Abstract
Mood disorders are associated with dysregulation of prefrontal cortex (PFC) function, circadian rhythms, and diurnal glucocorticoid (corticosterone [CORT]) circulation. Entrainment of clock gene expression in some peripheral tissues depends on CORT. In this study, we characterized over the course of the day the mRNA expression pattern of the core clock genes Per1, Per2, and Bmal1 in the male rat PFC and suprachiasmatic nucleus (SCN) under different diurnal CORT conditions. In experiment 1, rats were left adrenal-intact (sham) or were adrenalectomized (ADX) followed by 10 daily antiphasic (opposite time of day of the endogenous CORT peak) ip injections of either vehicle or 2.5 mg/kg CORT. In experiment 2, all rats received ADX surgery followed by 13 daily injections of vehicle or CORT either antiphasic or in-phase with the endogenous CORT peak. In sham rats clock gene mRNA levels displayed a diurnal pattern of expression in the PFC and the SCN, but the phase differed between the 2 structures. ADX substantially altered clock gene expression patterns in the PFC. This alteration was normalized by in-phase CORT treatment, whereas antiphasic CORT treatment appears to have eliminated a diurnal pattern (Per1 and Bmal1) or dampened/inverted its phase (Per2). There was very little effect of CORT condition on clock gene expression in the SCN. These experiments suggest that an important component of glucocorticoid circadian physiology entails CORT regulation of the molecular clock in the PFC. Consequently, they also point to a possible mechanism that contributes to PFC disrupted function in disorders associated with abnormal CORT circulation.
Optimal organismal function depends on the integrity of the circadian system (1). Circadian misalignment is associated with many physiological and psychological disease states including obesity, diabetes, some forms of cancer, cardiovascular disease, posttraumatic stress disorder (PTSD), and depression (2–4). The hypothalamic suprachiasmatic nucleus (SCN) is an autonomous circadian oscillator that is primarily entrained by the light-dark cycle and coordinates circadian function throughout the entire body (5).
Circadian rhythms are generated by the transcriptional/translational feedforward and feedback cycles of clock gene expression present not only in the SCN, but also in many other cells throughout the body. This molecular clock is comprised of a positive arm (Bmal1 and Clock gene products) which induces the transcription of a negative arm (Period and Cryptochrome genes) whose protein products inhibit their own production by preventing the actions of Brain and muscle ARNT-like protein (BMAL1) and circadian locomotor output cycles kaput (CLOCK) proteins (6). REV-ERBα and retinoic acid-like orphan receptor modulate the cycle by inhibiting and inducing Bmal1 transcription, respectively (7). The completion of this cycle takes approximately 24 hours (6). Although many extra-SCN tissues show oscillations in core clock gene expression, the phase of expression (ie, time of peak and trough expression) varies in a tissue specific manner (8–10). The SCN is necessary to maintain synchronized rhythmic clock gene expression throughout the body (11–13), but because it provides virtually no direct innervation to peripheral and central tissues outside of the hypothalamus (14), its mechanism of transduction remains to be established.
In recent years, corticosterone (CORT), the principal glucocorticoid hormone in rodents, has been found to serve as a clock gene expression entraining factor in the liver, kidney, lung, cornea, and salivary gland (8, 15). Glucocorticoid receptors (GRs) are ubiquitously expressed throughout the body with the noted exception of the SCN (16). The core clock gene Period1 (Per1) has a functional glucocorticoid-response element (GRE) in its promoter region (17–19) and is modulated by CORT in both central (20, 21) and peripheral tissues (8, 16, 22, 23). CORT has also been shown to directly modulate Per2 (18, 19) and Rev-erbα (24) mRNA expression in some peripheral cell types. CORT circulation is normally entrained by the SCN (25) and shows a robust diurnal rhythm with peak levels occurring around the time of waking (26). CORT therefore is a promising candidate as an entraining factor in extra-SCN tissues. Interestingly, normal diurnal and stress-induced CORT release also relies on the presence of a functional molecular clock within the adrenal gland itself (27, 28), supporting the importance of the interplay and communication between the circadian and glucocorticoid systems.
Although CORT-dependent clock gene entrainment has been extensively examined in the periphery, limited research has examined this process in the brain (20). The prefrontal cortex (PFC) is a key neural center for regulation of the emotional and physiological response to stress (29, 30). Many PFC-dependent functions such as attention (31), mood (1, 32), and conditioned fear extinction learning (33, 34) show diurnal variation. In addition, dendritic branching (which is also CORT-dependent) (35) of pyramidal cells in the infralimbic subregion of the PFC exhibits diurnal variation (36). PFC dysfunction is associated with depression and PTSD (37–39). These disorders are also characterized by impaired circadian function parameters such as sleep, diurnal glucocorticoid circulation, and PFC clock gene rhythmicity (40, 41). Chronic unpredictable stress can disrupt clock gene oscillation in brain regions associated with mood regulation (42), and successful treatment of psychiatric disorders is frequently associated with corrected circadian abnormalities (43–45). Interestingly, increasing the amplitude of diurnal CORT circulation in mice has anxiolytic effects (46). To date there has been no examination of CORT-dependent modulation of clock gene expression in the brain encompassing both the positive and negative arms of the molecular clock. We therefore examined the diurnal expression of 3 core clock genes (Per1, Per2, and Bmal1) in 4 subregions of the PFC (anterior cingulate cortex [ACC], prelimbic cortex [PL], infralimbic cortex [IL], and ventral orbital cortex [VO]) and the SCN under different chronic CORT conditions. In experiment 1, we compared these expression profiles in adrenal intact rats, adrenalectomized (ADX) rats, as well as ADX rats injected with CORT every day for 10 days at the opposite time of day (zeitgeber time [ZT]1) as the endogenous CORT peak (ZT12) (antiphasic CORT). In experiment 2, we compared the diurnal expression of the same clock genes in ADX rats, ADX rats treated with antiphasic CORT, and ADX rats treated with in-phase CORT (ie, a CORT injection at the time of the endogenous peak) daily for 13 days. We have previously found in adrenal intact male and female rats that all 3 clock genes have a fluctuating daily (“diurnal”) expression pattern in the PFC with peak expression of the positive arm (Bmal1) approximately antiphasic to the negative arm (Per1 and Per2) (10). We hypothesized that the normal phase relationship and robustness of 24-hour clock gene expression in the PFC depends on the presence of in-phase diurnal CORT. Consequently, we predicted that the phase, and possibly even the presence of diurnal PFC clock gene expression, would be altered by the absence of circulating CORT (ADX) and would be substantially disrupted in ADX rats treated with antiphasic CORT. We also predicted that clock gene expression in the SCN would not be affected by CORT status due to the SCN's lack of GR expression.
Materials and Methods
Animals
For experiment 1 (N = 72) and experiment 2 (N = 80) male Sprague-Dawley rats (250–280 g; Harlan Laboratories) were housed 2 per cage (polycarbonate tubs, 47 × 23 × 20 cm) and given food (Teklad Rodent Diet 8640; Harlan Laboratories) and either tap water (sham rats) or 0.9% saline (ADX rats) ad libitum. All rats were maintained on a 12-hour light, 12-hour dark cycle and were given either a 3-day (experiment 1) or 2 week (experiment 2) acclimation period before surgery. The time of manipulations and measures for each rat is expressed as ZT, the time (hours) after light phase onset. Rats were housed in 4 separate sound and light attenuated rooms to ensure proper entrainment to the light-dark cycle. All experiments were conducted in accordance to the guidelines found within the Guide for the Care and Use of Laboratory Animals (DHHS publication number [NIH] 80-23, revised 2010, 8th edition) and were approved by the University of Colorado Institutional Animal Care and Use Committee.
ADX surgery
For experiment 1, rats received either ADX or sham-ADX surgery. Briefly, rats were anesthetized with halothane during surgery, bilateral incisions were made through the dorsal lateral skin and peritoneal wall near the kidneys, and adrenal glands of ADX rats were excised. Sham-ADX rats went through the same procedure but adrenal glands were left in place. For experiment 2, all rats received ADX surgery as described above.
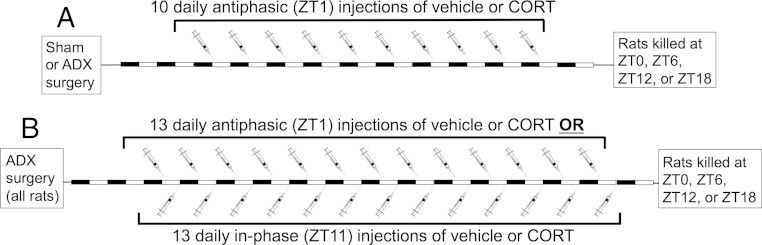
Experiment 1 procedure (Figure 1A)
Figure 1.
Experimental timelines. A, Experiment 1. Starting 2 days after ADX or sham surgery, rats were given 10 daily ip injections of either vehicle or CORT (2.5 mg/kg) at ZT1. On the 11th day, rats were killed at ZT0, ZT6, ZT12, or ZT18 (n = 6 per time of death for each CORT status condition). B, Experiment 2. Starting 2 days after ADX surgery, rats were given 13 daily ip injections of either vehicle or CORT (2.5 mg/kg) at either ZT1 (antiphasic) or ZT11 (in-phase). On the 14th day, rats were killed at ZT0, ZT6, ZT12, or ZT18 (n = 4 or n = 6 per time of death for each daily vehicle or CORT treatment time, respectively).
This experiment tested whether diurnal clock gene expression depends on the presence and/or time of day when daily CORT elevations occur by comparing clock gene expression of sham rats with ADX rats and ADX rats that received a daily pulse of antiphasic CORT. Starting 2 days after surgery, rats received an ip injection of either vehicle (60% sterile saline, 30% propylene glycol, 10% ethanol 1 mL/kg) or CORT (2.5 mg/kg; Steraloids) each day at ZT1 for 10 days. We have previously demonstrated that this CORT treatment produces peak plasma CORT levels (∼50 μg/100 mL) within 30 minutes after injection, and those levels return to preinjection levels within 2 hours (47). This manipulation produces a relatively short-lasting pulse of CORT levels within the moderate-to-high physiological range that is expected to occupy most GRs in the body (48). All sham rats received a vehicle injection (n = 24), and all ADX rats received either vehicle (n = 24) or CORT (n = 24). The day after 10 daily injections, groups of rats within each CORT status condition were decapitated at 4 time points throughout the day (ZT0, ZT6, ZT12, and ZT18; n = 6 for each ZT within a CORT status condition). For the time points ZT0 and ZT12, rats were decapitated approximately 15 minutes before the light transition in order to avoid any rapid effects the light transition may have on clock gene expression. Rats did not receive an injection on the day they were killed in order to avoid any acute effects of CORT on clock gene expression. Brains were extracted and flash-frozen in isopentane chilled to −30°C with dry-ice and then stored at −70°C. Trunk blood plasma was collected for CORT assay.
Experiment 2 procedure (Figure 1B)
The second experiment tested whether the altered diurnal clock gene expression in the PFC of ADX rats observed in experiment 1 (see Results) is normalized by daily in-phase (ZT11), but not antiphasic (ZT1) CORT injections. Starting 2 days after ADX surgery, rats received an ip injection of either vehicle or CORT (2.5 mg/kg) every day for 13 days at either ZT1 (antiphasic; n = 24 for CORT, n = 16 for vehicle) or ZT11 (in-phase; n = 24 for CORT, n = 16 for vehicle). The day after 13 daily injections, groups of rats within each CORT status condition were decapitated at 4 different time points through the day (ZT0, ZT6, ZT12, and ZT18; n = 4 or n = 6 for each ZT with daily vehicle or CORT treatment conditions, respectively). For the time points ZT0 and ZT12 rats were decapitated approximately 15 minutes before the light transition in order to avoid any rapid effects the light transition may have on clock gene expression. Rats did not receive an injection on the day they were killed in order to avoid any acute effects of CORT on clock gene expression. Brains were extracted and flash-frozen in isopentane chilled to −30°C with dry ice and then stored at −70°C.
CORT assay
Plasma CORT levels were measured using an enzyme-linked immunosorbant assay for CORT (Assay Design) in agreement with the manufacturer's protocol. Before use, plasma was diluted 1:50 in assay buffer and incubated at 65°C for 60 minutes in order to denature corticosteroid binding globulin. Intraassay coefficient of variation was 6.3%.
In situ hybridization and image analysis
Coronal brain slices (12 μm) were cut on a Leica Microsystems cryostat (model 1850) from the PFC (2.7 mm anterior to bregma) through the caudal extent of the SCN (1.3 mm posterior to bregma) (16), thaw mounted on Colorfrost Plus slides (VWR), and stored at −70°C.
In situ hybridization for Per1, Per2, and Bmal1 mRNA was performed as previously described (49). Briefly, tissue was hybridized with 35S-uridine triphosphate-labeled antisense riboprobes in a 50% formamide humidified atmosphere at 54°C for 16–18 hours. Slides were then treated with Ribonuclease A (Sigma) at 37°C for 1 hour, washed in decreasing concentrations of saline citrate solution (2×, 1×, 0.5×, and 0.1×), incubated in 0.1× saline citrate solution at 65°C for 1 hour, then dehydrated through a series of ethanol washes. Dried slides were then exposed to x-ray film for 2–4 weeks, after which films were digitized by use of Northern Light lightbox model B95 (Imaging Res, Inc), a Sony CCD video camera model XC-ST70 fitted with a Navitar 7000 zoom lens connected to a LG3–01 frame grabber (Scion Corp) inside a Dell Dimension 500, and captured with Scion Image Beta release 4.0.2. The cloned coding portion of each clock gene is as follows: Per1, nuclear transcript (nt) 974–1547, GenBank accession number NM_001034125; Per2, nt 2240–2869, GenBank accession number NM_031678; and Bmal1, nt 697–1278, GenBank accession number NM_024362. Densitometry measurements from left and right hemispheres on 4–6 sections per brain per region of interest (ROI) was performed by an individual blind to treatment condition. ROIs were positioned relative to the corpus callosum and longitudinal fissure for PFC subregions and relative to the optic chiasm for the SCN. ROIs were 12 × 12 pixels in size and were centered over the target brain region with reference to a rat brain atlas (50). Measurements were taken without differentiation between cortical layers, as gene expression was predominantly uniform throughout. Densitometry measurements (uncalibrated optical density) were taken using ImageJ software 1.46r (National Institutes of Health). Optical density values for a given ROI were averaged across each of the separate tissue sections/hemisphere measures for each brain to yield a single value for each brain.
Statistical analysis
Two-way ANOVA was used to examine overall main effects and interactions of time of death (ZT) and CORT status. Follow-up one-way ANOVA was used to test whether there was a significant time of death effect for clock gene expression within each CORT status group. Post hoc pair-wise comparisons used Fisher's least significant difference (FLSD). Statistical Package for the Social Sciences (SPSS v. 21.0, IBM) was used for ANOVA analysis; P < .05 was considered significant. There were some missing data due either to insufficient brain sections for a given ROI, or variable within brain hybridization signal, accounting for the variable degrees of freedom for the reported F tests. Data presented in figures are the mean ± SEM.
Results
Experiment 1. CORT status modulates Per1, Per2, and Bmal1 mRNA diurnal expression in the PFC but not in the SCN
Corticosterone
CORT levels in sham rats displayed a strong diurnal pattern of secretion (ZT effect: F3,23 = 12.0, P < .001) with peak circulation occurring around ZT12 (Figure 2). CORT levels were below assay detection limits for rats in both ADX and ADX+antiphasic CORT treatment groups. This is consistent with the very short half-life of CORT in the rat (≤15 min) (21, 51). Consequently, any effect that this daily antiphasic CORT treatment had on clock gene expression levels at the time of death was likely not due to an acute modulatory effect of concurrent GR activation by CORT.
Figure 2.
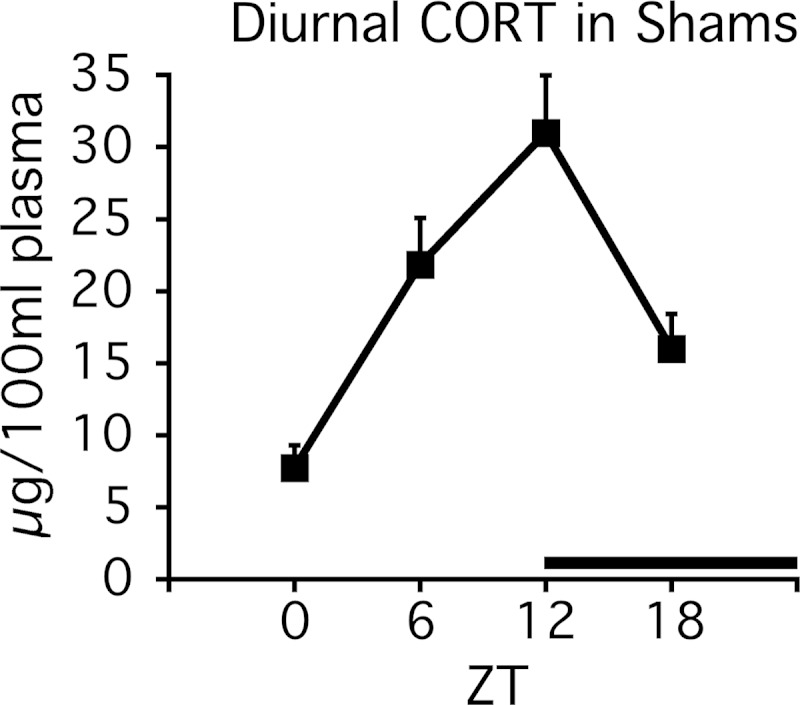
Experiment 1. Diurnal CORT profile in sham-ADX rats. There was a prominent diurnal CORT peak at ZT12 in trunk blood from sham-ADX rats (n = 6). Plasma CORT levels were undetectable in trunk blood of ADX and ADX+antiphasic CORT-treated rats. The black bar above the x-axis denotes dark phase.
Clock gene mRNA
There was a significant main effect of CORT status and a time of death by CORT status interaction for Per1 and Per2 mRNA expression in each of the PFC subregions examined (ACC, PL, IL, and VO) but not in the SCN (Table 1). Bmal1 mRNA levels appeared to also be affected by CORT status in the PFC, because its expression showed a significant effect of time of death only in sham rats (Figure 3 and Table 1). The specific effect of each CORT status condition on clock gene expression is outlined below.
Table 1.
Experiment 1
| Experiment 1. Rat Clock Gene Expression Two-Way ANOVA Results |
Peak ZT for CORT Status Groups With Significant Effect of Time of Death (P < .05, One-Way ANOVA) |
||||||
|---|---|---|---|---|---|---|---|
| Gene | ROI | Time of Death | CORT Status | Time of Death × CORT Status | Sham | ADX | Antiphasic CORT |
| Per1 | ACC | F3,45 = 13.6a | F2,45 = 3.3a | F6,45 = 3.8a | ZT18 | ZT18 | (P = .05) |
| PL | F3,41 = 14.2a | F2,41 = 17.1a | F6,41 = 5.8a | ZT18 | ZT12 | (P = .6) | |
| IL | F3,39 = 8.3a | F2,39 = 15.4a | F6,39 = 4.6a | ZT18 | ZT12 | (P = .6) | |
| VO | F3,44 = 17.6a | F2,44 = 6.6a | F6,44 = 4.6a | ZT18 | ZT12 | (P = .2) | |
| SCN | F3,50 = 44.6a | F2,50 = 0.67 | F6,50 = 0.43 | ZT6 | ZT6 | ZT6 | |
| Per2 | ACC | F3,55 = 47.8a | F2,55 = 40.7a | F6,55 = 80.1a | ZT12 | ZT18 | ZT0 |
| PL | F3,55 = 35.7a | F2,55 = 40.9a | F6,55 = 70.9a | ZT12 | ZT18 | ZT0 | |
| IL | F3,55 = 34.3a | F2,55 = 43.2a | F6,55 = 69.6a | ZT12 | ZT18 | ZT0 | |
| VO | F3,55 = 34.3a | F2,55 = 33.1a | F6,55 = 52.3a | ZT12 | ZT18 | (P = .06) | |
| SCN | F3,60 = 193.5a | F2,60 = 0.03 | F6,60 = 0.142 | ZT6 | ZT6 | ZT6 | |
| Bmal1 | ACC | F3,58 = 5.4a | F2,58 = 2.6 | F6,58 = 2.0 | ZT0 | (P = .3) | (P = .3) |
| PL | F3,53 = 1.2 | F2,53 = 1.9 | F6,53 = 1.2 | (P = .2) | (P = .2) | (P = .2) | |
| IL | F3,54 = 1.5 | F2,54 = 1.5 | F6,54 = 1.4 | (P = .3) | (P = .2) | (P = .4) | |
| VO | F3,58 = 9.0a | F2,58 = 0.88 | F6,58 = 1.8 | ZT0 | (P = .5) | (P = .2) | |
| SCN | F3,45 = 41.8a | F2,45 = 0.24 | F6,45 = 1.6 | ZT18 | ZT12 | ZT18 | |
Two- and one-way ANOVA results for clock gene expression in the PFC (ACC, PL, IL, and VO) and SCN. Factors were time of death (ZT0, ZT6, ZT12, or ZT18) and CORT status (sham, ADX, and ADX+antiphasic CORT).
P < .05.
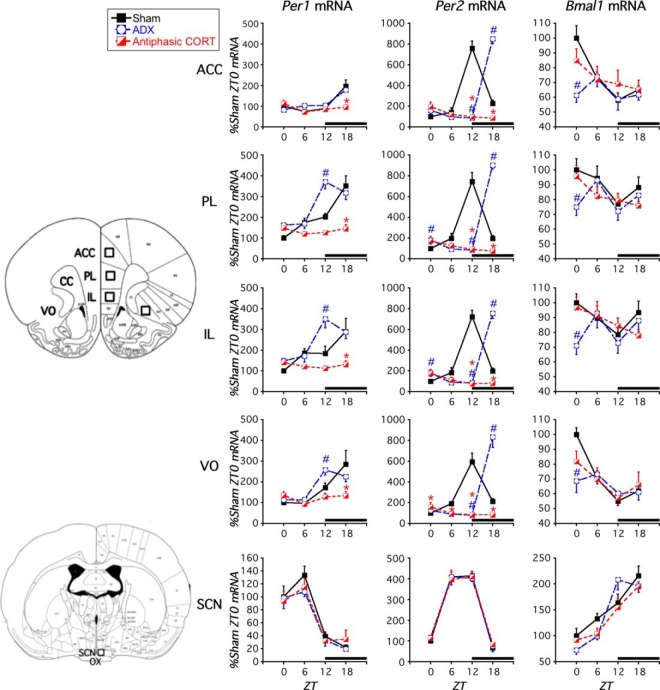
Figure 3.
Experiment 1. Effect of ADX and daily antiphasic CORT treatment on clock gene expression in PFC and SCN. In sham rats, Per1, Per2, and Bmal1 mRNA expression exhibited a diurnal pattern in the PFC and SCN, but the phase of peak expression differed between the PFC and SCN. ADX shifted the time of peak Per1 and Per2 mRNA expression and disrupted Bmal1 mRNA diurnal expression pattern in the PFC. Ten days of antiphasic CORT disrupted Per1, Per2, and Bmal1 mRNA diurnal expression. There was no effect of CORT status in the SCN. The black bar above the x-axis denotes dark phase. #, sham vs ADX difference at that ZT, P < .05; *, sham vs antiphasic CORT difference at that ZT P < .05 (FLSD, n = 4–6). Brain ROIs used for analyses are depicted on brain atlas images adapted from Paxinos and Watson (50).
Sham rats
After 10 daily vehicle injections at ZT1, Per1, Per2, and Bmal1 mRNA expression in adrenal-intact (sham) rats showed a diurnal pattern (ie, effect of time of death) in subregions of the PFC and the SCN as determined by one-way ANOVA (Figures 3 and 4; Table 1). Within both the SCN and some PFC subregions, positive (Bmal1) and negative (Per1 and Per2) regulatory clock genes had peak mRNA expression levels during opposite phases of the light-dark cycle (Table 1). Although the time of day for peak clock gene expression was similar across all PFC subregions, it varied considerably between the PFC and SCN, as previously reported (8, 10, 16, 22). Specifically, we found peak Per1 mRNA occurred at ZT18 in the PFC and ZT6 in the SCN, peak Per2 occurred around ZT12 in the PFC and ZT6 in the SCN, and peak Bmal1 mRNA occurred around ZT0 in the PFC and ZT18 in the SCN.
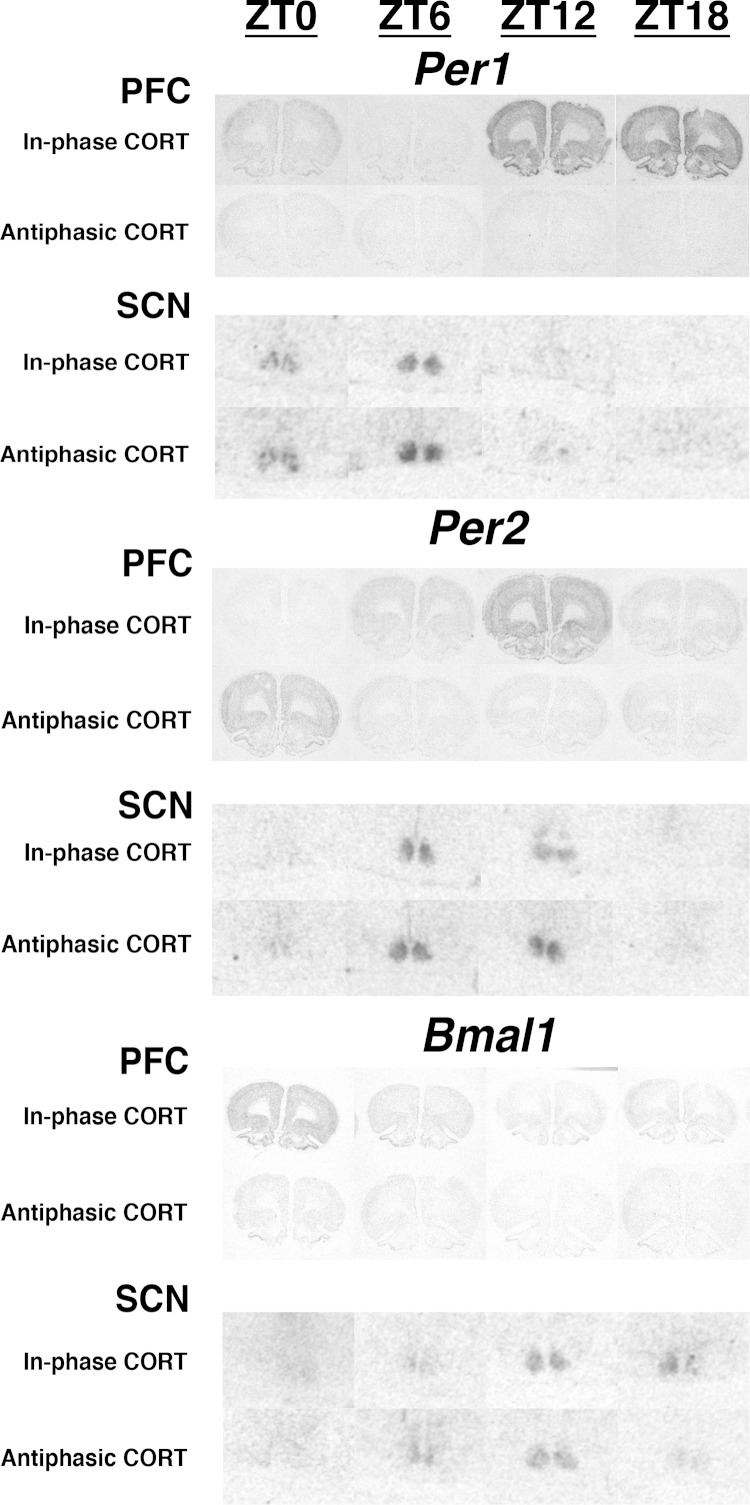
Figure 4.
Experiment 1. Representative autoradiogram images of Per1, Per2, and Bmal1 mRNA (in situ hybridization) in the PFC (coronal section) and SCN (ventral portion of coronal section) of sham and ADX rats across 4 times of day (ZT0, ZT6, ZT12, and ZT18). Note the different phase relationships of peak clock gene expression in the PFC of sham vs ADX rats but similar phase relationships of both treatment groups in the SCN.
ADX rats
After 10 daily vehicle injections at ZT1, Per1 and Per2 mRNA expression displayed a diurnal pattern in the PFC and the SCN of ADX rats as determined by one-way ANOVA (Figures 3 and 4; Table 1). However, in most PFC subregions Per1 peak expression was phase advanced (shift from ZT18 to ZT12 relative to sham rats), and across all PFC subregions Per2 peak expression was phase delayed (shift from ZT12 to ZT18) pointing towards a disruptive effect of ADX on normal PFC clock gene expression phase relationships. A diurnal pattern of Bmal1 mRNA expression was absent across each of the PFC subregions, further supporting disruption in clock gene expression. In contrast, there was no significant effect of ADX on the diurnal presence or phase of clock gene expression within the SCN.
ADX+antiphasic CORT rats
After treatment of ADX rats with 10 daily antiphasic CORT injections at ZT1, a diurnal pattern of Per1 and Bmal1 mRNA expression was absent across all PFC subregions but was maintained in the SCN as determined by one-way ANOVA (Figures 3 and 4; Table 1). In the PFC, Per2 mRNA expression displayed a significant effect of time of death supporting a diurnal pattern in its expression; however, its diurnal amplitude was substantially blunted, and the phase of its peak expression was inverted relative to sham rats (ZT0 as opposed to ZT12) (Figures 3 and 4; Table 1). In contrast, there was no significant effect of antiphasic CORT treatment in ADX rats on diurnal clock gene expression within the SCN.
Experiment 2. Diurnal pattern of Per1, Per2, and Bmal1 mRNA in the PFC of ADX rats is normalized by daily in-phase CORT treatment but not daily antiphasic CORT treatment; CORT status has no effect on clock gene mRNA in the SCN
There was no significant difference in clock gene expression in any brain area between ADX rats given a daily vehicle injection at ZT1 or ZT11 (two-way ANOVA, P > .05) (Supplemental Figure 1). Accordingly, data from vehicle-treated rats were pooled for subsequent statistical analyses and graphical presentation (ADX+vehicle group: total of 32 rats, with n = 8 for each ZT of death).
As in the first experiment, there was an overall significant main effect of CORT status for Per1 and Per2 mRNA expression in each of the PFC subregions examined (ACC, PL, IL, and VO), but not in the SCN (two-way ANOVA) (Table 2). There was also a significant time of death by CORT status interaction for each of the clock genes throughout the PFC, and there was a time of death by CORT status interaction for Per1 and Bmal1 mRNA expression in the SCN. However, post hoc tests and visual inspection of the graphs indicate that the interaction within the SCN, in contrast to the PFC, was due to small group differences at a single time point that did not reflect a CORT status dependent difference in clock gene expression pattern, amplitude, or phase relationship (Figures 5 and 6). The specific effect of each CORT status condition on clock gene expression is outlined below.
Table 2.
Experiment 2
| Experiment 2. Rat Clock Gene Expression Two-Way ANOVA Results |
Peak ZT for CORT Status Groups With Significant Effect of Time of Death (P < .05, One-Way ANOVA) |
||||||
|---|---|---|---|---|---|---|---|
| Gene | ROI | Time of Death | CORT Status | Time of Death × CORT Status | ADX + In-Phase CORT | ADX + Veh | ADX + Antiphasic CORT |
| Per1 | ACC | F3,52 = 5.8a | F2,52 = 3.6a | F6,52 = 4.7a | ZT12 | (P = .06) | (P =.5) |
| PL | F3,58 = 7.8a | F2,58 = 5.1a | F6,58 = 5.3a | ZT18 | (P = .05) | (P = .7) | |
| IL | F3,53 = 8.5a | F2,53 = 13.3a | F6,53 = 6.6a | ZT18 | ZT6/18 | (P = .4) | |
| VO | F3,58 = 7.9a | F2,58 = 3.3a | F6,58 = 5.5a | ZT12 | ZT18 | (P = .7) | |
| SCN | F3,67 = 165.2a | F2,67 = 2.0 | F6,67 = 2.9a | ZT6 | ZT6 | ZT6 | |
| Per2 | ACC | F3,53 = 6.1a | F2,53 = 7.2a | F6,53 = 6.5a | ZT12 | ZT18 | (P = .3) |
| PL | F3,55 = 1.5 | F2,55 = 4.5a | F6,55 = 2.8a | (P = .07) | ZT18 | (P = .3) | |
| IL | F3,56 = 1.4 | F2,56 = 4.5a | F6,56 = 5.3a | ZT12 | ZT18 | ZT0 | |
| VO | F3,54 = 9.9a | F2,54 = 3.5a | F6,54 = 6.3a | ZT12 | ZT18 | ZT0 | |
| SCN | F3.67 = 194.4a | F2,67 = 0.04 | F6,67 = 1.1 | ZT6 | ZT6 | ZT6 | |
| Bmal1 | ACC | F3,61 = 4.5a | F2,61 = 0.11 | F6,61 = 4.2a | ZT0 | ZT18 | (P = .4) |
| PL | F3,60 = 2.3a | F2,60 = 0.28 | F6,60 = 4.3a | ZT0 | ZT18 | (P = .5) | |
| IL | F3,62 = 2.4 | F2,62 = 0.29 | F6,62 = 5.5a | ZT0 | ZT18 | (P = .1) | |
| VO | F3,61 = 8.7a | F2,61 = 0.49 | F6,61 = 7.9a | ZT0 | ZT18 | (P = .4) | |
| SCN | F3,67 = 52.8a | F2,67 = 1.3 | F6,67 = 2.8a | ZT12 | ZT12 | ZT12 | |
Two-way ANOVA and one-way ANOVA results for clock gene expression in the PFC (ACC, PL, IL, and VO) and SCN. Factors were time of death (ZT0, ZT6, ZT12, or ZT18) and CORT status (ADX+vehicle, ADX+in-phase CORT, and ADX+antiphasic.
P < .05.
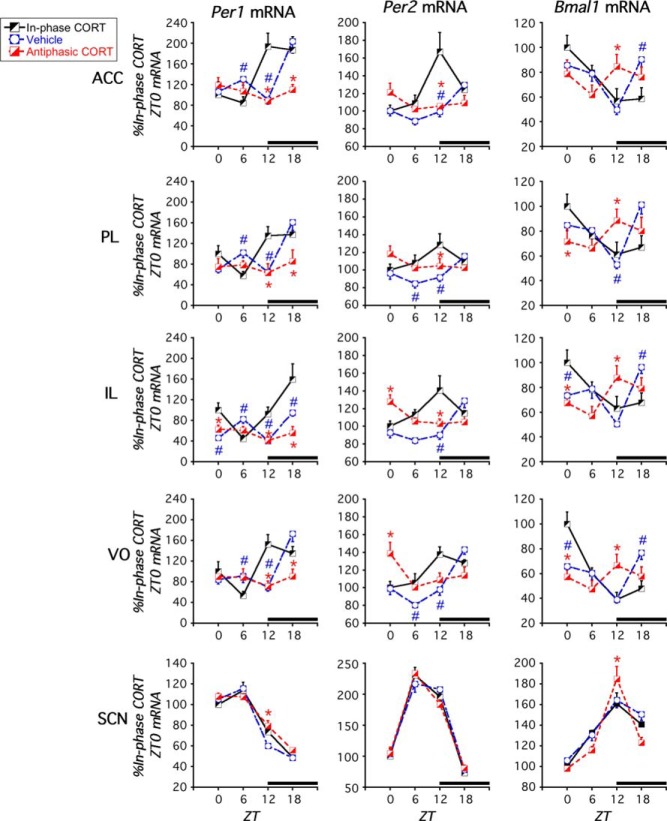
Figure 5.
Experiment 2. Effect of daily in-phase or antiphasic CORT treatment on clock gene expression in PFC and SCN of ADX rats. All rats were ADX and treated for 13 days with either vehicle or CORT at either ZT11 (in-phase CORT) or ZT1 (antiphasic CORT). In vehicle-treated rats (regardless of the time of daily injection) (Supplemental Figure 1) Per1, Per2, and Bmal1 mRNA in PFC subregions either lacked a significant diurnal expression pattern or the time of peak expression was shifted relative to sham rats in experiment 1, whereas 13 days of in-phase CORT treatment normalized PFC diurnal clock gene expression patterns compared with sham rats (Figure 3). Thirteen days of antiphasic CORT substantially disrupted Per1, Per2, and Bmal1 diurnal expression, and in the IL and VO, it inverted the diurnal expression profile of Per2. There were only minor effects of CORT status in the SCN. The black bar above the x-axis denotes dark phase. #, in-phase vs vehicle difference at that ZT, P < .05; *, in-phase vs antiphasic CORT difference at that ZT, P < .05 (FLSD, n = 4–8).
Figure 6.
Experiment 2. Representative autoradiogram images of Per1, Per2, and Bmal1 mRNA (in situ hybridization) in the PFC (coronal section) and SCN (ventral portion of coronal section) of in-phase CORT and antiphasic CORT-treated rats across 4 times of day (ZT0, ZT6, ZT12, and ZT18). Note the absent or phase shifted relationships of diurnal clock gene expression in the PFC of antiphasic CORT rats compared with in-phase CORT rats but similar phase relationships of both treatment groups in the SCN.
ADX+vehicle rats
The phase relationship for the peak of each clock gene mRNA in the SCN corresponded to that of all rats in experiment 1, with the exception that in this experiment we found Bmal1 mRNA peak expression at ZT12 instead of ZT18 (Figure 5 and Table 2). The positive (Bmal1) and negative (Per1 and Per2) arms of the molecular clock still displayed peak expression levels during opposite phases of the light-dark cycle. Similar to the first experiment, in the PFC of ADX rats Per2 mRNA expression was diurnal in nature but phase delayed relative to the sham rats in experiment 1 (Figure 5 and Table 2). In this second experiment, Per1 expression in the VO subregion of the PFC of ADX rats had a significant diurnal pattern with peak expression levels at ZT18 (Figure 5 and Table 2). There was also a significant time of death effect for Per1 mRNA levels in the IL, and a near significant trend in the ACC and PL; however, in each of those PFC subregions, there appeared to be 2 peaks of expression at ZT6 and ZT18 (Figure 5 and Table 2). For this experiment there was also a significant time of death effect for Bmal1 expression in all subregions of the PFC with a peak at ZT18 (Figures 3 and 5). Supportive of a disruptive nature of ADX on PFC clock gene expression, as in the first experiment, ADX+vehicle-treated rats lacked peak expression of the positive (Bmal1) and negative (Per1 and Per2) arms of the molecular clock during opposite phases of the light-dark cycle.
ADX+in-phase CORT rats
After 13 daily CORT (2.5 mg/kg) injections at ZT11, Per1, Per2, and Bmal1 mRNA expression displayed a diurnal pattern in the PFC and the SCN as determined by one-way ANOVA (Figures 5 and 6; Table 2). Within each subregion of the PFC, clock gene expression patterns were largely normalized in these ADX rats. That is, peak expression for the positive (Bmal1) and negative (Per1 and Per2) arms of the molecular clock occurred at opposite phases of the light-dark cycle with the same diurnal timing as observed in adrenal intact rats (Figure 3).
ADX+antiphasic CORT rats
After 13 daily CORT (2.5 mg/kg) injections at ZT1, Per1, Per2, and Bmal1 mRNA exhibited a diurnal expression pattern in the SCN but not in the PFC for Per1 and Bmal1 expression (Figures 5 and 6; Table 2). Consistent with the first experiment, Per2 mRNA expression in the PFC did exhibit a significant time of death effect, but it had a somewhat blunted diurnal peak amplitude and its peak expression was phase-inverted relative to Per2 mRNA expression in in-phase CORT-treated rats (ZT12) (Figure 5 and Table 2) or sham rats in experiment 1 (ZT12) (Figure 3 and Table 1). Consequently, as in the first experiment, antiphasic CORT treatment of ADX rats substantially disrupted the normal diurnal expression of both the positive and negative arms of PFC clock gene expression.
Discussion
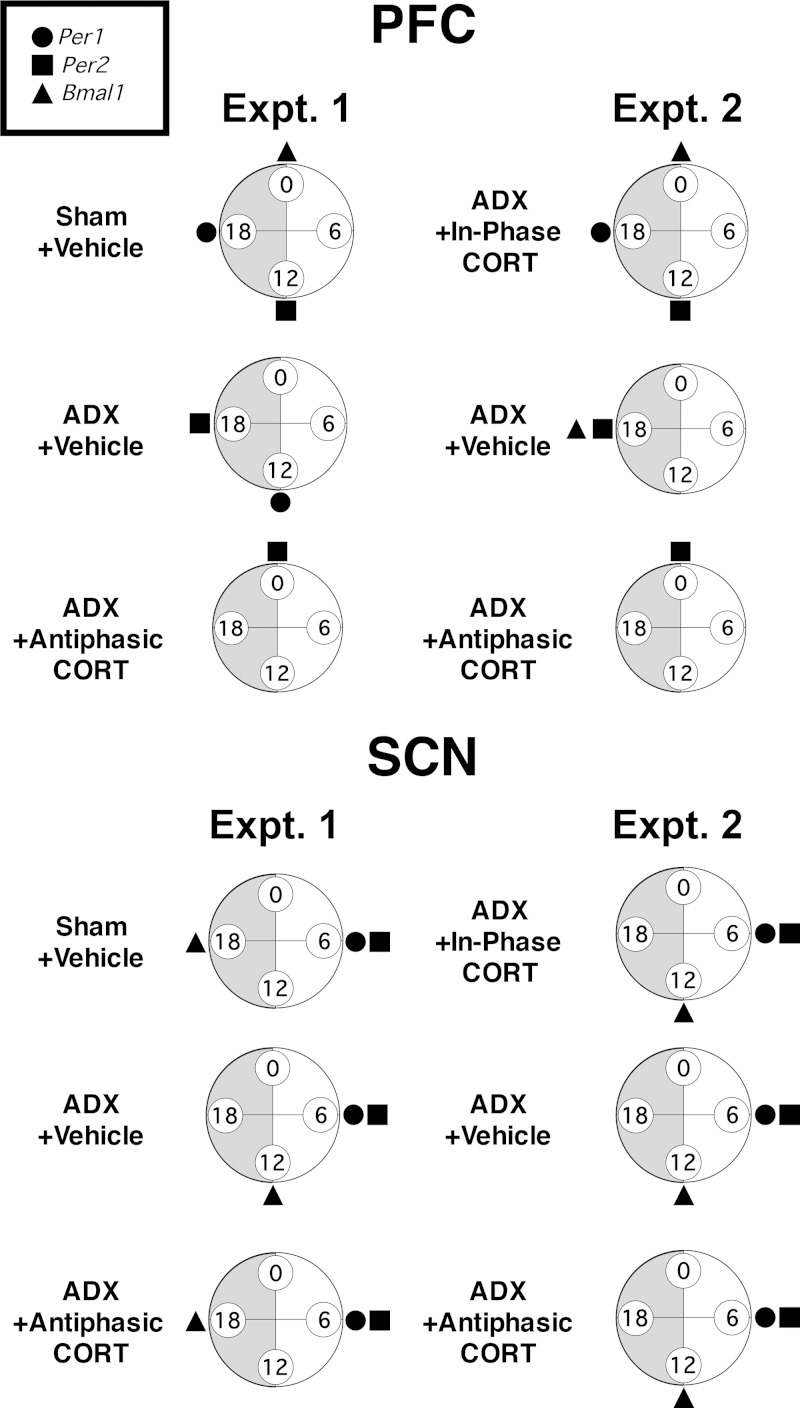
In these experiments, we demonstrated that diurnal clock gene expression in the PFC was strongly modulated by the presence and phase of daily fluctuations in circulating CORT. The diurnal variations in Per1, Per2, and Bmal1 mRNA expression that we observed in the SCN are consistent with the well-established rhythmic expression of these clock genes that constitutes a molecular clock (5, 6). The diurnal variation of clock gene expression in PFC subregions of adrenal-intact rats suggests that under normal conditions there may also be an operational molecular clock here as well. Interestingly, CORT manipulations substantially affected both the presence and timing of diurnal clock gene expression patterns in the PFC. In contrast, clock gene expression in the SCN was unaffected by CORT manipulations, likely due to the absence of GR expression in the SCN (16, 18, 19). To aid in the discussion of these data we have provided summary figures for each treatment group (Figure 7 and Supplemental Figure 2) that show whether there was a significant diurnal variation in clock gene expression and if so, the observed peak time of that expression. Comparison of the time of peak diurnal expression of these clock genes provides some indication of the phase relationships that would be expected in the case of sustained rhythmic expression.
Figure 7.
Phase comparisons of peak Per1, Per2, and Bmal1 mRNA diurnal expression within the PFC (most typical timing across all subregions is depicted) (Supplemental Figure 2) and SCN of each treatment group in experiments 1 and 2. Circles are oriented as clock faces with ZT labels, and shading of the dark phase. Time of peak expression is shown only where a significant effect of time of death was observed (Tables 1 and 2). Note in the SCN the lack of effect of CORT manipulations on clock gene expression phase relationships, but in the PFC the dramatic effect relative to sham rats of all manipulations except daily in-phase CORT treatment of ADX rats.
Adrenal-intact rats had diurnal expression patterns of Per1, Per2, and Bmal1 mRNA in both the PFC and SCN (Figure 7 and Supplemental Figure 2). The phase of peak clock gene mRNA that we observed in the SCN is similar to other reports of SCN clock gene expression in the rodent (8–10, 16, 24, 49). Sham rats displayed a different phase relationship for the expression of each clock gene within the PFC compared with the SCN, and this PFC-specific clock gene expression profile is in close agreement with our recent report of diurnal clock gene expression in the PFC of male and female rats (10). Importantly, there was an antiphasic relationship between the time of day for peak expression of a positive (Bmal1) and negative (Per1/Per2) arm of the molecular clock in both the SCN and PFC of sham rats, as would be expected for an operational intrinsic molecular clock (Figure 7).
ADX alters PFC diurnal clock gene expression
Although there was no significant difference in SCN clock gene expression between ADX and sham rats, PFC clock gene expression profiles were significantly altered by ADX. In the first experiment, Per1 and Per2 mRNA expression continued to exhibit a diurnal pattern in the PFC of ADX rats, but the apparent phase of that expression was altered with a delay of peak Per2 mRNA and an advance of peak Per1 mRNA relative to the expression patterns in sham rats. In the second experiment, ADX rats with vehicle treatment again displayed a phase delay in PFC Per2 mRNA expression relative to sham rats, and PFC Per1 mRNA expression lacked a reliable diurnal variation across the PFC subregions. ADX also affected PFC Bmal1 expression patterns in both experiments. The discrepancy between experiments in the specific effects of ADX on PFC clock gene expression patterns may be related to the longer duration of ADX in the second experiment (13 d) compared with the first experiment (10 d). CORT is known to stabilize circadian rhythms (8, 26, 52, 53), therefore the extended absence of endogenous CORT in experiment 2 may have been enough to further alter the clock gene expression patterns in the PFC. ADX has been found in other studies to disrupt diurnal clock gene expression in some but not all peripheral tissues (8, 20, 22) and to blunt diurnal PER2 immunoreactivity in subregions of the extended amygdala (20).
It should be noted that in the second experiment peak SCN Bmal1 mRNA occurred at ZT12 instead of ZT18 as seen in the first experiment. Although this may reflect an effect of ADX on molecular clock operation within the SCN, we believe that is not the case for the following reasons. First, we did not see this effect of ADX in the first experiment. Second, in contrast to clock gene expression in the PFC, neither in-phase nor antiphasic CORT treatment had an effect on SCN clock gene expression in ADX rats. Third, other studies report no effect of ADX on SCN clock gene expression (8, 20, 31, 54). Instead, we suspect that this difference in Bmal1 mRNA peak times reflects a cohort difference in timing that may appear more pronounced than it was due to the 6-hour interval between sampling times. Other studies with greater temporal resolution typically report a Bmal1 mRNA acrophase in the rat SCN around ZT16–ZT18 (9, 10, 49).
In-phase diurnal CORT normalizes PFC diurnal clock gene expression
Daily in-phase CORT pulses were sufficient to normalize the diurnal pattern in PFC clock gene expression of ADX rats (Figure 7 and Supplemental Figure 2). Similar in-phase glucocorticoid treatment (glucocorticoid in drinking water) of ADX rats has previously been shown to normalize ex vivo Per1 gene promoter rhythmic activity in some peripheral tissues (8) and normalize diurnal PER2 expression in select extended amygdala subregions of the brain (54). In contrast, treatment of ADX rats with constant glucocorticoid levels was not sufficient to restore normal brain PER2 expression patterns (54). Our study is the first to demonstrate a regulatory influence of CORT treatment on clock gene expression in the PFC. Importantly, our study also demonstrated that this influence was evident for both positive (Bmal1) and negative (Per1 and Per2) transcriptional regulatory components of the core molecular clock. We are the first to systematically test and report the extent of in-phase CORT normalization of negative and positive clock gene expression in extra-SCN tissue and to directly compare this with antiphasic CORT treatment of ADX rats. This result, combined with the general antiphasic relationship between Bmal1 mRNA (peak at onset of the light phase) and Per1/Per2 mRNA (peak during the early to midportion of the dark phase) in subregions of the PFC suggests that there is not only 24-hour rhythmic clock gene expression present within the PFC, but also intrinsic oscillatory control of that expression. Support for rhythmic and generally oscillatory clock gene expression in the PFC has also been observed in humans (41, 55). However, the clock gene expression phase relationship profile in the human PFC was opposite to what we have found in the rat, ie, the negative components of the clock tended to reach peak expression during the light phase and the positive components during the dark phase. This species difference may be related to the different awake/sleep activity phase profile of diurnal humans compared with nocturnal rats. If such is the case, then clock gene expression in the PFC of both species may depend more on entraining influences that align with activity patterns which are known to influence endogenous CORT circulation rather than light-dark cycle phase (56).
Antiphasic diurnal CORT substantially disrupts PFC diurnal clock gene expression
In contrast to the normalizing influence of daily in-phase CORT, antiphasic CORT, matched in dose and delivery procedure, produced a dramatic disruption of PFC clock gene expression in ADX rats (Figure 7 and Supplemental Figure 2). In both of our experiments, antiphasic CORT-treated rats lacked a significant diurnal variation in PFC Per1 and Bmal1 mRNA. This effect was especially pronounced for Per1 mRNA. Diurnal expression of Per2 mRNA was substantially blunted in most PFC subregions, at least for the specific sample times used in the study. These results are similar to what was observed in the liver of adrenal-intact mice after daily antiphasic, but not in-phase Prednisolone treatment (15). Interestingly, despite the blunted diurnal amplitude of PFC Per2 mRNA expression, we saw a distinct 12-hour phase shift of Per2 mRNA peak expression relative to its profile in sham rats or ADX rats with in-phase CORT treatment. A similar phase inversion of PER2 diurnal immunoreactivity in the extended amygdala was observed after daily antiphasic CORT injections of ADX rats (20). Despite the dramatic effect of antiphasic CORT treatment on clock gene expression in the PFC, it had no significant effect on clock gene expression in the SCN.
Possible mechanisms of glucocorticoid modulation of the PFC molecular clock
In our study, CORT was a strong modulatory factor for PFC diurnal Per1 mRNA levels, a clock gene with a well-characterized functional GRE (17–19). Glucocorticoids rapidly induce Per1 expression in a variety of cell lines and tissues (16, 17, 21). CORT manipulations in our study also effectively modulated PFC expression patterns of Per2 and Bmal1 mRNA. These clock genes are also associated with candidate GREs; however, there is much less evidence that these GREs are functional across a range of tissues and conditions (18, 19, 57). Thus, the entraining influence of CORT on PFC rhythmic clock gene expression may be due to the activation of GR within the PFC (which expresses a high level of GR) (58) by daily CORT surges/pulses, and subsequent induction of Per1 gene expression. In hepatocytes, the expression of GR is necessary for glucocorticoid modulation of the phase of liver rhythmic clock gene expression (16). Recent studies show direct protein-protein interactions between GR and several clock gene proteins, including CLOCK, BMAL1, and CRYPTOCHROME1, each of which modulates GR-dependent transcriptional activity (19, 59, 60). Glucocorticoids have also been shown to down-regulate Rev-erbα expression in both rat and human liver tissue (8, 15, 24). REV-ERBα is a negative controller of Bmal1 transcription, and the expression of Rev-erbα is suppressed by PERIOD proteins (7).
We also cannot rule out the possibility that CORT indirectly affects PFC clock gene expression by acting at other brain regions. For example, normal CORT secretion is necessary for diurnal fluctuations in tryptophan hydroxylase 2 expression in the dorsal raphe nuclei (61). Tryptophan hydroxylase 2 is the rate-limiting enzyme for serotonin synthesis, and the dorsal raphe nucleus provides a strong serotonergic input to the PFC (62).
Although our results demonstrate that diurnal fluctuations in circulating CORT had a strong influence on PFC diurnal clock gene expression, we do not believe that the results point to CORT as the sole entrainment factor of PFC clock gene expression. In ADX+vehicle rats we still observed a diurnal pattern of PFC clock gene mRNA levels, albeit a pattern that was altered relative to those observed in adrenal-intact rats. In addition, although treatment of ADX rats with in-phase CORT pulses normalized PFC clock gene expression, treatment with antiphasic CORT pulses inverted the phase only of PFC Per2 expression, and that diurnal expression amplitude was substantially blunted. If CORT is the sole entrainer for PFC clock gene expression, then one would expect that Per1 and Bmal1 expression would have been inverted as well. It is therefore likely that the SCN has some influence on PFC clock gene expression that is independent of circadian CORT rhythms. The SCN does not directly innervate the PFC, but it provides strong indirect input to the PFC IL via a relay through the paraventricular nucleus of the thalamus (14, 63). SCN-dependent input could then be exchanged throughout the subregions of the PFC which communicate extensively with each other (64). Consequently, the PFC may receive circadian entrainment input from multiple sources including the master clock in the SCN and diurnal CORT circulation. In instances when these inputs are not tightly coupled to each other, a “competition” for entrainment may occur and lead to a complete loss of normal circadian rhythmicity in the PFC, as appears to be the case with antiphasic CORT treatment in our study. On the other hand, the presence of daily in-phase CORT pulses combined with an indirect SCN influence may lead to robust rhythmic and oscillatory expression of Per1, Per2, and Bmal1 mRNA in the PFC.
Regardless of the process by which CORT modulates clock gene expression in the PFC, that process appears to be similar across PFC subregions. We observed very similar CORT treatment effects on the extent of diurnal clock gene expression patterns and peak expression timing in each of the 4 subregions that we examined. Although the functionality and connectivity of these various subregions of the PFC are similar in some regards, they also exhibit distinct differences (64–66). Perhaps CORT provides a similar modulatory influence in clock gene expression across PFC subregions by regulation of the indirect SCN input to the PFC. Alternatively, CORT may directly affect clock gene expression in each PFC subregion via the abundant presence of GR found throughout the PFC (58). The similarity across PFC subregions in diurnal clock gene expression profiles and their sensitivity to CORT suggest that this CORT modulatory influence is important for a shared and coordinated aspect of PFC subregion function.
Glucocorticoid circadian physiology and implications for PFC-dependent function
The PFC modulates many cognitive (29, 67) and physiological functions (68), and its operation and dendritic architecture are strongly affected by time of day and stress (36). It is noteworthy that many PFC-dependent behaviors show circadian variation (31–33). Mood and anxiety disorders are characterized by both abnormal PFC functioning (37, 38) and abnormal diurnal CORT circulation (4, 69). Intriguingly, Li et al (41) found reduced diurnal patterns of clock gene mRNA in the postmortem PFC of major depressive disorder (MDD) patients. Successful treatment of MDD is associated with normalization of both diurnal CORT circulation (70) and peripheral blood cell clock gene expression (69). Chen et al (55) have similarly found altered diurnal clock gene expression in the postmortem PFC of older humans compared with younger humans, potentially related to age-associated alterations in cognition and mood. Karatsoreos et al (71) have shown that a rodent model of circadian disruption produces sequelae of behaviors resembling those that occur after PFC lesion (decreased cognitive flexibility and altered emotionality). In addition, McClung and coworkers have shown that both mutation and knock down of Clock (a second component of the positive arm) lead to disruption in mood related behavior in mice (72, 73). That research group has also found that chronic stress leads to similar behavioral disruption as well as altered clock gene expression in several relevant brain areas (42).
Our results have important implications for some of the consequences of situations in which normal diurnal CORT circulation is no longer present, as can occur with hypothalamic adrenal pituitary axis dysregulation or pharmacological treatment with glucocorticoids. Disrupted clock gene oscillatory expression due to abnormal CORT circulation may be a mechanism that causes or exacerbates MDD, PTSD, and other PFC-related psychiatric disorders. This will be an important consideration in the future when optimizing treatment strategies for these disorders. It was because of these clinically relevant considerations that the primary aim of this study was to examine the effect that varying chronic patterns of diurnal CORT circulation have on clock gene expression in the PFC in the presence of a fully functioning master clock and a normal environmental light-dark cycle. It would be interesting, however, to examine the effects of various patterns of CORT circulation on PFC clock gene expression in the absence of the master clock (eg, SCN lesion) or under free running SCN conditions (eg, under constant darkness). Those studies will be useful in determining the mechanistic relationship between the SCN and CORT in control of PFC clock gene expression and circadian function.
Acknowledgments
We thank Sara Fardi and Nicolas Varra for technical assistance.
This work was supported the National Institutes of Health Grant MH075968 and the National Science Foundation Grant IOS-1456706.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACC
- anterior cingulate cortex
- ADX
- adrenalectomized
- BMAL
- brain and muscle ARNT-like protein
- CLOCK
- circadian locomotor output cycles kaput
- CORT
- corticosterone
- FLSD
- Fisher's least significant difference
- GR
- glucocorticoid receptor
- GRE
- glucocorticoid-response element
- IL
- infralimbic cortex
- MDD
- major depressive disorder
- nt
- nuclear transcript
- Per1
- Period1
- PFC
- prefrontal cortex
- PL
- prelimbic cortex
- PTSD
- posttraumatic stress disorder
- ROI
- region of interest
- Rev-erb
- NR1D1
- SCN
- suprachiasmatic nucleus
- VO
- ventral orbital cortex
- ZT
- zeitgeber time.
References
- 1. Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4(8):649–661. [DOI] [PubMed] [Google Scholar]
- 2. Barclay JL, Husse J, Bode B, et al. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. Mistlberger RE, ed. PLoS One. 2012;7(5):e37150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bunney JN, Potkin SG. Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br Med Bull. 2008;86(1):23–32. [DOI] [PubMed] [Google Scholar]
- 4. Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(suppl 17):41–46. [PubMed] [Google Scholar]
- 5. Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72(1):551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. [DOI] [PubMed] [Google Scholar]
- 7. Preitner N, Damiola F, Lopez-Molina L, et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110(2):251–260. [DOI] [PubMed] [Google Scholar]
- 8. Pezük P, Mohawk JA, Wang LA, Menaker M. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology. 2012;153(10):4775–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harbour VL, Weigl Y, Robinson B, Amir S. Phase differences in expression of circadian clock genes in the central nucleus of the amygdala, dentate gyrus, and suprachiasmatic nucleus in the rat. PLoS One. 2014;9(7):e103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chun LE, Woodruff ER, Morton S, Hinds LR, Spencer RL. Variations in phase and amplitude of rhythmic clock gene expression across prefrontal cortex, hippocampus, amygdala, and hypothalamic paraventricular and suprachiasmatic nuclei of male and female rats. J Biol Rhythms. 2015;30(5):417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101(15):5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amir S, Lamont EW, Robinson B, Stewart J. A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. J Neurosci. 2004;24(4):781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. [DOI] [PubMed] [Google Scholar]
- 14. Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258(2):204–229. [DOI] [PubMed] [Google Scholar]
- 15. Koyanagi S, Okazawa S, Kuramoto Y, et al. Chronic treatment with prednisolone represses the circadian oscillation of clock gene expression in mouse peripheral tissues. Mol Endocrinol. 2006;20(3):573–583. [DOI] [PubMed] [Google Scholar]
- 16. Balsalobre A. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. [DOI] [PubMed] [Google Scholar]
- 17. Reddy TE, Gertz J, Crawford GE, Garabedian MJ, Myers RM. The hypersensitive glucocorticoid response specifically regulates period 1 and expression of circadian genes. Mol Cell Biol. 2012;32(18):3756–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci USA. 2009;106(41):17582–17587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheon S, Park N, Cho S, Kim K. Glucocorticoid-mediated Period2 induction delays the phase of circadian rhythm. Nucleic Acids Res. 2013;41(12):6161–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Segall LA, Amir S. Glucocorticoid regulation of clock gene expression in the mammalian limbic forebrain. J Mol Neurosci. 2010;42(2):168–175. [DOI] [PubMed] [Google Scholar]
- 21. Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, et al. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol. 2010;22(10):1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sujino M, Furukawa K, Koinuma S, et al. Differential entrainment of peripheral clocks in the rat by glucocorticoid and feeding. Endocrinology. 2012;153(5):2277–2286. [DOI] [PubMed] [Google Scholar]
- 23. Leliavski A, Dumbell R, Ott V, Oster H. Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology. J Biol Rhythms. 2015;30(1):20–34. [DOI] [PubMed] [Google Scholar]
- 24. Torra IP, Tsibulsky V, Delaunay F, et al. Circadian and glucocorticoid regulation of Rev-erbα expression in liver. Endocrinology. 2000;141(10):3799–3806. [DOI] [PubMed] [Google Scholar]
- 25. Buijs RM, Kalsbeek A, van der Woude TP, van Heerikhuize JJ, Shinn S. Suprachiasmatic nucleus lesion increases corticosterone secretion. Am J Physiol. 1993;264(6 pt 2):R1186–R1192. [DOI] [PubMed] [Google Scholar]
- 26. Krieger DT, Allen W, Rizzo F, Krieger HP. Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab. 1971;32(2):266–284. [DOI] [PubMed] [Google Scholar]
- 27. Leliavski A, Shostak A, Husse J, Oster H. Impaired glucocorticoid production and response to stress in Arntl-deficient male mice. Endocrinology. 2014;155(1):133–142. [DOI] [PubMed] [Google Scholar]
- 28. Yoder JM, Brandeland M, Engeland WC. Phase-dependent resetting of the adrenal clock by ACTH in vitro. Am J Physiol Regul Integr Comp Physiol. 2014;306(6):R387–R393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herman JP, Figueiredo H, Mueller NK, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. [DOI] [PubMed] [Google Scholar]
- 30. Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18(8):2357–2364. [DOI] [PubMed] [Google Scholar]
- 31. Matchock RL, Mordkoff JT. Chronotype and time-of-day influences on the alerting, orienting, and executive components of attention. Exp Brain Res. 2009;192(2):189–198. [DOI] [PubMed] [Google Scholar]
- 32. Owens DS, Macdonald I, Tucker P, et al. Diurnal variations in the mood and performance of highly practised young women living under strictly controlled conditions. Br J Psychol. 2000;91(pt 1):41–60. [DOI] [PubMed] [Google Scholar]
- 33. Woodruff ER, Greenwood BN, Chun LE, Fardi S, Hinds LR, Spencer RL. Adrenal-dependent diurnal modulation of conditioned fear extinction learning. Behav Brain Res. 2015;286:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pace-Schott EF, Spencer RM, Vijayakumar S, et al. Extinction of conditioned fear is better learned and recalled in the morning than in the evening. J Psychiatr Res. 2013;47(11):1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci USA. 2011;108(38):16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perez-Cruz C, Simon M, Flügge G, Fuchs E, Czéh B. Diurnal rhythm and stress regulate dendritic architecture and spine density of pyramidal neurons in the rat infralimbic cortex. Behav Brain Res. 2009;205(2):406–413. [DOI] [PubMed] [Google Scholar]
- 37. VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garfinkel SN, Abelson JL, King AP, et al. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34(40):13435–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pizzagalli D, Pascual-Marqui RD, Nitschke JB, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158(3):405–415. [DOI] [PubMed] [Google Scholar]
- 40. Bunney WE, Bunney BG. Molecular clock genes in man and lower animals: possible implications for circadian abnormalities in depression. Neuropsychopharmacology. 2000;22(4):335–345. [DOI] [PubMed] [Google Scholar]
- 41. Li JZ, Bunney BG, Meng F, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci USA. 2013;110(24):9950–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu X, McClung CA. Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biol Psychiatry. 2015;78(4):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Manev H, Uz T. Clock genes: influencing and being influenced by psychoactive drugs. Trends Pharmacol. Sci. 2006;27(4):186–189. [DOI] [PubMed] [Google Scholar]
- 44. Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry. 2013;73(12):1164–1171. [DOI] [PubMed] [Google Scholar]
- 45. Szuba MP, Guze BH, Baxter LR. Electroconvulsive therapy increases circadian amplitude and lowers core body temperature in depressed subjects. Biol Psychiatry. 1997;42(12):1130–1137. [DOI] [PubMed] [Google Scholar]
- 46. Ikeda Y, Kumagai H, Skach A, Sato M, Yanagisawa M. Modulation of circadian glucocorticoid oscillation via adrenal opioid-CXCR7 signaling alters emotional behavior. Cell. 2013;155(6):1323–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pace TW, Cole MA, Ward G, Kalman BA, Spencer RL. Acute exposure to a novel stressor further reduces the habituated corticosterone response to restraint in rats. Stress. 2001;4(4):319–331. [DOI] [PubMed] [Google Scholar]
- 48. Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. [DOI] [PubMed] [Google Scholar]
- 49. Girotti M, Weinberg MS, Spencer RL. Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule. Am J Physiol Endocrinol Metab. 2009;296(4):E888–E897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd ed San Diego, CA: Academic Press; 1986. [Google Scholar]
- 51. Sainio EL, Lehtola T, Roininen P. Radioimmunoassay of total and free corticosterone in rat plasma: measurement of the effect of different doses of corticosterone. Steroids. 1988;51(5–6):609–622. [DOI] [PubMed] [Google Scholar]
- 52. Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest. 2010;120(7):2600–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sage D, Ganem J, Guillaumond F, et al. Influence of the corticosterone rhythm on photic entrainment of locomotor activity in rats. J Biol Rhythms. 2004;19(2):144–156. [DOI] [PubMed] [Google Scholar]
- 54. Segall LA, Perrin JS, Walker CD, Stewart J, Amir S. Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience. 2006;140(3):753–757. [DOI] [PubMed] [Google Scholar]
- 55. Chen CY, Logan RW, Ma T, et al. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci USA. 2016;113(1):206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200(1):3–22. [DOI] [PubMed] [Google Scholar]
- 57. Reddy AB, Maywood ES, Karp NA, et al. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology. 2007;45(6):1478–1488. [DOI] [PubMed] [Google Scholar]
- 58. McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66(4):1121–1188. [DOI] [PubMed] [Google Scholar]
- 59. Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 2009;23(5):1572–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lamia KA, Papp SJ, Yu RT, et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480(7378):552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Donner NC, Montoya CD, Lukkes JL, Lowry CA. Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology. 2012;37(5):645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lowry CA, Hale MW, Evans AK, et al. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann NY Acad Sci. 2008;1148(1):86–94. [DOI] [PubMed] [Google Scholar]
- 63. Sylvester CM, Krout KE, Loewy AD. Suprachiasmatic nucleus projection to the medial prefrontal cortex: a viral transneuronal tracing study. Neuroscience. 2002;114(4):1071–1080. [DOI] [PubMed] [Google Scholar]
- 64. Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212(2):149–179. [DOI] [PubMed] [Google Scholar]
- 65. Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28(7):771–784. [DOI] [PubMed] [Google Scholar]
- 66. Schoenbaum G, Setlow B, Ramus SJ. A systems approach to orbitofrontal cortex function: recordings in rat orbitofrontal cortex reveal interactions with different learning systems. Behav Brain Res. 2003;146(1–2):19–29. [DOI] [PubMed] [Google Scholar]
- 67. Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. [DOI] [PubMed] [Google Scholar]
- 68. Recabarren MP, Valdés JL, Farías P, Serón-Ferré M, Torrealba F. Differential effects of infralimbic cortical lesions on temperature and locomotor activity responses to feeding in rats. Neuroscience. 2005;134(4):1413–1422. [DOI] [PubMed] [Google Scholar]
- 69. Li SX, Liu LJ, Xu LZ, et al. Diurnal alterations in circadian genes and peptides in major depressive disorder before and after escitalopram treatment. Psychoneuroendocrinology. 2013;38(11):2789–2799. [DOI] [PubMed] [Google Scholar]
- 70. Gibbons JL. Cortisol secretion rate in depressive illness. Arch Gen Psychiatry. 1964;10:572–575. [DOI] [PubMed] [Google Scholar]
- 71. Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA. 2011;108(4):1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Roybal K, Theobold D, Graham A, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA. 2007;104(15):6406–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mukherjee S, Coque L, Cao JL, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68(6):503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]