb and Fshb mRNA expression in peripubertal WT mice but has no effect on FSH secretion or Lhb and Fshb mRNA expression in peripubertal KERαKO mice. Mean plasma LH (A) and FSH (B) levels, as well as relative Lhb (C) and Fshb (D) mRNA expression levels, for WT and KERαKO mice that were left intact (sham) or bilaterally OVX and implanted with a capsule containing vehicle (V) or E2 on P34 and then euthanized 2 days later (P36). All Lhb and Fshb mRNA expression levels are normalized to Rn18s and compared relative to expression levels in sham WT mice. Two-way ANOVA analyses indicated a significant interaction between genotype and treatment for all measurements (P < .05), except for LH levels (P = .08). Genotype significantly affected all measurements independent of treatment (P < .01), except for LH levels (P = .42), whereas treatment significantly affected all measurements independent of genotype (P < .05). *, P < .05; **, P < .01; ***, P < .001 as determined by post hoc comparisons. All data represented as mean ± SEM.
Unlike P16 mice, basal LH levels did not differ between P36 sham KERαKO and WT mice (Figure 2A). Nevertheless, similar to P16 KERαKO mice, LH levels did not change in response to ovariectomy or E2 treatment in P36 KERαKO mice (Figure 2A). As a result, LH levels were significantly lower in vehicle-treated KERαKO mice as compared with vehicle-treated WT mice (P < .05). Surprisingly, FSH and Fshb mRNA levels increased in P36 KERαKO mice after ovariectomy (FSH, P < .05; Fshb, P < .001 vs KERαKO sham) but not to levels exhibited by age-matched WT mice after ovariectomy (P < .001 vs WT OVX + V) (Figure 2, B and D). FSH and Fshb mRNA levels remained elevated in P36 KERαKO mice even after E2 treatment (P < .001 vs KERαKO sham) (Figure 2, B and D).
P36 sham KERαKO mice exhibited significantly lower Lhb mRNA levels than sham WT mice (P < .05) (Figure 2C). These levels increased after ovariectomy in KERαKO mice (P < .05 vs KERαKO sham), albeit not to levels exhibited by WT mice after ovariectomy (P < .001 vs WT OVX + V). E2 treatment did not affect Lhb mRNA levels in P36 KERαKO mice (Figure 2C).
Elevation of pituitary Gnrhr mRNA expression in prepubertal KERαKO mice
Gonadotropin hypersecretion and refractoriness to E2 in prepubertal KERαKO mice indicates a premature stimulation by GnRH secretion, and increased GnRH release has previously been shown to induce expression of GnRHR (13). Therefore, relative Gnrhr mRNA expression in the pituitary gland was examined in sham WT and KERαKO mice using qPCR. Gnrhr mRNA expression was higher in P16 sham KERαKO mice compared with age-matched WT mice (P < .05) (Figure 3). These elevated levels were similar to the levels exhibited by both P36 KERαKO and WT mice (Figure 3). Gnrhr mRNA expression and pituitary responsiveness to GnRH gradually increase through pubertal development in mice (14). Because KERαKO mice exhibit elevated levels of Gnrhr mRNA expression and LH secretion prematurely, pituitary responsiveness to GnRH is precociously attained in these mice.
Figure 3.
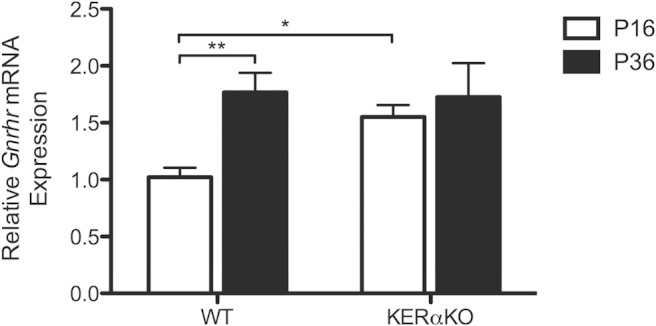
Gnrhr mRNA expression levels in the pituitary glands of prepubertal KERαKO mice are elevated relative to prepubertal WT mice. Relative Gnrhr mRNA expression levels for WT and KERαKO mice that were left intact (sham) on P14 or P34 and then euthanized 2 days later (P16 or P36, respectively). Gnrhr mRNA expression levels are normalized to Rn18s and compared relative to expression levels in P16 WT mice. Two-way ANOVA analysis indicated no significant interaction between genotype and age (P = .10). Age significantly affected Gnrhr mRNA expression independent of genotype (P < .05). *, P < .05; **, P < .01 as determined by post hoc comparisons. All data represented as mean ± SEM.
Absence of Kiss1 mRNA suppression by E2 in prepubertal KERαKO mice
E2 treatment suppressed Lhb and Fshb mRNA expression and gonadotropin secretion in P16 WT mice but had no effect on Lhb and Fshb mRNA expression or gonadotropin secretion in P16 KERαKO mice. To determine whether E2-mediated control of Kiss1 expression was also dysregulated in prepubertal KERαKO mice, Kiss1 mRNA expression levels in the MBH were measured at P16. In P16 WT mice, Kiss1 mRNA levels rose significantly after ovariectomy (P < .05 vs WT sham) and were suppressed with E2 treatment (P < .001 vs WT OVX + V) (Figure 4). Meanwhile, Kiss1 mRNA levels in P16 KERαKO mice did not change in response to ovariectomy or E2 treatment (Figure 4). These results mimic treatment effects on gonadotropin secretion in P16 WT mice, and Lhb and Fshb mRNA expression and gonadotropin secretion in P16 KERαKO mice.
Figure 4.
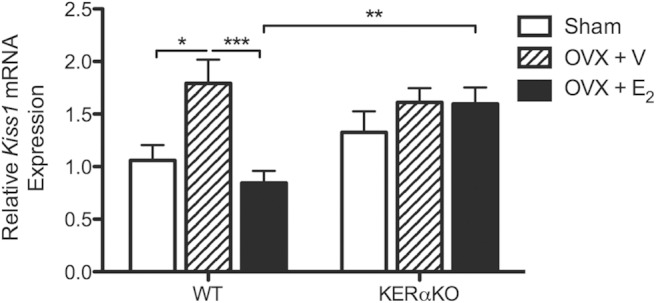
E2 suppresses Kiss1 mRNA expression in prepubertal WT mice but has no effect on Kiss1 mRNA expression in prepubertal KERαKO mice. Relative Kiss1 mRNA expression levels for WT and KERαKO mice that were left intact (sham) or bilaterally OVX and implanted with a capsule containing vehicle (V) or E2 on P14 and then euthanized 2 days later (P16). Kiss1 mRNA expression levels are normalized to Rn18s and compared relative to expression levels in sham WT mice. Two-way ANOVA analysis indicated a significant interaction between genotype and treatment (P < .05). Genotype and treatment significantly affected Kiss1 mRNA expression independent of each other (P < .05). *, P < .05; **P, < .01; ***, P < .001 as determined by post hoc comparisons. All data represented as mean ± SEM.
E2 levels in prepubertal and peripubertal WT and KERαKO mice
Single value determinations of E2 levels by LC/MS are provided in Table 2. Basal E2 levels in sham WT mice at both P16 and P36 were not detectable, whereas values in sham KERαKO mice at these ages were in the detectable range. The E2 levels were also undetectable in vehicle-treated mice at both ages, and in P16 E2-treated mice. E2 levels were low but detectable in E2-treated mice at P36, confirming that sc implants had sustained E2 levels at, or above, normal levels but below those apparent in sham KERαKO mice. The range of detectable E2 values among the groups is consistent with values recently reported for young adult female mice using gas chromatography and mass spectroscopy (2.7 ± 1.0 pg/mL) (15).
Table 2.
E2 Levels as Measured by LC/MS
| P16 |
P36 |
|||
|---|---|---|---|---|
| WT | KERαKO | WT | KERαKO | |
| Sham | <1.9 | 3.3 | <1.9 | 4.9 |
| OVX + V | <1.9 | <1.9 | <1.9 | <1.9 |
| OVX + E2 | <1.9 | <1.9 | 2.3 | 2.1 |
Discussion
Premature activation of central Kiss1-Kiss1r signaling pathways results in precocious puberty (2–4), suggesting that Kiss1 release is unstimulated or is actively restrained before pubertal onset. Prepubertal female KERαKO mice, which lack ERα specifically in Kiss1-expressing cells, also exhibit precocious pubertal onset (9, 16). Taken together, these observations suggest that E2 acts via ERα in Kiss1 neurons to restrain GnRH secretion before puberty. In the current studies, we tested this idea by comparing the ability of E2 treatment to suppress Kiss1 expression and gonadotropin secretion after ovariectomy in prepubertal WT and KERαKO mice. Our findings that E2 treatment inhibits Kiss1 mRNA expression and gonadotropin secretion in prepubertal WT, but not KERαKO, mice provide direct evidence that ERα is required in Kiss1 cells in female mice to restrain LH secretion before puberty. These results stand in contrast with our recently published observations in adult (postpubertal) mice, where E2-mediated negative feedback actions on LH secretion were observed even in the absence of ERα in Kiss1 neurons (11). Our findings thus support a role for ERα in Kiss1 neurons before puberty, whereby sensitivity of these receptors to low, prepubertal levels of E2 maintains a suppression of Kiss1 expression and hence, GnRH and LH release. The apparent diminishment of this feedback response in adulthood is consistent with the idea that waning sensitivity of Kiss1 neurons to E2-mediated negative feedback contributes to the pubertal activation of GnRH release.
Our findings suggest that E2 exerts its negative feedback effects through different neural pathways in prepubertal vs adult female mice. The transition to a different negative feedback mechanism in adulthood may explain the gradual developmental decrease in sensitivity of GnRH and LH secretion to E2, demonstrated in classic studies as the requirement of a higher dose of E2 to suppress GnRH and LH secretion in adults compared with prepubertal animals (17–19). Thus, decreased Kiss1 neuronal sensitivity to E2 would contribute to the pubertal activation of Kiss1 and GnRH release, and hence gonadotropin and ovarian steroid secretions; then the equilibration of the HPG axis at a higher level of activity in adulthood may be attained by the ability of adult-levels of E2 to engage other neuronal groups in mediating negative feedback. The identities of these non-Kiss1 neuronal targets remain unknown; however, evidence supports the involvement of ERα-expressing γ-aminobutyric acidergic and proopiomelanocortin-expressing neurons in the ARC (20–23).
A gradual switch from the prepubertal to an adult negative feedback pathway would likely begin at the onset of puberty and be completed towards the end of puberty. The failure of LH secretion levels to rise 2 days following ovariectomy in peripubertal KERαKO mice, which reflects the diminished rise in LH secretion after ovariectomy exhibited by adult KERαKO mice (9, 11), prohibits us from clearly determining whether E2 suppresses GnRH and LH secretion in these mice. However, similar to adult KERαKO mice, basal LH levels are similar to WT levels in ovary-intact peripubertal KERαKO mice. Therefore, we can speculate that E2-mediated negative feedback is, for the most part, intact in KERαKO mice at this age. Because WT mice in our colony exhibit vaginal opening at approximately P29 (9) and regular estrous cyclicity is only attained several days after vaginal opening (1), the transition to the adult E2-mediated negative feedback pathway appears to be largely complete around P36.
The loss of E2-mediated control of Kiss1 expression in the MBH in prepubertal KERαKO mice may result in a premature initiation or elevation in Kiss1 secretion. As a result of precocious activation of GnRH neurons, prepubertal KERαKO mice exhibit elevated levels of LH and E2, leading to early vaginal opening (9, 16). This reproductive phenotype mimics the precocious puberty phenotype exhibited by rodents centrally infused with Kiss1 prepubertally (4). E2 may also act through ERα signaling pathways to regulate the expression of other neuropeptides coexpressed in Kiss1 neurons in the ARC, namely neurokinin B and dynorphin (24–26). These neuropeptides also regulate GnRH secretion and pubertal development (8, 27, 28). Thus, the dysregulation of the release of neurokinin B and/or dynorphin may also contribute to the early onset of puberty in KERαKO mice.
ERα-expressing Kiss1 neurons in the ARC form functional connections with downstream Kiss1r-expressing GnRH neurons during fetal development (29), but it is currently not known when E2 begins to exert its inhibitory actions on this circuit. Female KERαKO mice exhibit vaginal opening at approximately P13 (9), suggesting that E2-mediated regulation of Kiss1 expression and Kiss1 secretion from the ARC begins at least a few days earlier. However, female hypogonadal and aromatase knockout mice, which lack the ability to convert testosterone to E2, do not exhibit higher levels of Kiss1 mRNA or Kiss1 protein expression, respectively, in the ARC compared with age-matched WT littermates until around P20 (8, 30). The absence of a difference in Kiss1 protein expression before P20 in aromatase knockout mice may be due to an increase in Kiss1 secretion that occurs in tandem with increased protein expression. Additional comprehensive studies are necessary to delineate when E2 suppression of Kiss1 mRNA and Kiss1 protein expression in the ARC occurs in neonatal and prepubertal female mice. Also, appreciable ovarian steroid production might not begin until the second week of postnatal development (31, 32), so it is possible that E2 synthesized elsewhere, such as the hypothalamus itself, could regulate Kiss1 release in the ARC perinatally. However, the relative contributions of these potential sources of E2 to prepubertal restraint await more sensitive estimates of endogenous E2 in ovary-intact and OVX animals at these ages.
Early activation of the GnRH neurons leads to precocious activation of the HPG axis only if the anterior pituitary gland can respond to the elevated incoming GnRH signal. During the first week of life, disorganized high-frequency GnRH release patterns, which may be independent of Kiss1 stimulation, appear to manifest as a lack of pituitary responsiveness during the neonatal period (14). Induction of pituitary responsiveness after this period appears to coincide with a switch to an organized, low frequency pattern of secretion, which is likely dependent upon Kiss1 stimulation (29). Thereafter, pituitary responsiveness to GnRH, along with Gnrhr mRNA expression in the anterior pituitary gland, gradually increases throughout puberty in mice, in parallel with the likely increase in GnRH release (14, 33, 34). Prepubertal female KERαKO mice exhibit elevated levels of Gnrhr mRNA expression and LH secretion, suggesting that female KERαKO mice precociously assume an organized pulsatile pattern of GnRH release and hence, LH responsiveness. GnRH is a primary mediator of GnRHR that stimulates Gnrhr mRNA and GnRHR protein expression in the pituitary glands of prepubertal and adult rodents (13, 33). E2 can act independently or in synergy with GnRH to increase Gnrhr mRNA expression in adult female rodents (13, 35). Thus, the premature development of Kiss1-dependent, pulsatile GnRH secretory patterns and the elevated E2 levels associated with this increase may both contribute to premature increase in Gnrhr mRNA expression and attainment of pituitary responsiveness to GnRH in female prepubertal KERαKO mice.
In addition to GnRH, other endocrine factors, including inhibin, activin and follistatin, regulate Fshb mRNA expression and FSH protein expression and release. The ovarian-derived glycoprotein inhibin acts directly on the pituitary gland to selectively down-regulate Fshb expression and suppress FSH secretion in adult animals (36). Passive immunoneutralization of the unique inhibin α-subunit induces an increase in circulating FSH levels in ovary-intact P20 female rats but has no effect on the FSH levels in rats 10 days of age or younger, suggesting that inhibin's ability to regulate FSH develops during puberty (37). Our findings indicate that the development of this inhibin-mediated mechanism during puberty coincides with a decreased sensitivity to E2 at the level of the gonadotrope that is specific to FSH. E2 fully suppresses Fshb mRNA expression and FSH secretion in OVX prepubertal WT mice but only partially suppresses Fshb mRNA expression and FSH secretion in OVX peripubertal WT mice; however, E2 completely suppresses LH secretion to ovary-intact levels in both OVX prepubertal and peripubertal WT mice. Also, although prepubertal and peripubertal KERαKO mice are insensitive to E2-mediated suppression of Fshb expression and FSH secretion, the switch to a predominantly inhibin-mediated mechanism during puberty likely remains intact in these mice because Fshb mRNA expression and FSH secretion increase after ovariectomy in peripubertal but not prepubertal KERαKO mice.
Ovariectomy evoked increases in LH secretion in both prepubertal and peripubertal WT mice but was accompanied by increased Lhb mRNA expression only in peripubertal animals. The reason for the failure to alter Lhb expression at the earlier age is not clear but may derive from an immaturity of the cellular pathways within gonadotropes that convey GnRHR-mediated signals for Lhb transcription. The trend toward higher Lhb mRNA expression in ovary-intact prepubertal KERαKO mice may reflect a cumulative effect of enhanced GnRH release over a longer duration than would occur over 2 days after ovariectomy in WT animals. In contrast to prepubertal KERαKO mice, ovary-intact peripubertal KERαKO mice exhibit relatively low levels of Lhb mRNA expression compared with WT mice. Although E2 acts primarily on the hypothalamus to regulate GnRH secretion and, in turn, Lhb expression and LH secretion, E2 also acts directly via ERα in gonadotropes in adult mice to suppress Lhb expression (38). Because ovary-intact prepubertal and peripubertal KERαKO mice exhibit elevated levels of circulating E2 compared with WT mice, but Lhb mRNA levels are only elevated in peripubertal KERαKO mice, the ability of E2 to act directly on the gonadotropes to suppress Lhb expression is likely acquired during puberty. This idea is further supported by our finding that E2 suppresses Lhb mRNA expression in OVX peripubertal but not OVX prepubertal WT mice.
Together with our previous findings, these studies demonstrate that E2 activates ERα in Kiss1 neurons to regulate Kiss1 mRNA expression in the ARC of both immature and adult female rodents. They also provide support for the role of ERα-expressing Kiss1 neurons in the ARC in E2-mediated negative feedback in prepubertal but not adult female mice. Further studies are required to delineate which neurons are necessary for E2-mediated negative feedback in adulthood and the mechanisms that trigger transition to the adult negative feedback pathway during puberty.
Acknowledgments
We thank the technical expertise of the Wisconsin National Primate Research Center's Assay Services.
This work was supported by National Institutes of Health Grants P01 HD21291, R01 HD68777, and P50 HD44405.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- arcuate nucleus
- E2
- 17β-estradiol
- ERα
- estrogen receptorα
- GnRHR
- GnRH receptor
- HPG
- hypothalamic pituitary gonadal
- KERαKO
- Kiss1 cell-specific ERα knockout
- Kiss1
- kisspeptin
- KISS1R/Kiss1R
- Kiss1 receptor
- LC/MS
- liquid chromatography and mass spectrometry
- MBH
- medial basal hypothalamus
- OVX
- ovariectomized
- P
- postnatal day
- qPCR
- quantitative real-time PCR
- sham
- sham operated
- V
- vehicle
- WT
- wild-type.
References
- 1. Ojeda SR, Skinner MK. Puberty in the rat. In: Neill JD, ed. Knobil and Neill's Physiology of Reproduction. vol 2 3rd ed Amsterdam, Boston: Elsevier; 2006:2061–2126. [Google Scholar]
- 2. Teles MG, Bianco SD, Brito VN, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silveira LG, Noel SD, Silveira-Neto AP, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95:2276–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Navarro VM, Fernández-Fernández R, Castellano JM, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pineda R, Garcia-Galiano D, Roseweir A, et al. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;151:722–730. [DOI] [PubMed] [Google Scholar]
- 6. Semaan SJ, Kauffman AS. Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice. Mol Cell Endocrinol. 2015;401:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology. 2009;150:3214–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gill JC, Navarro VM, Kwong C, et al. Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid-negative feedback than Kiss1. Endocrinology. 2012;153:4883–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayer C, Acosta-Martinez M, Dubois SL, et al. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107:22693–22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stephens SB, Tolson KP, Rouse ML, Jr, et al. Absent progesterone signaling in kisspeptin neurons disrupts the LH surge and impairs fertility in female mice. Endocrinology. 2015;156:3091–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dubois SL, Acosta-Martínez M, DeJoseph MR, et al. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology. 2015;156:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152:4443–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yasin M, Dalkin AC, Haisenleder DJ, Kerrigan JR, Marshall JC. Gonadotropin-releasing hormone (GnRH) pulse pattern regulates GnRH receptor gene expression: augmentation by estradiol. Endocrinology. 1995;136:1559–1564. [DOI] [PubMed] [Google Scholar]
- 14. Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014;34:15060–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nilsson ME, Vandenput L, Tivesten Å, et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology. 2015;156:2492–2502. [DOI] [PubMed] [Google Scholar]
- 16. Frazão R, Cravo RM, Donato J, Jr, et al. Shift in Kiss1 cell activity requires estrogen receptor α. J Neurosci. 2013;33:2807–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramirez DV, McCann SM. Comparison of the regulation of luteinizing hormone (LH) secretion in immature and adult rats. Endocrinology. 1963;72:452–464. [DOI] [PubMed] [Google Scholar]
- 18. Steele RE, Weisz J. Changes in sensitivity of the estradiol-LH feedback system with puberty in the female rat. Endocrinology. 1974;95:513–520. [DOI] [PubMed] [Google Scholar]
- 19. Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108:506–516. [DOI] [PubMed] [Google Scholar]
- 20. Yeo SH, Herbison AE. Estrogen-negative feedback and estrous cyclicity are critically dependent upon estrogen receptor-α expression in the arcuate nucleus of adult female mice. Endocrinology. 2014;155:2986–2995. [DOI] [PubMed] [Google Scholar]
- 21. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly MJ, Loose MD, Ronnekleiv OK. Estrogen suppresses mu-opioid- and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA. 2005;102:15682–15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology. 2004;145:736–742. [DOI] [PubMed] [Google Scholar]
- 25. Gottsch ML, Navarro VM, Zhao Z, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32:2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakahara T, Uenoyama Y, Iwase A, et al. Chronic peripheral administration of κ-opioid receptor antagonist advances puberty onset associated with acceleration of pulsatile luteinizing hormone secretion in female rats. J Reprod Dev. 2013;59:479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar D, Freese M, Drexler D, Hermans-Borgmeyer I, Marquardt A, Boehm U. Murine arcuate nucleus kisspeptin neurons communicate with GnRH neurons in utero. J Neurosci. 2014;34:3756–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brock O, Bakker J. The two kisspeptin neuronal populations are differentially organized and activated by estradiol in mice. Endocrinology. 2013;154:2739–2749. [DOI] [PubMed] [Google Scholar]
- 31. Greco TL, Payne AH. Ontogeny of expression of the genes for steroidogenic enzymes P450 side-chain cleavage, 3 β-hydroxysteroid dehydrogenase, P450 17 α-hydroxylase/C17–20 lyase, and P450 aromatase in fetal mouse gonads. Endocrinology. 1994;135:262–268. [DOI] [PubMed] [Google Scholar]
- 32. O'Shaughnessy PJ, Mannan MA. Development of cytochrome P-450 side chain cleavage mRNA levels in neonatal ovaries of normal and hypogonadal (hpg) mice. Mol Cell Endocrinol. 1994;104:133–138. [DOI] [PubMed] [Google Scholar]
- 33. Zapatero-Caballero H, Sanchez-Franco F, Fernandez-Mendez C, García-San Frutos M, Botella-Cubells LM, Fernandez-Vazquez G. Gonadotropin-releasing hormone receptor gene expression during pubertal development of female rats. Biol Reprod. 2004;70:348–355. [DOI] [PubMed] [Google Scholar]
- 34. Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology. 2001;142:2929–2936. [DOI] [PubMed] [Google Scholar]
- 35. Bauer-Dantoin AC, Weiss J, Jameson JL. Roles of estrogen, progesterone, and gonadotropin-releasing hormone (GnRH) in the control of pituitary GnRH receptor gene expression at the time of the preovulatory gonadotropin surges. Endocrinology. 1995;136:1014–1019. [DOI] [PubMed] [Google Scholar]
- 36. Bilezikjian LM, Blount AL, Donaldson CJ, Vale WW. Pituitary actions of ligands of the TGF-β family: activins and inhibins. Reproduction. 2006;132:207–215. [DOI] [PubMed] [Google Scholar]
- 37. Culler MD, Negro-Vilar A. Passive immunoneutralization of endogenous inhibin: sex-related differences in the role of inhibin during development. Mol Cell Endocrinol. 1988;58:263–273. [DOI] [PubMed] [Google Scholar]
- 38. Singh SP, Wolfe A, Ng Y, et al. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor α (ESR1). Biol Reprod. 2009;81:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]